Abstract
Objective
To determine the frequency and spectrum of myocardial dysfunction in patients with severe sepsis and septic shock using transthoracic echocardiography and to evaluate the impact of the myocardial dysfunction types on mortality.
Patients and Methods
A prospective study of 106 patients with severe sepsis or septic shock was conducted from August 1, 2007, to January 31, 2009. All patients underwent transthoracic echocardiography within 24 hours of admission to the intensive care unit. Myocardial dysfunction was classified as left ventricular (LV) diastolic, LV systolic, and right ventricular (RV) dysfunction. Frequency of myocardial dysfunction was calculated, and demographic, hemodynamic, and physiologic variables and mortality were compared between the myocardial dysfunction types and patients without cardiac dysfunction.
Results
The frequency of myocardial dysfunction in patients with severe sepsis or septic shock was 64% (n=68). Left ventricular diastolic dysfunction was present in 39 patients (37%), LV systolic dysfunction in 29 (27%), and RV dysfunction in 33 (31%). There was significant overlap. The 30-day and 1-year mortality rates were 36% and 57%, respectively. There was no difference in mortality between patients with normal myocardial function and those with left, right, or any ventricular dysfunction.
Conclusion
Myocardial dysfunction is frequent in patients with severe sepsis or septic shock and has a wide spectrum including LV diastolic, LV systolic, and RV dysfunction types. Although evaluation for the presence and type of myocardial dysfunction is important for tailoring specific therapy, its presence in patients with severe sepsis and septic shock was not associated with increased 30-day or 1-year mortality.
Abbreviations and Acronyms: APACHE, Acute Physiology and Chronic Health Evaluation; Fio2, fraction of inspired oxygen; LV, left ventricular; LVEF, left ventricular ejection fraction; Pao2, partial pressure of arterial oxygen; RV, right ventricular; SOFA, Sequential Organ Failure Assessment
Myocardial dysfunction in sepsis is one of the most complex organ failures to characterize because of the dynamic adaptation of the cardiovascular system to the disease process, host response, and resuscitation. The pathophysiology of this entity is complex and multifactorial. Systemic, extracellular, and cellular mechanisms have been described, including maldistribution of coronary blood flow, cytokine-induced (tumor necrosis factor α, interleukin 1β, interleukin 6) neutrophil activation and myocardial injury, complement (C5a)-triggered myocyte contractile failure, calcium handling dysregulation, and cytopathic hypoxia due to mitochondrial dysfunction.1,2
Numerous studies have described different types of myocardial dysfunction in sepsis. These efforts have evolved from focusing only on left ventricular (LV) systolic dysfunction to recognition of other types of myocardial dysfunction as a part of the spectrum of this organ failure, which may have different treatment options and prognostic implications. These variants include LV systolic dysfunction,3,4 LV diastolic dysfunction,5-9 and right ventricular (RV) dysfunction.10-12 All types of myocardial dysfunction can be present in isolation or combination and demonstrate reversibility on resolution of critical illness.5,13 Despite the fact that different types of myocardial dysfunction have been evaluated to some extent, there is lack of consensus on the definition and clinical spectrum of this entity. Therefore, its true frequency remains elusive. Moreover, the use of more sophisticated methods to evaluate myocardial tissue properties has improved recognition of more subtle myocardial function abnormalities. These methods include tissue Doppler and strain imaging.14,15 The use of tissue Doppler imaging (TDI) of the mitral valve annulus has gained interest for its relative independence of loading conditions and prognostic value in patients with various cardiac diseases.16-18
Currently, the accepted definition of myocardial dysfunction in sepsis is based solely on an LV ejection fraction (LVEF) of less than 45% to 50% in the absence of cardiac disease that demonstrates reversibility on remission.3,4,19 Moreover, there are conflicting data regarding the prognostic implications of myocardial dysfunction in sepsis and its impact on mortality.3,4,20
We sought to evaluate LV and RV performance with comprehensive echocardiography in patients with severe sepsis or septic shock. We determined the frequency of myocardial dysfunction, described the different types, and evaluated its impact on mortality.
Patients and Methods
This prospective study was approved by the Institutional Review Board of Mayo Clinic, and written informed consent was obtained from all patients or legally authorized representatives before enrollment.
Patients admitted to 3 adult intensive care units (ICUs) with a total of 62 beds at Mayo Clinic in Rochester, Minnesota, with severe sepsis or septic shock were eligible to participate in the study from August 1, 2007, to January 31, 2009. The characteristics of these ICUs have been previously described.21
For inclusion in the study, the patients had to meet criteria for new-onset sepsis as defined by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference.22 Severe sepsis was defined as sepsis associated with organ dysfunction, hypoperfusion, or hypotension. When severe sepsis was associated with hypotension resistant to intravenous fluid administration, it was considered septic shock. Hypotension was defined as systolic pressure less than 90 mm Hg or a decrease of 40 mm Hg below baseline. A lactate level exceeding 2.3 mmol/L (institutional high normal value) was considered indicative of hypoperfusion. Specific organ dysfunction was defined as a Sequential Organ Failure Assessment (SOFA) score of 2 or higher23 at the time of echocardiography, and the Acute Physiology and Chronic Health Evaluation (APACHE III) score was obtained on admission day. Exclusion criteria were age less than 18 years, pregnancy, and history of congenital heart disease, valvular stenosis or clinically significant valvular insufficiency, valvular prosthesis, and coronary artery disease without recent echocardiography or known abnormalities on recent echocardiography (within 6 months of enrollment).
