Abstract
Objective
To determine whether the free light chain (FLC) assay provides prognostic information relevant to the general population.
Methods
After excluding persons with a known plasma cell disorder, we studied 15,859 Olmsted County, Minnesota, residents 50 years or older in whom unmasked data and samples for FLC testing were available. Baseline information was obtained between March 13, 1995, and November 21, 2003, and follow-up status and cause of death were identified through June 30, 2009. The κ and λ FLC sum (Σ FLC) was evaluated for its ability to predict overall survival. Specific causes of death were also investigated.
Results
In 158,003 person-years of follow-up, 4348 individuals died. A high Σ FLC was significantly predictive of worse overall survival; the risk ratio for death for those with the highest decile of Σ FLC (ie, ≥4.72 mg/dL) was 4.4 (95% confidence interval, 4.1-4.7) relative to the remaining study participants. Multivariate analyses demonstrated that this excess risk of death was independent of age, sex, and renal insufficiency, with a corrected risk ratio of 2.1 (95% confidence interval, 1.9-2.2). The increased mortality was not restricted to any particular cause of death because the observed-to-expected risk of death from most causes was significantly higher among those individuals with an antecedent Σ FLC of 4.72 mg/dL or higher, which is near the upper limit of normal for the test.
Conclusion
A nonclonal elevation of Σ FLC is a significant predictor of worse overall survival in the general population of persons without plasma cell disorders.
Abbreviations and Acronyms: FLC, serum immunoglobulin free light chain; Σ FLC, κ and λ free light chain sum; ICD-9, International Classification of Diseases, Ninth Revision; ICD-10, International Statistical Classification of Diseases, 10th Revision; LC-MGUS, light chain monoclonal gammopathy of undetermined significance; MGUS, monoclonal gammopathy of undetermined significance; rFLC, κ-to-λ FLC ratio
The serum immunoglobulin free light chain (FLC) assay has been important in the management of plasma cell disorders, such as monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma, and amyloidosis, with respect to diagnosis, prognosis, and disease measurement.1 Additional uses for the assay include the identification of a premalignant condition called light chain MGUS (LC-MGUS)2 and prognostication in other hematologic conditions, such as chronic lymphocytic leukemia3,4 and non-Hodgkin lymphoma.5 When screening for clonal disease, the κ-to-λ FLC ratio (rFLC) is used to correct for both impaired catabolism (renal function) and nonspecific production (generalized immune overstimulation). This adjustment makes it possible to identify patients with a relative imbalance between κ and λ concentrations, which typically qualifies for clonal excess. Investigators have also used the absolute value of an involved FLC for various end points.5,6 Presumably by acting as a global marker of immune stimulation, nonclonal elevations of FLCs represent a risk of developing non-Hodgkin lymphoma among patients with human immunodeficiency virus infection7 and of short event-free survival among patients with diffuse large cell lymphoma.5 The purpose of the present study was to test the hypothesis that nonclonal elevation of serum FLCs, measured as the serum κ and λ FLC sum (Σ FLC), is a significant predictor of overall survival in the general population.
