Abstract
Iodinated contrast agents have been in use since the 1950s to facilitate radiographic imaging modalities. Physicians in almost all specialties will either administer these agents or care for patients who have received these drugs. Different iodinated contrast agents vary greatly in their properties, uses, and toxic effects. Therefore, clinicians should be at least superficially familiar with the clinical pharmacology, administration, risks, and adverse effects associated with iodinated contrast agents. This primer offers the non-radiologist physician the opportunity to gain insight into the use of this class of drugs.
Abbreviations and Acronyms: CT, computed tomography; ICA, iodinated contrast agent
Contrast agents have long been used for the imaging of anatomic boundaries and to explore normal and abnormal physiologic findings. These agents have included colorimetric contrast agents (eg, methylene blue and indocyanine green) and fluorescent contrast agents (eg, fluorescein). However, the introduction of increasingly faster and more discriminating radiographic imaging techniques has resulted in the need for radiation-attenuating contrast agents that can be used in traditional radiographic imaging or, more recently, in subtraction imaging, both of which can be projected and rotated in 3 dimensions.
By far the most successful and widely applied contrast agents in use today are the iodinated contrast agents (ICAs), first introduced into clinical practice in the 1950s. It is estimated that approximately 75 million doses of ICAs are given worldwide each year.1 The ICAs fall into 4 broad groups, each possessing unique chemical, physical, and biologic properties. These various ICAs are needed to address the demands of a wide variety of imaging modalities (Table 1).
TABLE 1.
Indications for Use of Iodinated Contrast Media
| Intravascular |
| Intravenous |
| Computed tomography |
| Digital subtraction angiography |
| Intravenous urography |
| Venography (phlebography) |
| Inferior vena cava and its tributaries |
| Superior vena cava and its tributaries |
| Extremities |
| Other venous sites |
| Epidural venography |
| Intra-arterial |
| Angiocardiography |
| Computed tomography |
| Coronary angiography |
| Pulmonary angiography |
| Aortography |
| Visceral and peripheral arteriography |
| Digital subtraction angiography |
| Central nervous system |
| Cerebral, vertebral, and spinal angiography |
| Intrathecal (Use US Food and Drug Administration–approved contrast media only) |
| Myelography (myelographic nonionic only) |
| Cisternography (myelographic nonionic only) |
| Other |
| Oral, rectal, or ostomy – gastrointestinal tract |
| Conventional fluoroscopy |
| Computed tomography |
| Therapeutic uses |
| Body cavity use |
| Herniorrhaphy |
| Peritoneography |
| Vaginography |
| Hysterosalpingography |
| Arthrography |
| Endoscopic retrograde cholangiopancreatography |
| Cholangiography |
| Nephrostography |
| Pyelography – antegrade, retrograde |
| Urethrography – voiding, retrograde |
| Cystography |
| Sialography |
| Ductography (breast) |
| Miscellaneous |
| Sinus tract injection |
| Cavity delineation (including urinary diversions, such as loop and pouch) |
Reprinted with permission of the American College of Radiology.17
Given the frequent use of ICAs and the diversity of images and patients requiring an ICA, it is not surprising that physicians from virtually every specialty will encounter patients scheduled to receive, or who have recently received, an ICA. Because the ICAs collectively have the potential to meaningfully alter patient physiologic features, produce immunologic reactions, and affect patient outcomes (in concert with, or independent of, other disease processes), it is important that all physicians have an appreciation for the indications for, selection of, and consequences of ICA use. For these reasons, we provide a primer on ICA use for the non-radiologist.
Clinical Pharmacology
All ICAs share a similar function group—a tri-iodinated benzene ring (Figure 1). Iodine plays a key role in the attenuation of x-rays. The atomic radius of a covalently bonded iodine atom is approximately 133 picometers, which falls within the range of the wavelengths of x-rays: 10 to 10,000 picometers; thus, x-rays are easily attenuated by the iodine atoms.2 Furthermore, 3 iodine atoms covalently bonded to a benzene ring offer 2 major advantages: (1) 3 large atoms located in such close proximity increase the effective molecular size, thus attenuating longer-wavelength x-rays, and (2) covalent bonding to a stable organic functional group (ie, benzene) reduces the risk of toxic effects from free iodide.
FIGURE 1.
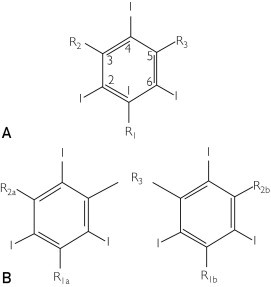
Basic molecular structural units of iodinated contrast agents. A, Monomeric form. B, Dimeric form. Benzene rings are trisubstituted at the 2, 4, and 6 positions with iodine atoms. Substitution at site R1 in the monomeric form or R1a in the dimeric form with a carboxylate (-COO-)-containing group results in “ionic” compounds; otherwise, substitution at this site with non–carboxylate-containing functional group results in “nonionic” compounds. Sites R2, R2a, R2b, and R3 have non–carboxylate-containing functional groups.
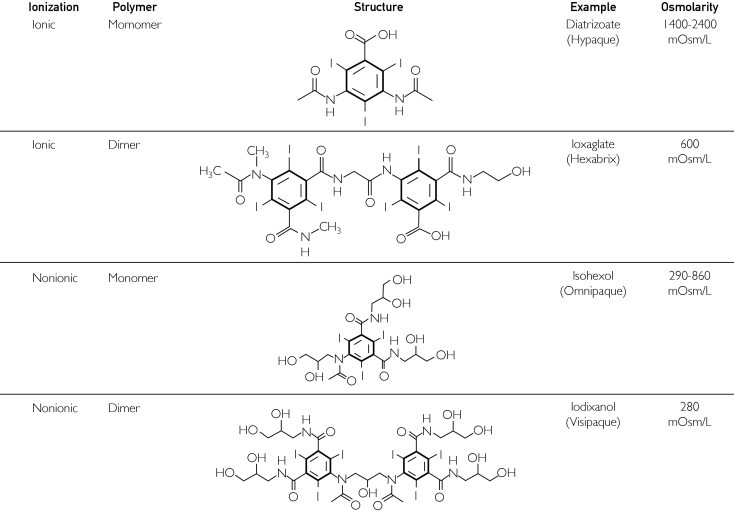
Two major chemical variations result in 4 classes of ICAs (Figure 2). Compounds consist of either 1 tri-iodinated benzene ring (ie, monomers) or 2 tri-iodinated benzene rings linked by an organic functional group (ie, dimers). In addition, ionic tendency is governed by the presence (ie, ionic) or absence (ie, nonionic) of a carboxylate (-COO-) functional group contained on an organic side chain. Typically, because the carboxylate moiety adds a net negative charge to the molecule, these anionic agents are usually available as salts of sodium, calcium, or methylglucamine cations. Hence, the 4 major classes of iodinated contrast agents are as follows:
-
1
Ionic monomer: single tri-iodinated benzene ring with a carboxylate-containing benzene substituent.
-
2
Ionic dimer: 2 linked tri-iodinated benzene rings in which at least 1 carboxylate-containing group is substituted on at least 1 benzene ring.
-
3
Nonionic monomer: single tri-iodinated benzene ring without a carboxylate-containing benzene substituent.
-
4
Nonionic dimer: 2 linked tri-iodinated benzene rings that do not contain a carboxylate functional group within any benzene substituent.
FIGURE 2.
Properties of the 4 classes of iodinated contrast agents.
