Abstract
Objective
To identify the change in the incidence of cutaneous melanoma over time among young adults.
Patients and Methods
Using Rochester Epidemiology Project data, we identified patients aged 18 to 39 years who had a first lifetime diagnosis of melanoma from January 1, 1970, through December 31, 2009, in Olmsted County, Minnesota. Demographic and clinical information, including survival, was abstracted, and estimates of the incidence of melanoma and overall and disease-specific survival were generated.
Results
From 1970 to 2009, the incidence of melanoma increased by 8-fold among young women and 4-fold among young men. Overall and disease-specific survival seemed to improve over time; hazard ratios comparing year of diagnosis with mortality were 0.92 and 0.91, respectively.
Conclusion
The incidence of cutaneous melanoma among young adults is rapidly increasing, especially among women. Continued close monitoring of this high-risk population is necessary.
Abbreviations and Acronyms: CI, confidence interval; REP, Rochester Epidemiology Project; SEER, Surveillance, Epidemiology, and End Results
Melanoma remains a major cause of morbidity and mortality in the United States. It is the fifth most common cancer in men and the seventh most common in women.1 Among young adults, melanoma is the second most common invasive cancer, behind only breast cancer.2 The lifetime risk of melanoma is 1.5 times higher in males than in females.3 This tendency to male predominance is reversed in young adults; in some younger age ranges, the female-male ratio is as high as 1.8.4
The incidence of nonmelanoma skin cancer has been increasing among young adults.5 This finding raises the question of whether the incidence of cutaneous melanoma is also increasing in this age group. The National Cancer Institute recently reported from the Surveillance, Epidemiology, and End Results (SEER) database that in males aged 15 to 39 years the age-adjusted annual incidence of melanoma increased from 4.7 cases per 100,000 persons in 1973 to 7.7 cases per 100,000 persons in 2004. In females of similar age, the age-adjusted annual incidence increased from 5.5 cases per 100,000 persons in 1973 to 13.9 cases per 100,000 persons in 2004.6 Interestingly, although the incidence is increasing, the disease-specific mortality rate seems to be decreasing in young patients in whom melanoma develops.6
Little is known about the natural history, distribution of subtypes, and disease-related mortality of melanoma among young adults. Studies addressing these issues have been unable to provide useful data because they are too small,7 not performed in well-defined populations,8 or biased by the underreporting and delayed reporting that characterize registry-based epidemiology studies.9-11 Some studies12 have suggested that younger age at the time of diagnosis of melanoma is correlated with increased risk of nodal disease.
The primary objective of this study was to estimate the age- and sex-specific incidence of melanoma in Olmsted County, Minnesota, in patients aged 18 to 39 years from 1970 through 2009. The secondary objectives were to describe the clinical presentation, histologic subtypes, anatomic distribution, and rates of recurrence, metastasis, and death among this population.
Patients and Methods
The Rochester Epidemiology Project (REP) was started in 1966, when indexes of diagnoses were created for use by the medical professionals in Olmsted County, Minnesota. The result is linkage of medical data from almost all sources of medical care available to the local population of the county. This data resource provides the ability to conduct population-based analytic studies for almost any disease.13
This study was approved by the institutional review boards of Olmsted Medical Center and Mayo Clinic. Only records of those patients who had previously provided consent for research were studied.
Potential cases of cutaneous melanoma were identified through the REP databases using the appropriate codes adapted from the International Classification of Diseases, Ninth Revision (ICD-9) and Hospital International Classification of Diseases. Patients with noncutaneous melanoma were excluded. Confirmation of diagnosis of cutaneous melanoma and residency status in Olmsted County, Minnesota, was conducted. The diagnosis was established on the basis of consensus dermatopathology diagnosis from skin biopsy specimens. For all confirmed cases, a date of diagnosis was established based on the date of biopsy. Inclusion criteria were a confirmed first lifetime diagnosis of cutaneous melanoma, age between 18 and 39 years, Olmsted County residency, and date of diagnosis between January 1, 1970, and December 31, 2009. Once the inclusion criteria were confirmed, the medical record was abstracted to determine patient demographics, melanoma location, pathologic stage, tumor subtype, Breslow depth, clinical course, and final outcome. Available pathology slides were reviewed by a single author (L.E.G.) to confirm the diagnosis of melanoma and, in some cases, to determine the histologic subtype of the melanoma.