Recruitment was facilitated by a validated computerized sepsis “sniffer” that scanned patients' data in the electronic medical record and notified the research coordinator on call of potential patients meeting physiologic criteria.24
All patients enrolled received a transthoracic echocardiographic evaluation within 24 hours of meeting sepsis criteria. Physiologic parameters including hemodynamic data and current vasoactive medications were recorded at the time of echocardiography. To determine reversibility, patients with LV systolic or RV dysfunction received a follow-up echocardiogram at study day 5 or at the day of dismissal from the ICU.
Echocardiographic Evaluation and Definitions of Myocardial Dysfunction Types
Transthoracic echocardiography was performed in the ICU with a commercial echocardiographic instrument (Vivid 7, GE Medical Systems, Milwaukee, WI). A comprehensive M-mode, 2-dimensional, and Doppler echocardiographic study was performed in all patients from the parasternal long- and short-axis views; apical 4-chamber, 2-chamber, and long-axis views; and subcostal views.
Left ventricular end-diastolic volume, LV end-systolic volume, and LVEF were assessed using the modified Simpson method as recommended by the American Society of Echocardiography.25 Measurements were taken during 3 cardiac cycles and then averaged. Systolic dysfunction was defined as mild (LVEF, 41%-50%), moderate (LVEF, 31%-40%), and severe (LVEF, <30%). Whenever suboptimal endomyocardial border definition was encountered for volumetric assessment, M-mode imaging and expert evaluation (M.M., T.Y., and J.K.O.) determined the final LVEF. Diastolic function evaluation was performed in accordance with the American Society of Echocardiography guidelines15 and graded as normal, mild diastolic dysfunction (grade I), moderate (grade II), severe (grade III), and indeterminate with or without evidence of increased filling pressures. All patients with indeterminate grade who had evidence of increased filling pressures were categorized as having diastolic dysfunction (indeterminate). Mitral inflow pulsed wave Doppler measurement of peak E and A waves, E/A ratio, and deceleration time was obtained with the sample volume between mitral leaflet tips during diastole. Mitral annulus peak velocities were obtained with TDI and included septal and lateral peak e′, a′, and s′ velocities. The E/e′ ratio was then obtained. Increased LV filling pressure was defined as E/e′ greater than 15.15,26 A multimodal approach was used to evaluate RV function, which was graded as mild, moderate, or severe RV dysfunction. Lateral tricuspid annulus peak systolic velocity (RV s′) by TDI was used20,27 in association with the relative RV-to-LV size, motion of the RV wall, and expert evaluation (J.K.O., M.M., T.Y.). Right ventricular s′ less than 15 cm/s was considered diminished lateral RV systolic motion consistent with RV dysfunction. Patients were categorized by myocardial dysfunction type and analyzed against patients without any myocardial dysfunction.
Patient Follow-up
Patient follow-up was obtained through the medical record system at Mayo Clinic. Progress notes and correspondence were reviewed in all patients, and for those who were lost to follow-up in the medical record, confirmation of survival or time of death was obtained by telephone or obituaries.
Statistical Analyses
Statistical analyses were performed with JMP version 9.0.1 (SAS Institute, Cary, NC) and SPSS software version 11.5.0 (SPSS Inc, Chicago, IL). Descriptive data are summarized as the mean ± SD, median (interquartile range [IQR]), or percentages. Unpaired t tests were used to compare continuous variables with normal distribution, and Mann-Whitney U test for variables with skewed distribution. We used a χ2 test to compare categorical variables. P value less than .05 was considered statistically significant.
Results
A total of 106 patients were enrolled. The mean ± SD age was 65±15 years, and 53 patients (50%) were female. Documented microbial infection with positive source cultures was present in 53 patients (50%), and 36% of the study population had positive blood culture results.
The frequency of any myocardial dysfunction was 64% (n=68). Left ventricular diastolic dysfunction was found in 39 patients (37%), LV systolic dysfunction in 29 (27%), and RV dysfunction in 33 (31%) (Figure, A). Thirty-eight patients (36%) had normal LV diastolic, LV systolic, and RV function and were categorized as patients with normal cardiac function. There was overlap of myocardial dysfunction types, with 4 patients (4%) demonstrating diastolic dysfunction along with LV and RV systolic dysfunction, and 11 patients (10%) had biventricular systolic dysfunction with normal diastolic evaluation (Figure, A).
FIGURE.
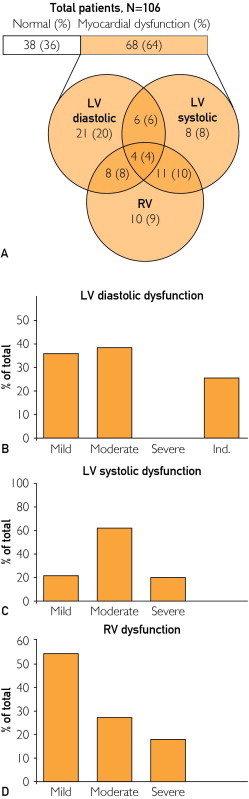
Frequency and clinical spectrum of myocardial dysfunction in severe sepsis and septic shock. A, Distribution of myocardial dysfunction types. B, Severity of LV diastolic dysfunction (n=39). C, Severity of LV systolic dysfunction (n=29). D, Severity of RV dysfunction (n=33). LV = left ventricular; Ind. = indeterminate; RV = right ventricular.
Thirty-eight patients (36%) died within 30 days, and 60 (57%) were dead at 1 year. The group with normal myocardial function had a 30-day mortality of 42% and 1-year mortality of 55%, compared with 32% and 57% in patients with any myocardial dysfunction (P=.31 and P=.83, respectively). No patients were lost to follow-up.
