Abstract
There is concern that the rate of environmental change is now exceeding the capacity of many populations to adapt. Mitigation of biodiversity loss requires science that integrates both ecological and evolutionary responses of populations and communities to rapid environmental change, and can identify the conditions that allow the recovery of declining populations. This special issue focuses on evolutionary rescue (ER), the idea that evolution might occur sufficiently fast to arrest population decline and allow population recovery before extinction ensues. ER emphasizes a shift to a perspective on evolutionary dynamics that focuses on short time-scales, genetic variants of large effects and absolute rather than relative fitness. The contributions in this issue reflect the state of field; the articles address the latest conceptual developments, and report novel theoretical and experimental results. The examples in this issue demonstrate that this burgeoning area of research can inform problems of direct practical concern, such as the conservation of biodiversity, adaptation to climate change and the emergence of infectious disease. The continued development of research on ER will be necessary if we are to understand the extent to which anthropogenic global change will reduce the Earth's biodiversity.
Keywords: extinction, rapid evolution, population, environmental change, genetics, experimental evolution
1. The scope
Rates of biodiversity loss are greater than at any other time in human history. There is concern that the rate of environmental change is now exceeding the capacity of populations to adapt; in general, we do not know the conditions that will predict when contemporary evolution will intervene and preclude population extinction. There is also concern that rapid evolution may be involved in the emergence and spread of new infectious diseases, the perennial resistance of established diseases, and foster the colonization and invasion of exotic species transported by human trade. The factors that set the pace of short-term (less than 100 years) evolution in response to environmental change are known but current theory suggests that evolution may have either no effect, or enhance or even hamper long-term population persistence in the face of environmental change [1]. The aim of this special issue is to present our current understanding about when evolution can ensure population persistence over short time-scales. This particular eco-evolutionary outcome has been termed evolutionary rescue (ER): scenarios where evolution can reverse demographic threats due to environmental stress, and so prevent otherwise inevitable extirpation. Remarkably little is known about the prevalence of ER in nature, and even less about how to predict when it might occur. In part, this is because evolutionary biology has tended to ignore extinction prone populations, because long-term population persistence is a prerequisite for studying evolutionary processes. Rapid environmental change has now forced a greater focus on the dynamic link between evolution and demography and on the evolutionary potential of declining populations under strong selection.
Historically, global change biology has assumed that only ecological responses are sufficiently rapid to be relevant, but evidence to the contrary is now mounting. Natural environments, especially those with a strong human influence can generate strong selection pressures, sufficient to allow rapid evolution if the appropriate genetic variation is either present or supplied at a sufficient rate. Microbial populations, for instance, often adapt rapidly to highly stressful environments, the classic example being antibiotic resistance in pathogenic bacteria [2]. Animals and plants may also adapt rapidly to anthropogenic stress, including heavy metal pollution [3], smoke pollution [4], herbicides [5], pesticides [6] and over-fishing [7]. Thompson [8] and Hendry et al. [9] have reviewed and synthesized this literature.
On the other hand, populations sometimes fail to adapt. For example, fish populations in lakes that have been rapidly acidified by smelter fall-out usually go extinct. In highly acidified lakes with a concomitant burden of heavy metals, even large microbial populations are eliminated [10]. The evolution of heavy metal tolerance among plant populations growing on old mine tailings is a classic example of rapid evolution due to natural selection. Nevertheless, Bradshaw [11] emphasized that only a minority of species in the original community consistently evolve high levels of tolerance, almost certainly through the selection of pre-existing genetic variation. Many species lack this variation, and despite their large populations, become extinct at heavily polluted sites. For this reason, Bradshaw [11] was sceptical that many species would succeed in adapting to global change.
A general understanding of ER requires a research programme that combines demography, ecology, environment, genetics and evolutionary processes. The theoretical foundations have been laid [12–15] and are under development, as can be seen by the various theoretical contributions to this issue. An experimental programme to test this theory, based on laboratory methods in experimental evolution and high throughput technology, is burgeoning [16–21], whereas field experiments are more challenging [22,23] and only just starting. Applications are numerous and range from conservation of declining species to strategies for combating antibiotic resistance [24].
2. Evolutionary rescue: concept and definition
Although the idea of ER has existed in the literature since at least Haldane [25], it was only recently formalized mathematically and become a focus for evolutionary biologists [12,13]. A growing number of papers have elaborated on the theoretical outcomes under a wide range of demographic and genetic systems [14,26] and have reported experimental tests of the assumptions and predictions of these models [16–20,27,28]. ER is broader than the idea of genetic rescue [29] because it emphasizes the demographic effects of genetic variation, whether the source of variation is via immigration (i.e. genetic rescue sensu stricto, [29,30]) or arises within a population by mutation. With various uses of the term population ‘rescue’ present in the ecological and evolutionary literature, we take the opportunity to briefly clarify the baseline definition as used in this special issue.
ER occurs when genetic adaptation allows a population to recover from demographic effects initiated by environmental change that would otherwise cause extirpation. Explicit in this definition is the idea that environmental change moves the population away from its fundamental niche to a set of conditions where few, if any, individuals in the population are capable of surviving and reproducing. Environmental change is thus a key component of ER, because it potentially drives population declines.