Methods
This study was performed in accordance with the Declaration of Helsinki and with approval of the Mayo Clinic Institutional Review Board and the Olmsted Medical Group. The Olmsted County MGUS prevalence cohort was composed of 21,462 of the 28,038 enumerated individuals 50 years and older who were living in Olmsted County, Minnesota, between January 1, 1995, and November 21, 2003.8 Because it captured more than 75% of the resident population in this age range, the cohort is considered to be representative of the general population living in a defined geographic area. Of the total 21,462 individuals in the cohort, we excluded 710 persons with known plasma cell disorders, including those with MGUS and LC-MGUS, because they represent conditions in which clonal elevations of FLCs occur and in which the prognostic value of increased FLCs is well established.2,9 A relatively newly described entity, LC-MGUS is defined as the presence of an abnormal rFLC with an elevation in at least one of the FLCs.2 We also excluded 4096 individuals who declined to provide authorization to participate in research,10 758 with an inadequate sample to perform the FLC assay, and 39 whose cause of death was recorded using a coding scheme other than that in the International Classification of Diseases, Ninth Revision (ICD-9) or International Statistical Classification of Diseases, 10th Revision (ICD-10) format. Thus, the final study cohort for the primary analyses to determine the prognostic value of nonclonal FLC elevation on overall survival comprised 15,859 individuals. Baseline information was obtained for these individuals between March 13, 1995, and November 21, 2003, and follow-up status and cause of death information were identified through June 30, 2009. For the multivariate analyses, 2772 additional individuals were excluded because of lack of a coincident serum creatinine measurement. Individuals were followed up using the data resources of the Rochester Epidemiology Project.11,12 Deaths and causes of death were ascertained using death certificate ICD-9 and ICD-10 codes. All codes were converted to ICD-10 (http://www.cdc.gov/nchs/icd/icd10cm.htm) and analyzed at the chapter level.9
Measurements were performed using the FLC assay (Binding Site, Birmingham, England). The reference ranges for κ FLCs and λ FLCs are 0.33 to 1.94 mg/dL and 0.57 to 2.63 mg/dL (to convert to mg/L, multiply by 10), respectively, and the reference range for the rFLC is 0.26 to 1.65.13 To calculate the reference range for the Σ FLC, we used κ FLC and λ FLC concentrations from the original reference range study13 and estimated standard errors of the quantile regression parameters using a bootstrap resampling procedure with replacement in 10,000 replicates.14 The resulting overall 95% reference range was 0.93 to 4.33 mg/dL. The Σ FLC was significantly associated with age at the 95th percentile (P<.001) but not at the 25th percentile. The association of Σ FLC with age disappeared when the FLC concentrations were normalized by cystatin C concentrations.13 Because the Σ FLC increased at 65 years of age, the age-specific reference ranges were 0.72 to 3.55 mg/dL among individuals 65 years or younger and 1.35 to 5.95 mg/dL among individuals older than 65 years (J.A.K., personal communication).
Survival time was calculated from the time of sample ascertainment using the method of Kaplan and Meier, and differences in survival were assessed using log-rank tests. The adjusted curves are based on a direct adjusted survival estimate.15 The risk of death (risk ratio) associated with Σ FLC, creatinine, age, and sex was estimated using both univariate and multivariate Cox proportional hazards models. In these models, age (per 10 years) and serum creatinine (per 1-mg/dL unit) were incorporated as continuous variables. The individuals in the Olmsted research cohort were further classified according to whether they had a Σ FLC in the top decile (≥4.72 mg/dL) or not, and the risk of death was calculated as a standardized mortality ratio (observed number of deaths divided by the expected number of deaths). The expected deaths were based on the white population of Minnesota from 1979 to 2002, recorded by calendar year, sex, age group, and ICD-9– or ICD-10–coded cause of death.14,16 The P values to determine differences between time to death among patients in the first 9 deciles compared with the 10th decile were calculated using the test of Gray.17
Results
At baseline, the median age of the community sample of 15,859 individuals was 63 years (range, 50-109 years), and 55.3% (n=8753) were women. As of June 2009, median follow-up was 12.7 years (range, 1 day to 14.3 years per person) from sample acquisition, and 4348 individuals (27.4%) had died and had an ICD-coded cause of death. For the purposes of the multivariate analyses, 13,087 individuals (82.5%) had a known creatinine value at the time of their FLC sampling.