The ICAs among the 4 groups have differing properties, clinical uses, and toxicity profiles, and to some degree these differences influence which types of agents are used by the imaging department for specific indications. For example, unlike nonionic agents, which are uncharged, the charged ionic species tend to disrupt the electrical potential of cell membranes, accounting for their increased toxicity.3 Also, ionic monomers have the weakest ability to attenuate x-rays and thus need to be administered in high concentrations that are hyperosmolar (approximate osmolarity = 1500-2000 mOsm/L) compared with blood (approximate osmolarity = 280-290 mOsm/L). As such, ionic monomers are also referred to as high-osmolarity agents. Low-osmolarity agents include ionic dimers and nonionic monomers with osmolarities in the range of 290 to 860 mOsm/L. Nonionic dimers are iso-osmolar with blood, with an osmolarity of 290 mOsm/L.
All ICAs demonstrate low protein binding. Distribution from the intravascular compartment to highly perfused organs, such as brain, liver, and kidney, is rapid, whereas distribution to less perfused organs and tissues, such as bone and fat, is much slower. As such, the half-life of redistribution of the intravascular compartment is still rapid (2-5 minutes for most agents). Currently, no available agent undergoes clinically significant metabolism because all are eliminated unchanged by the kidneys via glomerular filtration with no significant tubular reabsorption.4 The elimination half-life of most agents falls within the range of 90 to 120 minutes in patients with normal renal function and can be delayed, on the order of weeks, in patients with renal insufficiency.
Clinical Uses of ICAs
The use of iodinated contrast media is essential to the practice of radiology. Because the administration of these agents carries some increased risk for patients in certain clinical settings, it is important to weigh risks and benefits of contrast administration and choose the appropriate type and amount of contrast for each patient. Ideally, patients should be informed of these risks. In patients at high risk for an adverse event, the need for administration of an ICA should be considered, as well as alternative imaging modalities or the use of other contrast agents, such as gadolinium.5 Although the various ICAs differ in a number of physical and chemical properties, such as viscosity, osmolarity, and immunogenicity, their imaging characteristics are entirely based on the ability to attenuate x-rays.6 The ability of an ICA to attenuate x-rays depends on the number of iodine molecules present in the tissue to be imaged and is inversely related to the x-ray energy used for imaging. Therefore, the type and amount of contrast media used for a given purpose also depend on the imaging characteristics needed. In general, there are 3 routes of administration for ICAs: intravascular, enteric, and direct injection (see Table 1 for a complete list).
Intravascular Contrast Administration
Intravascular administration of contrast is by far the most common use of iodinated contrast media and can be further subdivided into intra-arterial and intravenous injection. Intra-arterial injection is the primary method of contrast delivery used in diagnostic catheter angiography and catheter-directed arterial intervention, such as percutaneous angioplasty and stent placement. Factors that affect the choice of type and amount of contrast to be administered include viscosity, iodine concentration, and osmolarity.
The imaging modality used for most intra-arterial injections is fluoroscopy. This modality requires higher rates of contrast administration to opacify the target vessels (up to 30 mL/s) as opposed to intravenous injections used for computed tomography (CT) scanning (typically 2-6 mL/s). Therefore, viscosity plays a significant role in the delivery of intra-arterial contrast media for angiography, and contrast agents are routinely warmed to 37°C so that adequate flow rates can be attained during catheter injection. In addition, iodine concentration can be an important factor for adequate opacification, and the use of high-iodine contrast media can be helpful, particularly in larger patients. Finally, osmolarity of intra-arterial contrast media has been shown to affect patient comfort during extremity angiography.7 For this reason, iso-osmolar contrast is frequently used for upper- and lower-extremity runoff studies.
Intravenous contrast administration for the purpose of CT scanning is the most common use of iodinated contrast media. Intravenous injection of contrast is also used for studies of the genitourinary tract, such as intravenous pyelography, and the venous system, such as direct venography; however, these studies have been decreasing in use during the last 2 decades, whereas the number of contrast-enhanced CT scans has increased significantly during that same period.
As with catheter arteriography, CT uses iodinated contrast media for arterial opacification and parenchymal enhancement. After intravenous injection, arterial contrast opacification is observed first, followed by parenchymal contrast enhancement. The enhancement observed is directly proportional to the local concentration of iodine; however, depending on the indication, the requirements for amount and rate of contrast administration may differ significantly.8,9
The primary goal of CT angiography is arterial opacification. This opacification is proportional to the iodine delivery rate, which is determined primarily by the rate of contrast injection and is influenced by the concentration of iodine in the contrast medium.10 Because arterial opacification is the primary goal, the total amount of contrast media used is frequently less of an issue than how quickly the contrast can be injected. Therefore, as with catheter angiography, viscosity and iodine concentration have an effect on the selection of the appropriate contrast media to be injected, particularly when intravenous access is limited. More viscous contrast media, such as high-iodine concentration and iso-osmolar contrast agents, are routinely warmed to 37°C so that adequate flow rates can be attained during intravenous injection.
When the primary goal of CT scanning is evaluation of a solid organ, such as the liver or pancreas, the situation changes. Parenchymal organ enhancement is more dependent on total amount of iodine administered than on iodine delivery rate.6 Therefore, lesion conspicuity within a solid organ may require a larger volume of contrast media to be injected, even if that contrast media cannot be administered very quickly. Therefore, viscosity tends to be less an issue for nonvascular CT, and choice of contrast media is primarily based on safety profile.
Complications of intravascular injection of iodinated contrast include anaphylactoid contrast reaction and contrast-induced nephropathy, each of which is discussed later in this article. An additional risk of intravenous contrast injection is contrast media extravasation. Although uncommon, contrast extravasation can lead to local edema and erythema. Rarely, serious local effects, such as skin and subcutaneous ulceration and tissue necrosis, can occur. Most published series suggest an extravasation rate of less than 1% for intravenous injection of iodinated contrast media for CT scanning.11,12 Most contrast extravasations are small volume, and symptoms resolve with conservative treatment. Unfortunately, the ultimate degree of injury cannot be determined at initial examination. Although no consensus exists regarding the management of contrast media extravasation, careful monitoring is recommended, and persistent pain, skin blistering, or evidence of altered tissue perfusion is considered to be evidence of serious injury.13 As a rule, the radiology department at our institution recommends plastic surgery consultation for large-volume extravasation (>100 mL).
Enteric Contrast Administration
Although oral or enteric contrast examinations traditionally are performed with barium suspensions, iodinated contrast media is also frequently used for this purpose. In general, iodinated contrast is preferred to barium when there is a risk of bowel leak or obstruction because extraintestinal barium can cause inflammation and adhesions. Diatrizoate meglumine (GastroView) and diatrizoate sodium (Hypaque) are examples of iodinated contrast used for fluoroscopic examinations, typically at a 20% concentration. These agents can also be used in a more dilute form for CT examinations (usually 2% to 3% concentration). Similarly, nonionic contrast agents, such as iohexol (Omnipaque), can be used for oral administration. At the dilutions used for CT scanning, these agents are nearly tasteless when dissolved in water.
The amount and route used for enteric contrast depend on the bowel segment that needs to be opacified. Fluoroscopic gastrointestinal studies are performed under direct visualization with real-time guidance; however, CT studies with enteric contrast may require preparation time to allow passage of oral contrast material into the corresponding bowel segment. If the esophagus and stomach are to be opacified, the patient can undergo imaging immediately after drinking the appropriate contrast mixture. Opacification of the small bowel, including the terminal ileum, can be accomplished using 750 mL of oral contrast with a delay time to imaging of approximately 90 minutes. Opacification of the entire small and large bowel frequently requires a second administration of 750 mL of oral contrast with imaging at 2 to 3 hours. Frequently, 200 to 300 mL of “top-off” drinks may be given at the time of the CT scan to reopacify the stomach and duodenum. In rare circumstances, 200 to 300 mL of rectal contrast may need to be administered to completely opacify the colon.
