Abstract
The past decade has brought important advances in the understanding of rheumatoid arthritis and its management and treatment. New classification criteria for rheumatoid arthritis, better definitions of treatment outcome and remission, and the introduction of biologic response-modifying drugs designed to inhibit the inflammatory process have greatly altered the approach to managing this disease. More aggressive management of rheumatoid arthritis early after diagnosis and throughout the course of the disease has resulted in improvement in patient functioning and quality of life, reduction in comorbid conditions, and enhanced survival.
Abbreviations and Acronyms: ACPA, anti–citrullinated protein antibody; ACR, American College of Rheumatology; BeSt, Behandel-Strategieën [trial]; CDAI, Clinical Disease Activity Index; CRP, C-reactive protein; CTLA-4:Ig, cytotoxic T lymphocyte–associated antigen 4:immunoglobulin fusion protein; DAS28, Disease Activity Score in 28 joints; DMARD, disease-modifying antirheumatic drug; EULAR, European League Against Rheumatism; HCQ, hydroxychloroquine; MTX, methotrexate; SDAI, Simplified Disease Activity Index; SSZ, sulfasalazine; TEAR, Treatment of Early Aggressive Rheumatoid Arthritis [study]; TNF, tumor necrosis factor
Rheumatoid arthritis is the most common autoimmune disease that affects the joints. Worldwide, approximately 1% of the population is affected, with higher prevalence in persons of European or Asian ancestry.1 Rheumatoid arthritis can develop in persons of any age, with the typical age at onset of about 55 years. The prevalence of rheumatoid arthritis increases considerably with age, affecting approximately 6% of the white population older than 65 years. In the United States, the lifetime risk of developing rheumatoid arthritis is 3.6% in women and 1.7% in men. There is some indication that the risk of developing rheumatoid arthritis has increased somewhat in recent years, at least in women.2
Clinical features of rheumatoid arthritis typically include symmetric polyarthritis with joint swelling, especially of the hands and feet, although any of the appendicular joints may become involved. Patients with rheumatoid arthritis experience morning stiffness that lasts 1 hour or longer. Characteristic subcutaneous nodules and other extra-articular disease manifestations including interstitial lung disease, vasculitis, and various forms of inflammatory eye disease are markers of severe disease.
Arthritis in general, and rheumatoid arthritis in particular, is a common cause of disability. More than a third of patients eventually experience work disability because of the disease.3 The loss of ability to maintain employment begins early after disease onset; 80% of patients are working at 2 years, and 68% are working at 5 years.4 Life expectancy is shortened by up to 3 to 5 years, especially in patients with extra-articular disease and those who develop serious treatment-related adverse effects including infections, tumors, and gastrointestinal toxicity from drugs used to treat rheumatoid arthritis.5,6 Furthermore, patients with rheumatoid arthritis are at 50% greater risk of heart attack and more than 2-fold increased risk of heart failure.7-11
A major advance in management of rheumatoid arthritis is the recognition that early diagnosis and prompt aggressive treatment substantially improve patient functional outcome and morbidity. The American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) 2010 classification criteria for rheumatoid arthritis represent an improvement in the diagnostic approach to the disease.12 These organizations have recognized that older criteria are insufficient to differentiate patients in whom rheumatoid arthritis eventually will develop from those in whom arthritis will have a limited course. Patients classified as having rheumatoid arthritis according to the previous criteria already have established and advanced disease. The new criteria promote the goal of early intervention to improve outcomes, prevent joint damage, and limit functional decline.
Recently, the ACR13 and the EULAR14 have published evidence-based recommendations for management of rheumatoid arthritis. However, these general guidelines permit considerable leeway for interpretation and in themselves are not sufficient for rheumatologists to use to devise a practical and standardized management approach. This clinical review describes our management approach, assimilating the current evidence, expert opinion, and clinical experience. It is intended to serve as a pragmatic approach for use in the treatment and management of rheumatoid arthritis.