There was no difference in LVEF (56.8%±16% vs 57.7%±14%; P=.75), cardiac output (6.59±2.56 vs 6.63±1.82 L/min; P=.94), or E/e′ (11.6±5 vs 13.7±7; P=.09) between 30-day survivors and nonsurvivors (Table 1). Physiologic parameters that showed statistical difference between survivors and nonsurvivors at 30 days included age, APACHE III score, SOFA score, and median ratio of Pao2 to fraction of inspired oxygen (Fio2) (Pao2/Fio2) (Table 1). At 1 year, age (62±16 vs 67±15 years; P=.05), SOFA score (9.8±3.7 vs 12±3.9; P=.003), and median (IQR) Pao2/Fio2 (270 [180-322] vs 166 [118-291] mm Hg; P=.01) maintained statistical significance.
TABLE 1.
| Marker | Survivors (n=68) | Nonsurvivors (n=38) | P value |
|---|---|---|---|
| Demographic | |||
| Age (y), mean ± SD | 63±15 | 69±15 | .07 |
| Weight (kg), mean ± SD | 86.9±26 | 86.6±25 | .97 |
| Physiologic | |||
| APACHE III, mean ± SD | 83.8±28 | 96.3±31 | .04 |
| SOFA, mean ± SD | 9.9±3.9 | 13.2±3.3 | .001 |
| MAP, mean ± SD | 61±12 | 58±8 | .17 |
| Hemoglobin (g/dL), mean ± SD | 10.3±1.7 | 9.6±1.8 | .07 |
| Scvo2 (%), mean ± SD | 70±11 | 72±10 | .43 |
| Lactate (mmol/L), median (IQR) | 1.9 (1.1-3.4) | 2.5 (1.5-4.3) | .05 |
| Troponin T (ng/mL), median (IQR) | 0.04 (0.01-0.19) | 0.04 (0.01-0.11) | .69 |
| Pao2/Fio2 (mm Hg), median (IQR) | 256 (153-315) | 151 (111-216) | .006 |
| Creatinine (mg/dL), median (IQR) | 1.9 (1.3-2.5) | 1.6 (1.2-2.4) | .54 |
| Fluids (mL/kg), median (IQR) | 76 (57-132) | 86 (51-131) | .93 |
| NE dose, median (IQR) | 0.16 (0.05-0.38) | 0.2 (0.14-0.4) | .35 |
| Echocardiographic | |||
| LVDD (mm), mean ± SD | 46.7±6.3 | 42.5±15.0 | .05 |
| LVSD (mm), mean ± SD | 32.1±7.8 | 28.5±11.2 | .05 |
| CO (L/min), mean ± SD | 6.59±2.56 | 6.63±1.82 | .94 |
| LVEF (%), mean ± SD | 56.8±16 | 57.7±14 | .75 |
| E/e′, mean ± SD | 11.6±5 | 13.7±7 | .09 |
APACHE = Acute Physiology and Chronic Health Evaluation score; CO = cardiac output; E = peak E velocity by pulsed wave Doppler; e′ = peak e′ velocity by tissue Doppler imaging; Fio2 = fraction of inspired oxygen; IQR = interquartile range; LVDD = left ventricular diastolic diameter; LVEF = left ventricular ejection fraction; LVSD = left ventricular systolic diameter; MAP = mean arterial pressure; NE dose = norepinephrine dose in μg/kg/min; Scvo2 = central venous oxygen saturation; SOFA = Sequential Organ Failure Assessment score.
SI conversion factors: To convert creatinine values to μmol/L, multiply by 88.4; to convert hemoglobin values to g/L, multiply by 10.0; to convert troponin T values to μg/L, multiply by 100.
LV Diastolic Dysfunction
Left ventricular diastolic dysfunction was present in 39 patients (37%). Twenty-one patients had isolated diastolic dysfunction, representing 20% of the entire cohort. Eighteen patients (17%) had a combination of LV diastolic and systolic dysfunction and/or RV dysfunction, as shown in Figure, A. Within the LV diastolic dysfunction group, 14 patients (36%) had grade I (mild) dysfunction, 15 (38%) had grade II (moderate) dysfunction, and 10 (26%) had increased filling pressures with indeterminate classification (Figure, B). Compared with patients with normal myocardial function, patients with LV diastolic dysfunction were older (64±16 vs 72±11 years; P=.007), more had hypertension (48% vs 79%; P=.003), and more had coronary artery disease but normal findings on previous echocardiograms (13% vs 38%; P=.01), as shown in Table 2. Most physiologic characteristics were similar between groups, including vasoactive medication use, norepinephrine dose, fluid administered, and cardiac output. However, hemoglobin concentration was lower in those with normal myocardial function (9.6±1.7 vs 10.5±1.9 g/dL; P=.03) (Table 2). There was no statistically significant difference in 30-day mortality (15 [38%] vs 16 [42%]; P=.74) or 1-year mortality (26 [67%] vs 21 [55%]; P=.30) when the diastolic dysfunction group was compared with the normal myocardial function group.
TABLE 2.