Incidence rates per 100,000 person-years were calculated overall and by decade using the incident cases of melanoma as the numerator and age- and sex-specific estimates of the population of Olmsted County as the denominator. The population at risk was estimated using US Census data from 1970, 1980, 1990, and 2000, with linear interpolation for intercensal years. Incidence rates were age and sex adjusted to the structure of the 2000 US white population.
The associations between the incidence of melanoma and age at diagnosis, sex, and calendar year of diagnosis were assessed by fitting generalized linear models using the SAS procedure GENMOD (SAS Institute Inc, Cary, NC). Incident cases were grouped into 4 age intervals (18-24, 25-29, 30-34, and 35-39 years) and 4 calendar year intervals (1970-1979, 1980-1989, 1990-1999, and 2000-2009). The models fit the natural logarithm of crude incidence rates as a linear function of age at diagnosis, sex, and calendar year of diagnosis, with a Poisson distribution used to model the error structure. The significance of the linear trends for the features of interest and interaction terms among these features was assessed using likelihood ratio statistics.
Overall survival and disease-specific survival were estimated using the Kaplan-Meier method. The duration of follow-up was calculated from the date of diagnosis to the date of death or last follow-up. Associations with death from any cause and death from disease were evaluated using Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% confidence intervals.
All analyses were performed using the SAS software package (version 9.2), and P<.05 was considered statistically significant.
Results
Using REP resources, we identified 256 young adults between the ages of 18 and 39 years who were residents of Olmsted County, Minnesota, at their first lifetime diagnosis of melanoma between 1970 and 2009. Characteristics of the 256 young adults under study are summarized in Table 1.
TABLE 1.
Summary of Characteristics of 256 Young Adults With Melanomaa
| Characteristic | Findingb |
|---|---|
| Age at diagnosis, mean (median; range), y | 29.9 (30; 18-39) |
| Breslow thickness, mean (median; range), mm (n=202) | 0.82 (0.53; 0.11-19.70) |
| Sex | |
| Female | 179 (70) |
| Male | 77 (30) |
| Decade of diagnosis | |
| 1970-1979 | 16 (6) |
| 1980-1989 | 44 (17) |
| 1990-1999 | 67 (26) |
| 2000-2009 | 129 (50) |
| Site, F/M (n=179 women and 76 men)c | |
| Lower extremity | 55 (31)/8 (11) |
| Back | 34 (19)/23 (30) |
| Upper extremity | 37 (21)/12 (16) |
| Neck, shoulder | 14 (8)/10 (13) |
| Chest, breast | 14 (8)/7 (9) |
| Head | 9 (5)/10 (13) |
| Abdomen | 10 (6)/6 (8) |
| Foot | 6 (3)/0 (0) |
| Location (n=254) | |
| Left | 122 (48) |
| Right | 111 (44) |
| Central | 21 (8) |
| Breslow thickness, mm (n=202) | |
| ≤1.00 | 167 (83) |
| 1.01-2.00 | 23 (11) |
| 2.01-4.00 | 9 (4) |
| >4.00 | 3 (1) |
| Histogenic type (n=223) | |
| SS | 155 (70) |
| MIS | 45 (20) |
| Nodular | 15 (7) |
| Spitzoid | 6 (3) |
| Acral lentiginous | 1 (<1) |
| Lentigo maligna | 1 (<1) |
| Pathologic stage (n=250) | |
| 0 | 45 (18) |
| IA | 161 (64) |
| IB | 20 (8) |
| Pathologic stage (n=250) (continued) | |
| IIA | 7 (3) |
| IIB | 1 (<1) |
| IIIA | 2 (1) |
| IIIB | 1 (<1) |
| IIIC | 1 (<1) |
| IV | 12 (5) |
| Overall stage (n=250) | |
| I | 226 (90) |
| II, III, or IV | 24 (10) |
| Disease type (n=250) | |
| Noninvasive | 45 (18) |
| Invasive | 205 (82) |
MIS = melanoma in situ; SS = superficial spreading.