Prognostic Effect of Σ FLC
The Σ FLC was used to study the effect of FLC elevation on survival in persons without evidence of a plasma cell disorder. The relationship between Σ FLC and rFLC is shown in Figure 1; no individual with a high Σ FLC had an abnormal rFLC because these patients were excluded from the study because they were deemed to have LC-MGUS. When Σ FLC was separated into deciles (Figure 2), there was progressively worse survival with increasing values of Σ FLC (P<.001). Because the range of Σ FLC was relatively small—0.09 to 48.2 mg/dL—and because the 10th decile (highest Σ FLC) was near the sum of the upper reference ranges for κ FLC and λ FLC, subsequent analyses were collapsed into 2 groups: 10th decile (Σ FLC ≥4.72 mg/L) vs deciles 1 through 9 (Σ FLC <4.72 mg/L). As indicated in Table 1, significant differences were found between these 2 groups in terms of mean age (72 vs 62 years; P<.001), sex (49.5% [n=782] vs 44.3% [n=6324] male; P<.001), and mean serum creatinine level (1.2 vs 1.0 mg/dL; P<.001).
FIGURE 1.
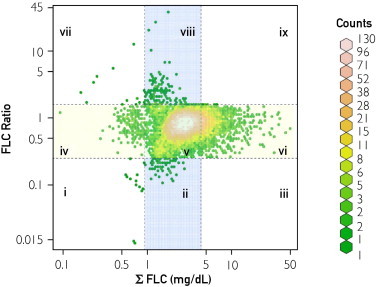
Relationship between the κ and λ free light chain sum (Σ FLC) and the free light chain ratio (rFLC). Most study participants had both normal Σ FLC and rFLC (section v, 86.3%). By definition of light chain monoclonal gammopathy of undetermined significance,2 no participant had both a high Σ FLC and an abnormal rFLC (low [section iii] or high [section ix]). The distribution of the remaining participants by section was as follows: i, 0.1%; ii, 0.4%; iv, 1.2%; vi, 11.2%; vii, 0.1%; and viii, 0.8%. Blue represents the reference range for Σ FLC (0.95-4.35 mg/dL), yellow represents the reference range for rFLC (0.26-1.65), and green represents those study participants in whom both the Σ FLC and rFLC were normal.
FIGURE 2.
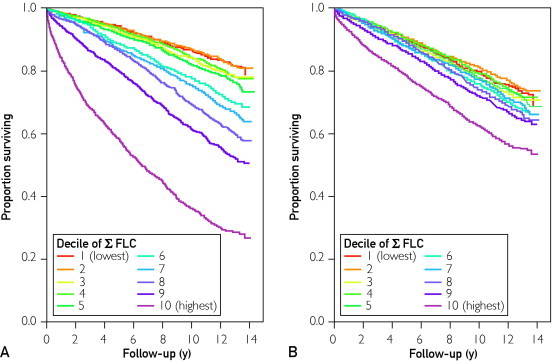
Risk of death by κ and λ free light chain sum (Σ FLC) decile. The higher the Σ FLC, the lower the overall survival from the time of sample ascertainment. A, All patients, crude data. B, A patients, corrected for age and creatinine level based on the overall distribution in the study.
TABLE 1.
Characteristics of Olmsted County, Minnesota, Residents by Decile of Σ FLCa
| Characteristic | All residents (N=15,859) | Top decile (n=1581) | Lower 9 deciles (n=14,278) | P value |
|---|---|---|---|---|
| Age (y) | 63 (50-109) | 73 (50-109) | 62 (50-101) | <.001 |
| Male, No. (%) | 7106 (44.8) | 782 (49.5) | 6324 (44.3) | <.001 |
| Creatinine (mg/dL)b | 1.0 (0.4-14.2) | 1.2 (0.4-14.2) | 1.0 (0.4-10.8) | <.001 |
| No. (%) of individuals by creatinine level | ||||
| ≥1.5 mg/dL | 597 (4.5) | 355 (25.4) | 232 (2.0) | <.001 |
| ≥2.0 mg/dL | 173 (1.3) | 141 (10.1) | 32 (0.3) | <.001 |
| Σ FLC (mg/dL) | 2.8 (0.1-48.2) | 5.8 (4.7-48.2) | 2.7 (0.1-4.7) | <.001 |
| κ FLC (mg/dL) | 1.3 (0.01-20.5) | 2.8 (1.1-20.5) | 1.2 (0.01-2.8) | <.001 |
| λ FLC (mg/dL) | 1.5 (0.04-28.2) | 3.11 (1.8-28.2) | 1.4 (0.04-3.6) | <.001 |
| rFLC | 0.8 (0.01-40.9) | 0.9 (0.3-1.6) | 0.8 (0.01-40.9) | <.001 |
Data are presented as median (range) unless otherwise specified. Σ FLC = κ and λ free light chain sum; FLC = free light chain; rFLC = κ to λ free light chain ratio.