Clinical and Physiologic Characteristics of Septic Patients With Normal Myocardial Function vs Left Ventricular Diastolic Dysfunctiona,b
| Characteristic | Normal myocardial function (n=38) | LV diastolic dysfunction (n=39) | P value |
|---|---|---|---|
| Clinical | |||
| Age (y), mean ± SD | 64±16 | 72±11 | .007 |
| Weight (kg), mean ± SD | 84.1±27 | 87±23 | .66 |
| Female, No. (%) | 19 (50) | 22 (56) | .56 |
| HTN, No. (%) | 18 (47) | 31 (79) | .003 |
| Arrhythmias, No. (%)c | 8 (21) | 12 (31) | .33 |
| CAD, No. (%) | 5 (13) | 15 (38) | .01 |
| ALI/ARDS, No. (%) | 6 (16) | 11 (28) | .18 |
| CKD, No. (%) | 6 (16) | 8 (21) | .61 |
| AKI, No. (%) | 17 (38) | 15 (47) | .57 |
| DM, No. (%) | 10 (26) | 12 (31) | .67 |
| Physiologic | |||
| APACHE III, mean ± SD | 84±30 | 88±29 | .56 |
| SOFA, mean ± SD | 11±4 | 10±4 | .19 |
| MAP (mm Hg), mean ± SD | 58±7 | 58±10 | .99 |
| HR (beats/min), mean ± SD | 105±18 | 98±16 | .09 |
| CVP (mm Hg), median (IQR) | 6 (2-11) | 8 (4-11) | .47 |
| Scvo2 (%), mean ± SD | 72±9 | 69±10 | .23 |
| CO (L/min), mean ± SD | 6.97±1.85 | 6.24±0.56 | .18 |
| Fluids (mL/kg), median (IQR) | 93 (64-137) | 80 (54-125) | .34 |
| Mechanical ventilation, No. (%) | 28 (74) | 24 (62) | .25 |
| PEEP (cm H2O), mean ± SD | 9±4 | 9±5 | .83 |
| Pao2/Fio2 (mm Hg), median (IQR) | 208 (120-311) | 195 (144-325) | .73 |
| Hemoglobin (g/dL), mean ± SD | 9.6±1.7 | 10.5±1.9 | .03 |
| Creatinine (mg/dL), median (IQR) | 1.8 (1.2-2.4) | 1.5 (1.3-3.1) | .56 |
| Troponin T (ng/mL), median (IQR) | 0.02 (0.01-0.08) | 0.13 (0-0.22) | .18 |
| Lactate (mmol/L), median (IQR) | 1.9 (1.1-3.5) | 1.9 (1.3-3.1) | .36 |
| Arterial pH, mean ± SD | 7.13±0.10 | 7.31±0.1 | .79 |
| Vasoactive medications | |||
| Vasopressor use, No. (%)d | 34 (90) | 32 (82) | .34 |
| NE dose, median (IQR) | 0.15 (0.06-0.32) | 0.15 (0.05-0.33) | .88 |
| Inotrope use (%)e | 6 (16) | 7 (20) | .59 |
AKI = acute kidney injury; ALI = acute lung injury; APACHE = Acute Physiology and Chronic Health Evaluation score; ARDS = acute respiratory distress syndrome; arrhythmias = atrial fibrillation/flutter, supraventricular tachycardia; CAD = coronary artery disease; CKD = chronic kidney disease; CO = cardiac output measured by transthoracic echocardiography; CVP = central venous pressure; DM = diabetes mellitus; Fio2 = fraction of inspired oxygen; HR = heart rate; HTN = hypertension; IQR = interquartile range; MAP = mean arterial pressure; NE dose = norepinephrine dose in μg/kg/min; PEEP = positive end-expiratory pressure; Scvo2 = central venous oxygen saturation; SOFA = Sequential Organ Failure Assessment score.
SI conversion factors: To convert creatinine values to μmol/L, multiply by 88.4; to convert hemoglobin values to g/L, multiply by 10.0; to convert troponin T values to μg/L, multiply by 100.
Atrial fibrillation/flutter, supraventricular tachycardia.
Norepinephrine, phenylephrine, vasopressin, dopamine.
Dobutamine, epinephrine, milrinone.
LV Systolic Dysfunction
Twenty-nine patients (27%) had LV systolic dysfunction. Eight patients had isolated LV systolic dysfunction, representing 8% of all patients. Twenty-one patients had a combination of LV systolic and diastolic dysfunction and/or RV dysfunction (Figure, A). Within the group, 6 patients (21%) had mild, 18 (62%) had moderate, and 5 (17%) had severe LV systolic dysfunction (Figure, C). There was no statistically significant difference in clinical characteristics when this group was compared with patients with normal myocardial function. However, the LV systolic dysfunction group had a higher median (IQR) lactate level (2.9 [1.7-4.3] vs 1.9 [1.0-3.5] mmol/L; P=.02) and higher mean ± SD arterial blood pressure (64±15 vs 58±7 mm Hg; P=.02) (Table 3). Both groups had similar numbers of patients receiving vasoactive medications and homogeneous fluid administration, but patients in the LV systolic dysfunction group had higher median (IQR) doses of norepinephrine (0.27 [0.19-0.65] vs 0.15 [0.06-0.32] μg/kg per min; P=.007) (Table 3). There was no difference in 30-day mortality (9 [31%] vs 16 [45%]; P=.35] or 1-year mortality (14 [48%] vs 21 [55%]; P=.57) between the groups.
TABLE 3.