There have been two main scenarios of ER that have been particularly studied from a theoretical point-of-view. In the first, the population's persistence is compromised by some abrupt environmental change [13], such as the addition of antibiotics to a bacterial population. In this case, the population must either have resistant variants present, or produce them rapidly if it is to recover and avoid extinction. In the second, evolution is necessary to keep track of a gradually changing environment [12], such as progressive climate warming, and failure to do so eventually compromises the population's persistence. These two theoretical views of ER show interesting conceptual differences in (i) the type of environmental change (abrupt versus gradual), (ii) threat (an actual demographic deficit versus a risk of demographic deficit), and (iii) rescue pathway (returning to demographic balance versus avoiding demographic imbalance). Interestingly, ER might be harder to detect in the second situation (see discussion in [22]), because the demographic challenges may not be immediately observed (see [23] for the use of models to explore this question in an empirical system). Other less studied forms of environmental change can be accommodated by this definition of ER, in particular low frequency periodic variation, stochastic variation with high variance or high autocorrelation [26]. Environmental vagaries may be sufficient in amplitude and duration to prevent apparently tolerant and fertile individuals from initiating a population recovery. ER is therefore a probabilistic process that may not occur even if appropriate phenotypic variation is present in the population.
Furthermore, rapid population decline is associated with a limited window of opportunity for individuals with appropriate phenotypic solutions to the novel environment to reproduce fast enough to begin population recovery. These viable individuals are the source of an incipient rescue event: they may exist at a low frequency in the declining population, but they also arise de novo by mutation or arrive by immigration from elsewhere, should the population be spatially structured (i.e. a metapopulation).
3. The objectives and themes of this issue
A special issue dedicated to ER has never been more opportune. Although a handful of articles have firmly established its theoretical basis, ER has not received widespread attention from theoreticians and empiricists. This situation is changing. Numerous research groups worldwide are currently studying ER from new angles. The theory is being extended to more complex scenarios of ecological and environmental change, new experiments and field data are appearing, and important new applications of the concept to conservation and the realms of biological control and human health (e.g. microbial antibiotic resistance) are now recognized.
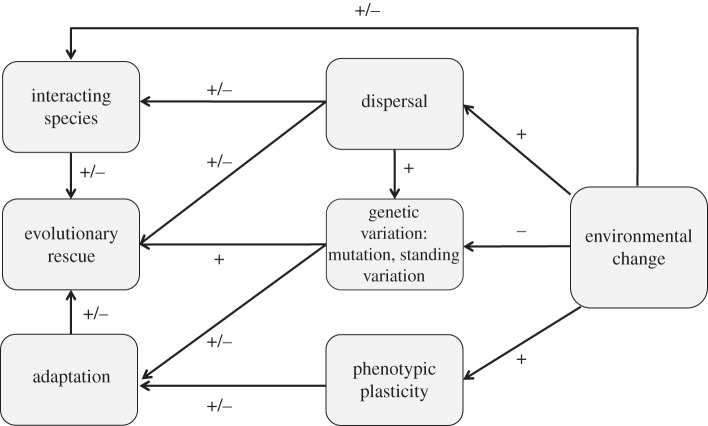
The idea for this special issue stemmed from a workshop held in Montpellier, France in 2011. The talks and discussions lead us to realize that the field was sufficiently mature to publish recent breakthroughs and syntheses in an integrated special issue. The issue has three facets: (i) conceptual and theoretical developments, (ii) experiments in the laboratory, and (iii) evidence from nature. By publishing these contributions together in this theme issue, we hope they will accelerate the development of the research on ER (figure 1).
Figure 1.
A simplified diagram of the factors influencing ER as represented by the contributions to this special issue.
Bell [31] situates the topic of ER within the historical context of the development of ideas in evolutionary biology. He reviews a diverse array of theoretical and empirical findings on the limits of adaptation. Among factors limiting adaptation, he discusses the demographic costs of natural selection and the lack of genetic variation in ecologically relevant traits, as initially indicated, respectively, by Haldane [32], and subsequently by Bradshaw [11]. Bell also argues that ER challenges how we view the process of adaptation. Population genetics has traditionally developed around the concept of relative fitness and ignored population dynamics; ER is about the demography of a population undergoing changes in absolute fitness. The conditions that lead to ER require that we combine population genetics, population dynamics and ecology. ER is also likely to depend on the presence of rare rescue genotypes that are able to withstand levels of stress lethal to most of the population, thus shifting our attention to the extremes of the fitness distribution, and raising many theoretical and empirical questions about the shape and the tails of the distribution in different environments and genetic backgrounds [33]. As found by several theoretical and empirical contributions to this issue, initial population size is a critical determinant of ER because larger size increases the likelihood of obtaining rescue variants, and allows more severe demographic decline to be sustained before the rescue variant emerges. Finally, Bell discusses the pace of habitat deterioration and how it sets the scope for ER: slow deterioration is more conducive to ER for diverse genetic and demographic reasons, which has recently been illustrated through experimental evolution [19].
ER can only occur if beneficial mutations not only become established but also rise in frequency to the point where they increase a population's size and/or growth rate. An integrated approach to the fixation of new beneficial mutations and demographic rescue is taken by Martin et al. [21]. They generalize previous work [15] to broader demographic assumptions (in the absence of density dependence). The theory focuses on a single population confronted with a new environment that causes its decline, such as bacteria exposed to antibiotics. The basic problem is to compute the probability that such a population initially declining at rate r0 < 0 is rescued from extinction by a mutant genotype with growth rate r > 0. A rescue genotype may be present at the onset of stress, or arise by mutation afterward. Martin et al. derive analytical predictions from stochastic population models, using branching processes and diffusion approximations. This new theory allows the integration of the probability of rescue over an arbitrary distribution of mutation effects on demographic parameters. Remarkably, the probability of rescue can be approximated in most cases by a similar expression, depending on the initial population size confronted with a stress, and a per capita rate of rescue that depends on the exact demographic and genetic scenario.