Data are presented as number (percentage) unless indicated otherwise.
Site not available for 1 male patient.
Histologic slides were available for 220 of the 256 melanomas. On the basis of reanalysis, the diagnosis of melanoma was confirmed for all tumors. The histologic subtype was changed from unknown to a known category in 12 melanomas. Two tumors were changed from superficial spreading to spitzoid type.
The overall age- and sex-adjusted incidence of melanoma for these young adults was 16.9 cases per 100,000 person-years (Table 2). The age-adjusted incidence was higher for women than men (23.2 vs 10.8 cases per 100,000 person-years; P<.001). The incidence of melanoma increased with age at diagnosis (P=.05) and by calendar year of diagnosis (P<.001) for both women and men. Median age and female-male sex distribution at time of diagnosis did not change over time.
TABLE 2.
Incidence of Melanoma for Young Adults Stratified by Advanced and Invasive Disease, 1970-2009
| Type and decade | Women |
Men |
Overall |
|||
|---|---|---|---|---|---|---|
| No. | Ratea | No. | Ratea | No. | Rateb | |
| All | ||||||
| 1970-2009 | 179 | 23.2 | 77 | 10.8 | 256 | 16.9 |
| 1970-1979 | 10 | 5.4 | 6 | 4.3 | 16 | 4.8 |
| 1980-1989 | 30 | 16.1 | 14 | 7.6 | 44 | 11.8 |
| 1990-1999 | 47 | 23.2 | 20 | 10.1 | 67 | 16.6 |
| 2000-2009 | 92 | 43.5 | 37 | 18.6 | 129 | 30.8 |
| Stage II, III, or IV | ||||||
| 1970-2009 | 8 | 1.0 | 16 | 2.2 | 24 | 1.6 |
| 1970-1979 | 0 | 0.0 | 2 | 1.4 | 2 | 0.7 |
| 1980-1989 | 1 | 0.4 | 6 | 3.2 | 7 | 1.8 |
| 1990-1999 | 5 | 2.3 | 5 | 2.6 | 10 | 2.4 |
| 2000-2009 | 2 | 1.0 | 3 | 1.6 | 5 | 1.3 |
| Stage I | ||||||
| 1970-2009 | 168 | 21.8 | 58 | 8.1 | 226 | 14.9 |
| 1970-1979 | 9 | 4.7 | 3 | 2.3 | 12 | 3.5 |
| 1980-1989 | 28 | 15.2 | 6 | 3.3 | 34 | 9.2 |
| 1990-1999 | 41 | 20.3 | 15 | 7.6 | 56 | 13.9 |
| 2000-2009 | 90 | 42.5 | 34 | 17.1 | 124 | 29.6 |
| Invasive | ||||||
| 1970-2009 | 140 | 18.1 | 65 | 9.1 | 205 | 13.6 |
| 1970-1979 | 8 | 3.7 | 4 | 3.0 | 12 | 3.3 |
| 1980-1989 | 27 | 14.5 | 11 | 5.7 | 38 | 10.1 |
| 1990-1999 | 32 | 15.9 | 18 | 9.0 | 50 | 12.4 |
| 2000-2009 | 73 | 34.8 | 32 | 16.2 | 105 | 25.3 |
| Noninvasive | ||||||
| 1970-2009 | 36 | 4.7 | 9 | 1.3 | 45 | 3.0 |
| 1970-1979 | 1 | 1.0 | 1 | 0.7 | 2 | 0.8 |
| 1980-1989 | 2 | 1.2 | 1 | 0.7 | 3 | 0.9 |
| 1990-1999 | 14 | 6.7 | 2 | 1.2 | 16 | 3.9 |
| 2000-2009 | 19 | 8.6 | 5 | 2.5 | 24 | 5.5 |
Incidence per 100,000 person-years age adjusted to the 2000 US white population.