Treatment Principles
Goals of Therapy
The goal of present-day therapy for rheumatoid arthritis is to control the underlying inflammatory disease. Attainment of this goal will alleviate pain, restore patients' quality of life, and ultimately, preserve their independence and ability to perform activities of daily living and vocational and avocational pursuits. Major long-term goals of treatment are to prevent joint destruction and prevent comorbidities of disease and treatment, including heart disease and osteoporosis.
Early Referral and Diagnosis
Timely intervention and accurate diagnosis reduce the burden of disease and the progression of rheumatoid arthritis, with the result that outcomes have globally improved, with more patients able to work and less need for joint reconstructive surgery than in previous decades. The expectations for disease management have become more rigorous as the effects of the disease have become better understood and treatments have improved. Critical to these expectations has been a fundamental change in the mindset of rheumatologists and their patients, who now expect complete abrogation of disease activity and remission or near remission as treatment goals.
The classification of “definite rheumatoid arthritis” is based on the confirmed presence of synovitis in at least 1 joint, absence of an alternative diagnosis that better accounts for the synovitis, and a score of 6 or higher in 4 individual score domains (Table 1).12,15 These domains and their score ranges are as follows: number and site of involved joints (0-5), serologic abnormality (0-3), increased acute-phase response (score, 0-1), and symptom duration (2 levels; score, 0-1). They incorporate the more specific anti–citrullinated protein antibody (ACPA; formerly, anticyclic citrullinated peptide antibody) serologic test, which has high specificity (>90%) and moderate sensitivity (∼60%) for rheumatoid arthritis. Rheumatoid factor may also be used as a serologic marker of the disease, although it has considerably lower specificity (<70%), with comparable sensitivity as ACPA, which increases to about 80% percent with prolonged disease.
TABLE 1.
2010 ACR/EULAR Classification Criteria for Rheumatoid Arthritisa
| Variable | Score |
|---|---|
| Target population: Who should be tested? | |
| Patients who have at least 1 joint with definite clinical synovitis (swelling)b | |
| Patients with synovitis not better accounted for by another diseasec | |
| Classification criteria for RA (score-based algorithm: add score of categories A-D. A score ≥6/10 is needed for definite classification)d | |
| A) Joint involvemente | |
| 1 large jointf | 0 |
| 2-10 large joints | 1 |
| 1-3 small joints (with or without involvement of large joints)g | 2 |
| 4-10 small joints (with or without involvement of large joints) | 3 |
| >10 joints, including at least 1 small jointh | 5 |
| B) Serologic findings (at least one test result is needed for classification)i | |
| Negative RF and negative ACPA | 0 |
| Low positive RF or low positive ACPA | 2 |
| High positive RF or high positive ACPA | 3 |
| C) Acute phase reactants (at least one test result is needed for classification)j | |
| Normal CRP and normal ESR | 0 |
| Abnormal CRP or abnormal ESR | 1 |
| D) Duration of symptoms (wk)k | |
| <6 | 0 |
| ≥6 | 1 |
ACPA = anti-citrullinated protein antibody; ACR = American College of Rheumatology; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; EULAR = European League Against Rheumatism; RA = rheumatoid arthritis; RF = rheumatoid factor; ULN = upper limit of normal.
The criteria are for classification of new patients. In addition, patients with erosive disease typical of RA with a history compatible with fulfillment of the 2010 criteria should be classified as having RA. Patients with long-standing disease including those with inactive disease (with or without treatment) who, on the basis of retrospectively available data, have previously fulfilled the 2010 criteria should be classified as having RA.
Differential diagnoses vary among patients with different clinical findings but may include conditions such as systemic lupus erythematosus, psoriatic arthritis, and gout. If it is unclear which relevant differential diagnoses to consider, an expert rheumatologist should be consulted.
Although patients with a score of <6/10 are not classifiable as having RA, their status can be reassessed, and the criteria might be fulfilled cumulatively over time.