So far ER has been formalized by imagining genetic variants with positive growth rates rescuing a declining population in a stressful environment. Osmond & Mazancourt [34] study a different model where environmental stress affects the carrying capacity of the population, and genetic variants differ not by their growth rate at low density but by their equilibrium population size in the stressful environment (see also appendix in [35]). Despite different assumptions, Osmond & Mazancourt recover a number of conclusions of previous theory on ER: namely that rescue is more likely with large initial population size, larger mutation rates and moderate shifts in environmental conditions. Interestingly, they predict that intermediate levels of selection in the new environment maximize the probability of rescue, by allowing fast evolutionary responses without too much demographic cost. Finally, using a very general formulation, they provide explanations for why interspecific competition might sometimes impede ER, or sometimes accelerate it. Their model thus extends the range of demographic conditions used to study ER. Their study thus also raises questions requiring experiments: does genetic variation for different life-history traits affect the likelihood of rescue? How is such genetic variation expressed at different densities? How can we experimentally discriminate between these situations? The theoretical study by Martin et al. [21] also shows that genetic variation affecting birth rates, mortality or variance in reproduction could potentially have distinct effects on ER. Thus, a detailed understanding of life-history variation seems to be necessary to quantitatively predict the probability of rescue.
Climate change imposes new adaptive challenges on natural populations. The process of adaptation and ER in this case is complicated by the fact that selection on genetic variation varies both in time, through yearly variation in climate and longer-term warming trends, and in space along latitudinal or altitudinal gradients. Climate change is in particular often modelled by imagining a shifting spatial gradient of selection [36–38]. Kirkpatrick & Peischl [39] address the question: ‘When and where do mutations occur that allow adaptation to environments that change in space and time?’ Specifically, they extend population genetics theory to mathematically study the probability of fixation of new beneficial mutations in changing environments. They remind us that population genetic models have provided key insights into this issue, but that there is lack of analytical results for environments that vary both in time and space. In this and a companion paper [40], Kirpatrick & Peischl [39] use mathematical approximations to derive simple analytical results for the cases of a single habitat that changes in time, a constant spatial gradient and a shifting spatial gradient. They show that whether a beneficial mutation becomes established is largely determined by chance events that occur in the first few generations after it appears. In an environment that changes in space and time, fixation further requires that the mutation occurs either near or in front of the region where it is currently favoured, and that it improves fitness enough to establish a travelling wave that advances along with the favourable habitat. They find an upper limit to the rate of environmental change, above which no mutation can become established. This rate is shown to be proportional to the mutation's maximum fitness; it sets an absolute constraint on the opportunity of locally adapted mutations to contribute to ER. Although, Kirkpatrick & Peischl's model does not describe the impact of new mutations on demography, it represents an important step in understanding the constraints on ER in more complex environments, when standing genetic variation for key ecological characters is not available and fixation of beneficial mutations is the main driver of adaptation.
Schiffers et al. [41] also model ER in the context of a spatio-temporally varying environment, examining the antagonistic effects of dispersal on the process of adaptation. They use an individual-based model to simulate a plant population confronted with the double challenge of adapting to increasing temperature through time and to fine-scale spatial heterogeneity in soil conditions. Dispersal in this scenario allows beneficial mutations conferring increased thermal tolerance to spread more quickly through space and rescue the population from extinction. However, dispersal also disrupts local adaptation to soil conditions imposing a demographic load depressing mean fitness, and thus compromising persistence. As a result, the probability of ER is maximized at some intermediate dispersal distance. Interestingly, some of the ER events they observed were ‘partial’, that is, only a limited range of sites in the metapopulation persisted in the long term after climate warming. In these cases, environmental change in one dimension of the niche (e.g. climate) results in the shrinkage of another dimension of the niche (local adaptation to some soil conditions is lost). More generally, this study illustrates the need to take into account the multivariate nature of adaptation: while other studies have shown that genetic correlations between traits can both accelerate or impede the evolutionary response to environmental change [38], the study by Shiffers et al. shows that evolution of genetically independent phenotypic traits can also interact through their effects on demography (here through their effect on gene flow).
An important theme in this special issue is the role phenotypic plasticity plays in modifying the likelihood of ER (see also [40]). Kovach-Orr & Fussman [42] investigated the potential effectiveness of genetic diversity and phenotypic plasticity in an inducible defence trait to allow ER within the context of a simple plankton food web. ER may occur at different trophic levels in this community context, and where it occurs may have different effects on dynamical stability and food web persistence. Their system is complex because it models direct and indirect trophic interactions and the two major sources of intraspecific variation mentioned above. Because of this a range of results emerge. Their basic findings are that plasticity promotes rescue more than genetically based mechanisms and that intraspecific variation tends to promote rescue when it occurs in the herbivore population alone. Their study builds on previous work showing the dynamical effects of eco-evolutionary dynamics in plankton foodwebs [43], but takes the important step of showing that ER may play a role in the dynamics of complex communities.
Ferriere & Legendre [44] remind us that evolution by natural selection does not necessarily lead to increased prospects of persistence. For a range of situations where ecological interactions make selection frequency-dependent, evolution does not optimize any obvious quantity, including population size or growth rate. This means that, in addition to environmental degradation, evolution can take a population into a zone where extinction is more likely than were evolution not to occur. This is, in particular, the case in many ‘tragedy of the commons’ scenarios where selection favours more competitive genotypes increasing in density and frequency but compromising the persistence of the population as a whole. An extreme case of evolution acting against population persistence has been dubbed ‘evolutionary suicide’: situations where a population is deterministically driven to extinction by invasion of competitively superior, yet demographically lethal, genetic variants. Theoretical scenarios of evolutionary suicide have been predicted to occur when there are Allee effects, but clear empirical examples remain scarce. More generally, Ferriere & Legendre highlight that the adaptive dynamics approach, by taking into account the feedbacks between ecology and evolution which generate frequency dependence, can reveal when ER is more complex than a simple race between environmental deterioration and genetic improvement: genetic changes may fail to rescue a population from extinction not because they are not fast enough, but because they aggravate the consequences of environmental change. Contrary to the baseline predictions of ER theory, small population sizes and/or low genetic variation for traits, such as dispersal or cooperation, may in theory actually help a population resist evolutionary suicide and escape ‘evolutionary traps’. Empirical tests of the prevalence of these scenarios in populations under environmental stress are needed.