Incidence per 100,000 person-years age and sex adjusted to the 2000 US white population.
Among the 250 patients with available pathologic stage, 24 (10%) were classified as having pathologic stage IIA or higher disease and 205 (82%) had invasive disease, classified as pathologic stage IA or higher. Incidence rates for patients with and without stage II, III, or IV disease and patients with and without invasive disease by sex and decade are summarized in Table 2.
A comparison of features by decade is given in Table 3. No statistically significant change in Breslow thickness by decade was found (P=.12; Kruskal-Wallis test). However, evidence indicated that stage II, III, or IV disease decreased by decade (P=.007; Fisher exact test), particularly in the 2000-2009 period compared with the earlier decades combined (P=.002; χ2 test).
TABLE 3.
| Characteristic | 1970-1979 | 1980-1989 | 1990-1999 | 2000-2009 | P Value |
|---|---|---|---|---|---|
| Age at diagnosis, mean (median; range), y | 26.6 (24.5; 19-36) | 30.4 (31.5; 19-39) | 30.4 (31; 18-39) | 29.8 (29; 19-39) | .09 |
| Breslow mean (median; range), mm (n=202) | 0.44 (0.47; 0.18-0.70) | 0.83 (0.58; 0.15-3.20) | 0.89 (0.58; 0.11-5.00) | 0.80 (0.47; 0.20-19.70) | .12 |
| Sex (N=256) | .90 | ||||
| Female | 10/16 (63) | 30/44 (68) | 47/67 (70) | 92/129 (71) | |
| Male | 6/16 (37) | 14/44 (32) | 20/67 (30) | 37/129 (29) | |
| Site (n=255) | .02 | ||||
| Lower extremity | 2/16 (13) | 16/43 (37) | 14/67 (21) | 31/129 (24) | |
| Back | 1/16 (6) | 4/43 (9) | 12/67 (18) | 40/129 (31) | |
| Upper extremity | 4/16 (25) | 8/43 (19) | 12/67 (18) | 25/129 (19) | |
| Neck, shoulder | 3/16 (19) | 5/43 (12) | 8/67 (12) | 8/129 (6) | |
| Chest, breast | 1/16 (6) | 3/43 (7) | 6/67 (9) | 11/129 (9) | |
| Head | 3/16 (19) | 5/43 (12) | 6/67 (9) | 5/129 (4) | |
| Abdomen | 1/16 (6) | 2/43 (5) | 4/67 (6) | 9/129 (7) | |
| Foot | 1/16 (6) | 0/43 | 5/67 (7) | 0/129 | |
| Location (n=254) | .54 | ||||
| Left | 8/15 (53) | 25/43 (58) | 28/67 (42) | 61/129 (47) | |
| Right | 5/15 (33) | 17/43 (40) | 32/67 (48) | 57/129 (44) | |
| Central | 2/15 (13) | 1/43 (2) | 7/67 (10) | 11/129 (9) | |
| Breslow thickness, mm (n=202) | .27 | ||||
| ≤1.00 | 6/6 (100) | 31/40 (78) | 38/51 (75) | 92/105 (88) | |
| 1.01-2.00 | 0/6 | 5/40 (13) | 8/51 (16) | 10/105 (10) | |
| 2.01-4.00 | 0/6 | 4/40 (10) | 4/51 (8) | 1/105 (1) | |
| >4.00 | 0/6 | 0/40 | 1/51 (2) | 2/105 (2) | |
| Histogenic type (n=223) | .05 | ||||
| SS | 5/9 (56) | 28/40 (70) | 39/60 (65) | 83/114 (73) | |
| MIS | 1/9 (11) | 3/40 (8) | 17/60 (28) | 24/114 (21) | |
| Nodular | 2/9 (22) | 6/40 (15) | 3/60 (5) | 4/114 (4) | |
| Spitzoid | 1/9 (11) | 2/40 (5) | 1/60 (2) | 2/114 (2) | |
