Abstract
Laboratory model systems and mathematical models have shed considerable light on the fundamental properties and processes of evolutionary rescue. But it remains to determine the extent to which these model-based findings can help biologists predict when evolution will fail or succeed in rescuing natural populations that are facing novel conditions that threaten their persistence. In this article, we present a prospectus for transferring our basic understanding of evolutionary rescue to wild and other non-laboratory populations. Current experimental and theoretical results emphasize how the interplay between inheritance processes and absolute fitness in changed environments drive population dynamics and determine prospects of extinction. We discuss the challenge of inferring these elements of the evolutionary rescue process in field and natural settings. Addressing this challenge will contribute to a more comprehensive understanding of population persistence that combines processes of evolutionary rescue with developmental and ecological mechanisms.
Keywords: eco-evolutionary models, environmental change, absolute fitness, population growth, genetic variation, aster models
1. Introduction
Populations that face harsh environments tend to be severely short-lived. Indeed, without evolution, a declining population of 10x individuals, isolated from immigration, is expected to persist on the order of only x generations before extinction [1,2]. However, if, in a shrinking population, heritable variation in fitness is present or arises quickly, then adaptive evolution may extend the population's existence over the immediate term, and perhaps indefinitely. Improvement in the expected growth rate via adaptive evolution of a population in deterministic decline to extinction defines the phenomenon of ‘evolutionary rescue’. Studies of evolutionary rescue seek to understand the role of adaptive evolution in prolonging the persistence of an isolated, declining population.
The intellectual roots of evolutionary rescue trace to Bradshaw [3], who in his 1991 Croonian lecture noted that, of many plant species presumably exposed to equivalent selective challenges (herbicides or heavy metal contamination), very few surmount them and persist in the affected habitats. His concept of ‘genostasis’ posited that adaptive evolution often fails, resulting in extinction, and he hypothesized that most failures were due to a lack of sufficient genetic variability (see [4] for a more recent treatment of this idea). During the same year, Macnair [5] emphasized that species evolving in the presence of anthropogenic toxins avoid extinction only when they adapt rapidly. He hypothesized that sufficiently fast evolution could only be achieved via variation of genes with major effects on fitness.
These early treatments attempted to comprehend evolutionary rescue versus failure largely in terms of heuristic arguments inspired by empirical observations of natural populations exposed to unnatural stressors. Formal mathematical models of evolutionary rescue that join population genetics with demography appeared soon after and have since provided a rigorous foundation for evolutionary rescue [6–21]. These models consider both gradual [6] and abrupt [7,8] modes of environmental change, various sources of environmental, genetic and demographic stochasticity [9–16] and intra- and interspecific interactions [18,19]. The relative influences of the genetic details underlying fitness variation (number of loci, gene effect sizes, initial allele frequencies) on evolutionary rescue have also been analysed [20]. These models were formulated without reference to any specific organism, but a recent study [21] used data from laboratory strains of Drosophila birchii to parametrize computer simulations of evolution and extinction in a hypothetical changing environment.
The biological plausibility of this theory has been supported by empirical studies of evolutionary rescue in model organisms [22–24]. Using an automated liquid handling system, Bell & Gonzalez [22] abruptly exposed replicate populations of Saccharomyces cerevisiae to normally lethal concentrations of salt in the first empirical test of evolutionary rescue theory. They confirmed its general predictions, including the U-shaped trajectories of rescued populations, and the importance of population size at the time of environmental change for the probability of rescue. These experiments were extended to examine evolutionary rescue in meta-populations of yeast distributed over a spatial gradient of salinity and variable rates of environmental change [24]. Their results showed that evolutionary rescue depends on both the speed of environmental change and the degree to which populations are connected by migration.
Predicted features of evolutionary rescue have also been observed in a model higher eukaryote. In a greenhouse experiment, Bodbyl Roels & Kelly [23] removed bee pollinators from Mimulus gutatus, which is partially self-fertile. Although the manipulated populations did not decline in size, they did show precipitous reductions in population growth rates immediately after bee removal followed by recovery of mean fitness, a pattern that is consistent with expectations of evolutionary rescue theory. The authors also showed that recovery of these Mimulus populations was achieved by rapid evolution of self-fertilization rates, which they believe caused sizable allele frequency changes observed at two major polymorphisms.
These investigations of mathematical models and model organisms provide the basis for a robust understanding of evolutionary rescue. While important, they leave a far more crucial question unresolved: how important is evolutionary rescue for natural populations, like those that inspired Bradshaw and Macnair? Addressing this question will be important for interpreting the past, understanding the present and predicting the future fates of populations subject to harsh environmental conditions. In this essay, we attempt to map out some of the main scientific challenges that will need to be met to extend our current model-based knowledge of evolutionary rescue to natural populations, past, present and future. The demand for progress on this front is especially acute, given growing concerns about how changing climates will affect the fate of our planet's biodiversity [25].
2. Detecting and predicting evolutionary rescue
Conceptually, evolutionary rescue of a population is characterized by a deterministic decline in abundance followed by recovery via adaptive evolution of its capacity to grow. On an empirical level, extinction is a sure sign of failed evolutionary rescue, but not necessarily of a deterministic decline because small populations with positive intrinsic growth rates can become extinct by random variation in vital rates. A major advantage of empirical model studies is the ability to account for this ‘demographic stochasticity’ through use of replicate populations.
Empirical verification of successful evolutionary rescue is perhaps even less clear-cut than establishing its failure since a population in evident decline can regain the capacity to persist in a number of ways that need not involve adaptive evolution. These include any return of the extrinsic abiotic or biotic environment to favourable conditions, environment-induced phenotypic or behavioural responses that enhance individual fitness, attenuated intraspecific competition and the addition of new immigrants. Many of these processes can be easily eliminated, controlled or measured in laboratory and greenhouse experiments but not for most studies of populations in the wild. Investigations of evolutionary rescue in natural populations must always be mindful of such complications when interpreting results. Indeed, the search for evidence of evolutionary rescue in the field is challenging compared with the laboratory or greenhouse even when non-evolutionary sources of persistence are absent or known. Below, we detail those specific challenges. We emphasize that our focus is on evolution over the immediate term, within a dozen or so generations, that stabilizes the abundance of a declining population.