The contributions of this special issue thus illustrate how the theory of ER has recently expanded and became more sophisticated by incorporating density and frequency dependence, diverse patterns of spatial and temporal variation in selection, plasticity and community interactions. In order to make progress towards incorporating ER in forecasts of future biodiversity loss, we need models to be empirically validated and tested. Experimental evolution offers a powerful opportunity to study ER in well-replicated experiments. Yet, to facilitate the quantitative comparison between models and data, theoretical predictions should use empirically measurable parameters. Models also need to account for certain peculiarities of experimental populations, such as overlapping generations for microbes, bottlenecks during transfers, low levels of polymorphism, asexual reproduction, etc. The model presented by Martin et al. [21] attempts to make a step in this direction. Remarkably, they are able to compare their mathematical predictions with experimental data. They evaluate the fit of their model by using data from previous experiments, one on bottlenecked populations of yeast subject to salt stress [18], and the other on declining bacterial populations exposed to antibiotics [45]. They also suggest several experimental perspectives to refine the quantitative comparison of their models with data.
Gonzalez & Bell [46] extend their recent series of experiments on ER [18,19] to consider the importance of historical selection. Using high throughput, robot-based methods, in experimental evolution, they study the ability of two species of yeast (Saccharomyces cerevisiae and Saccharomyces paradoxus) to adapt to a gradient of salinity over many generations, and then examine whether adaptation to chronic salt stress facilitated ER following transfer to concentrates of salt lethal to the ancestor. They found that the species differed in their capacity to adapt to chronic salinity, and S. cerevisiae adapted to higher salt concentration than S. paradoxus. The probability of ER was positively correlated with salt concentration for S. cerevisiae, but negatively correlated for S. paradoxus. This is the first indication that the probability of ER was an indirect response to historical selection.
Chevin et al. [47] like Kovach-Orr & Fussman [42], make the important point that the fate of a population in a changing environment can be affected by phenotypic plasticity (where the phenotype of a given genotype changes with its environment of development) and that this may alter the likelihood of ER. Phenotypic plasticity may also evolve in response to selection further complicating the task of attributing demographic rescue to genetic adaptation, most especially under field conditions where population replication is limited, environmental conditions are not easily controlled, and the measuring of selection and inheritance of traits affecting fitness is difficult at best. Chevin et al. [48] summarize the findings from an augmented theory of ER that incorporates plasticity, and that predicts that plasticity is likely to foster ER if (i) plasticity is initially adaptive because it decreases the severity of effects stemming from environmental change and (ii) because evolution of plasticity can accelerate adaptation. They argue that experimental evolution in the laboratory is the most promising place to start to test this theory. The article closes with some open questions requiring further research and some suggestions for how to test them with an experiment and the most appropriate model organism.
Moving from theory and experimental evolution, Vander Wal et al. [22] review evidence for ER in wild vertebrate populations. Their first conclusion is that such evidence is scarce and documented cases of ER restricted to short-lived fecund animals with large population sizes, such as rats and rabbits when confronted with attempts at eradication using pathogens and pesticides. Still, there is now building evidence of genetic changes in wild vertebrate populations tracking environmental change, even though the consequences of such adaptive tracking, or lack thereof, on population persistence are rarely documented [49]. Conversely, there are now several cases of successful genetic rescue in the wild, where artificial introduction of genetic diversity through immigration helped populations rebound from dangerously low numbers. Mechanisms of rescue are then thought to reduce inbreeding depression and deleterious mutation accumulation, and it is open to discussion whether it is desirable to include such instances as special cases of ER. Absence of clear evidence for ER in species of conservation concern could be explained by the difficulty of tracking genetic changes in wild populations and, in parallel, evaluating the demographic consequences of such changes for persistence. However, spontaneous ER is unlikely to save small, genetically depauperate populations of organisms with long generation times, facing many simultaneous threats, as is typically the case for endangered species. ER cannot be seen as a panacea to solve the many threats on biodiversity. Yet, Vander Wal and colleagues defend the idea that research on ER could still profitably inform conservation biology of vertebrate populations by (i) drawing attention to the necessity to preserve evolutionary potential (combining its demographic and genetic components) in large populations confronted with fast changing environments, (ii) stimulating innovative interventions to foster increased evolutionary potential in rare endangered species, in a manner similar in spirit to genetic rescue. To achieve these goals, they propose a research programme mapping genotypes, phenotypes, demography and fitness and offer different priorities for research depending on the conservation issue.
In the spirit of such a research programme, Gienapp et al. [49] used data from a long-term field study and powerful modelling approach to estimate the critical rate of climate change beyond which micro-evolution of breeding time in the great tit (Parus major) would be insufficient to prevent population decline and extirpation. This article shows the unique value of long-term population studies. With it they were able to obtain the genetic, ecological and demographic data required to parametrize a stochastic dynamic programming model to predict the optimal breeding time (trait under selection) of great tits in the future, and a survival model to predict future egg-laying dates as a function of temperatures predicted by various climate change scenarios. With this information, they parametrized two theoretical evolution models, to predict the critical rates of environmental change likely to prevent ER. The bottom line is that even with phenotypic plasticity, mild rates of climate change would approach the critical rate, and scenarios close to the upper limit of the IPCC climate change projections would exceed the critical rate for population persistence.