Clinical and Physiologic Characteristics of Septic Patients With Normal Myocardial Function vs Left Ventricular Systolic Dysfunctiona,b
| Characteristic | Normal myocardial function (n=38) | LV systolic dysfunction (n=29) | P value |
|---|---|---|---|
| Clinical | |||
| Age (y), mean ± SD | 64±16 | 59±16 | .18 |
| Weight (kg), mean ± SD | 84.1±27 | 89.1±27 | .45 |
| Female, No. (%) | 19 (50) | 10 (35) | .20 |
| HTN, No. (%) | 18 (47) | 16 (55) | .52 |
| Arrhythmias, No. (%)c | 8 (21) | 6 (21) | .97 |
| CAD, No. (%) | 5 (13) | 5 (17) | .64 |
| ALI/ARDS, No. (%) | 6 (16) | 4 (14) | .82 |
| CKD, No. (%) | 6 (16) | 7 (24) | .39 |
| AKI, No. (%) | 17 (45) | 9 (31) | .25 |
| DM, No. (%) | 10 (26) | 6 (21) | .59 |
| Physiologic | |||
| APACHE III, mean ± SD | 84±30 | 92±27 | .22 |
| SOFA, mean ± SD | 11.2±3.7 | 12.7±3.7 | .11 |
| MAP (mm Hg), mean ± SD | 58±7 | 64±15 | .02 |
| HR (beats/min), mean ± SD | 105±18 | 113±21 | .09 |
| CVP (mm Hg), median (IQR) | 6 (2-11) | 8 (5-11) | .35 |
| Scvo2 (%), mean ± SD | 72±9 | 70±11 | .42 |
| CO (L/min), mean ± SD | 6.97±1.85 | 5.99±2.53 | .09 |
| Fluids (mL/kg), median (IQR) | 93 (64-137) | 99 (59-156) | .63 |
| Mechanical ventilation, No. (%) | 28 (74) | 25 (86) | .25 |
| PEEP (cm H2O), mean ± SD | 9±4 | 9±4 | .81 |
| Pao2/Fio2 (mm Hg), median (IQR) | 208 (120-311) | 190 (138-308) | .93 |
| Hemoglobin (g/dL), mean ± SD | 9.6±1.7 | 9.9±1.7 | .47 |
| Creatinine (mg/dL), median (IQR) | 1.8 (1.2-2.4) | 1.9 (1.4-2.6) | .42 |
| Troponin T (ng/mL), median (IQR) | 0.02 (0.01-0.08) | 0.05 (0.02-0.13) | .16 |
| Lactate (mmol/L), median (IQR) | 1.9 (1.0-3.5) | 2.9 (1.7-4.3) | .02 |
| Arterial pH, mean ± SD | 7.30±0.08 | 7.27±0.12 | .26 |
| Vasoactive medications | |||
| Vasopressor use, No. (%)d | 34 (90) | 24 (83) | .42 |
| NE dose, median (IQR) | 0.15 (0.06-0.32) | 0.27 (0.19-0.65) | .007 |
| Inotrope use, No. (%)e | 6 (16) | 6 (21) | .60 |
AKI = acute kidney injury; ALI = acute lung injury; APACHE = Acute Physiology and Chronic Health Evaluation score; ARDS = acute respiratory distress syndrome; CAD = coronary artery disease; CKD = chronic kidney disease; CO = cardiac output measured by transthoracic echocardiography; CVP = central venous pressure; DM = diabetes mellitus; Fio2 = fraction of inspired oxygen; HR = heart rate; HTN = hypertension; IQR = interquartile range; LV = left ventricular; MAP = mean arterial pressure; NE dose = norepinephrine dose in μg/kg/min; PEEP = positive end-expiratory pressure; Scvo2 = central venous oxygen saturation; SOFA = Sequential Organ Failure Assessment score.
SI conversion factors: To convert creatinine values to μmol/L, multiply by 88.4; to convert hemoglobin values to g/L, multiply by 10.0; to convert troponin T values to μg/L, multiply by 100.
Atrial fibrillation/flutter, supraventricular tachycardia.
Norepinephrine, phenylephrine, vasopressin, dopamine.
Dobutamine, epinephrine, milrinone.
RV Dysfunction
Thirty-three patients (31%) had evidence of RV dysfunction. Ten patients had isolated RV dysfunction, representing 9% of all patients. The rest had concomitant LV diastolic and/or systolic dysfunction (Figure, A). Within the group of patients with RV dysfunction, 18 (55%) had mild, 9 (27%) had moderate, and 6 (18%) had severe RV dysfunction. There was no statistically significant difference in clinical or hemodynamic characteristics when this group was compared with patients with normal myocardial function, except for lower cardiac output (5.69±2.01 vs 6.97±1.85 L/min; P=.01) and higher median (IQR) norepinephrine doses (0.26 [0.15-0.4] vs 0.15 [0.06-0.32] μg/kg per min; P=.02) in patients with RV dysfunction. Median (IQR) troponin T (0.08 [0.02-0.17] vs 0.02 [0.01-0.08] ng/mL; P=.05) and lactate (2.6 [1.6-4.1] vs 1.9 [1-3.5] mmol/L; P=.02) levels were also higher in the RV dysfunction group compared with patients with normal myocardial function. There was no difference in 30-day mortality (16 [42%] vs 12 [36%]; P=.62) or 1-year mortality (21 [55%] vs 19 [58%]; P=.84) between the groups.
Reversibility
Of the 47 patients (44%) with either LV systolic or RV dysfunction, 10 died in the initial 5 days. Reimaging was not possible in 9 patients, and 28 received a follow-up echocardiogram to document reversibility at day 5 or at discharge from the ICU if ICU length of stay was less than 5 days. There was significant improvement of LVEF (42%±15% vs 61%±9%; P=.001), E/e′ (12±5 vs 9.4±3; P=.01), and RV systolic pressure (44±11 vs 34±13 mm Hg; P=.04) compared with the initial echocardiographic examination. Twenty patients had complete normalization of myocardial function, 7 patients improved, and only 1 patient did not show significant change of LV systolic function on follow-up.