Although extremely rare, anaphylactoid-type contrast reactions have been reported after the oral administration of iodinated contrast. Typically, these reactions occur in the same time frame as reactions to intravascular contrast, can be similar in severity, and are treated using the same methods.14,15 Because of the rarity of these reactions, corticosteroid premedication before oral contrast administration is not considered the standard of care for patients with a history of a reaction to intravenous contrast. In a to intravenous contrast. In addition, the potential deleterious effects of iodinated contrast media on the kidney are not believed to occur in a clinically significant manner for nonvascular routes of administration.
Direct Contrast Injection
Direct injection of iodinated contrast comes in 2 basic forms: injection via percutaneous needle access, such as direct arthrography, and injection via an indwelling catheter or tube, such as cystography or sinography. Contrast injection of this type differs from intravascular injection in that the contrast is not rapidly cleared by the kidneys after image acquisition but is evacuated back through the catheter or by natural drainage. In some cases, such as articular injection and myelography, the contrast is absorbed slowly back into the body via the lymphatic system.
As with enteric contrast administration, contrast reactions have been reported but are extremely rare. Contrast-related complications from such procedures are far more frequently associated with adverse local reactions.16 Therefore, the contrast preparation for each of these procedures should be specifically formulated for the appropriate indication (Supplemental Appendix, reprinted with permission of the American College of Radiology, available online at http://www.mayoclinicproceedings.org).17 As with oral contrast administration, the potential effects of iodinated contrast on the kidney are not believed to be significant for direct contrast injection.
Adverse Reactions to ICAs
The incidence of adverse reactions is more common after the use of high-osmolarity agents: approximately 15% with a high-osmolarity agent vs only 3% with a low-osmolarity ICA.18 Therefore, the use of high-osmolarity agents has decreased significantly in recent years. The causes of most adverse effects and adverse reactions to ICAs are multifactorial and are probably due to a combination of direct chemotoxicity, the ionic state (ie, ionic vs nonionic), or the osmolarity of the injected ICA preparation. A summary of adverse reactions to ICAs, including signs, symptoms, diagnosis, preventive measures, and treatment options, can be found in Table 2.
TABLE 2.
Summary of Adverse Reactions to Iodinated Contrast Agents
| Definition | Signs and symptoms | Risk factors | Preventive measures | Treatment |
|---|---|---|---|---|
| Acute reaction | ||||
|
|
|
|
|
| Delayed reaction | ||||
|
|
|
|
|
| Contrast-induced nephropathy | ||||
|
|
|
|
|
ACE = angiotensin-converting enzyme inhibitor; IM = intramuscular; IV = intravenous.
Acute Contrast Reactions
Acute adverse reactions to ICAs occur within 1 hour of use. The overall incidence of severe acute reactions to ICAs depends on the class of agent administered. Specifically, severe acute reactions are 5 times more common after the administration of an ionic, monomeric, high-osmolarity agent vs a low- or iso-osmolar agent with a risk of 0.22% vs 0.04%.19 Overall mortality from acute reactions to ICAs is 1:13,000 to 1:169,000.19-21 The severity of these reactions can range from mild to severe and life-threatening. Common benign reactions include pain on intravascular injection, nausea and vomiting, rash, and hemodynamic changes. Pain after injection is most commonly encountered with ionic monomers, such as diatrizoate,22 and is thought to be related to the high osmolarity and greater association of endothelial injury with ionic agents.23,24 Nausea and vomiting occur in up to 6.7% of patients who receive ICAs and, as with pain on injection, are more common with the high-osmolarity ionic monomeric agents.25 Vagal episodes can occur and manifest as bradycardia with hypotension.
Severe acute reactions typically manifest as pruritus and urticaria in addition to bronchospasm, dyspnea, hypotension, laryngeal edema, and cardiovascular collapse. Although the clinical appearance may be similar to that of a type I hypersensitivity anaphylaxis reaction, most reactions are probably anaphylactoid (ie, nonspecific activation of the complement system) in nature because prior exposure to an ICA is not necessary, IgE antibodies cannot be consistently demonstrated in patients who experience these episodes, and the reactions do not consistently recur in the same patient.26-29 However, Laroche et al30 showed that serum tryptase (an enzyme released by mast cells along with histamine during degranulation) and anti-ICA–specific IgE levels are elevated in patients who experienced a severe acute reaction to an ICA; this finding supports an anaphylaxis-type reaction. These reactions typically involve the release of inflammatory mediators, such as histamine, serotonin, bradykinin, adenosine, prostaglandins, and leukotrienes, which accounts for the development of symptoms, and tend to be unpredictable and non–dose dependent. Therefore, both anaphylactic and anaphylactoid reactions probably occur; however, signs, symptoms, and treatment are similar.
Management of acute reactions should begin by identifying patients who are at risk and preventing these events from occurring in the first place. The 3 most common risk factors for an acute reaction to ICAs are a history of asthma, a prior reaction to an ICA, and atopy. These 3 factors increase the risk of developing an acute reaction to an ICA by 6- to 10-fold, 5-fold, and 3-fold, respectively.31,32 Given that the risk of severe acute reactions is increased after the use of ionic monomeric ICAs, these agents should be avoided in high-risk patients, if possible. Also, the use of imaging studies that do not involve the use of ICAs altogether (eg, ultrasonography and magnetic resonance imaging) should be considered if these studies are feasible alternatives. Prior consultation with an allergist can prove helpful in identifying patients who may be at risk for anaphylactic reactions; however, the clinician should be aware that allergy testing, although rarely performed, will not be predictive of the development of future anaphylactoid reactions.
In patients at risk for an acute reaction, pretreatment with corticosteroids, with or without concomitant use of histamine receptor antagonists, is a common practice. However, for the prevention of severe and life-threatening reactions, treatment with methylprednisolone, 32 mg orally, 2 hours before ICA exposure was not effective at reducing the risk of an adverse reaction (0.5% vs 0.2% in the corticosteroid and control groups; P>.05).33 Patients who received 2 doses of oral methylprednisolone, 32 mg each (one 6-24 hours before and another 2 hours before administration of an ICA), had a 4.5-fold reduction in the incidence of severe acute reactions (0.2% vs 0.9% in the corticosteroid and control groups; P=.005).33,34 Furthermore, the use of a nonionic monomeric agent provides greater protection from a severe reaction than the use of an ionic monomer with corticosteroid prophylaxis35; however, severe reaction can still occur despite corticosteroid pretreatment and the use of low-osmolarity contrast media.3,33,34 In patients at risk for acute reaction, pretreatment with corticosteroids is a common practice. For adults at Mayo Clinic in Rochester, Minnesota, this consists of methylprednisolone, 32 mg orally, both 12 hours and 2 hours before the administration of an ICA (ie, “the Lasser prep”).34 “The Greenberger prep” is a commonly used alternative and consists of triple therapy with prednisone, 50 mg, administered orally 13 hours, 7 hours, and again at 1 hour before ICA administration, and both diphenhydramine, 50 mg, and ephedrine, 25 mg, administered orally 1 hour before contrast.36
For mild reactions, because they are usually self-limiting and often present no major threat to the patient's well-being and long-term outcome, treatment is often symptomatic. Antiemetics such as prochlorperazine, 12.5 mg intravenously, or ondansetron, 4 mg intravenously, can be used to treat nausea and vomiting. Diphenhydramine, 25 to 50 mg intravenously, with hydrocortisone, 100 mg intravenously, or equivalent can be administered if the patient develops rash and pruritus. In those with mild hypotension, intravenous fluids and mild pressors (eg, ephedrine or phenylephrine) can be given. If the hypotension occurs with bradycardia (ie, a vagal episode), 0.2 to 0.4 mg of atropine intravenously should be administered and repeated if necessary.