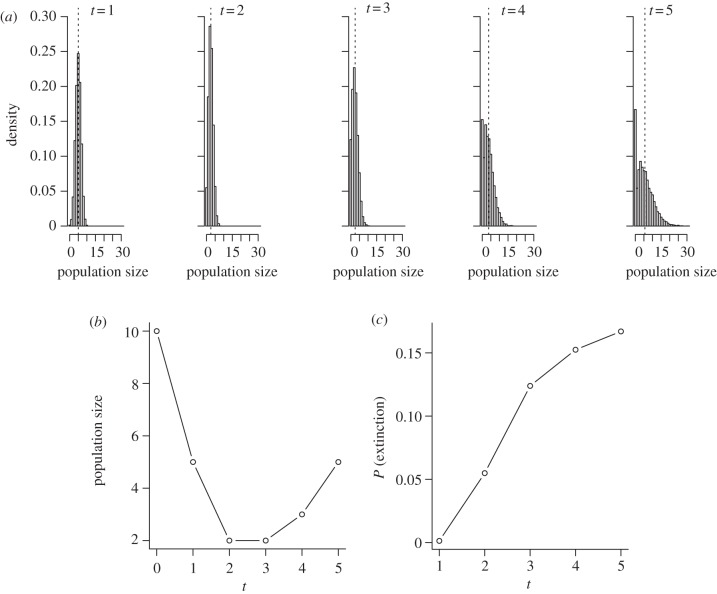
Continued persistence of a once declining population is prerequisite for evolutionary rescue, but for how long and at what abundances? An ideal analysis of population viability would describe the probability distribution of population sizes at any future time, given the nature of environmental degradation and relevant attributes of the population (figure 1a). The probability of extinction at any time (figure 1c) is sufficient for many purposes, but this itself can be challenging to predict or estimate without adopting mathematically convenient assumptions that may be of dubious, or limited, biological relevance [9,10,14]. Rather than consider probabilities explicitly, early theoretical analyses of evolutionary rescue focused on the deterministic time to reach a so-called critical population size, which was used as mathematically convenient shorthand for ‘high’ immediate extinction risk. Besides the time to real or pseudo-extinction, both probabilistic and deterministic theory predict U-shaped population trajectories for rescued populations (figure 1b), a qualitative prediction that has been confirmed by experiments with model organisms [22].
Figure 1.
Alternative depictions of evolutionary rescue. Probability distributions of (a) population sizes, (b) median population sizes and (c) extinction probabilities at five successive future times for a hypothetical population of initial size 10. Mean population sizes are indicated by vertical dashed lines in panel (a). On the basis of 10 000 realizations, implemented in the R statistical language [26], of a branching process model that assumes each individual leaves a binomially distributed number of offspring with parameters p = 1/2 and N = 1, 1, 2, 3, 3 at the successive time steps, which correspond to mean absolute fitnesses (finite growth rates) of 0.5, 0.5, 1.0, 1.5 and 1.5, respectively.
Non-evolutionary processes, such as phenotypic plasticity [27] or density dependence, could enhance a declining population's capacity to endure and recover, and so the role of adaptive evolution in rescue should not be assumed. In principle, this could be addressed by comparing an evolving population in a stressful environment to an otherwise identical population that cannot evolve. While such a comparison might be possible in theory [7], it would obviously be difficult—if not outright impossible—for organisms in the wild. A more practical approach would be to develop a tailored model of how adaptive evolution might affect the dynamics of a population and the probability it would endure for a given length of time, obtain empirical data from the population to parametrize the model and use the fitted model to evaluate the dependence of the population's persistence on evolution or to partition the impacts of evolution and other processes on population rescue.
Analyses of mathematical models suggest that evolutionary rescue is largely determined by a core set of properties. The first is the form of environmental change, be it abrupt, gradual or erratic. This includes the rate, pattern and degree of environmental degradation and the magnitude of any fluctuations [7,9,17]. The second core property is the size of the population experiencing the environmental change. Indeed, as mentioned at the outset of this article, larger populations take longer to go extinct than small ones, but they can also harbour more genetic variants and incur more genetic variation de novo [28]. The third core property is the rate of population growth or decline, because populations in steeper decline have less time to be rescued from extinction than those in slow decline. These growth rates may often reflect the form of environmental change, be it mild or catastrophic, gradual or sudden. The fourth core property is the amount of heritable genetic variation in absolute fitness segregating in the population, since this standing variation largely determines a population's short-term capacity to increase its mean absolute fitness through natural selection [29]. In addition to the four core properties, it would be helpful to have information about mutational contributions to genetic variation in absolute fitness, how traits (such as selfing rates) mediate absolute fitness in the stressful environment, behavioural or phenotypic plasticity affecting fitness, and ecological properties that affect population growth or decline such as density dependence, spatial heterogeneity or interspecific interactions [16,18,19].
Experiments with model organisms show that it is possible to obtain meaningful measurements of many of these properties and verify their predicted central importance for evolutionary rescue. In §3, we discuss what we see as the main impediments to the study of evolutionary rescue in natural populations.
3. Noise, statistical and practical challenges
Linking the theory of evolutionary rescue to wild populations entails evaluating key population attributes in conditions under which they are declining (or will in the future). Among these key attributes is the number of individuals in a population. Unlike in a laboratory or greenhouse, defining the extent of a natural population is not simple, because the spatial scale of interaction among individuals may differ depending on the process considered; for example, mating versus competition [30]. If we leave aside this issue by focusing on the individuals occupying an area of designated extent, obtaining a count may be straightforward if the organism of interest is conspicuous or sessile, but many are small, reclusive, highly vagile or sparsely distributed. In such cases, determining abundance alone poses a significant challenge [31,32].