Joint involvement refers to any swollen or tender joint on examination, which may be confirmed by imaging evidence of synovitis. Distal interphalangeal joints, first carpometacarpal joints, and first metatarsophalangeal joints are excluded from assessment. Categories of joint distribution are classified according to location and number of involved joints, with placement in the highest category possible on the basis of pattern of joint involvement.
Large joints include shoulders, elbows, hips, knees, and ankles.
Small joints include the metacarpophalangeal joints, proximal interphalangeal joints, second through fifth metatarsophalangeal joints, thumb interphalangeal joints, and wrists.
In this category, at least one of the involved joints must be a small joint; the other joints can include any combination of large and additional small joints and other joints not specifically listed elsewhere (eg, temporomandibular, acromioclavicular, sternoclavicular joints).
Negative refers to international unit values that are less than or equal to ULN for the laboratory and assay; low positive refers to international unit values that are higher than ULN but 3 times ULN or less for the laboratory and assay; high positive refers to international unit values that are more than 3 times ULN for the laboratory and assay. When RF information is available only as positive or negative, a positive result should be scored as low positive for RF.
Normal or abnormal is determined by local laboratory standards.
Patient self-report of duration of signs or symptoms of synovitis (eg, pain, swelling, tenderness) of joints that are clinically involved at the time of assessment, regardless of treatment status.
From Arthritis Rheum,12 with permission.
Application of these new criteria facilitates earlier referral of patients with early inflammatory arthritis to rheumatologists and earlier diagnosis of rheumatoid arthritis. For example, patients with early-morning stiffness, a swollen wrist joint, and strongly positive ACPA test results for 6 weeks (or less with an abnormal C-reactive protein [CRP] concentration) fulfill the criteria for rheumatoid arthritis, and disease-modifying therapy should be initiated. We advocate use of “early arthritis clinics” or, at the least, triage of appointment indications so that patients with suspected early rheumatoid arthritis can be seen in urgent appointment slots within 1 to 2 weeks of referral.
In some patients with early rheumatoid arthritis, clinical examination may not yield evidence of synovitis, in particular in those who test seronegative for the disease. Advanced imaging techniques such as high-resolution ultrasonography and magnetic resonance imaging can be useful in such cases. Identification of synovitis, bone edema, and bone erosions not evident at clinical examination alone can facilitate early diagnosis when these findings otherwise support clinical judgment.16-19
Assessment of Disease Activity
Clinical evaluation of rheumatoid arthritis should include quantitative assessment of disease activity, and treatment decisions should hinge on this assessment.13,14 Routine evaluation includes assessment of patient-reported outcomes including pain, the patient Global Assessment of Disease Activity score, and the Health Assessment Questionnaire Disability Index score. The physician, trained nurse, or physician assistant performs the evaluator global assessment. These assessments are performed using visual analog scales, either in paper or electronic format. The evaluator also physically examines the joints and evaluates the number of tender and swollen joints on the basis of the 28-joint count, which includes the proximal interphalangeal joints (first through fifth), metacarpophalangeal joints (first through fifth), wrists, elbows, shoulders, and knees, on both sides of the body.20 We also routinely assess the serum CRP concentration, which is the most clinically useful biomarker.21 We prefer the CRP concentration to the sedimentation rate because the test is simple, more reliable, and not age dependent. Measurement of both acute-phase reactants offers no additional clinical value.22 A list of our standard clinical assessment tools is provided in the Supplemental Table (available online at http://www.mayoclinicproceedings.com).