For patients who develop a severe acute reaction to ICAs, such as anaphylaxis or an anaphylactoid reaction, treatment is generally guided by the ABCs of airway, breathing, and circulation. One should begin by calling for help while ensuring a patent airway and adequate oxygen delivery. The World Allergy Organization recommends epinephrine as the drug of choice for the treatment of anaphylaxis and anaphylactoid reactions.37 α-Adrenergic receptor agonism by epinephrine reverses the vasodilatation during anaphylaxis and alleviates hypotension, urticaria, and angioedema. β-Adrenergic agonism produces bronchodilatation, increases cardiac output, and can serve to reduce the further release of inflammatory mediators by mast cells and basophils.38,39 Epinephrine should be administered intramuscularly, 0.2 to 0.5 mg (ie, 0.2-0.5 mL of 1:1000 [1 mg per 1 mL] solution) every 5 to 15 minutes as necessary. Alternatively, epinephrine may be administered intravenously by clinicians familiar with intravenous dosing of epinephrine (titrated to clinical response); however, specific dosing guidelines for intravenous epinephrine are not available.37 Liberal use of intravenous fluids during this period will also help offset the relative hypovolemia that occurs secondary to vasodilatation and movement of fluids from the intravascular compartment into the extracellular space. In addition, hydrocortisone, 100 mg intravenously, or equivalent should be administered after the patient is oxygenating well and hemodynamically stable. The patient should be monitored for further decompensation after the effects of epinephrine dissipate. Therefore, equipment used for the treatment of such reactions (ie, airway devices, supplemental oxygen, intravenous fluids, and epinephrine) should be readily available and the staff appropriately trained in the use of such equipment in all locations where ICAs are administered.
Delayed Reactions to Contrast Agents
Delayed reactions to contrast agents are those that occur at least 1 hour after but within 1 week of receiving an ICA. Typical delayed reactions can manifest with signs and symptoms similar to an acute reaction, such as rash, pruritus, nausea, vomiting, diarrhea, and, occasionally, hypotension; however, reactions with cutaneous manifestations are most common. These cutaneous manifestations can be diverse in nature but typically occur as a pruritic maculopapular rash or urticaria.40 Severe skin reactions, such as toxic epidermal necrolysis and Stevens-Johnson syndrome, have also been reported.41,42 Delayed reactions tend to be milder in nature than acute reactions. The overall incidence of delayed reactions after the administration of an ICA can be as high as 14%.40 Iso-osmolar agents (ie, nonionic dimers) are associated with the highest risk of causing a delayed reaction. Specifically, the incidence of a delayed cutaneous reaction after a nonionic dimer is 3 times greater than after the use of a nonionic monomer or an ionic dimer.43 Some data suggest an association between sun exposure and the risk of developing a delayed cutaneous reaction to ICAs, due to a possible photosensitizing effect by ICAs.44
Cutaneous delayed reactions are thought to be due to a T-cell–mediated type IV hypersensitivity reaction and are believed to be a reaction generated against the ICA molecule itself, not iodine.45 Delayed reactions are more common in patients treated with interleukin 2 because interleukin 2 is a potent stimulator of T lymphocytes.46 As with acute reactions, the management goal for delayed reactions is to identify patients at risk and minimize the risk of a reaction. Patients at greater risk for a delayed reaction to ICAs are those who had a prior reaction to an ICA agent, who are to receive a nonionic dimeric ICA, and who are being treated with interleukin 2. Although skin testing may be used to confirm a reaction to an ICA, the sensitivity of the test may depend on the duration of the administration of the test compared with the timing of initial ICA exposure.47 Furthermore, a negative predictive value of 97% suggests that skin testing may be more useful to rule out a reaction to ICAs.48 In patients with a prior reaction, ICAs should be avoided, if possible. However, in those with a prior reaction who still require the use of ICAs, nonionic dimeric ICAs should be avoided and corticosteroid prophylaxis should be considered.49 In those who develop a reaction, treatment is usually based on symptoms. For cutaneous reactions, treatment with corticosteroids can be helpful. For severe cutaneous reactions or those that do not resolve quickly, consultation with a dermatologist should be considered.
Contrast-Induced Nephropathy
Contrast-induced nephropathy refers to a reduction in renal function after the administration of an ICA. The standard diagnostic criteria for contrast-induced nephropathy is a greater than 25% increase in baseline serum creatinine concentration within 3 days of receiving an ICA after other possible causes have been ruled out. Serum creatinine will usually peak within 3 to 7 days and return to baseline (or a new baseline) within 14 days. In many patients, the course is usually benign; however, the development of contrast-induced nephropathy can prolong hospital stay, increases the need for dialysis, and increases overall mortality.50,51
The major risk factors for contrast-induced nephropathy can be found in Table 2. Preexisting renal dysfunction is the greatest risk factor for developing contrast-induced nephropathy, and the risk becomes greater with increasing baseline renal impairment. The incidence of contrast-induced nephropathy is less than 5% in patients with normal renal function but can be as high as 50% in those with preexisting renal dysfunction.52 Compared with those with normal renal function, patients with a baseline serum creatinine concentration of 1.2 through 1.9 mg/dL, 2.0 through 2.9 mg/dL, and 3.0 mg/dL or more (to convert to μmol/L, multiply by 88.4) have a 2.4, 7.4, and 12.8 increased odds of developing contrast-induced nephropathy after administration of an ICA for a coronary intervention.53 In one report, the risk of developing contrast-induced nephropathy was 2%, 10.4%, and 62% for patients with a preprocedural baseline serum creatinine concentration of 1.2 mg/dL or less, 1.3 through 1.9 mg/dL, and 2.0 mg/dL or higher, respectively.54 Other patient factors include age, systemic diseases that predispose patients to renal dysfunction (eg, diabetes mellitus and hypertension), and factors that contribute to a reduction in cardiac output (eg, severe hemodynamic instability, dehydration, congestive heart failure, and myocardial infarction within 24 hours of receiving an ICA). Recent use of nephrotoxic drugs (eg, aminoglycosides) or drugs that alter renal hemodynamics (eg, nonsteroidal anti-inflammatory agents and angiotensin-converting enzyme inhibitors) may also predispose patients to the development of contrast-induced nephropathy.55
The nature of the ICA and volume administered can also influence the risk of developing contrast-induced nephropathy and appears to be greatest after the use of high-osmolar ionic monomers, especially in patients with preexisting renal disease. However, it is still unclear whether there is a difference in the risk of developing contrast-induced nephropathy after the use of either nonionic dimers or nonionic monomers.32 For a given ICA preparation, increased volume of ICA administered clearly increases the risk of nephropathy. In patients undergoing coronary angiography, each additional 100 mL of ICA increased the risk of subsequent development of nephropathy by 12%.53
The route of contrast administration may also have an effect on the development of nephropathy. Most clinical studies of contrast-induced nephropathy relate to intraarterial injection of contrast media, whereas corresponding studies of nephropathy after an intravenous contrast injection are somewhat lacking. Recently, some investigators have questioned the existence of clinically significant contrast-induced nephropathy from intravenous injection of iodinated contrast.56 Additional studies with adequate control groups will be necessary to answer this question.