Beyond this, ubiquitous environmental variability in nature and the inherently large sampling variation of estimates of quantitative genetic parameters together seriously hinder evaluation of the potential for evolutionary rescue. This is true for experiments in nature as well as for observational studies of unmanipulated wild populations. For example, Stanton-Geddes et al. [33] estimated a 95% confidence interval of 0.47–1.42 for the finite growth rate of Chamaecrista fasciculata at a central location within its range. The corresponding estimate for a site at the western edge of the species' range was −0.17 to 1.19. In both locations, but particularly the latter, the evidence is not definitive about whether a population would persist or decline, whereas beyond the western edge of the range, the 95% CI of −0.01 to 0.03 makes clear that, barring adaptation, the population would decline deterministically. Observational studies have the further problem that confounding of genetic and environmental effects compromises the accuracy of estimates [34,35]. The extent of variation in nature and the consequent noise of estimates must be taken into account in understanding evolution in nature. Experiments in natural populations that are carefully designed, with due attention to randomization and replication, can elucidate the potential and prevalence of evolutionary rescue.
As crucial influences on a population's extinction risk and its potential for evolutionary rescue, both the mean and genetic variance of its absolute fitness in the habitat it occupies are of particular interest. Absolute fitness is a notoriously difficult property to measure. In individual-based studies, this minimally entails complete records of recruitment, survival and reproduction throughout the lifetime, which may, of course, extend for many years. Even for organisms whose active lives span less than a year (annual plants; planktonic microcrustacea, like Daphnia), completion of the full life cycle through seed/propagule dormancy may take many years. Moreover, all aspects of the life history that contribute to fitness are sensitive to environmental influences that, in the wild, often vary temporally and spatially on multiple scales. Consequently, individual variation in fitness is typically substantial.
The frequency distribution of fitness is a compound distribution, combining the sampling variation at each stage of the life history, and is characteristically right-skewed, in part because most individuals die without reproducing (figure 2). These aspects of fitness have impeded (though not prevented) its evaluation. Aster modelling [36] was developed to enable precise estimation of mean absolute fitness, with statistical power for conclusive inference about population decline [33] by explicitly modelling the compound nature of lifetime fitness. Even so, because environmental variation over years can be substantial [37], assessment of population decline would appropriately consider multiple cohorts ([38] is a rare example). Moreover, absolute fitness estimated for successive cohorts would reveal the extent to which the environment changes gradually, abruptly or erratically, another aspect that is critical to prediction of evolutionary rescue. Indeed, theory shows that unpredictable fluctuations in fitness can increase a population's risk of extinction considerably and the inevitable time lags of adaptive responses to constantly shifting patterns of selection may actually further exacerbate that risk [9].
Figure 2.
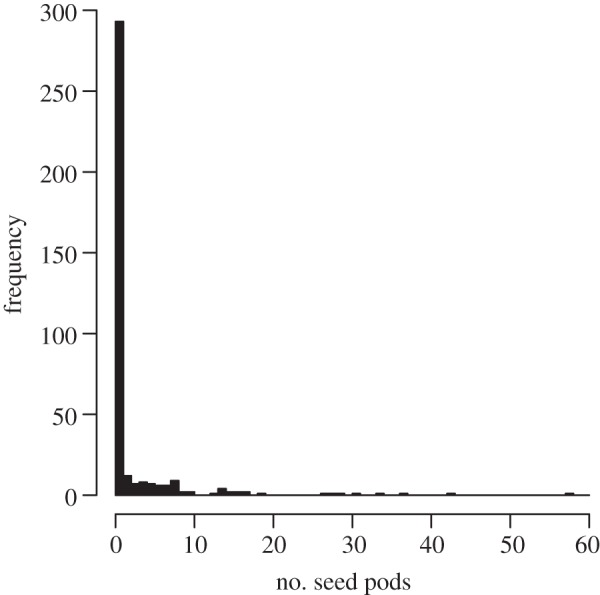
The distribution of the number of seed pods produced by 372 Chamaecrista fasciculata plants at Conard Environmental Research Area (Grinnell College) in 2009 [33].
Complete evaluation of absolute fitness in nature has rarely been accomplished even for populations in their typical environmental conditions, prior to a major degradation. This may be, in part, because evolutionary theory has, until recently, emphasized variation in relative fitness as the explanatory basis for natural selection, but certainly also because the challenges of obtaining complete fitness records impose considerable demands. Nevertheless, as the examples cited above show, it is feasible to assess absolute fitness in nature, even for species with lifespans that approach a decade [39,40].
The further challenge of evaluating the potential for evolutionary rescue in conditions that are expected to impinge on populations in the future would ideally entail both prediction of those conditions and the capacity to generate them experimentally. However, considering changing climate as an example, there is an extreme degree of uncertainty in detailed climate prediction and, even if precise predictions of climate were available, its many dimensions (including temperature, rainfall, humidity and insolation, all varying through the year) would preclude establishing realistic future environments (see, for example, discussion in [41]). As an alternative, Etterson [42,43] used a chronosequence, substituting latitudinal differences in climate as approximate analogues for climatic differences projected into the future. Transplants of populations can demonstrate whether species are limited by habitat that is a sink beyond their range [33,44]. Investigation of the effect of particular environmental aspects on fitness can be undertaken in conjunction with experiments that manipulate properties of the environment—for example, free-air carbon enrichment (FACE) studies [45,46].
Inferences about genetic variation in absolute fitness can be based on the same experimental populations used to assess the rate of growth or decline if they comprise large numbers of individuals of known pedigree. In that case, the phenotypic variation in absolute fitness can, in principle, be partitioned into components of variance attributable to different sources [34,47]. Of particular relevance to the process of evolutionary rescue in sexual populations is the variance of breeding values for fitness, i.e. the additive genetic variance in fitness. Although this component is expected to be small relative to the environmental component of variance, it represents the population's potential to adapt to current conditions [29,48]. The characteristically problematic features of fitness distributions noted above also interfere with estimation of genetic variance in fitness, for which the usual statistical methodology is especially deeply rooted in theory that assumes normally distributed variation. Aster analysis accommodating random genetic effects with an arbitrary distribution has recently been implemented [49]. Although there are formidable challenges to assessing evolutionary rescue or its determinants in natural populations and settings, a handful of empirical studies show that progress is possible. In §4, we highlight a few case studies as exemplars of approaches to gathering dependable information that could be used to evaluate evolutionary rescue in wild populations.