Composite measures of these individual factors should be used to determine the absolute state of clinical disease activity.13,14 Such composite measures are more sensitive to changes in disease activity than are the aforementioned individual assessments.23 Care driven by aggressive treatment modification to achieve targets based on a composite disease activity score, such as the Disease Activity Score, leads to superior clinical outcomes.24,25 The Disease Activity Score using 28 joint counts (DAS28) is recommended by EULAR for assessing disease activity and treatment response.20,26,27
The Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI) are alternative composite disease activity measures that have salutary advantages in clinical practice.28 These measures do not require any calculation based on a complicated formula, and the CDAI does not require measurement of an acute-phase reactant.29,30 The SDAI and CDAI provide a more stringent definition of clinical remission.31,32 As defined by power Doppler ultrasonography, achievement of remission according to the SDAI signifies true lack of joint inflammation more accurately than with the DAS28.33 Further, the SDAI is among the most sensitive to change in the various composite measures of disease activity, which justifies use of this measure to evaluate response.23
We use the SDAI primarily in defining treatment response and remission (Table 2). The SDAI should be used in patients with a history of increased CRP concentration, and the CDAI may be used in patients with repeatedly undetectable CRP concentration (<0.3 mg/dL; to convert CRP mg/dL value to nmol/L, multiply by 95.24). Systematic monitoring of these disease activity measures provides a longitudinal view of treatment effects and outcomes, which will greatly facilitate evaluation of the success of our treatment approach for managing rheumatoid arthritis.
TABLE 2.
| Formulas for calculation of recommended composite measures |
| SDAI = TJC + SJC + PGA + EGA + CRP |
| CDAI = TJC + SJC + PGA + EGA |
| Disease activity cutoffs | ||||
|---|---|---|---|---|
| Absolute disease activity level |
||||
| Index | Remission | Low | Moderate | High |
| SDAI | ≤3.3 | ≤11 | ≤26 | >26 |
| CDAI | ≤2.8 | ≤10 | ≤22 | >22 |
CDAI = Clinical Disease Activity Index; CRP = C-reactive protein (mg/dL); EGA = Evaluator Global Assessment (0-100 mm); PGA = Patient Global Assessment (0-100 mm); SDAI = Simplified Disease Activity Index; SJC = No. of swollen joints using a 28-joint count; TJC = No. of tender joints using a 28-joint count.
Measurement of disease activity at each point in time should be based on quantitative assessments and summarized using a composite disease activity instrument. We use the SDAI for patients with increased acute-phase reactant levels, and for all others we use the CDAI. Note that the SDAI requires that CRP values be given in milligrams per deciliter. When calculating the SDAI for a patient with CRP reported as less than the detectable range (ie, <0.3 mg/dL), we input a value of 0.29 for the calculation. To convert CRP mg/dL value to nmol/L, multiply by 95.24.
Adapted from Best Pract Res Clin Rheumatol,28 with permission.
Treat-to-Target Principle
A recent consensus statement by the EULAR indicated that the primary target of therapy in rheumatoid arthritis is remission, defined as absence of signs or symptoms of inflammatory disease activity.14,34 Remission is a realistic and achievable target in the current era of rheumatoid arthritis therapy, in particular when treat-to-target strategies are used and when the disease is diagnosed and treated early in its course.35-38 Use of a tight-control treatment strategy, with the goal of a DAS28 score lower than 2.6, leads to faster and more frequent remission than does treatment according to current “usual” clinical care.25
The ACR and EULAR have recently published more stringent criteria for remission.39 Rheumatoid arthritis now is defined as in remission when the number of tender joints, swollen joints (both using 28-joint counts), CRP concentration (in milligrams per deciliter), and patient global assessment (on a 10-cm visual analog scale) are all 1 or less, or, alternatively, when the SDAI score is 3.3 or lower. In a relevant post hoc analysis of the ASPIRE (Active-Controlled Study of Patients Receiving Infliximab for the Treatment of Rheumatoid Arthritis of Early Onset) trial, achievement of remission (SDAI ≤3.3 or CDAI ≤2.8) by week 14 was predictive of radiographic nonprogression at 1 year, irrespective of therapy with methotrexate (MTX) alone or in combination with infliximab.40
Remission may not be attainable in all patients, in particular those with established disease that has been refractory to many therapies. There is also uncertainty about the validity of the remission criteria in clinical practice inasmuch as they were designed for use in clinical trials.41
In view of this dilemma, the EULAR guidelines acknowledge that an alternative target that is more viable in clinical practice is low disease activity,14 as defined by an SDAI score of 11 or lower or CDAI score of 10 or lower. Patients who achieve this target overall do well and have low mean progression of joint damage, especially those patients receiving biologic therapy.40 However, we are concerned that uncritical use of the remission target might lead to overtreatment and inappropriate exposure of some patients to expensive and potentially risky biologic therapies. Although remission is the most appropriate target in the long term, we recognize that physicians must individualize treatment goals on the basis of prognostic characteristics and patient preferences.