Discussion
We found that myocardial dysfunction is common in severe sepsis and septic shock, affecting 64% of patients. With standard echocardiography, these abnormalities can be further divided into LV diastolic (37% of all patients), LV systolic (27%), and RV dysfunction (31%), which demonstrates the importance of going beyond LVEF when categorizing myocardial dysfunction in sepsis. There was significant overlap between the different types, as well as a wide range of severity within the groups (Figure). Despite the obvious importance of these echocardiographic findings, the presence of any myocardial dysfunction was not associated with increased mortality at 30 days or 1 year. Nevertheless, survivors had larger and proportional LV end-diastolic diameter (46.7±6.3 vs 42.5±15.0 mm; P=.05) and LV end-systolic diameter (32.1±7.8 vs 28.5±11.2 mm; P=.05) compared with nonsurvivors at 30 days, with resultant similar mean LVEF (56.8%±16% vs 57.7%±14%; P=.75). This observation of reversible compensatory LV dilatation has been described previously,28,29 suggesting that LV diameters and volumes could be better markers of prognosis than LVEF. Arguably, this difference could be secondary to discrepancies in resuscitation and loading conditions; patients having less aggressive fluid resuscitation could demonstrate lower ventricular volumes, which could translate to poorer prognosis. Nonetheless, we did not find significant differences in fluid administration between survivors and nonsurvivors or between the groups (Tables 1-4).
TABLE 4.
Clinical and Physiologic Characteristics of Septic Patients With Normal Myocardial Function vs Right Ventricular Dysfunctiona,b
| Characteristic | Normal myocardial function (n=38) | RV dysfunction (n=33) | P value |
|---|---|---|---|
| Clinical | |||
| Age (y), mean ± SD | 64±16 | 62±16 | .72 |
| Weight (kg), mean ± SD | 84.1±27 | 88.5±31 | .52 |
| Female, No. (%) | 19 (50) | 17 (52) | .89 |
| HTN, No. (%) | 18 (47) | 17 (52) | .72 |
| Arrhythmias, No. (%)c | 8 (21) | 8 (24) | .74 |
| CAD, No. (%) | 5 (13) | 5 (15) | .81 |
| ALI/ARDS, No. (%) | 6 (16) | 6 (18) | .78 |
| CKD, No. (%) | 6 (16) | 9 (27) | .23 |
| AKI, No. (%) | 17 (45) | 9 (27) | .12 |
| DM, No. (%) | 10 (26) | 9 (27) | .92 |
| Physiologic | |||
| APACHE III, mean ± SD | 84±30 | 94±26 | .14 |
| SOFA, mean ± SD | 11.2±3.7 | 12.2±3.3 | .28 |
| MAP (mm Hg), mean ± SD | 58±7 | 63±14 | .06 |
| HR (beats/min), mean ± SD | 105±18 | 109±21 | .36 |
| CVP (mm Hg), median (IQR) | 6 (2-11) | 9 (5-15) | .10 |
| Scvo2 (%), mean ± SD | 72±9 | 70±12 | .34 |
| CO (L/min), mean ± SD | 6.97±1.85 | 5.69±2.01 | .01 |
| Fluids (mL/kg), median (IQR) | 93 (64-137) | 80 (54-145) | .83 |
| Mechanical ventilation, No. (%) | 28 (74) | 27 (82) | .41 |
| PEEP (cm H2O), mean ± SD | 9±4 | 9±4 | .80 |
| Pao2/Fio2 (mm Hg), median (IQR) | 208 (120-311) | 178 (106-267) | .17 |
| Hemoglobin (g/dL), mean ± SD | 9.6±1.7 | 10.3±1.8 | .07 |
| Creatinine (mg/dL), median (IQR) | 1.8 (1.2-2.4) | 1.8 (1.2-2.6) | .72 |
| Troponin T (ng/dL), median (IQR) | 0.02 (0.01-0.08) | 0.08 (0.02-0.17) | .05 |
| Lactate (mmol/dL), median (IQR) | 1.9 (1.0-3.5) | 2.6 (1.6-4.1) | .02 |
| Arterial pH, mean ± SD | 7.30±0.08 | 7.26±0.12 | .07 |
| Vasoactive medications | |||
| Vasopressor use, No. (%)d | 34 (90) | 30 (91) | .84 |
| NE dose, median (IQR) | 0.15 (0.06-0.32) | 0.26 (0.15-0.4) | .02 |
| Inotrope use, No. (%)e | 6 (16) | 10 (30) | .14 |
AKI = acute kidney injury; ALI = acute lung injury; APACHE = Acute Physiology and Chronic Health Evaluation score; ARDS = acute respiratory distress syndrome; CAD = coronary artery disease; CKD = chronic kidney disease; CO = cardiac output measured by transthoracic echocardiography; CVP = central venous pressure; DM = diabetes mellitus; Fio2 = fraction of inspired oxygen; HR = heart rate; HTN = hypertension; IQR = interquartile range; MAP = mean arterial pressure; NE dose = norepinephrine dose in μg/kg/min; PEEP = positive end-expiratory pressure; RV = right ventricular; Scvo2 = central venous oxygen saturation; SOFA = Sequential Organ Failure Assessment score.
SI conversion factors: To convert creatinine values to μmol/L, multiply by 88.4; to convert hemoglobin values to g/L, multiply by 10.0; to convert troponin T values to μg/L, multiply by 100.
Atrial fibrillation/flutter, supraventricular tachycardia.
Norepinephrine, phenylephrine, vasopressin, dopamine.
Dobutamine, epinephrine, milrinone.