4. Stepping beyond the models: instructive case studies
The challenges of detecting evolutionary rescue in wild populations are daunting, but a few pioneering empirical studies represent important precedents on which future investigations can build. Elsewhere in this special issue, Vander Wal et al. [50] review investigations of evolutionary rescue in natural vertebrate populations, and Gandon et al. [51] discuss evidence for the role of evolution in the emergence of infectious diseases. Below we present a selection of case studies involving insects and plants that, while not expressly designed to evaluate evolutionary rescue, nonetheless demonstrate the practicability of collecting data that would contribute to prediction of how evolution might affect the destinies of wild populations in degraded environments. For each case, we suggest some empirical ‘next steps’ beyond the study that would be particularly useful for generating inferences or predictions about evolutionary rescue.
(a). Studies assessing individual fitness in future environments
Etterson [42] transplanted populations of a widespread native annual legume, Chamaecrista fasciculata, in field sites along a spatial aridity gradient to mimic environmental conditions that these populations might one day face. The objective of the study was to assess future patterns of selection. Fitness components were measured for individual plants, and these were used to estimate multivariate patterns of selection on leaf number, leaf thickness and reproductive stage at each field site. These data have been re-analysed using aster models [36,49]. This experiment also yielded estimates of additive-genetic variances and co-variances among the three focal plant traits within each field site and covariation in trait expression among the sites [43], indicating the potential for adaptive evolution in response to the novel environmental conditions.
Next steps. Individuals in the study were planted as seedlings; complete estimates of absolute fitness would require measurements of seed production through seedling recruitment. Although populations were transplanted to foreign sites, those sites were apparently not ‘demographic sinks’, i.e. habitats in which population size tends to decline. So, by definition, evolutionary rescue at those sites of the populations considered is not an issue. An investigation of evolutionary rescue per se would include field sites that are genuine sinks, whether for particular populations or for all populations of a focal species.
A later study of C. fasciculata by Stanton-Geddes et al. [33] tested the extent to which absolute plant fitness decreases at and beyond the species' northern and western range edges, and how the community context influences vital rates at different life stages. The study assessed individual plant lifetime fitness (including seedling recruitment) at various locations, including sites that are demographic sinks, and estimated population growth rates over a complete generation using aster models. The study demonstrates reliable estimation of individual absolute fitness and population growth rates under conditions that bar positive population growth and, in particular, revealed important effects of biotic interactions on population persistence. In fact, neighbour removal treatments showed in most cases that neighbours reduced fertility of reproductive adults but enhanced early season survival with a net positive effect of neighbours on lifetime fitness.
Next steps. It would be useful to estimate the amount of genetic variability for absolute fitness that is available for adaptive evolution in the sink environments. In addition, multi-year studies would reveal the nature of temporal environmental change, which is of value for generating realistic predictions since among-year patterns of environmental variability at any particular site might strongly affect the potential for evolutionary rescue or failure [9,52].
(b). Study of the relation between genetic diversity and persistence
Newman & Pilson [53] manipulated the genetic diversity of replicate Clarkia pulchella populations while removing population size differences among their treatments. They varied genetic diversity by altering the overall relatedness of individuals in founder and descendent populations. The populations were propagated over multiple seasons, but mean population fitness was estimated only over the final generation. Some replicate populations within each treatment went extinct during the course of the experiment and a statistically significant association between genetic variability and extinction was found.
Next steps. It is unclear that environmental conditions during the experiments were demographic sinks despite extinction of some replicate populations. There is a good possibility that the extinctions were due to the small population sizes that were used. Indeed, low abundances cause populations to be highly vulnerable to rapid chance extinction by demographic stochasticity in even benign environments [1]; so it would be more informative for a study of evolutionary rescue to use larger replicate populations. Also, experimental approaches that directly manipulate (or measure) heritable variation in absolute fitness would be illuminating. Estimates of mean population fitness over multiple seasons would be valuable, given the evident temporal variation that populations experienced during the study.
(c). Study extrapolating species-level fitness estimates
Deutsch et al. [54] surveyed thermal performance curves (i.e. fitnesses across a gradient of temperature environments) for insect species worldwide and used cross-species average performance curves to compare the future relative fitness of temperate versus tropical species given projected levels of global temperature increases over the next century. They predicted that the relative performance of temperate species would increase while that of tropical species would decrease sharply.
Next steps. Measurements of absolute fitness, rather than just relative fitness, are needed to assess the extent to which insect species facing warmer environments are dependent on evolutionary rescue for persistence. The severe reduction in relative fitness of the tropical species predicted by the study does not necessarily imply future population declines, but perhaps just lower, but still positive, growth rates. Estimated thermal performance curves for species and individuals (including heritable variation in absolute fitness) would be needed to project both the risks of extinction caused by anticipated rising temperatures for insect populations and their capacities for evolutionary rescue.
(d). Population growth study using transition matrices
In a study of two Mimulus species, Angert [55] used demographic observations of populations located at central and marginal sites along an elevation gradient to examine differences in individual vital and population growth rates over multiple years. Analyses were based on life table and population transition matrix methods, and compared the contributions of various transition components to differences in asymptotic growth rates among the populations. The study found, unexpectedly, that central populations of M. cardinalis had unsustainable growth rates contrary to results of a reciprocal transplant involving the same species [44]. One key contributor to this discordance may have been the dynamics of flooding affecting the observational study [55].
Next steps. Aster models [36] could be used to refine estimates of vital rates for individual life stages and population growth rates. Neighbour removal experiments (as in Stanton-Geddes et al. [33]) could also illuminate the influences of biotic interactions on different transition components. More work is needed to identify the types of information revealed by alternative analyses of population growth, especially for accurate population projection. In addition, there may be practical differences between analyses for studying evolutionary rescue. (For example, it may be difficult to obtain reliable estimates of transition matrices in sink environments since populations subject to those conditions might tend to be small or highly ephemeral.) Analyses of the estimated transition matrices in the Mimulus study focused on asymptotic rather than on short-term growth rates, but the fates of sink populations are probably determined primarily on the basis of short-term dynamics [56]. Finally, as in our other examples, it would be useful to obtain estimates of genetic variation in absolute fitness associated with transition matrix elements that could be used to project joint population and adaptive evolutionary dynamics [57,58].