Assessment of Disease Severity and Prognosis
The clinician must assess the probability of disease progression and of complications in patients with rheumatoid arthritis. At diagnosis, useful predictors of severe disease and poor prognosis include positive results of blood tests for rheumatoid factor and/or ACPA, greater disability, increased acute-phase reactant concentrations, and presence of radiographic joint erosions at baseline. The presence of rheumatoid factor, ACPA, or the major risk allele for rheumatoid arthritis, the human leukocyte antigen DRB1 “shared epitope,” is not useful for predicting treatment response in the context of current treatment approaches.42 Tobacco smoking is a modifiable predictor of adverse outcomes.43-46 There is some evidence that clinical prediction tools might be useful in assessing the risk of radiographic disease progression47,48; however, we do not advocate use of these in clinical practice at this time. The role of commercially available biomarker panels remains to be defined. Development of more useful biomarkers for predicting treatment response and the likelihood of adverse outcomes in personalized treatment strategies will advance the management of rheumatoid arthritis.
Nonpharmacologic Principles
Consideration of nonpharmacologic principles is crucial, in our opinion, to optimal management of rheumatoid arthritis. Education of patients about the pathophysiologic characteristics of the disease, self-management skills, and principles of joint protection lead to improved health and physical function.49,50 Occupational therapy, in particular, is beneficial for instruction about joint protection and prescription of assistive devices, orthotics, and splints, which can substantially improve function and reduce pain.51-53 We advise patients that adequate rest reduces fatigue associated with active rheumatoid arthritis and that resting joints during periods of poorly controlled inflammation will lessen the symptoms of the disease. Cognitive behavioral therapy can also benefit patients with fatigue by enhancing self-management and reducing their sense of helplessness.54 Dynamic exercise programs that incorporate both aerobic exercise and progressive resistance training improve fitness and strength, have salutary benefits on lean body mass, and are safe.55,56 All of these approaches to patient management are best undertaken in a patient-centered manner by a multidisciplinary care team that includes a rheumatologist, nurses, physical and occupational therapists, psychologists, and a skilled primary care physician.
Treatment Approach
Initial Treatment Approach
There is a strong rationale for MTX monotherapy for newly diagnosed rheumatoid arthritis (Figure 1). A recent 3E Initiative Consensus Group recommendation (No. 7) states that in patients who are naïve to disease-modifying antirheumatic drugs (DMARDs), “the balance of efficacy/toxicity favours methotrexate monotherapy over combination with other conventional DMARDs.”57 This conclusion is supported by a 2010 Cochrane systematic review that emphasized lack of evidence of a statistically significant advantage for initial combination therapy using MTX and other conventional DMARDs over monotherapy with MTX.58
FIGURE 1.