| Acral lentiginous | 0/9 | 0/40 | 0/60 | 1/114 (1) | |
| Lentigo maligna | 0/9 | 1/40 (3) | 0/60 | 0/114 | |
| Pathologic stage (n=250) | .006 | ||||
| 0 | 2/14 (14) | 3/41 (7) | 16/66 (24) | 24/129 (19) | |
| IA | 8/14 (57) | 28/41 (68) | 37/66 (56) | 88/129 (68) | |
| IB | 2/14 (14) | 3/41 (7) | 3/66 (5) | 12/129 (9) | |
| IIA | 0/14 | 3/41 (7) | 2/66 (3) | 2/129 (2) | |
| IIB | 0/14 | 0/41 | 0/66 | 1/129 (1) | |
| IIIA | 0/14 | 0/41 | 1/66 (2) | 1/129 (1) | |
| IIIB | 0/14 | 1/41 (2) | 0/66 | 0/129 | |
| IIIC | 1/14 (7) | 0/41 | 0/66 | 0/129 | |
| IV | 1/14 (7) | 3/41 (7) | 7/66 (11) | 1/129 (1) | |
| Overall stage (n=250) | .007 | ||||
| I | 12/14 (86) | 34/41 (83) | 56/66 (85) | 124/129 (96) | |
| II, III, or IV | 2/14 (14) | 7/41 (17) | 10/66 (15) | 5/129 (4) | |
| Disease type (n=250) | .17 | ||||
| Noninvasive | 2/14 (14) | 3/41 (7) | 16/66 (24) | 24/129 (19) | |
| Invasive | 12/14 (86) | 38/41 (93) | 50/66 (76) | 105/129 (81) |
MIS = melanoma in situ; SS = superficial spreading.
Data are presented as No. (percentage) unless indicated otherwise.
Site of disease varied significantly between men and women (P=.003; χ2 test) but not by histologic subtype (P=.07; χ2 test) (Table 4).
TABLE 4.
| Site | SS (n=154) | MIS (n=43) | Nodular (n=15) | Acral (n=1) | Spitzoid (n=1) |
|---|---|---|---|---|---|
| Lower extremity | 41 (27) | 11 (26) | 2 (13) | 0 | 1 (100) |
| Back | 39 (25) | 7 (16) | 3 (20) | 0 | 0 |
| Upper extremity | 25 (16) | 14 (33) | 3 (20) | 1 (100) | 0 |
| Neck, shoulder | 13 (8) | 4 (9) | 4 (27) | 0 | 0 |
| Chest, breast | 12 (8) | 3 (7) | 0 | 0 | 0 |
| Head | 10 (6) | 1 (2) | 3 (20) | 0 | 0 |
| Abdomen | 12 (8) | 1 (2) | 0 | 0 | 0 |
| Foot | 2 (1) | 2 (5) | 0 | 0 | 0 |
MIS = melanoma in situ; SS = superficial spreading.
Data are presented as No. (percentage).
At last follow-up, 12 patients were dead, with a mean survival of 5.2 years after diagnosis (median, 3.1 years; range, 0.3-21.1 years). Among the 244 patients still alive at last follow-up, the mean duration of follow-up was 7.7 years (median, 4.4 years; range, 0.0-36.8 years). Among the 12 patients who died, 8 died of melanoma, 1 died of another cause, and 3 had unknown causes of death and were therefore excluded from analyses of disease-specific survival.
Each 1-year increase in calendar year of diagnosis was associated with a significantly decreased risk of death from any cause (HR, 0.92; 95% CI, 0.86-0.97; P=.005). Similarly, each 1-year increase in calendar year of diagnosis was associated with a significantly decreased risk of death due to metastatic melanoma (HR, 0.91; 95% CI, 0.85-0.98; P=.01). Kaplan-Meier curves comparing survival by decade of diagnosis are shown in the Figure.
FIGURE.