Data available for only 13,087 patients. (To convert to μmol/L, multiply by 88.4).
On univariate analysis, a Σ FLC result near the upper limit of normal was significantly predictive for worse overall survival (Table 2). The risk ratio for death for those with the highest decile of Σ FLC relative to the other 90% of residents was 4.4 (95% confidence interval, 4.1-4.7). Multivariate analyses, also detailed in Table 2, were performed to exclude the possibility that Σ FLC was merely a surrogate for age, sex, and/or renal insufficiency. With these other variables included, Σ FLC remained a significant independent predictor of worse survival, with a relative risk for death with the highest Σ FLC of 2.1 (95% confidence interval, 1.9-2.2).
TABLE 2.
Risk of Death Among Olmsted County, Minnesota, Residents: Univariate and Multivariate Modelsa
| Term | Risk ratio (95% confidence interval) | P value |
|---|---|---|
| Univariate model | ||
| Σ FLC, top decile | 4.38 (4.08-4.70) | <.001 |
| Creatinineb | 1.44 (1.40-1.48) | <.001 |
| Male | 1.07 (1.01-1.14) | .02 |
| Age (10-y increase) | 3.02 (2.93-3.11) | <.001 |
| Multivariate model | ||
| 2 Variables | ||
| Σ FLC, top decile | 3.88 (3.59-4.19) | <.001 |
| Creatinineb | 1.25 (1.21-1.29) | <.001 |
| 3 Variables | ||
| Σ FLC, top decile | 2.12 (1.96-2.29) | <.001 |
| Creatinineb | 1.30 (1.25-1.35) | <.001 |
| Age (10-y increase) | 2.77 (2.68-2.86) | <.001 |
| 4 Variables | ||
| Σ FLC, top decile | 2.07 (1.91-2.24) | <.001 |
| Creatinineb | 1.26 (1.20-1.31) | <.001 |
| Age (10-y increase) | 2.85 (2.76-2.95) | <.001 |
| Male sex | 1.34 (1.25-1.43) | <.001 |
Σ FLC = κ and λ free light chain sum.
Data available for only 13,087 individuals.
Relationship Between Σ FLC and Cause of Death
We next focused on whether Σ FLC predicted particular causes of death. A total of 4348 Olmsted County residents had an informative death. Table 3 gives the distribution of deaths by cause at the ICD-10 chapter level, with the leading cause of death being circulatory disease (n=363) followed by neoplasms (n=197).
TABLE 3.
Observed-to-Expected Risk of Death Among Olmsted County, Minnesota, Residents for Participants With Top Decile Σ FLCa,b
| Cause of death by ICD-10 chapter | Observed, No. | Expected, No. | RR | P value |
|---|---|---|---|---|
| Circulatory | 363 | 168.5 | 1.9 | <.001 |
| Neoplasms | 197 | 96.8 | 2.0 | <.001 |
| Respiratory | 80 | 47.2 | 1.7 | <.001 |
| Mental | 68 | 20.9 | 3.3 | <.001 |
| Nervous system | 42 | 25.6 | 1.7 | <.001 |
| Endocrine | 34 | 18.4 | 1.6 | .02 |
| Genitourinary | 31 | 11.4 | 2.1 | <.001 |
| Digestive | 41 | 13.0 | 3.0 | <.001 |
| External causes | 22 | 11.0 | 2.1 | .001 |
| Injury or poisoning | 18 | 3.4 | 6.4 | <.001 |
| Infectious | 16 | 4.4 | 3.9 | <.001 |
| Skin | 3 | 0.6 | 5.3 | .004 |
| Ill-defined | 8 | 13.7 | 0.6 | .13 |
| Musculoskeletal and connective tissue | 7 | 3.8 | 1.8 | .11 |
| Congenital | 2 | 0.4 | 5.2 | .02 |
| Blood | 0 | 2.1 | 0.0 | .99 |
| Total | 932 | 441.2 | 2.0 | <.001 |
Σ FLC = κ and λ free light chain sum; ICD-10 = International Statistical Classification of Diseases, 10th Revision (ICD-10); RR = risk ratio.