The specific cause of contrast-induced nephropathy is not known; however, multiple factors are thought to contribute to renal impairment associated with ICA use. The ICAs can produce transient vasodilatation followed by prolonged vasoconstriction, which can last 1 to 2 hours, thus reducing renal perfusion. This vasoconstriction is hypothesized to be due to a direct effect of ICAs on the renal endothelial cells, causing an increased production of endothelin, prostaglandins, and adenosine. The ICA molecules are filtered at the glomerulus but not reabsorbed within the renal tubules. Most notable with the high-osmolarity ionic monomers, this results in increased tubular flow and, because of tubuloglomerular feedback (and direct renal vasoconstriction), in a decrease in glomerular filtration. The ICAs are also toxic to renal epithelial cells, causing an increase in the production of free radicals and activating apoptosis in the cells of the thick ascending limb of the loop of Henle.18 One must also note that a reduction in renal function after a radiologic study may not necessarily be due to contrast-induced nephropathy because another disorder included in this differential diagnosis is renal atheroembolic disease due to the use of an aortic catheter (ie, with coronary or cerebral angiography after femoral artery cannulation), which usually occurs days to weeks after a procedure; it is often associated with other manifestations of embolic events, such as digital or mesenteric ischemia.
Treatment should begin by preventing contrast-induced nephropathy in patients who are at increased risk57 (Table 2). In these patients, one should consider the possibility of alternate testing that would not involve the use of an ICA. If that is not possible, the total volume of low- or iso-osmolar ICA should be minimized. Contrast volume should be adjusted to body weight and serum creatinine as when total volume (in milliliters) of ICA exceeded 5 times (body weight [in kilograms]/baseline serum creatinine [in milligrams per deciliter]) the risk of the need for dialysis increased by a factor of 12.58 One should also consider avoiding the concurrent administration of nephrotoxic drugs with ICAs, and intravascular volume depletion should be treated. Other treatments include volume expansion, sodium bicarbonate, diuretics, N-acetylcysteine, dopamine receptor agonists, renal vasodilators, and prophylactic hemofiltration; we will discuss each of these.
Adequate hydration, and especially avoidance of dehydration, is critical for attenuating the risk of contrast-induced nephropathy.59 However, some patients may require careful hydration, such as those with congestive heart failure. At-risk patients may be hydrated with oral or intravenous fluids, at least 100 mL/h starting at least 4 hours before receiving contrast and continuing for at least 24 hours after contrast administration.59,60 Furthermore, hydration with isotonic solutions (ie, 0.9% sodium chloride) has been shown to be superior at reducing the incidence of contrast-induced nephropathy (especially in women, those with diabetes mellitus, and those who received more than 250 mL of contrast), compared with hypotonic solutions (ie, 0.45% sodium chloride).61 Hydration with an isotonic sodium bicarbonate solution (154 mEq/L) might be superior to an equiosmolar solution of sodium chloride (154 mEq/L) at further reducing the incidence of contrast-induced nephropathy; in patients with chronic renal insufficiency, the reported incidence of contrast-induced nephropathy was 13.6% with sodium chloride hydration and 1.7% with sodium bicarbonate hydration (P=.02).62 A sodium bicarbonate solution can be prepared by adding 150 mL of 1-mEq/mL sodium bicarbonate to 850 mL of 5% dextrose in water. This solution is administered at 3.5 mL/kg for 1 hour before contrast exposure and 1.18 mL/kg/per hour for 6 hours after contrast exposure. Because free radical formation is promoted in an acidic environment, sodium bicarbonate is believed to attenuate contrast-induced nephropathy by increasing the pH of renal tubular fluid, thus decreasing free radical formation.62 Recently, From et al63 retrospectively reviewed 11,516 contrast exposures in 7977 patients and reported that sodium bicarbonate use increases the risk of contrast-induced nephropathy, in contradistinction to the prospective data obtained from 199 patients reported by Merten et al.62 Further study will be needed to sort out this discrepancy and determine whether sodium bicarbonate is beneficial in only certain subgroups of patients.64 Still, many recommend prophylactic sodium bicarbonate administration in high-risk patients.
Forced diuresis can have an adverse effect on the risk of developing contrast-induced nephropathy. Rates of contrast-induced nephropathy have been reported to be either similar65 or worse66 after use of furosemide compared with saline hydration alone. Addition of mannitol to saline hydration has shown benefit in reducing the risk of contrast-induced nephropathy.65,66 These data are not surprising because furosemide and mannitol both increase tubular fluid flow, which can further decrease glomerular filtration via tubuloglomerular feedback, and both agents can also result in dehydration. In addition, mannitol increases renal oxygen consumption and is a renal vasoconstrictor. Thus, forced diuresis should be avoided in patients at risk for contrast-induced nephropathy.
Use of N-acetylcysteine, a free radical scavenger and renal vasodilator,67 has produced mixed results in the prevention of contrast-induced nephropathy. The largest and most recent meta-analyses showed a benefit from the use of N-acetylcysteine in high-risk patients; however, there was considerable variability in both dosing and efficacy.68,69 As such, the authors refrained from making clinical recommendations based on their pooled data. N-acetylcysteine may show the greatest benefit when used in patients with severe preexisting renal dysfunction.70,71 Furthermore, N-acetylcysteine has a relatively safe toxicity profile. Because its use is unlikely to cause harm and may provide protection against contrast-induced nephropathy, N-acetylcysteine could be considered. Generally, N-acetylcysteine is administered to adults orally as 600 mg twice per day for 2 days before receipt of an ICA, although doses up to 1200 mg twice per day have also been reported and might be more efficacious.69
Low-dose dopamine (<2 μg/kg/per minute) increases renal blood flow and reduces sodium reabsorption, thus inducing diuresis. Use of low-dose dopamine to prevent contrast-induced nephropathy has demonstrated mixed results and may be harmful in patients with diabetes mellitus.55,72 Recent findings derived from larger investigations have shown that fenoldopam can reduce the incidence of ICA-induced nephropathy, especially if administered directly into the renal arteries.73-75 Direct administration into the renal vasculature has been shown to reduce the risk of systemic hypotension, possibly allowing higher doses of fenoldopam to reach the kidneys while avoiding systemic hypotension. Other agents, such as adenosine receptor antagonists and calcium channel antagonists, show mixed and inconsistent results in reducing the incidence of contrast-induced nephropathy.55 As such, the currently available literature does not support the use of low-dose dopamine, adenosine receptor antagonists, or calcium channel antagonists to reduce the incidence of contrast-induced nephropathy.
Hemodialysis is effective at removing the ICA from circulation, but the use of hemodialysis after exposure to an ICA has not been shown to reduce the risk of the subsequent development of nephropathy in patients with preexisting renal insufficiency.76 However, Marenzi et al77 showed that hemofiltration use before, during, and after the administration of ICAs in patients with severe renal dysfunction may show promise for reducing the incidence of contrast-induced nephropathy and in-hospital adverse events. Therefore, additional studies will be required to determine the utility of hemodialysis or hemofiltration as a preventive measure against contrast-induced nephropathy in at-risk patients.
In patients who develop contrast-induced nephropathy, the treatment is similar to the methods for prevention. Adequate hydration remains the mainstay of therapy with additional protective measures, such as avoiding other nephrotoxic drugs and possibly instituting hemodialysis, can be considered. Early consultation with a nephrologist might prove helpful. Fortunately, in most circumstances, contrast-induced nephropathy follows a benign course and often spontaneously resolves.