Paralleling the prediction that past evolution should affect the probability of contemporary ER [46], past niche evolution might be expected to influence species’ responses to global change. To test this idea, Lavergne et al. [50] used a large dataset on more than 400 European bird species that combined information on phylogeny, contemporary demographic trends, life history, habitats, and trophic and climatic niches to identify species and lineages that have shown either niche conservatism or niche evolution. Past rates of evolution were compared among species with declining, increasing or stable population sizes in recent decades. They found that species and lineages characterized by a history of rapid niche evolution could be more resilient to current-day global changes than species that have experienced slower niche evolution. This study is a first step in linking macroevolutionary and microevolutionary patterns and provides a promising way forward to incorporate ER into to forecasts of biodiversity change at broad geographical and taxonomic scales.
Gandon et al. [51] provide a thorough review of evolutionary epidemiology theory that can contribute to our understanding of ER. They emphasize that ER is likely to be a recurrent and widespread feature of the co-evolutionary dynamics of hosts and pathogens. When a pathogen is exposed to a new host, an epidemic might occur even if its basic reproduction rate R0 < 1 initially, because mutations might arise that make R0 > 1 before extinction occurs. Thus, there is a race between the process of extinction and the process of adaptation to the new host. There is ‘evolutionary emergence’ of a new disease in the host population when the pathogen wins the race. Evolutionary epidemiology theory has been developed to identify the key factors that govern the probability of evolutionary emergence. In effect, much of this theory is relevant to ER. Insights gained by exploiting the analogy between the evolutionary emergence of pathogens and general ER will help us understand the effect of migration and reintroductions (here there are direct links with theory of adaptation to sink habitats), the effect of life history (the same growth rate r0 < 0 can be realized by very different life histories and that life history may influence which rescuing r > 0 value can be reached by mutation), the effect of host (habitat) heterogeneity and the effect of the mutation process (distribution of fitness effects, distribution of life-history effects and mutation rates).
Gomulkiewicz & Shaw [23] close with a challenge: can recent theoretical and experimental results be extended to inform when natural populations will undergo ER if faced with novel environmental conditions? Their article addresses how this might be made possible. The main obstacle to this is the study of long-lived organisms, because low replication and multiple interacting selective pressures limit the power to infer the genetic basis of the changes in absolute fitness responsible for a putative ER event. An important solution involves coupling long-term field studies with carefully parametrized eco-evolutionary models [49,52]. But even then, an environmental event is required to drive population decline and elicit ER, the likelihood of which may be low. Field experiments involving controlled and sustained environmental press conditions coupled with relatively fast reproducing wild populations of animals (e.g. Poecilid fishes, or small rodents such Peromyscus sp.) or plants (e.g. aquatic plants in the genus Lemna) will be required to demonstrate the conditions leading to ER in nature. Field evidence will identify conditions that can be used for the management of wild populations, including those of conservation value, and those that are not, such as invasive species.
4. Concluding thoughts and future steps
This special issue provides an interesting vantage point to survey promising future directions for the research programme on ER. Contributions to the issue consistently show the value of linking theoretical and experimental methods. Theoretical models have used widely different techniques and assumptions, each with their own merits and drawbacks, but despite this the theory of ER has now provided a core set of conclusions upon which to build. To date, the common prediction is that ER is an unlikely outcome for natural populations, because rescue is most likely in large populations under moderate rates and magnitudes of environmental change, and persistence is not necessarily increased by higher levels of variation in traits linked to fitness [1,44]. It remains to be seen how robust this conclusion is when major assumptions are relaxed and when greater ecological and evolutionary complexity is incorporated into the models. Interesting biological questions that have been little explored from a theoretical point-of-view include the effect of mating system [53], age structure and dispersal mode [54] on the probability of ER. We also need to further our theoretical understanding of general effects of biotic interactions. Finally, we need operational predictors of when adaptation will promote persistence or compromise it.
A first wave of experimental tests of this theory is underway, but for the most part they have been restricted to the laboratory. The laboratory constitutes a promising ‘halfway house’ between mathematical models and field experiments. Experimental evolution has already yielded valuable insights about how different forms of environmental change and population parameters (e.g. population size and genetic variation) may contribute to the probability of ER [18,19]. A new generation of laboratory experiments can provide guidelines for new theory. Some issues for experimental evolution to tackle include:
— Experiments are needed to reveal the genetic mechanisms (e.g. changes in genome size, gene expression and regulation, mutation rate) and the developmental phenotypic plasticity in key traits responsible for changes in fitness that underlie the demographic recovery associated with ER. High throughput and real-time sequencing techniques coupled to new approaches in phenomics [55] are now making this goal a possibility.
— Tests of quantitative theoretical predictions using models tailored to the empirical system. Martin et al. [21] and Gienapp et al. [49] in this issue point to progress in this direction. Tighter links between models and experiments will improve the applicability of current theory beyond the laboratory. Better models will lead to more accurate predictions. In most cases, we will be entirely reliant on models to identify critical rates of environmental change, where and when populations are most likely to be at risk of extinction and thus most in need of management to foster a rapid adaptive response and recovery by ER.
— Ultimately ER occurs within the context of a complex biotic environment and so experiments that incorporate multispecies interactions in micro- or mesocosms are needed to guide the new theory in this context. To incorporate more trophic complexity, experiments should be extended to rapidly reproducing plants (e.g. Arabidopsis and Lemna) and animals (e.g. Daphnia and Drosophila). This development will also allow experiments with organisms of sufficient complexity to allow tests of ER theory with organisms more akin to those of conservation concern. It would then be a small step to experiments and sustained genetic monitoring [56] of populations in the field; this would provide much needed tests of the applied value of ER for biodiversity conservation, restoration of ecosystem function and services, disease management and pest control [23].