(e). Resurrection studies
The ultimate empirical test of evolutionary rescue would directly compare ancestral and descendent populations. This is routine in many laboratory systems, particularly microbial ones, where one can easily preserve and revive individuals from any generation. One might presume that such comparisons are impossible for natural populations, but in fact, a few cases have been reported where ancestral seeds, spores or eggs were serendipitously preserved (e.g. in tundra soils, lake sediments or human collections) in a condition allowing them to be grown directly alongside modern descendants [59–64]. This ‘resurrection approach’ is exemplified by Franks et al. ([63], see also [64]) who re-grew seeds of Brassica rapa that had been stored prior to an extended drought in southern California alongside post-drought descendants of the same population in a greenhouse. This approach allowed elimination of phenotypic plasticity as a cause of population differences, thereby lending strong support for the conclusion that the observed divergence in flowering time was due to genetically based evolution in a direction consistent with predictions based on estimates of selection under drought conditions.
Next steps. It would be ideal if resurrection studies of wild populations were available more often than just by accident. This step is already being taken for plant species by a new initiative called Project Baseline, whose goal is to systematically collect and bank seeds of current populations so that they will be available to biologists for future studies of evolutionary responses that may occur in the coming decades [65,66]. An especially fascinating use of Project Baseline and the resurrection approach would be to compare contemporaneous ancestors of populations that have survived or gone extinct in the wild.
5. Beyond evolutionary rescue
Our discussion has stressed the formidable practical and statistical challenges of investigating evolutionary rescue in natural populations and realistic settings. It is important, however, to recognize that current theory, which has been broadly aimed at establishing a well-founded conceptual basis for evolutionary rescue, is also not yet ready to turn reliable estimates into useful assessments or forecasts of evolutionary success and failure in the wild. To move the science from general concepts to detailed inferences or predictions, it will be necessary to coordinate empirical, statistical and theoretical investigations of evolutionary rescue for natural systems of interest. Those studies would likely need to consider a number of new processes and perspectives.
Given the difficulties of inferring the genetic basis and variability of fitness directly, one might envision a more prominent future role for empirically based mechanistic models that relate phenotype and environment to fitness. While these descriptions might eventually enable better estimates of variation in absolute fitness and more accurate predictions of population and evolutionary dynamics, robustly connecting phenotype and environment to fitness is itself a highly daunting challenge.
This review has concentrated on the role of natural selection in population rescue, but other evolutionary processes could certainly impact the outcome in any given situation. Foremost among these are immigration and spatial variation. There is a rather extensive theoretical literature on gene flow and the evolution of species' fundamental niches or species' geographical ranges that is of relevance to evolutionary rescue in open populations (reviewed in [13]). A recent empirical study based on the yeast model system has demonstrated some of the impacts that spatial variability and gene flow can have on evolutionary rescue [24]. The processes of mutation and random genetic drift, mating systems (e.g. asexuality, self-fertilization and outcrossing) and the genetic underpinnings of absolute fitness (major genes, polygenes, ploidy level), are all theoretically capable of influencing the efficiency of adaptation and the prospects of extinction of populations in stressful environments [11,14,67] but the real-world importance of these genetic considerations remains an open question that will need to be settled to mature our understanding of evolutionary rescue in natural populations.
To a great extent, evolutionary rescue is decided by short-term, chance outcomes, and so it will be imperative to develop a greater understanding of—and capacity to predict—stochastic transient population and evolutionary dynamics. Because mathematical analyses of even deterministic transient dynamics tend to be forbidding, theoretical predictions for particular species will most likely rely on numerical solution and simulation. While it may be relatively straightforward to implement computational approaches, these methods are less amenable to discovery of general principles and results can be hard to interpret in a biologically meaningful way or to verify empirically. Whenever analytical and computational approaches can be applied in concert, greater insight will emerge [58,68,69].
Evolutionary rescue shares much in common with studies of species' responses to climate change, the evolution of range limits and niche evolution in open populations. Evolutionary rescue's singular focus on populations headed to extinction, however, distinguishes it from all these fields, because not all climate change imperils persistence and any population outside its species' core range or fundamental niche is saved from permanent extinction by recurrent migration. Still, the question of evolutionary rescue arises automatically when considering responses to any kind of climate change that does endanger populations and also within the evolution of species limits because the expansion of a species' borders involves adaptation to harsh environmental conditions (abiotic or biotic) that lie outside its current core range. And a definitive empirical test of successful niche evolution in a non-isolated population must show that the ancestral population would become extinct in the absence of migration and that its descendant has evolved to become a self-sustaining population (i.e. is expected to persist in the absence of migration). These are the same starting and ending conditions as evolutionary rescue, which is defined in terms of constantly isolated populations. Finally, investigations of adaptation to climate change, evolution of range limits or niche evolution in wild populations all require sound estimates of heritable variation in absolute fitness in novel environments and, as such, these studies face many of the same empirical challenges as those we have outlined for evolutionary rescue [25].
Consistent with the theme of the special volume of this journal, our essay has focused on adaptive evolution and its role in determining the lot of isolated populations encountering stressful habitats. But those fates are also affected by a number of mechanisms that do not require evolution. These include phenotypic plasticity [27], bet hedging [70], behavioural responses and dispersal syndromes, cross-generational maternal and epigenetic effects, and ecological interactions with both conspecifics and heterospecifics. We expect that well-designed empirical studies of evolutionary rescue in wild populations using powerful statistical methods that parametrize appropriately formulated theoretical models will forge the foundation for a complete understanding of species persistence, diversification and extinction in harsh environments.
Acknowledgements
Support for R.G. was provided by US National Science Foundation grant DEB-0919376 and for R.S. by US National Science Foundation grant DEB-0544970. We thank J. Stanton-Geddes for preparing figure 2 and allowing us to use it. We also gratefully acknowledge constructive comments from him and other members of the Minnesota Center for Community Genetics, and from two anonymous reviewers.