Conclusion
Adverse reactions to ICAs are common; however, severe reactions are rare. Most severe acute reactions and contrast-induced nephropathy occur with high-osmolarity ionic monomeric ICAs. Delayed reactions are most common after nonionic dimers. The best means to treat all adverse reactions is to prevent them from occurring. Identification of patients who are at risk for adverse reactions and minimizing risk by following preventive measures or using alternative (ie, ICA independent) diagnostic techniques can be helpful in reducing the incidence of adverse effects. Furthermore, physicians responsible for ordering imaging tests that require the use of ICAs should consider addressing risks, benefits, and alternatives with their patients given the potential for adverse outcomes associated with this class of drugs. Equipment and drugs used to treat severe and life-threatening reactions should be readily available in all clinical locations where ICAs are administered.
Article Highlights.
-
•
Major reactions to iodinated contrast agents (ICAs) include contrast-induced nephropathy and both acute and delayed reactions. Common minor adverse reactions include rash, pain at the injection site, nausea, vomiting, and minor hemodynamic changes, which are all usually self-limiting.
-
•
Acute severe reactions are likely anaphylactoid in nature, and patients with asthma, atopy, or a history of an acute reaction to an ICA are at greatest risk.
-
•
Treatment of acute severe reactions should focus on prevention. In patients at high risk, alternate imaging modalities should be considered to avoid the use of ICAs. If that is not possible, ionic monomeric ICAs should be avoided. Pretreatment with corticosteroids can reduce the risk of, but not prevent, severe reactions. If a reaction occurs, the airway, breathing, and circulation should be managed. Administration of epinephrine and intravenous fluids is critical.
-
•
Route of administration of an ICA can affect the risk of developing contrast-induced nephropathy. In patients at high risk, alternate imaging should be considered. If ICA administration is required, the volume of the ICA should be reduced and use of other nephrotoxic drugs should be minimized or avoided. Patients should also receive intravenous hydration, and the administration of both sodium bicarbonate and N-acetylcysteine should be considered. Forced diureses with furosemide, mannitol, or dopamine (especially in patients with diabetes mellitus) should be avoided.
Supplemental Online Material
Author Interview Video
References
- 1.Christiansen C. X-ray contrast media: an overview. Toxicology. 2005;209(2):185–187. doi: 10.1016/j.tox.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Dean J.A., editor. Lange's Handbook of Chemistry. 14th ed. McGraw Hill; New York, NY: 1992. 4.18. [Google Scholar]
- 3.Thomsen H.S., Morcos S.K. Radiographic contrast media. BJU Int. 2000;86(suppl 1):1–10. doi: 10.1046/j.1464-410x.2000.00586.x. [DOI] [PubMed] [Google Scholar]
- 4.Katzberg R.W. Urography into the 21st century: new contrast media, renal handling, imaging characteristics, and nephrotoxicity. Radiology. 1997;204(2):297–312. doi: 10.1148/radiology.204.2.9240511. [DOI] [PubMed] [Google Scholar]
- 5.Kalsch H., Kalsch T., Eggebrecht H., Konorza T., Kahlert P., Erbel R. Gadolinium-based coronary angiography in patients with contraindication for iodinated x-ray contrast medium: a word of caution. J Interv Cardiol. 2008;21(2):167–174. doi: 10.1111/j.1540-8183.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- 6.Fleischmann D., Kamaya A. Optimal vascular and parenchymal contrast enhancement: the current state of the art. Radiol Clin North Am. 2009;47(1):13–26. doi: 10.1016/j.rcl.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Justesen P., Downes M., Grynne B.H., Lang H., Rasch W., Seim E. Injection-associated pain in femoral arteriography: a European multicenter study comparing safety, tolerability, and efficacy of iodixanol and iopromide. Cardiovasc Intervent Radiol. 1997;20(4):251–256. doi: 10.1007/s002709900147. [DOI] [PubMed] [Google Scholar]
- 8.Bae K.T. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology. 2010;256(1):32–61. doi: 10.1148/radiol.10090908. [DOI] [PubMed] [Google Scholar]
- 9.Dawson C., Corry D.A., Bowsher W.G., Nockler I.B., Whitfield H.N. Use of image enhancement during lithotripsy. J Endourol. 1996;10(4):335–339. doi: 10.1089/end.1996.10.335. [DOI] [PubMed] [Google Scholar]
- 10.Weininger M., Barraza J.M., Kemper C.A., Kalafut J.F., Costello P., Schoepf U.J. Cardiothoracic CT angiography: current contrast medium delivery strategies. AJR Am J Roentgenol. 2011;196(3):W260–W272. doi: 10.2214/AJR.10.5814. [DOI] [PubMed] [Google Scholar]
- 11.Wang C.L., Cohan R.H., Ellis J.H., Adusumilli S., Dunnick N.R. Frequency, management, and outcome of extravasation of nonionic iodinated contrast medium in 69,657 intravenous injections. Radiology. 2007;243(1):80–87. doi: 10.1148/radiol.2431060554. [DOI] [PubMed] [Google Scholar]
- 12.Wienbeck S., Fischbach R., Kloska S.P. Prospective study of access site complications of automated contrast injection with peripheral venous access in MDCT. AJR Am J Roentgenol. 2010;195(4):825–829. doi: 10.2214/AJR.09.3739. [DOI] [PubMed] [Google Scholar]
- 13.Schaverien M.V., Evison D., McCulley S.J. Management of large volume CT contrast medium extravasation injury: technical refinement and literature review. J Plast Reconstr Aesthet Surg. 2008;61(5):562–565. doi: 10.1016/j.bjps.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Ridley L.J. Allergic reactions to oral iodinated contrast agents: reactions to oral contrast. Australas Radiol. 1998;42(2):114–117. doi: 10.1111/j.1440-1673.1998.tb00585.x. [DOI] [PubMed] [Google Scholar]
- 15.Seymour C.W., Pryor J.P., Gupta R., Schwab C.W. Anaphylactoid reaction to oral contrast for computed tomography. J Trauma. 2004;57(5):1105–1107. doi: 10.1097/01.ta.0000133578.57031.97. [DOI] [PubMed] [Google Scholar]
- 16.Rosati G., Leto di Priolo S., Tirone P. Serious or fatal complications after inadvertent administration of ionic water-soluble contrast media in myelography. Eur J Radiol. 1992;15(2):95–100. doi: 10.1016/0720-048x(92)90131-r. [DOI] [PubMed] [Google Scholar]
- 17.American College of Radiology Manual on Contrast Media . 2010. Appendix A.http://www.acr.org/SecondaryMainMenuCategories/quality_safety/contrast_manual.aspx Accessed July 2, 2011. [Google Scholar]
- 18.Dickinson M.C., Kam P.C. Intravascular iodinated contrast media and the anaesthetist. Anaesthesia. 2008;63(6):626–634. doi: 10.1111/j.1365-2044.2008.05479.x. [DOI] [PubMed] [Google Scholar]
- 19.Katayama H., Yamaguchi K., Kozuka T., Takashima T., Seez P., Matsuura K. Adverse reactions to ionic and nonionic contrast media: a report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175(3):621–628. doi: 10.1148/radiology.175.3.2343107. [DOI] [PubMed] [Google Scholar]