We believe the tight links between theory and experiments evident in this special issue will provide the firmest basis for rapid progress in the ongoing synthesis between ecology and evolution: this is necessary if we are to understand the extent to which anthropogenic global change will reduce the Earth's biodiversity.
Acknowledgements
We thank all the authors for their hard work and excellent contributions to this issue. We are grateful to the many anonymous reviewers, who often provided prompt and in-depth reviews. We also thank Helen Eaton for her advice and help throughout. A.G. is supported by the Canada Research Chair programme. M.E.H. is supported by the Agence Nationale de la Recherche Scientifique (EvolStress ANR-09-BLAN-099-01) and the McDonnell Foundation (JSMF 220020294/SCS-Research Award). We thank Nicolas Mouquet and Fadela Tamoune for their instrumental help in co-organizing the open conference ‘ER in a changing world’, Montpellier 2011. We thank all participants to this conference, who stimulated us to put together this special issue. We thank many funders of this event: Agence Nationale de la Recherche (project EVORANGE, ANR-09-PEXT-011), Région Languedoc Roussillon, Conseil Scientifique Université Montpellier 2, Société Française d'Ecologie, Institut des Sciences de l'Evolution de Montpellier, Centre d'Ecologie Fonctionnelle et Evolutive, SFR Montpellier Environnement Biodiversité, Central National de la Recherche Scientifique, Canada Foundation for Innovation, Canada Research Chairs, Natural Sciences and Engineering Research Council of Canada. This is publication ISEM 2012-137.
References
- 1.Ferrière R, Dieckmann U, Couvet D. (eds) 2004. Evolutionary conservation biology. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Levin BR, Lipsitch M, Perrot V, Schrag S, Antia R, Simonsen L, Moore Walker N, Stewart FM. 1997. The population genetics of antibiotic resistance. Clin. Infect. Dis. 24, S9–S16 10.1093/clinids/24.Supplement_1.S9 (doi:10.1093/clinids/24.Supplement_1.S9) [DOI] [PubMed] [Google Scholar]
- 3.McNeilly T, Bradshaw AD. 1968. Evolutionary processes in populations of copper tolerant Agrostis tenuis Sibth. Evolution 22, 108–118 10.2307/2406655 (doi:10.2307/2406655) [DOI] [PubMed] [Google Scholar]
- 4.Kettlewell HBD. 1973. The evolution of melanism: the study of a recurring necessity. Oxford, UK: Oxford University Press [Google Scholar]
- 5.Jasieniuk M, Brule-Babel AL, Morrison IN. 1996. The evolution and genetics of herbicide resistance in weeds. Weed Sci. 44, 176–193 [Google Scholar]
- 6.Georghiou GP. 1972. The evolution of resistance to pesticides. Ann. Rev. Ecol. Syst. 3, 133–168 10.1146/annurev.es.03.110172.001025 (doi:10.1146/annurev.es.03.110172.001025) [DOI] [Google Scholar]
- 7.Handford P, Bell G, Reimchen T. 1977. A gillnet fishery considered as an experiment in artificial selection. J. Fish. Res. Can. 34, 954–961 10.1139/f77-148 (doi:10.1139/f77-148) [DOI] [Google Scholar]
- 8.Thompson JN. 1998. Rapid evolution as an ecological process. Trends Ecol. Evol. 13, 329–332 10.1016/S0169-5347(98)01378-0 (doi:10.1016/S0169-5347(98)01378-0) [DOI] [PubMed] [Google Scholar]
- 9.Hendry AP, Farrugia T, Kinnison MT. 2008. Human influences on rates of phenotypic change in wild animal populations. Mol. Ecol. 17, 20–29 10.1111/j.1365-294X.2007.03428.x (doi:10.1111/j.1365-294X.2007.03428.x) [DOI] [PubMed] [Google Scholar]
- 10.Kwiatkowski RE, Roff JC. 1976. Effects of acidity on the phytoplankton and primary productivity of selected northern Ontario lakes. Can. J. Bot. 54, 2546–2561 10.1139/b76-274 (doi:10.1139/b76-274) [DOI] [Google Scholar]
- 11.Bradshaw AD. 1991. Genostasis and the limits to evolution. Phil. Trans. R. Soc. Lond. B 333, 289–305 10.1098/rstb.1991.0079 (doi:10.1098/rstb.1991.0079) [DOI] [PubMed] [Google Scholar]
- 12.Lynch M, Lande R. 1993. Evolution and extinction in response to environmental change. In Biotic interactions and global change (eds Kareiva PM, Kingsolver JG, Huey RB.), pp. 234–250 Sunderland, MA: Sinauer [Google Scholar]
- 13.Gomulkiewicz R, Holt RD. 1995. When does evolution by natural selection prevent extinction? Evolution 49, 201–207 10.2307/2410305 (doi:10.2307/2410305) [DOI] [PubMed] [Google Scholar]
- 14.Holt RD, Gomulkiewicz R. 2004. Conservation implication of niche conservatism and evolution in heterogeneous environments. In Evolutionary conservation biology (eds Ferrière R, Dieckmann U, Couvet D.), pp. 244–264 Cambridge, UK: Cambridge University Press [Google Scholar]
- 15.Orr HA, Unckless RL. 2008. Population extinction and the genetics of adaptation. Am. Nat. 172, 160–169 10.1086/589460 (doi:10.1086/589460) [DOI] [PubMed] [Google Scholar]
- 16.Agashe D. 2009. The stabilizing effect of intraspecific genetic variation on population dynamics in novel and ancestral environment. Am. Nat. 174, 255–267 10.1086/600085 (doi:10.1086/600085) [DOI] [PubMed] [Google Scholar]