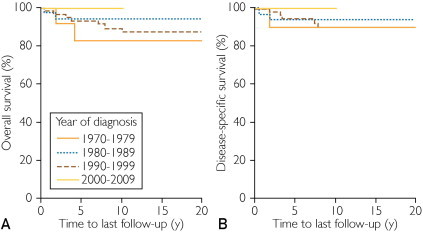
Kaplan-Meier curves comparing overall survival (A) and disease-specific survival (B) by decade of diagnosis.
Sex and histologic subtype were not significantly associated with mortality. Patients with stage II, III, or IV disease were more than 35 times more likely to die compared with patients with stage I disease (HR, 35.61; 95% CI, 7.68-165.13; P<.001), a difference that persisted after adjusting for year of diagnosis (HR, 28.53; 95% CI, 6.09-133.59; P<.001). Nine of the 24 patients (38%) with stage II, III, or IV disease died compared with only 2 of the 226 patients (1%) with stage I disease. Patients with Breslow thickness greater than 2.0 mm were more than 9 times more likely to die compared with patients with Breslow thickness of 2.0 mm or less (HR, 9.42; 95% CI, 2.52-35.20; P<.001), a difference that persisted after adjusting for year of diagnosis (HR, 8.45; 95% CI, 2.26-31.62; P=.002).
Discussion
Our study confirms that the incidence of cutaneous melanoma is increasing among young adults, with this incidence increasing more than 6-fold during the past 40 years in these patients. The lifetime risk of melanoma is higher in males than females, although the opposite is true in young adults and adolescents, with the female-male incidence ratio being as high as 1.8 in young adults aged 20 to 24 years.4 The present study confirms this trend, but we observed that the rate of increase of the incidence of melanoma in young women is greater than that of young men. Comparing the 1970s to the 2000s, the incidence for men increased more than 4-fold; for women, the incidence increased more than 8-fold.
This finding may be explained by some sex-specific behaviors that lead to different UV light exposure. Young women are more likely than young men to participate in activities that increase the risk of melanoma, including voluntary exposure to artificial sunlamps.14 In 1996, a telephone survey conducted by the American Academy of Dermatology found that, despite increased public awareness of the harms of exposure to UV light, the frequency of severe sunburns and tanning bed use had increased and was higher among women.15 Results from a recent telephone survey reveal that young women are much more likely than young men to participate in indoor tanning.16 In addition to UV exposure in adulthood, tanning bed use and sunburns in childhood and adolescence may contribute to melanoma development. High-risk behavior is increasingly common among children17 and adolescents.18 Despite public health education campaigns designed to decrease behaviors that lead to excessive UV light exposure, children, adolescents, and adults continue to put themselves at risk.
Some studies show a strong association between UV light exposure and the risk of melanoma. A survey of patients from an academic dermatology clinic found that exposure to indoor tanning beds was a significant risk factor for the development of melanoma and that this risk was even greater among women younger than 45 years.19 A meta-analysis of other studies addressing this topic confirmed the association between use of tanning beds and melanoma.20
Although the incidence of melanoma among young adults is clearly increasing over time, the disease-specific mortality is actually decreasing. This finding is consistent with SEER data.6 Many possible explanations exist for the improved survival of young patients with melanoma over time. Long-term follow-up is not yet available for all patients whose melanomas were diagnosed in the most recent decade (2000-2009), so we do not yet have an estimate for the true survival rate for these patients.
Mortality, as expected, is associated strongly with increased Breslow thickness and advanced-stage disease at the time of presentation. It is not surprising to find in this study that as the incidence of advanced-stage disease decreased in the most recent decade, the rate of disease-specific death also decreased.
We have demonstrated that although the incidence of cutaneous melanoma in young adults is increasing markedly, advanced-stage disease at the time of first presentation is decreasing. Residents of Olmsted County, Minnesota, have relatively effortless access to health care, which may explain why most patients have early-stage disease at the time of presentation.