- 20.Hartman G.W., Hattery R.R., Witten D.M., Williamson B., Jr Mortality during excretory urography: Mayo Clinic experience. AJR Am J Roentgenol. 1982;139(5):919–922. doi: 10.2214/ajr.139.5.919. [DOI] [PubMed] [Google Scholar]
- 21.Shehadi W.H. Death following intravascular administration of contrast media. Acta Radiol Diagn (Stockh) 1985;26(4):457–461. doi: 10.1177/028418518502600416. [DOI] [PubMed] [Google Scholar]
- 22.Kinnison M.L., Powe N.R., Steinberg E.P. Results of randomized controlled trials of low-versus high-osmolality contrast media. Radiology. 1989;170(2):381–389. doi: 10.1148/radiology.170.2.2643140. [DOI] [PubMed] [Google Scholar]
- 23.Beynon H.L., Walport M.J., Dawson P. Vascular endothelial injury by intravascular contrast agents. Invest Radiol. 1994;29(suppl 2):S195–S197. doi: 10.1097/00004424-199406001-00064. [DOI] [PubMed] [Google Scholar]
- 24.Sumimura T., Sendo T., Itoh Y. Calcium-dependent injury of human microvascular endothelial cells induced by a variety of iodinated radiographic contrast media. Invest Radiol. 2003;38(6):366–374. [PubMed] [Google Scholar]
- 25.Oowaki K., Saigusa H., Ojiri H. Relationship between oral food intake and nausea caused by intravenous injection of iodinated contrast material. Nihon Igaku Hoshasen Gakkai Zasshi. 1994;54(6):476–479. [in Japanese] [PubMed] [Google Scholar]
- 26.Almen T. The etiology of contrast medium reactions. Invest Radiol. 1994;29(suppl 1):S37–S45. [PubMed] [Google Scholar]
- 27.Lieberman P., Nicklas R.A., Oppenheimer J. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126(3):477–480. doi: 10.1016/j.jaci.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Simons F.E., Ardusso L.R., Bilo M.B. World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol. 2011;127(3):587–593. doi: 10.1016/j.jaci.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 29.Vervloet D., Durham S. Adverse reactions to drugs. BMJ. 1998;316(7143):1511–1514. doi: 10.1136/bmj.316.7143.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laroche D., Aimone-Gastin I., Dubois F. Mechanisms of severe, immediate reactions to iodinated contrast material. Radiology. 1998;209(1):183–190. doi: 10.1148/radiology.209.1.9769830. [DOI] [PubMed] [Google Scholar]
- 31.Lang D.M., Alpern M.B., Visintainer P.F., Smith S.T. Increased risk for anaphylactoid reaction from contrast media in patients on beta-adrenergic blockers or with asthma. Ann Intern Med. 1991;115(4):270–276. doi: 10.7326/0003-4819-115-4-270. [DOI] [PubMed] [Google Scholar]
- 32.Morcos S.K., Thomsen H.S. Adverse reactions to iodinated contrast media. Eur Radiol. 2001;11(7):1267–1275. doi: 10.1007/s003300000729. [DOI] [PubMed] [Google Scholar]
- 33.Lasser E.C., Berry C.C., Talner L.B. Pretreatment with corticosteroids to alleviate reactions to intravenous contrast material. N Engl J Med. 1987;317(14):845–849. doi: 10.1056/NEJM198710013171401. [DOI] [PubMed] [Google Scholar]
- 34.Lasser E.C., Berry C.C., Mishkin M.M., Williamson B., Zheutlin N., Silverman J.M. Pretreatment with corticosteroids to prevent adverse reactions to nonionic contrast media. AJR Am J Roentgenol. 1994;162(3):523–526. doi: 10.2214/ajr.162.3.8109489. [DOI] [PubMed] [Google Scholar]
- 35.Wolf G.L., Mishkin M.M., Roux S.G. Comparison of the rates of adverse drug reactions: ionic contrast agents, ionic agents combined with steroids, and nonionic agents. Invest Radiol. 1991;26(5):404–410. doi: 10.1097/00004424-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Greenberger P.A., Patterson R. The prevention of immediate generalized reactions to radiocontrast media in high-risk patients. J Allergy Clin Immunol. 1991;87(4):867–872. doi: 10.1016/0091-6749(91)90135-b. [DOI] [PubMed] [Google Scholar]
- 37.Kemp S.F., Lockey R.F., Simons F.E. Epinephrine: the drug of choice for anaphylaxis: a statement of the World Allergy Organization. Allergy. 2008;63(8):1061–1070. doi: 10.1111/j.1398-9995.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 38.Barach E.M., Nowak R.M., Lee T.G., Tomlanovich M.C. Epinephrine for treatment of anaphylactic shock. JAMA. 1984;251(16):2118–2122. [PubMed] [Google Scholar]
- 39.Lieberman P. Use of epinephrine in the treatment of anaphylaxis. Curr Opin Allergy Clin Immunol. 2003;3(4):313–318. doi: 10.1097/00130832-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Loh S., Bagheri S., Katzberg R.W., Fung M.A., Li C.S. Delayed adverse reaction to contrast-enhanced CT: a prospective single-center study comparison to control group without enhancement. Radiology. 2010;255(3):764–771. doi: 10.1148/radiol.10091848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savill J.S., Barrie R., Ghosh S., Muhlemann M., Dawson P., Pusey C.D. Fatal Stevens-Johnson syndrome following urography with iopamidol in systemic lupus erythematosus. Postgrad Med J. 1988;64(751):392–394. doi: 10.1136/pgmj.64.751.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood B.P., Lane A.T., Rabinowitz R. Cutaneous reaction to contrast material. Radiology. 1988;169(3):739–740. doi: 10.1148/radiology.169.3.2973079. [DOI] [PubMed] [Google Scholar]
- 43.Sutton A.G., Finn P., Grech E.D. Early and late reactions after the use of iopamidol 340, ioxaglate 320, and iodixanol 320 in cardiac catheterization. Am Heart J. 2001;141(4):677–683. doi: 10.1067/mhj.2001.113570. [DOI] [PubMed] [Google Scholar]
- 44.Mikkonen R., Vehmas T., Granlund H., Kivisaari L. Seasonal variation in the occurrence of late adverse skin reactions to iodine-based contrast media. Acta Radiol. 2000;41(4):390–393. doi: 10.1080/028418500127345532. [DOI] [PubMed] [Google Scholar]
- 45.Scherer K., Harr T., Bach S., Bircher A.J. The role of iodine in hypersensitivity reactions to radio contrast media. Clin Exp Allergy. 2010;40(3):468–475. doi: 10.1111/j.1365-2222.2009.03361.x. [DOI] [PubMed] [Google Scholar]
- 46.Shulman K.L., Thompson J.A., Benyunes M.C., Winter T.C., Fefer A. Adverse reactions to intravenous contrast media in patients treated with interleukin-2. J Immunother Emphasis Tumor Immunol. 1993;13(3):208–212. doi: 10.1097/00002371-199304000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Brockow K., Romano A., Aberer W. Skin testing in patients with hypersensitivity reactions to iodinated contrast media: a European multicenter study. Allergy. 2009;64(2):234–241. doi: 10.1111/j.1398-9995.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- 48.Caimmi S., Benyahia B., Suau D. Clinical value of negative skin tests to iodinated contrast media. Clin Exp Allergy. 2010;40(5):805–810. doi: 10.1111/j.1365-2222.2010.03493.x. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe H., Sueki H., Nakada T., Akiyama M., Iijima M. Multiple fixed drug eruption caused by iomeprol (Iomeron), a nonionic contrast medium. Dermatology. 1999;198(3):291–294. doi: 10.1159/000018133. [DOI] [PubMed] [Google Scholar]