- 17.Agashe D, Falk JJ, Bolnick DI. 2011. Effects of founding genetic variation on adaptation to a novel resource. Evolution 65, 2481–2491 10.1111/j.1558-5646.2011.01307.x (doi:10.1111/j.1558-5646.2011.01307.x) [DOI] [PubMed] [Google Scholar]
- 18.Bell G, Gonzalez A. 2009. Evolutionary rescue can prevent extinction following environmental change. Ecol. Lett. 12, 942–948 10.1111/j.1461-0248.2009.01350.x (doi:10.1111/j.1461-0248.2009.01350.x) [DOI] [PubMed] [Google Scholar]
- 19.Bell G, Gonzalez A. 2011. Adaptation and evolutionary rescue in metapopulations experiencing environmental deterioration. Science 332, 1327–1330 10.1126/science.1203105 (doi:10.1126/science.1203105) [DOI] [PubMed] [Google Scholar]
- 20.Samani P, Bell G. 2010. Adaptation of experimental yeast populations to stressful conditions in relation to population size. J. Evol. Biol. 23, 791–796 10.1111/j.1420-9101.2010.01945.x (doi:10.1111/j.1420-9101.2010.01945.x) [DOI] [PubMed] [Google Scholar]
- 21.Martin G, Aguilée R, Ramsayer J, Kaltz O, Ronce O. 2012. The probability of evolutionary rescue: towards a quantitative comparison between theory and evolution experiments. Phil. Trans. R. Soc. B 368, 20120088. 10.1098/rstb.2012.0088 (doi:10.1098/rstb.2012.0088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vander Wal E, Garant D, Festa-Bianchet M, Pelletier F. 2012. Evolutionary rescue in vertebrates: evidence, applications, and uncertainty. Phil. Trans. R. Soc. B 368, 20120090. 10.1098/rstb.2012.0090 (doi:10.1098/rstb.2012.0090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomulkiewicz R, Shaw RG. 2012. Evolutionary rescue beyond the models. Phil. Trans. R. Soc. B 368, 20120093. 10.1098/rstb.2012.0093 (doi:10.1098/rstb.2012.0093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baquero F, Coque TM, De La Cruz F. 2011. Eco-Evo drugs and strategies: the need for novel tools to fight antibiotic resistance. Antimicrob. Agents Chemother. 55, 3649–3660 10.1128/AAC.00013-11 (doi:10.1128/AAC.00013-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haldane JBS. 1939. The effect of variation of fitness. Am. Nat. 71, 337–349 10.1086/280722 (doi:10.1086/280722) [DOI] [Google Scholar]
- 26.Bürger R, Krall C. 2004. Quantitative-genetic models and changing environments. In Evolutionary conservation biology (eds Ferrière R, Dieckmann U, Couvet D.), pp. 171–187 Cambridge, UK: Cambridge University Press [Google Scholar]
- 27.Perron GG, Gonzalez A, Buckling A. 2007. Source-sink dynamics shape the evolution of antibiotic resistance and its pleiotropic fitness cost. Proc. R. Soc. B 274, 2351–2356 10.1098/rspb.2007.0640 (doi:10.1098/rspb.2007.0640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lachapelle J, Bell G. 2012. Evolutionary rescue of sexual and asexual populations in a deteriorating environment. Evolution. 10.1111/j.1558-5646.2012.01697.x (doi:10.1111/j.1558-5646.2012.01697.x) [DOI] [PubMed] [Google Scholar]
- 29.Tallmon DA, Luikart G, Waples RS. 2004. The alluring simplicity and complex reality of genetic rescue. Trends Ecol. Evol. 19, 489–496 10.1016/j.tree.2004.07.003 (doi:10.1016/j.tree.2004.07.003) [DOI] [PubMed] [Google Scholar]
- 30.Hedrick PW, Adams JR, Vucetish JA. 2011. Reevaluating and broadening the definition of genetic rescue. Conserv. Biol. 25, 1069–1070 10.1111/j.1523-1739.2011.01751.x (doi:10.1111/j.1523-1739.2011.01751.x) [DOI] [PubMed] [Google Scholar]
- 31.Bell G. 2012. Evolutionary rescue and the limits of adaptation . Phil. Trans. R. Soc. B 368, 20120080. 10.1098/rstb.2012.0080 (doi:10.1098/rstb.2012.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haldane JBS. 1957. The cost of natural selection. J. Genet. 55, 511–524 10.1007/BF02984069 (doi:10.1007/BF02984069) [DOI] [Google Scholar]
- 33.Joyce P, Rokyta DR, Beisel CJ, Orr HA. 2008. A general extreme value theory model for the adaptation of DNA sequences under strong selection and weak mutation. Genetics 180, 1627–1643 10.1534/genetics.108.088716 (doi:10.1534/genetics.108.088716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osmond MM, de Mazancourt C. 2012. How competition affects evolutionary rescue. Phil. Trans. R. Soc. B 368, 20120085. 10.1098/rstb.2012.0085 (doi:10.1098/rstb.2012.0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uecker H, Hermisson J. 2011. On the fixation process of a beneficial mutation in a variable environment. Genetics 188, 915–930 10.1534/genetics.110.124297 (doi:10.1534/genetics.110.124297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pease CM, Lande R, Bull JJ. 1989. A model of population growth, dispersal and evolution in a changing environment. Ecology 70, 1657–1664 10.2307/1938100 (doi:10.2307/1938100) [DOI] [Google Scholar]
- 37.Polechova J, Barton NH, Marion G. 2009. Species range: adaptation in space and time. Am. Nat. 174, E186–E204 10.1086/605958 (doi:10.1086/605958) [DOI] [PubMed] [Google Scholar]