Another possible explanation for the decrease in mortality among this patient population is a change over time in the histologic criteria for the diagnosis of melanoma. This concept of “melanoma inflation” suggests that patients who were believed to have melanoma were never at risk for morbidity or mortality because the lesion removed was in fact biologically benign. One board-certified dermatopathologist (L.E.G.) reviewed all pathologic slides that were available (220 in all). On the basis of this review, we found no evidence of a change in the diagnostic criteria for melanoma over time.
Our results suggest a much greater absolute incidence and increase in incidence of melanoma among young adults than that reported from the SEER database by Purdue et al.6 Population-based cancer registries rely on the accurate and comprehensive reporting of all incident cases of disease to be able to generate estimates of incidence. Numerous studies have documented the underreporting of melanoma to large cancer registries. An Iowa group found that, based on a survey of state dermatologists, between 10% and 17% of cases were not reported to a state cancer registry. They reported that 25% of all cases of melanoma were reported from independent laboratories, which may contribute to the discrepancy.21 Koh et al22 estimated that between 12% and 19% of new cases of melanoma were not reported to the Massachusetts Cancer Registry. These estimates of large discrepancies occur because of the wide variety of nonhospital settings in which melanoma is diagnosed and treated.
An interesting finding in our study was that the location of cutaneous melanoma among young adults differs between sexes. The most common location for melanoma among women in this population was the lower extremity, followed by the upper extremity. For men, melanoma was most commonly found on the back, followed by the upper extremity.
The results presented in this article must be considered in light of the following factors related to the study. We relied on the complete and accurate reporting of cases of melanoma in the medical record. Because the final diagnosis from pathologic specimens is abstracted into REP records, it is unlikely that any cases were missed. It is possible that some cases of melanoma among Olmsted County residents were not included because the patients sought care elsewhere. Another potential limitation is the demographic makeup of the population studied; the incidence of melanoma among this largely white, highly educated population may not be applicable to young adults throughout the United States. Populations of young adults who do not have a reliable source of health care may present with more advanced disease.
Conclusion
This study demonstrates an increase in the incidence of melanoma among young adults in Olmsted County, Minnesota, with young women being at higher risk than young men. Although the incidence is increasing, the mortality from this disease seems to be decreasing. Our results emphasize the importance of active interventions to decrease risk factors associated with melanoma in young individuals. In addition, skin cancer screening examinations in young adults are strongly recommended.
Acknowledgments
Christine M. Lohse, MS, performed the statistical analyses.
Footnotes
Grant Support: A portion of this work was supported in party by National Institutes of Health grant and the Rochester Epidemiology Project (grant number R01-AG034676; Principal Investigator: Walter A. Rocca, MD, MPH). Dr Brewer is a recipient of a Dermatology Foundation Career Development Award for the study of lymphoma-associated skin cancer.
Supplemental Online Material
Author Interview Video
References
- 1.Jemal A., Siegel R., Xu J., Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [published correction appears in CA Cancer J Clin. 2011;61(2):133-134] [DOI] [PubMed] [Google Scholar]
- 2.Bleyer A., Barr R. Cancer in young adults 20 to 39 years of age: overview. Semin Oncol. 2009;36(3):194–206. doi: 10.1053/j.seminoncol.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Ries L.A., Wingo P.A., Miller D.S. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer. 2000;88(10):2398–2424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Bleyer A., O'Leary M., Barr R., Ries L.A.G. National Cancer Institute; Bethesda, MD: 2006. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. NIH publication 06-5767. [Google Scholar]