LV Diastolic Dysfunction
Left ventricular diastolic dysfunction is frequent in severe sepsis and septic shock and can complicate hemodynamic management because of a lower therapeutic index for fluid resuscitation. We found that 37% of patients had diastolic dysfunction. Because all patients with coronary artery disease had a recent echocardiogram showing normal diastolic function, sepsis likely represents a significant stressor that can unmask and/or precipitate diastolic heart failure. Moreover, because fluid resuscitation is the backbone of hemodynamic management in patients with sepsis, the presence of diastolic dysfunction should alert the caregiver to have a more conservative approach during resuscitation. Diastolic dysfunction should be considered a variant of myocardial dysfunction in sepsis, given that a sizable proportion of these patients (20%) have preserved LVEF and RV function. The E/e′ should be included in the evaluation of patients with severe sepsis and septic shock because of the inherent load-independent characteristics. However, despite recent evidence linking E/e′ and diastolic dysfunction with mortality in septic shock,9,20 we found no statistical difference in E/e′ (11.6±5 vs 13.7±7; P=.09) between survivors and nonsurvivors, and the presence of diastolic dysfunction had no impact on early or late mortality.
LV Systolic Dysfunction
Although often referred as the “classic” myocardial dysfunction in sepsis, isolated LV systolic dysfunction was found in only 8% of our patients, and concomitant LV diastolic and RV dysfunction was a more common scenario. As mentioned before, LVEF was no different in survivors than in nonsurvivors, despite the greater LV diameters in survivors at 30 days. These findings, along with the relatively high frequency of diastolic and RV function abnormalities, beg the question of appropriateness of the current definition of myocardial dysfunction in sepsis. Most patients with documented LV systolic dysfunction who were alive at day 5 underwent follow-up echocardiography that showed significant improvement of LVEF, E/e′ ratio, and estimated pulmonary pressures, confirming reversibility of this organ dysfunction.
RV Dysfunction
The adaptation of the RV to sepsis is complex, and the presence of positive pressure ventilation complicates further its objective evaluation. Because of the known sensitivity of the RV to changes in pulmonary vascular resistance induced by a variety of factors, including mechanical ventilation, positive end-expiratory pressure, hypoxemia, acidosis, and vasoactive medications, we compared these physiologic parameters within all groups and found no statistical difference when compared with patients with normal myocardial function (Table 4). Although there was no difference in early or late mortality, patients with RV dysfunction had lower cardiac output, higher norepinephrine dose, and higher troponin T and lactate levels compared with patients with normal myocardial function, suggesting greater severity of illness.
Limitations
Because of the dynamic nature of sepsis, variability in host response, and underlying disease, as well as the complex interaction between the cardiovascular and respiratory systems, the evaluation of myocardial dysfunction is limited to isolated “snapshots” in time during the disease process and treatment. Furthermore, the variability in time from initial presentation to echocardiogram, discrepancies in resuscitation, and difference in vasoactive medication dose could alter echocardiographic measurements and therefore the results. This could contribute to different echocardiographic results in a single patient over time and variation in study results. Despite the current challenges in critical care research, we had a somewhat homogeneous practice in resuscitation during septic shock,30 and there was no difference in fluid administration. Even though we used a computerized sepsis sniffer pager to optimize time to enrollment and time to initial echocardiogram,24 because of the variability in practice, we cannot ensure that patients were enrolled at similar times during their disease process. Furthermore, our sample size was not large enough to provide definite conclusions about mortality. Despite these limitations, this study provides the general spectrum of myocardial dysfunction in severe sepsis and septic shock.
Conclusion
Myocardial dysfunction is frequent in patients with severe sepsis and septic shock and presents in a wide spectrum including LV diastolic, LV systolic, and/or RV dysfunction. Decreased LVEF as the sole criterion for diagnosis of myocardial dysfunction in sepsis is inaccurate and misleading. We found no difference in mortality at 30 days or 1 year between patients with any myocardial dysfunction and patients with normal results on echocardiography. Despite these findings, echocardiography is a useful tool to diagnose and categorize the type of myocardial dysfunction in sepsis and may aid in the management of these patients. Our findings question the appropriateness of the current definitions of this entity and advocate for the addition of these variants to more accurately describe cardiac dysfunction during sepsis beyond LVEF because this marker lacks prognostic value.
Acknowledgments
We thank Melissa Passe, RRT, Richard Hinds, RRT, and the Anesthesia Clinical Research Unit personnel who were instrumental in patient recruitment and the Mayo Clinic Echocardiography Laboratory for allowing the use of the echocardiography instruments.
Footnotes
Grant Support: This study was funded by the Mayo Clinic Critical Care Research Committee.