- 38.Duputié A, Massol F, Chuine I, Kirkpatrick M, Ronce O. 2012. How do genetic correlations affect species range shifts in a changing environment? Ecol. Lett. 15, 251–259 10.1111/j.1461-0248.2011.01734.x (doi:10.1111/j.1461-0248.2011.01734.x) [DOI] [PubMed] [Google Scholar]
- 39.Kirkpatrick M, Peischl S. 2012. Evolutionary rescue by beneficial mutations in environments that change in space and time. Phil. Trans. R. Soc. B 368, 20120082. 10.1098/rstb.2012.0082 (doi:10.1098/rstb.2012.0082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peischl S, Kirkpatrick M. 2012. Establishment of new mutations in changing environments. Genetics 191, 895–906 10.1534/genetics.112.140756 (doi:10.1534/genetics.112.140756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiffers K, Bourne EC, Lavergne S, Thuiller W, Travis JMJ. 2012. Limited evolutionary rescue of locally adapted populations facing climate change. Phil. Trans. R. Soc. B 368, 20120083. 10.1098/rstb.2012.0083 (doi:10.1098/rstb.2012.0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovach-Orr C, Fussman GF. 2012. Evolutionary and plastic rescue in multitrophic model communities. Phil. Trans. R. Soc. B 368, 20120084. 10.1098/rstb.2012.0084 (doi:10.1098/rstb.2012.0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida T, Jones LE, Ellner SP, Fussmann GF, Hairston NG., Jr 2003. Rapid evolution drives ecological dynamics in a predator–prey system. Nature 424, 303–306 10.1038/nature01767 (doi:10.1038/nature01767) [DOI] [PubMed] [Google Scholar]
- 44.Ferriere R, Legendre S. 2012. Eco-evolutionary feedbacks, adaptive dynamics, and evolutionary rescue theory. Phil. Trans. R. Soc. B 368, 20120081. 10.1098/rstb.2012.0081 (doi:10.1098/rstb.2012.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramsayer J, Kaltz O, Hochberg ME. In press. Evolutionary rescue in populations of Pseudomonas fluorescens across an antibiotic gradient. Evol. Appl . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez A, Bell G. 2012. Evolutionary rescue and adaptation to abrupt environmental change depends upon the history of stress. Phil. Trans. R. Soc. B 368, 20120079. 10.1098/rstb.2012.0079 (doi:10.1098/rstb.2012.0079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chevin L-M, Gallet R, Gomulkiewicz R, Holt RD, Fellous S. 2012. Phenotypic plasticity in evolutionary rescue experiments. Phil. Trans. R. Soc. B 368, 20120089. 10.1098/rstb.2012.0089 (doi:10.1098/rstb.2012.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chevin L-M, Lande R. 2010. When do adaptive plasticity and genetic evolution prevent extinction of a density-regulated population? Evolution 64, 1143–1150 10.1111/j.1558-5646.2009.00875.x (doi:10.1111/j.1558-5646.2009.00875.x) [DOI] [PubMed] [Google Scholar]
- 49.Gienapp P, Lof M, Reed T, Verhulst S, Visser M. 2012. Predicting demographically sustainable rates of adaptation: can great tit breeding time keep pace with climate change? Phil. Trans. R. Soc. B 368, 20120289. 10.1098/rstb.2012.0289 (doi:10.1098/rstb.2012.0289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lavergne S, Evans MEK, Burfield IJ, Jiguet F, Thuiller W. 2012. Are species’ responses to global change predicted by past niche evolution? Phil. Trans. R. Soc. B 368, 20120091. 10.1098/rstb.2012.0091 (doi:10.1098/rstb.2012.0091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gandon S, Hochberg ME, Holt RD, Day T. 2012. What limits the evolutionary emergence of pathogens? Phil. Trans. R. Soc. B 368, 20120086. 10.1098/rstb.2012.0086 (doi:10.1098/rstb.2012.0086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pelletier F, Clutton-Brock T, Pemberton J, Tuljapurkar S, Coulson T. 2007. The evolutionary demography of ecological change: linking trait variation and population growth. Science 315, 1571–1574 10.1126/science.1139024 (doi:10.1126/science.1139024) [DOI] [PubMed] [Google Scholar]
- 53.Glémin S, Ronfort J. 2012. Adaptation and maladaptation in selfing and outcrossing species: new mutations versus standing variation. Evolution. 10.1111/j.1558-5646.2012.01778.x (doi:10.1111/j.1558-5646.2012.01778.x) [DOI] [PubMed] [Google Scholar]
- 54.Aguilée R, Shaw FH, Rousset F, Shaw RG, Ronce O. 2012. How does pollen versus seed dispersal affect niche evolution? Evolution 367 10.1111/j.1558-5646.2012.01816.x (doi:10.1111/j.1558-5646.2012.01816.x) [DOI] [PubMed] [Google Scholar]
- 55.Warringer J, Ericson E, Fernandez L, Nerman O, Blomberg A. 2003. High-resolution yeast phenomics resolves different physiological features in the saline response. Proc. Natl Acad. Sci. USA 100, 15 724–15 729 10.1073/pnas.2435976100 (doi:10.1073/pnas.2435976100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansen MM, Olivieri I, Waller DM, Nielsen EE; GeM Working Group 2012. Monitoring adaptive genetic responses to environmental change. Mol. Ecol. 21, 1311–1329 10.1111/j.1365-294X.2011.05463.x (doi:10.1111/j.1365-294X.2011.05463.x) [DOI] [PubMed] [Google Scholar]