Corrected for age, sex, and serum creatinine level.
Also indicated in Table 3, the increased mortality in patients with a top decile Σ FLC was not restricted to any specific cause of death. Almost universally, chapter by chapter, those with a higher Σ FLC had a higher risk of death with the exceptions of “blood,” “musculoskeletal/connective tissue,” “genitourinary,” “congenital,” and “ill-defined” conditions. The time from FLC ascertainment to death was also calculated and plotted according to Σ FLC decile (Figure 3). The causes of death most proximal to FLC ascertainment were musculoskeletal, circulatory, neoplasm, respiratory, mental, and digestive disorders.
FIGURE 3.
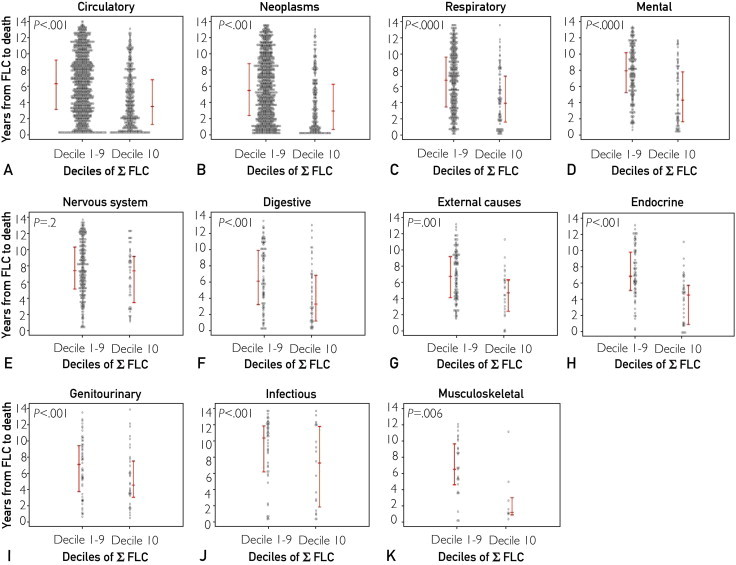
Time to death as a function of cause of death and κ and λ free light chain sum (Σ FLC) decile. Time between sample ascertainment to death among 4348 dead Olmsted County residents by International Statistical Classification of Diseases, 10th Revision (ICD-10) chapter. Those with the highest Σ FLC deciles died sooner than those with lower (deciles 1-9) Σ FLC deciles. A, Circulatory. B, Neoplasms. C, Respiratory. D, Mental. E, Nervous system. F, Digestive. G, External causes. H, Endocrine. I, Genitourinary. J, Infectious. K, Musculoskeletal. Data for the chapters “ill-defined” and “external causes” are not shown because of small numbers and no significant differences between groups. The red bars represent the 25th, 50th, and 75th percentiles.
Discussion
In this study, we found that a nonclonal elevation of Σ FLC is a significant predictor of worse overall survival in persons without plasma cell disorders. This is a remarkable finding in a general population of individuals 50 years or older who do not have MGUS or LC-MGUS or any other known plasma cell disorder. The increased risk of death was independent of renal function, sex, and age. However, the increased risk of death was not restricted to any particular cause of death; the observed-to-expected risk of death from most causes, including cardiac and neoplastic conditions, was significantly higher among individuals with an antecedent Σ FLC equal to or greater than 4.72 mg/dL.