- 5.Christenson L.J., Borrowman T.A., Vachon C.M. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294(6):681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 6.Purdue M.P., Freeman L.E., Anderson W.F., Tucker M.A. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J Invest Dermatol. 2008;128(12):2905–2908. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berk D.R., LaBuz E., Dadras S.S., Johnson D.L., Swetter S.M. Melanoma and melanocytic tumors of uncertain malignant potential in children, adolescents and young adults: the Stanford experience 1995-2008. Pediatr Dermatol. 2010;27(3):244–254. doi: 10.1111/j.1525-1470.2009.01078.x. [DOI] [PubMed] [Google Scholar]
- 8.Amerio P., Manzoli L., Auriemma M. Epidemiology and clinical and pathologic characteristics of cutaneous malignant melanoma in Abruzzo (Italy) Int J Dermatol. 2009;48(7):718–722. doi: 10.1111/j.1365-4632.2009.03974.x. [DOI] [PubMed] [Google Scholar]
- 9.Clegg L.X., Feuer E.J., Midthune D.N., Fay M.P., Hankey B.F. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537–1545. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 10.Hall H.I., Jamison P., Fulton J.P., Clutter G., Roffers S., Parrish P. Reporting cutaneous melanoma to cancer registries in the United States. J Am Acad Dermatol. 2003;49(4):624–630. doi: 10.1067/s0190-9622(03)00885-5. [DOI] [PubMed] [Google Scholar]
- 11.Paterson I.C., Beer H., Adams Jones D. Under-registration of melanoma in Wales in 1998: use of the capture-recapture method to estimate the ‘true’ incidence. Melanoma Res. 2001;11(2):141–145. doi: 10.1097/00008390-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Faries M.B., Wanek L.A., Elashoff D., Wright B.E., Morton D.L. Predictors of occult nodal metastasis in patients with thin melanoma. Arch Surg. 2010;145(2):137–142. doi: 10.1001/archsurg.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melton L.J., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.Coelho S.G., Hearing V.J. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2010;23(1):57–63. doi: 10.1111/j.1755-148X.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson J.K., Rigel D.S., Amonette R.A. Trends in sun exposure knowledge, attitudes, and behaviors: 1986 to 1996. J Am Acad Dermatol. 1997;37(2, pt 1):179–186. doi: 10.1016/s0190-9622(97)80122-3. [DOI] [PubMed] [Google Scholar]
- 16.Choi K., Lazovich D., Southwell B., Forster J., Rolnick S.J., Jackson J. Prevalence and characteristics of indoor tanning use among men and women in the United States. Arch Dermatol. 2010;146(12):1356–1361. doi: 10.1001/archdermatol.2010.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandi P., Cokkinides V.E., Weinstock M.A., Ward E. Sunburns, sun protection and indoor tanning behaviors, and attitudes regarding sun protection benefits and tan appeal among parents of U.S. adolescents—1998 compared to 2004. Pediatr Dermatol. 2010;27(1):9–18. doi: 10.1111/j.1525-1470.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- 18.Cokkinides V., Weinstock M., Glanz K., Albano J., Ward E., Thun M. Trends in sunburns, sun protection practices, and attitudes toward sun exposure protection and tanning among US adolescents, 1998-2004. Pediatrics. 2006;118(3):853–864. doi: 10.1542/peds.2005-3109. [DOI] [PubMed] [Google Scholar]
- 19.Ting W., Schultz K., Cac N.N., Peterson M., Walling H.W. Tanning bed exposure increases the risk of malignant melanoma. Int J Dermatol. 2007;46(12):1253–1257. doi: 10.1111/j.1365-4632.2007.03408.x. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher R.P., Spinelli J.J., Lee T.K. Tanning beds, sunlamps, and risk of cutaneous malignant melanoma. Cancer Epidemiol Biomarkers Prev. 2005;14(3):562–566. doi: 10.1158/1055-9965.EPI-04-0564. [DOI] [PubMed] [Google Scholar]
- 21.Merlino L.A., Sullivan K.J., Whitaker D.C., Lynch C.F. The independent pathology laboratory as a reporting source for cutaneous melanoma incidence in Iowa, 1977-1994. J Am Acad Dermatol. 1997;37(4):578–585. doi: 10.1016/s0190-9622(97)70175-0. [DOI] [PubMed] [Google Scholar]
- 22.Koh H.K., Clapp R.W., Barnett J.M. Systematic underreporting of cutaneous malignant melanoma in Massachusetts: possible implications for national incidence figures. J Am Acad Dermatol. 1991;24(4):545–550. doi: 10.1016/0190-9622(91)70079-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview Video