References
- 1.MacArthur RH, Wilson EO. 1967. Island biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Lande R. 1993. Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am. Nat. 142, 911–927 10.1086/285580 (doi:10.1086/285580) [DOI] [PubMed] [Google Scholar]
- 3.Bradshaw AD. 1991. The Croonian Lecture 1991: genostasis and the limits to evolution. Phil. Trans. R. Soc. Lond. B 333, 289–305 10.1098/rstb.1991.0079 (doi:10.1098/rstb.1991.0079) [DOI] [PubMed] [Google Scholar]
- 4.Gomulkiewicz R, Houle D. 2009. Demographic and genetic constraints on evolution. Am. Nat. 174, E218–EE29 10.1086/645086 (doi:10.1086/645086) [DOI] [PubMed] [Google Scholar]
- 5.Macnair MR. 1991. Why the evolution of resistance to anthropogenic toxins normally involves major gene changes: the limits to natural selection. Genetica 84, 213–219 10.1007/BF00127250 (doi:10.1007/BF00127250) [DOI] [Google Scholar]
- 6.Lynch M, Lande R. 1993. Evolution and extinction in response to environmental change. In Biotic interactions and global climate change (eds Kareiva PM, Kingsolver JG, Huey RB.), pp. 234–250 Sunderland, MA: Sinauer Associates [Google Scholar]
- 7.Gomulkiewicz R, Holt RD. 1995. When does evolution by natural selection prevent extinction? Evolution 49, 201–207 10.2307/2410305 (doi:10.2307/2410305) [DOI] [PubMed] [Google Scholar]
- 8.Boulding EG, Hay T. 2001. Genetic and demographic parameters determining population persistence after a discrete change in the environment. Heredity 86, 313–324 10.1046/j.1365-2540.2001.00829.x (doi:10.1046/j.1365-2540.2001.00829.x) [DOI] [PubMed] [Google Scholar]
- 9.Bürger R, Lynch M. 1995. Evolution and extinction in a changing environment: a quantitative-genetic analysis. Evolution 49, 151–163 10.2307/2410301 (doi:10.2307/2410301) [DOI] [PubMed] [Google Scholar]
- 10.Holt RD, Gomulkiewicz R. 1997. The evolution of species’ niches: a population dynamic perspective. In Case studies in mathematical modeling—ecology, physiology, and cell biology (eds Othmer HG, Adler FR, Lewis MA.), pp. 25–50 New York, NY: Prentice-Hall [Google Scholar]
- 11.Antia R, Regoes RR, Koella JC, Bergstrom CT. 2003. The role of evolution in the emergence of infectious diseases. Nature 426, 658–661 10.1038/nature02104 (doi:10.1038/nature02104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt RD, Gomulkiewicz R. 2004. Conservation implications of niche conservatism and evolution in heterogeneous environments. In Evolutionary conservation biology (eds Ferrier R, Dieckmann U, Couvet D.), pp. 244–264 Cambridge, UK: Cambridge University Press [Google Scholar]
- 13.Holt RD, Barfield M, Gomulkiewicz R. 2005. Theories of niche conservatism and evolution: could exotic species be potential tests? In Species Invasions: Insights into ecology, evolution, and biogeography (eds Sax DF, Stachowicz JJ, Gaines SD.), pp. 259–290 Sunderland, MA: Sinauer Associates [Google Scholar]
- 14.Orr HA, Unckless RL. 2008. Population extinction and the genetics of adaptation. Am. Nat. 172, 160–169 10.1086/589460 (doi:10.1086/589460) [DOI] [PubMed] [Google Scholar]
- 15.Baskett ML, Gomulkiewicz R. 2011. Introgressive hybridization as a mechanism for species rescue. Theor. Ecol. 4, 223–239 10.1007/s12080-011-0118-0 (doi:10.1007/s12080-011-0118-0) [DOI] [Google Scholar]
- 16.de Mazencourt C, Johnson E, Barraclough TG. 2008. Biodiversity inhibits species’ evolutionary responses to changing environments. Ecol. Lett. 11, 380–388 10.1111/j.1461-0248.2008.01152.x (doi:10.1111/j.1461-0248.2008.01152.x) [DOI] [PubMed] [Google Scholar]
- 17.Björklund M, Ranta E, Kaitala V, Bach LA, Lundberg P, Stenseth NC. 2009. Quantitative trait evolution and environmental change. PLoS ONE 4, e4521. 10.1371/journal.pone.0004521 (doi:10.1371/journal.pone.0004521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones AG. 2008. A theoretical quantitative genetic study of negative ecological interactions and extinction times in changing environments. BMC Evol. Biol. 8, 119–128 10.1186/1471-2148-8-119 (doi:10.1186/1471-2148-8-119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanarek AR, Webb CT. 2010. Allee effects, adaptive evolution, and invasion success. Evol. Appl. 3, 122–135 10.1111/j.1752-4571.2009.00112.x (doi:10.1111/j.1752-4571.2009.00112.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomulkiewicz R, Holt RD, Barfield M, Nuismer SL. 2010. Genetics, adaptation, and invasion in harsh environments. Evol. Appl. 3, 97–108 10.1111/j.1752-4571.2009.00117.x (doi:10.1111/j.1752-4571.2009.00117.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willi Y, Hoffman AA. 2009. Demographic factors and genetic variation influence population persistence under environmental change. J. Evol. Biol. 22, 124–133 10.1111/j.1420-9101.2008.01631.x (doi:10.1111/j.1420-9101.2008.01631.x) [DOI] [PubMed] [Google Scholar]
- 22.Bell G, Gonzalez A. 2009. Evolutionary rescue can prevent extinction following environmental change. Ecol. Lett. 12, 942–948 10.1111/j.1461-0248.2009.01350.x (doi:10.1111/j.1461-0248.2009.01350.x) [DOI] [PubMed] [Google Scholar]
- 23.Bodbyl Roels SA, Kelly JK. 2011. Rapid evolution caused by pollinator loss in Mimulus guttatus. Evolution 65, 2541–2552 10.1111/j.1558-5646.2011.01326.x (doi:10.1111/j.1558-5646.2011.01326.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell G, Gonzalez A. 2011. Adaptation and evolutionary rescue in metapopulations experiencing environmental deterioration. Science 332, 1327–1330 10.1126/science.1203105 (doi:10.1126/science.1203105) [DOI] [PubMed] [Google Scholar]