Polyclonal increases in FLC are seen with renal dysfunction; are elevated in diseases such as rheumatoid arthritis, Sjögren syndrome, and systemic lupus erythematosus18; and have been shown to be a risk factor for the development of non-Hodgkin lymphoma among patients with human immunodeficiency virus infection.7 However, the mechanism by which Σ FLC predicts survival in this large population-based cohort is uncertain. Conditions resulting in overactivation of the immune system may have contributed to the excess risk of death, but inflammatory rheumatologic deaths accounted for less than 1% of all deaths observed in this cohort. However, the observation of a 2-fold risk of cardiovascular death compared with those with lower Σ FLC fits nicely with the emerging connections between inflammation and atherosclerosis.19 Another possibility is that Σ FLC excess is one manifestation of immunosenescence, which results in increased susceptibility to infection, malignant neoplasms, and autoimmunity.20,21
Our data add to the growing body of literature that connects inflammation, aging, and chronic disease,21 but they do not provide insight into whether inflammation served as a surrogate for an underlying life-threatening disease entity or whether the underlying inflammation predisposes individuals for conditions such as cancer through free radical–induced injury. Because approximately 20% of all cancers in adults result from chronic inflammatory states or have an inflammatory origin,22,23 it is not surprising that there was a nearly 2-fold higher observed-to-expected rate of deaths from malignant neoplasms among the patients with high Σ FLC in our cohort. It is intriguing, however, that a Σ FLC measurement taken 3 to 4 years before death has the power to portend a worse outcome for nearly all cause-of-death categories.
Limitations of this study include a lack of other inflammatory markers with which to compare our Σ FLC results and the fact that causes of death were ascertained retrospectively by death certificates. These potential shortcomings are outweighed by the size of the study cohort and its population-based structure, as well as by the simplicity and availability of the FLC assay. Although the FLC assay is able to predict for worse overall survival in an unselected general population, it is not yet clear whether the Σ FLC adds above and beyond other conventional, nonspecific inflammatory markers, such as erythrocyte sedimentation rate or C-reactive protein. However, erythrocyte sedimentation rate and C-reactive protein are acute and chronic inflammatory markers, respectively, rather than markers of the adaptive immune system, which includes highly specialized immune responses. To date, no study has shown such a significant influence with respect to such a breadth of causes of death based on any one assay. Until additional studies are performed on other cohorts with a more expansive inflammatory marker panel, these data are not strong enough to support a recommendation of routine FLC measurement as part of day-to-day clinical practice. Short of that, however, this assay could serve as a means of normalizing the expected risk of death among large patient populations and as a hypothesis-generating tool to study culprits believed to contribute to inflammation, cancer, and aging.
Conclusion
A nonclonal elevation of Σ FLC predicts inferior overall survival in a general population that has been depleted of patients with plasma cell disorders. The observed increased risk of death introduced by a nonclonal elevation of Σ FLC was independent of renal function, sex, and age and maintained its predictive value regardless of the eventual cause of death.
Acknowledgments
We thank Tara Phelps for her maintenance of the peripheral blood sample bank.
Footnotes
For editorial comment, see page 505
Grant Support: This work was supported in part by grants CA62242 (A.D, R.A.K. S.V.R.), CA107476 (A.D., S.V.R., J.A.K., R.A.K.), and CA91561 (A.D) from the National Cancer Institute, The JABBS Foundation, and The Predolin Foundation. Binding Site provided the serum immunoglobulin free light chain reagent. This study was supported in part by National Institutes of Health grant R01 AG034676 and the Rochester Epidemiology Project (grant number R01-AG034676; Principal Investigator: Walter A. Rocca, MD).
Potential Competing Interests: Drs Dispenzieri and Katzmann have received a travel grant from Binding Site, to present data. Dr Mead is an employee of Binding Site. Free light chain reagent was provided by Binding Site. There are no other potential conflicts of interest.