- 50.From A.M., Bartholmai B.J., Williams A.W., Cha S.S., McDonald F.S. Mortality associated with nephropathy after radiographic contrast exposure. Mayo Clin Proc. 2008;83(10):1095–1100. doi: 10.4065/83.10.1095. [DOI] [PubMed] [Google Scholar]
- 51.Levy E.M., Viscoli C.M., Horwitz R.I. The effect of acute renal failure on mortality: a cohort analysis. JAMA. 1996;275(19):1489–1494. [PubMed] [Google Scholar]
- 52.Thomsen H.S., Morcos S.K. Contrast media and the kidney: European Society of Urogenital Radiology (ESUR) guidelines. Br J Radiol. 2003;76(908):513–518. doi: 10.1259/bjr/26964464. [DOI] [PubMed] [Google Scholar]
- 53.Rihal C.S., Textor S.C., Grill D.E. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105(19):2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 54.Hall K.A., Wong R.W., Hunter G.C. Contrast-induced nephrotoxicity: the effects of vasodilator therapy. J Surg Res. 1992;53(4):317–320. doi: 10.1016/0022-4804(92)90054-4. [DOI] [PubMed] [Google Scholar]
- 55.Goldenberg I., Matetzky S. Nephropathy induced by contrast media: pathogenesis, risk factors and preventive strategies. CMAJ. 2005;172(11):1461–1471. doi: 10.1503/cmaj.1040847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao Q.A., Newhouse J.H. Risk of nephropathy after intravenous administration of contrast material: a critical literature analysis. Radiology. 2006;239(2):392–397. doi: 10.1148/radiol.2392050413. [DOI] [PubMed] [Google Scholar]
- 57.Rundback J.H., Nahl D., Yoo V. Contrast-induced nephropathy. J Vasc Surg. 2011;54(2):575–579. doi: 10.1016/j.jvs.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 58.Freeman R.V., O'Donnell M., Share D. Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. Am J Cardiol. 2002;90(10):1068–1073. doi: 10.1016/s0002-9149(02)02771-6. [DOI] [PubMed] [Google Scholar]
- 59.Morcos S.K., Thomsen H.S., Webb J.A., Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR) Contrast-media-induced nephrotoxicity: a consensus report. Eur Radiol. 1999;9(8):1602–1613. doi: 10.1007/s003300050894. [DOI] [PubMed] [Google Scholar]
- 60.Taylor A.J., Hotchkiss D., Morse R.W., McCabe J. PREPARED: Preparation for Angiography in Renal Dysfunction: a randomized trial of inpatient vs outpatient hydration protocols for cardiac catheterization in mild-to-moderate renal dysfunction. Chest. 1998;114(6):1570–1574. doi: 10.1378/chest.114.6.1570. [DOI] [PubMed] [Google Scholar]
- 61.Mueller C., Buerkle G., Buettner H.J. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162(3):329–336. doi: 10.1001/archinte.162.3.329. [DOI] [PubMed] [Google Scholar]
- 62.Merten G.J., Burgess W.P., Gray L.V. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291(19):2328–2334. doi: 10.1001/jama.291.19.2328. [DOI] [PubMed] [Google Scholar]
- 63.From A.M., Bartholmai B.J., Williams A.W., Cha S.S., Pflueger A., McDonald F.S. Sodium bicarbonate is associated with an increased incidence of contrast nephropathy: a retrospective cohort study of 7977 patients at Mayo Clinic. Clin J Am Soc Nephrol. 2008;3(1):10–18. doi: 10.2215/CJN.03100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trivedi H., Nadella R., Szabo A. Hydration with sodium bicarbonate for the prevention of contrast-induced nephropathy: a meta-analysis of randomized controlled trials. Clin Nephrol. 2010;74(4):288–296. doi: 10.5414/cnp74288. [DOI] [PubMed] [Google Scholar]
- 65.Stevens M.A., McCullough P.A., Tobin K.J. A prospective randomized trial of prevention measures in patients at high risk for contrast nephropathy: results of the P.R.I.N.C.E. Study; Prevention of Radiocontrast Induced Nephropathy Clinical Evaluation. J Am Coll Cardiol. 1999;33(2):403–411. doi: 10.1016/s0735-1097(98)00574-9. [DOI] [PubMed] [Google Scholar]
- 66.Solomon R., Werner C., Mann D., D'Elia J., Silva P. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331(21):1416–1420. doi: 10.1056/NEJM199411243312104. [DOI] [PubMed] [Google Scholar]
- 67.Drager L.F., Andrade L., Barros de Toledo J.F., Laurindo F.R., Machado Cesar L.A., Seguro A.C. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant. 2004;19(7):1803–1807. doi: 10.1093/ndt/gfh261. [DOI] [PubMed] [Google Scholar]
- 68.Kshirsagar A.V., Poole C., Mottl A. N-acetylcysteine for the prevention of radiocontrast induced nephropathy: a meta-analysis of prospective controlled trials. J Am Soc Nephrol. 2004;15(3):761–769. doi: 10.1097/01.asn.0000116241.47678.49. [DOI] [PubMed] [Google Scholar]
- 69.Trivedi H., Daram S., Szabo A., Bartorelli A.L., Marenzi G. High-dose N-acetylcysteine for the prevention of contrast-induced nephropathy. Am J Med. 2009;122(9):874. doi: 10.1016/j.amjmed.2009.01.035. e9-15. [DOI] [PubMed] [Google Scholar]
- 70.Fishbane S., Durham J.H., Marzo K., Rudnick M. N-acetylcysteine in the prevention of radiocontrast-induced nephropathy. J Am Soc Nephrol. 2004;15(2):251–260. doi: 10.1097/01.asn.0000107562.68920.92. [DOI] [PubMed] [Google Scholar]
- 71.Goldenberg I., Shechter M., Matetzky S. Oral acetylcysteine as an adjunct to saline hydration for the prevention of contrast-induced nephropathy following coronary angiography: a randomized controlled trial and review of the current literature. Eur Heart J. 2004;25(3):212–218. doi: 10.1016/j.ehj.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 72.Weisberg L.S., Kurnik P.B., Kurnik B.R. Dopamine and renal blood flow in radiocontrast-induced nephropathy in humans. Ren Fail. 1993;15(1):61–68. doi: 10.3109/08860229309065574. [DOI] [PubMed] [Google Scholar]
- 73.Kini A.S., Mitre C.A., Kim M., Kamran M., Reich D., Sharma S.K. A protocol for prevention of radiographic contrast nephropathy during percutaneous coronary intervention: effect of selective dopamine receptor agonist fenoldopam. Catheter Cardiovasc Interv. 2002;55(2):169–173. doi: 10.1002/ccd.10038. [DOI] [PubMed] [Google Scholar]
- 74.Teirstein P.S., Price M.J., Mathur V.S., Madyoon H., Sawhney N., Baim D.S. Differential effects between intravenous and targeted renal delivery of fenoldopam on renal function and blood pressure in patients undergoing cardiac catheterization. Am J Cardiol. 2006;97(7):1076–1081. doi: 10.1016/j.amjcard.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 75.Weisz G., Filby S.J., Cohen M.G. Safety and performance of targeted renal therapy: the Be-RITe! Registry. J Endovasc Ther. 2009;16(1):1–12. doi: 10.1583/08-2515.1. [DOI] [PubMed] [Google Scholar]
- 76.Vogt B., Ferrari P., Schonholzer C. Prophylactic hemodialysis after radiocontrast media in patients with renal insufficiency is potentially harmful. Am J Med. 2001;111(9):692–698. doi: 10.1016/s0002-9343(01)00983-4. [DOI] [PubMed] [Google Scholar]
- 77.Marenzi G., Marana I., Lauri G. The prevention of radiocontrast-agent-induced nephropathy by hemofiltration. N Engl J Med. 2003;349(14):1333–1340. doi: 10.1056/NEJMoa023204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview Video