- 25.Shaw RG, Etterson JR. 2012. Tansley review: rapid climate change and the rate of adaptation: insight from experimental quantitative genetics. New Phytol. 195, 752–765 10.1111/j.1469-8137.2012.04230.x (doi:10.1111/j.1469-8137.2012.04230.x) [DOI] [PubMed] [Google Scholar]
- 26.R Development Core Team 2006. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.r-project.org [Google Scholar]
- 27.Chevin L-M, Gallet R, Gomulkiewicz R, Holt R, Fellous S. 2012. Phenotypic plasticity in evolutionary rescue experiments. Phil. Trans. R. Soc. B 368, 20120089. 10.1098/rstb.2012.0089 (doi:10.1098/rstb.2012.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber KE. 1990. Increased selection response in larger populations. I. Selection for wing-tip height in Drosophila melanogastor at three population sizes. Genetics 125, 579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: Clarendon Press [Google Scholar]
- 30.Antonovics J, Levin DA. 1980. The ecological and genetical consequences of density dependent regulation in plants. Annu. Rev. Ecol. Syst. 11, 411–452 10.1146/annurev.es.11.110180.002211 (doi:10.1146/annurev.es.11.110180.002211) [DOI] [Google Scholar]
- 31.Seber GAF. 2002. Estimation of animal abundance and related parameters. Caldwell, NJ: Blackburn Press [Google Scholar]
- 32.Slade NA, Alexander HM, Kettle WD. 2003. Estimation of population size and probabilities of survival and detection in Mead's milkweed. Ecology 84, 791–797 10.1890/0012-9658(2003)084[0791:EOPSAP]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[0791:EOPSAP]2.0.CO;2) [DOI] [Google Scholar]
- 33.Stanton-Geddes J, Tiffin P, Shaw RG. 2012. Role of climate and competitors in limiting fitness across range edges of an annual plant. Ecology 93, 1604–1613 10.1890/11-1701.1 (doi:10.1890/11-1701.1) [DOI] [PubMed] [Google Scholar]
- 34.Ozgul A, Tuljapurkar S, Benton TG, Pemberton JM, Clutton-Brock TH, Coulson T. 2009. The dynamics of phenotypic change and the shrinking sheep of St. Kilda. Science 325, 464–467 10.1126/science.1173668 (doi:10.1126/science.1173668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pemberton JM. 2010. Evolution of quantitative traits in the wild: mind the ecology. Phil. Trans. R. Soc. Lond. B 365, 2431–2438 10.1098/rstb.2010.0108 (doi:10.1098/rstb.2010.0108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw RG, Geyer CJ, Wagenius S, Hangelbroek HH, Etterson JR. 2008. Unifying life history analyses for inference of fitness and population growth. Am. Nat. 172, E35–E47 10.1086/588063 (doi:10.1086/588063) [DOI] [PubMed] [Google Scholar]
- 37.Venable DL. 2007. Bet hedging in a guild of desert annuals. Ecology 88, 1086–1090 10.1890/06-1495 (doi:10.1890/06-1495) [DOI] [PubMed] [Google Scholar]
- 38.Price MV, Campbell DR, Waser NM, Brody AK. 2008. Bridging the generation gap in plants: pollination, parental fecundity, and offspring demography. Ecology 89, 1596–1604 10.1890/07-0614.1 (doi:10.1890/07-0614.1) [DOI] [PubMed] [Google Scholar]
- 39.Campbell DR, Waser NM. 2007. Evolutionary dynamics of an Ipomopsis hybrid zone: confronting models with lifetime fitness data. Am. Nat. 169, 298–310 10.1086/510758 (doi:10.1086/510758) [DOI] [PubMed] [Google Scholar]
- 40.Waser NM, Campbell DR, Price MV. 2010. Density-dependent demographic responses of a semelparous plant to natural variation in seed rain. Oikos 119, 1929–1935 10.1111/j.1600-0706.2010.18429.x (doi:10.1111/j.1600-0706.2010.18429.x) [DOI] [Google Scholar]
- 41.Price MV, Waser NM. 2000. Responses of subalpine meadow vegetation to four years of experimental warming. Ecol. Appl. 10, 811–823 10.1890/1051-0761(2000)010[0811:ROSMVT]2.0.CO;2 (doi:10.1890/1051-0761(2000)010[0811:ROSMVT]2.0.CO;2) [DOI] [Google Scholar]
- 42.Etterson JR. 2004. Evolutionary potential of Chamaecrista fasciculata in relation to climate change. I. Clinal patterns of selection along an environmental gradient in the Great Plains. Evolution 58, 1446–1458 [DOI] [PubMed] [Google Scholar]
- 43.Etterson JR. 2004. Evolutionary potential of Chamaecrista fasciculata in relation to climate change. II. Genetic architecture of three populations reciprocally planted along an environmental gradient in the Great Plains. Evolution 58, 1459–1471 [DOI] [PubMed] [Google Scholar]
- 44.Angert AL, Schemske DW. 2005. The evolution of species’ distributions: reciprocal transplants across the elevation ranges of Mimulus cardinalis and M. lewisii. Evolution 59, 1671–1684 [PubMed] [Google Scholar]
- 45.Lau JA, Shaw RG, Reich PB, Tiffin P. 2007. Strong ecological but weak evolutionary effects of elevated CO2 on a recombinant inbred population of Arabidopsis thaliana. New Phytol. 175, 351–362 10.1111/j.1469-8137.2007.02108.x (doi:10.1111/j.1469-8137.2007.02108.x) [DOI] [PubMed] [Google Scholar]
- 46.Lau JA, Shaw RG, Reich PB, Tiffin P. 2010. Species interactions in a changing environment: elevated CO2 alters the ecological and potential evolutionary consequences of competition. Evol. Ecol. Res. 12, 435–455 [Google Scholar]