Supplemental Online Material
Author Interview Video
References
- 1.Flierl M.A., Rittirsch D., Huber-Lang M.S., Sarma J.V., Ward P.A. Molecular events in the cardiomyopathy of sepsis. Mol Med. 2008;14(5-6):327–336. doi: 10.2119/2007-00130.Flierl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochstadt A., Meroz Y., Landesberg G. Myocardial dysfunction in severe sepsis and septic shock: more questions than answers? J Cardiothorac Vasc Anesth. 2011;25(3):526–535. doi: 10.1053/j.jvca.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Parker M.M., Shelhamer J.H., Bacharach S.L. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100(4):483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 4.Vieillard-Baron A., Caille V., Charron C., Belliard G., Page B., Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008;36(6):1701–1706. doi: 10.1097/CCM.0b013e318174db05. [DOI] [PubMed] [Google Scholar]
- 5.Bouhemad B., Nicolas-Robin A., Arbelot C., Arthaud M., Feger F., Rouby J.J. Isolated and reversible impairment of ventricular relaxation in patients with septic shock. Crit Care Med. 2008;36(3):766–774. doi: 10.1097/CCM.0B013E31816596BC. [DOI] [PubMed] [Google Scholar]
- 6.Munt B., Jue J., Gin K., Fenwick J., Tweeddale M. Diastolic filling in human severe sepsis: an echocardiographic study. Crit Care Med. 1998;26(11):1829–1833. doi: 10.1097/00003246-199811000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Poelaert J., Declerck C., Vogelaers D., Colardyn F., Visser C.A. Left ventricular systolic and diastolic function in septic shock. Intensive Care Med. 1997;23(5):553–560. doi: 10.1007/s001340050372. [DOI] [PubMed] [Google Scholar]
- 8.Jafri S.M., Lavine S., Field B.E., Bahorozian M.T., Carlson R.W. Left ventricular diastolic function in sepsis. Crit Care Med. 1990;18(7):709–714. doi: 10.1097/00003246-199007000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Sturgess D.J., Marwick T.H., Joyce C. Prediction of hospital outcome in septic shock: a prospective comparison of tissue Doppler and cardiac biomarkers. Crit Care. 2010;14(2):R44. doi: 10.1186/cc8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker M.M., McCarthy K.E., Ognibene F.P., Parrillo J.E. Right ventricular dysfunction and dilatation, similar to left ventricular changes, characterize the cardiac depression of septic shock in humans. Chest. 1990;97(1):126–131. doi: 10.1378/chest.97.1.126. [DOI] [PubMed] [Google Scholar]
- 11.Liu D., Du B., Long Y., Zhao C., Hou B. Right ventricular function of patients with septic shock: clinical significance. Zhonghua Wai Ke Za Zhi. 2000;38(7):488–492. [PubMed] [Google Scholar]
- 12.Chan C.M., Klinger J.R. The right ventricle in sepsis. Clin Chest Med. 2008;29(4):661–676. doi: 10.1016/j.ccm.2008.07.002. ix. [DOI] [PubMed] [Google Scholar]
- 13.Burns J.R., Menapace F.J. Acute reversible cardiomyopathy complicating toxic shock syndrome. Arch Intern Med. 1982;142(5):1032–1034. [PubMed] [Google Scholar]
- 14.Oh J.K., Park S.J., Nagueh S.F. Established and novel clinical applications of diastolic function assessment by echocardiography. Circ Cardiovasc Imaging. 2011;4(4):444–455. doi: 10.1161/CIRCIMAGING.110.961623. [DOI] [PubMed] [Google Scholar]
- 15.Nagueh S.F., Appleton C.P., Gillebert T.C. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10(2):165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 16.Troughton R.W., Prior D.L., Frampton C.M. Usefulness of tissue Doppler and color M-mode indexes of left ventricular diastolic function in predicting outcomes in systolic left ventricular heart failure (from the ADEPT study) Am J Cardiol. 2005;96(2):257–262. doi: 10.1016/j.amjcard.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 17.Wang M., Yip G.W., Wang A.Y. Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value. J Am Coll Cardiol. 2003;41(5):820–826. doi: 10.1016/s0735-1097(02)02921-2. [DOI] [PubMed] [Google Scholar]
- 18.Hillis G.S., Moller J.E., Pellikka P.A. Noninvasive estimation of left ventricular filling pressure by E/e' is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol. 2004;43(3):360–367. doi: 10.1016/j.jacc.2003.07.044. [DOI] [PubMed] [Google Scholar]
- 19.Parrillo J.E. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993;328(20):1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 20.Furian T, Aguiar C, Prado K, et al. Ventricular dysfunction and dilation in severe sepsis and septic shock: relation to endothelial function and mortality [published online ahead of print August 18, 2011]. J Crit Care. PMID: 21855287 [DOI] [PubMed]
- 21.Afessa B., Keegan M.T., Hubmayr R.D. Evaluating the performance of an institution using an intensive care unit benchmark. Mayo Clin Proc. 2005;80(2):174–180. doi: 10.4065/80.2.174. [DOI] [PubMed] [Google Scholar]
- 22.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 23.Vincent J.L., de Mendonca A., Cantraine F. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study: working group on ”sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Herasevich V., Pieper M.S., Pulido J., Gajic O. Enrollment into a time sensitive clinical study in the critical care setting: results from computerized septic shock sniffer implementation. J Am Med Inform Assoc. 2011;18(5):639–644. doi: 10.1136/amiajnl-2011-000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiller N.B., Shah P.M., Crawford M. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography: American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 26.Ommen S.R., Nishimura R.A., Appleton C.P. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102(15):1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 27.Wahl A., Praz F., Schwerzmann M. Assessment of right ventricular systolic function: comparison between cardiac magnetic resonance derived ejection fraction and pulsed-wave tissue Doppler imaging of the tricuspid annulus. Int J Cardiol. 2011;151(1):58–62. doi: 10.1016/j.ijcard.2010.04.089. [DOI] [PubMed] [Google Scholar]
- 28.Zanotti Cavazzoni S.L., Guglielmi M., Parrillo J.E., Walker T., Dellinger R.P., Hollenberg S.M. Ventricular dilation is associated with improved cardiovascular performance and survival in sepsis. Chest. 2010;138(4):848–855. doi: 10.1378/chest.09-1086. [DOI] [PubMed] [Google Scholar]
- 29.Bouhemad B., Nicolas-Robin A., Arbelot C., Arthaud M., Feger F., Rouby J.J. Acute left ventricular dilatation and shock-induced myocardial dysfunction. Crit Care Med. 2009;37(2):441–447. doi: 10.1097/CCM.0b013e318194ac44. [DOI] [PubMed] [Google Scholar]
- 30.Schramm G.E., Kashyap R., Mullon J.J., Gajic O., Afessa B. Septic shock: a multidisciplinary response team and weekly feedback to clinicians improve the process of care and mortality. Crit Care Med. 2011;39(2):252–258. doi: 10.1097/CCM.0b013e3181ffde08. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview Video