Supplemental Online Material
Author Interview Video
References
- 1.Dispenzieri A., Kyle R., Merlini G. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215–224. doi: 10.1038/leu.2008.307. [DOI] [PubMed] [Google Scholar]
- 2.Dispenzieri A., Katzmann J.A., Kyle R.A. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375(9727):1721–1728. doi: 10.1016/S0140-6736(10)60482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pratt G., Harding S., Holder R. Abnormal serum free light chain ratios are associated with poor survival and may reflect biological subgroups in patients with chronic lymphocytic leukaemia. Br J Haematol. 2009;144(2):217–222. doi: 10.1111/j.1365-2141.2008.07456.x. [DOI] [PubMed] [Google Scholar]
- 4.Yegin Z.A., Ozkurt Z.N., Yağci M. Free light chain: a novel predictor of adverse outcome in chronic lymphocytic leukemia. Eur J Haematol. 2010;84(5):406–411. doi: 10.1111/j.1600-0609.2010.01412.x. [DOI] [PubMed] [Google Scholar]
- 5.Maurer M.J., Micallef I.N., Cerhan J.R. Elevated serum free light chains are associated with event-free and overall survival in two independent cohorts of patients with diffuse large B-cell lymphoma. J Clin Oncol. 2011;29(12):1620–1626. doi: 10.1200/JCO.2010.29.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dispenzieri A., Lacy M.Q., Katzmann J.A. Absolute values of immunoglobulin free light chains are prognostic in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2006;107(8):3378–3383. doi: 10.1182/blood-2005-07-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landgren O., Goedert J.J., Rabkin C.S. Circulating serum free light chains as predictive markers of AIDS-related lymphoma. J Clin Oncol. 2010;28(5):773–779. doi: 10.1200/JCO.2009.25.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyle R.A., Therneau T.M., Rajkumar S.V. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362–1369. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 9.Rajkumar S.V., Kyle R.A., Therneau T.M. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106(3):812–817. doi: 10.1182/blood-2005-03-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melton L.J., III The threat to medical-records research. N Engl J Med. 1997;337(20):1466–1470. doi: 10.1056/NEJM199711133372012. [DOI] [PubMed] [Google Scholar]
- 11.St Sauver J.L., Grossardt B.R., Yawn B.P., Melton L.J., III, Rocca W.A. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melton L.J., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 13.Katzmann J.A., Clark R.J., Abraham R.S. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437–1444. [PubMed] [Google Scholar]
- 14.Compressed Mortality File (CMF) 1989-1998. http://www.cdc.gov/nchs/data_access/cmf.htm Accessed May 3, 2012.
- 15.Gail M.H., Byar D.P. Variance calculations for direct adjusted survival curves, with applications to testing for no treatment effect. Biometric J. 1986;28:587–599. [Google Scholar]
- 16.Compressed Mortality File (CMF) 1999-2002. http://www.cdc.gov/nchs/data_access/cmf.htm Accessed May 3, 2012.
- 17.Gray R.J. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 18.Gottenberg J.E., Aucouturier F., Goetz J. Serum immunoglobulin free light chain assessment in rheumatoid arthritis and primary Sjogren's syndrome. Ann Rheum Dis. 2007;66(1):23–27. doi: 10.1136/ard.2006.052159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puntmann V.O., Taylor P.C., Mayr M. Coupling vascular and myocardial inflammatory injury into a common phenotype of cardiovascular dysfunction: systemic inflammation and aging—a mini-review. Gerontology. 2011;57(4):295–303. doi: 10.1159/000316577. [DOI] [PubMed] [Google Scholar]
- 20.Busse P.J., Mathur S.K. Age-related changes in immune function: effect on airway inflammation. J Allergy Clin Immunol. 2010;126(4):690–700. doi: 10.1016/j.jaci.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad A., Banerjee S., Wang Z., Kong D., Majumdar A.P., Sarkar F.H. Aging and inflammation: etiological culprits of cancer. Curr Aging Sci. 2009;2(3):174–186. doi: 10.2174/1874609810902030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu H., Ouyang W., Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4(4):221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 23.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview Video