- 47.Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE. 2010. Coupled dynamics of body mass and population growth in response to environmental change. Nature 466, 482–485 10.1038/nature09210 (doi:10.1038/nature09210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ewens WJ. 2004. In Mathematical population genetics. I. Theoretical introduction, 2nd edn (eds Antman SS, Marsden JE, Sirovich L, Wiggins S.). Interdisciplinary Applied Mathematics, vol. 27, pp. 259–261. New York, NY: Springer [Google Scholar]
- 49.Geyer CJ, Ridley CE, Latta RG, Etterson JR, Shaw RG. 2012. Aster models with random effects via penalized likelihood. Technical Report no. 692. School of Statistics, University of Minnesota, MN, USA
- 50.Vander Wal EGD, Garant D, Festa-Bianchet M, Pelletier F. 2012. Evolutionary rescue in vertebrates: evidence, applications and uncertainty. Phil. Trans. R. Soc. B 368, 20120090. 10.1098/rstb.2012.0090 (doi:10.1098/rstb.2012.0090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gandon S, Hochberg M, Holt RD, Martin G, Day T. 2012. What limits the evolutionary emergence of pathogens? Phil. Trans. R. Soc. B 368, 20120086. 10.1098/rstb.2012.0086 (doi:10.1098/rstb.2012.0086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holt RD, Barfield M, Gomulkiewicz R. 2004. Temporal variation can facilitate niche evolution in harsh sink environments. Am. Nat. 164, 187–200 10.1086/422343 (doi:10.1086/422343) [DOI] [PubMed] [Google Scholar]
- 53.Newman D, Pilson D. 1997. Increased probability of extinction due to decreased genetic effective population size: experimental populations of Clarkia pulchella. Evolution 51, 354–362 10.2307/2411107 (doi:10.2307/2411107) [DOI] [PubMed] [Google Scholar]
- 54.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 10.1073/pnas.0709472105 (doi:10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angert AL. 2006. Demography of central and marginal populations of monkeyflowers (Mimulus cardinalis and M. lewisii). Ecology 87, 2014–2025 10.1890/0012-9658(2006)87[2014:DOCAMP]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[2014:DOCAMP]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 56.Caswell H. 2001. Matrix population models: construction, analysis, and interpretation, 2nd edn Sunderland, MA: Sinauer Associates [Google Scholar]
- 57.Charlesworth B. 1994. Evolution in age-structured populations, 2nd edn. Cambridge, UK: Cambridge University Press [Google Scholar]
- 58.Barfield M, Holt RD, Gomulkiewicz R. 2011. Evolution in stage-structured populations. Am. Nat. 177, 397–409 10.1086/658903 (doi:10.1086/658903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennington CC, McGraw JB, Vavrek MC. 1991. Ecological genetic variation in seed banks. II. Phenotypic and genetic differences between young and old subpopulations of Luzula parviflora. J. Ecol. 79, 627–644 10.2307/2260658 (doi:10.2307/2260658) [DOI] [Google Scholar]
- 60.Vavrek MC, McGraw JB, Bennington CC. 1991. Ecological genetic variation in seed banks. III. Phenotypic and genetic differences between plants from young and old seed subpopulations of Carex biglowii. J. Ecol. 79, 645–662 10.2307/2260659 (doi:10.2307/2260659) [DOI] [Google Scholar]
- 61.Hairston NG, Lampert W, Cacres CE, Holtmeier CL, Weider LJ, Gaedke U, Fischer JM, Fox JA, Post DM. 1999. Rapid evolution revealed by dormant eggs. Nature 410, 446. 10.1038/46731 (doi:10.1038/46731) [DOI] [Google Scholar]
- 62.Kerfoot WC, Robbins JA, Weider LJ. 1999. A new approach to historical reconstruction: combining descriptive and experimental paleolimnology. Limnol. Oceanogr. 44, 1232–1247 10.4319/lo.1999.44.5.1232 (doi:10.4319/lo.1999.44.5.1232) [DOI] [Google Scholar]
- 63.Franks S, Sim S, Weis AE. 2007. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc. Natl Acad. Sci. USA 104, 1278–1282 10.1073/pnas.0608379104 (doi:10.1073/pnas.0608379104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franks SJ. 2011. Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytol. 190, 249–257 10.1111/j.1469-8137.2010.03603.x (doi:10.1111/j.1469-8137.2010.03603.x) [DOI] [PubMed] [Google Scholar]
- 65.Franks SJ, Avise JC, Bradshaw WE, Conner JK, Etterson JR, Mazer SJ, Shaw RG, Weis AE. 2008. The resurrection initiative: storing ancestral genotypes to capture evolution in action. BioScience 58, 870–873 10.1641/B580913 (doi:10.1641/B580913) [DOI] [Google Scholar]
- 66.Etterson JR, Franks S, Mazer SJ, Shaw RG, Updegraff K. 2012. Project Baseline: a seed bank to study evolution. (30 March 2012). See http://www.baselineseedbank.org/
- 67.Lynch M, Conery J, Bürger R. 1995. Mutation accumulation and the extinction of small populations. Am. Nat. 146, 489–518 10.1086/285812 (doi:10.1086/285812) [DOI] [Google Scholar]
- 68.Holt RD, Gomulkiewicz R, Barfield M. 2003. The phenomology of niche evolution via quantitive traits in a ‘black-hole’ sink. Proc. R. Soc. Lond. B 270, 215–224 10.1098/rspb.2002.2219 (doi:10.1098/rspb.2002.2219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopez S, Rousset F, Shaw FH, Shaw RG, Ronce O. 2009. Joint effects of inbreeding and local adaptation on the evolution of genetic load after fragmentation. Conserv. Biol. 23, 1618–1627 10.1111/j.1523-1739.2009.01326.x (doi:10.1111/j.1523-1739.2009.01326.x) [DOI] [PubMed] [Google Scholar]
- 70.Simons AM. 2011. Modes of response to environmental change and the elusive empirical evidence for bet hedging. Proc. R. Soc. B 278, 1601–1609 10.1098/rspb.2011.0176 (doi:10.1098/rspb.2011.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]