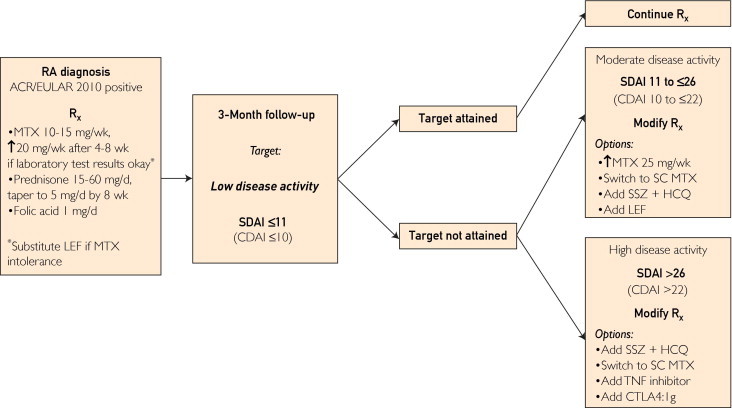
Our treatment approach to newly diagnosed rheumatoid arthritis (RA) from baseline to 6 months of follow-up. ACR/EULAR 2010 = American College of Rheumatology/European League Against Rheumatism 2010 Classification Criteria for RA; CDAI = Clinical Disease Activity Index; CTLA4:Ig = cytotoxic T lymphocyte–associated antigen 4:immunoglobulin fusion protein; HCQ = hydroxychloroquine; LEF = leflunomide; MTX = methotrexate; Rx = prescription; SC = subcutaneous; SDAI = Simplified Disease Activity Index; SSZ = sulfasalazine; TNF = tumor necrosis factor.
A number of randomized, placebo-controlled clinical trials have tested the efficacy and safety of MTX, biologic agents (including various tumor necrosis factor [TNF] inhibitors or the T-cell costimulation blocker cytotoxic T lymphocyte–associated antigen 4:immunoglobulin fusion protein [CTLA-4:Ig]), or their combination, in patients with early MTX-naïve rheumatoid arthritis including many with poor prognostic signs.37,59-64 The results of these studies suggest that the combination of MTX with a TNF inhibitor or CTLA-4:Ig (abatacept) has greater efficacy than MTX monotherapy insofar as both clinical and radiographic outcomes are concerned.
In our opinion, these trials have a number of limitations. Disease severity in patients participating in clinical trials is systematically different from that in patients we see in the outpatient clinic. In many studies, treatment with MTX, TNF inhibitors, or other biologic agents, or combinations thereof, is administered according to fixed protocols that do not reflect longitudinal clinical care, which often requires modification of the treatment regimen sooner than is typically permitted in trials with patients in whom the disease fails to improve sufficiently. Influential to our thinking is the TEAR (Treatment of Early Aggressive Rheumatoid Arthritis) trial, an investigator-initiated, randomized, double-blind, placebo-controlled trial of 4 treatment strategies, performed in the United States, that compared not only initial monotherapy vs combination therapy but also treatment with conventional DMARDs vs a TNF inhibitor (etanercept), published thus far in abstract form.65,66 The results of this trial do not support any advantages of initial combination therapy incorporating etanercept in either clinical or radiographic outcomes at 2 years over initial MTX monotherapy with step-up to combination therapy at 6 months because of inadequate response.
The likely response to MTX cannot be reliably predicted on the basis of current clinical assessments.67 Certainly, practical and cost considerations favor initial MTX therapy over combinations of DMARDs or biologic agents. Thus, at this time, we favor MTX therapy in most patients unless there are contraindications. Our approach is to initiate MTX at a dose of 15 mg/wk along with folic acid at 1 mg/d. Lower doses of MTX are required in some elderly patients and in patients with chronic kidney disease. Folic acid supplementation reduces mucosal and gastrointestinal toxicity, and likely liver toxicity, without reducing MTX efficacy.68
Numerous clinical trials have reported the salutary benefits of high-dose prednisone therapy in early rheumatoid arthritis. Two European studies, COBRA (Combination Therapy in Rheumatoid Arthritis) and BeSt (Behandel-Stratieën) trial, found that a high-dose oral prednisone regimen (60 mg, tapering to 7.5 mg by week 6, then stopping after week 12) in combination with other conventional DMARDs substantially inhibited the progression of radiographic joint damage, and this effect was sustained over many years.35,69 In particular, the BeSt study findings suggest that the addition of high-dose prednisone therapy may mitigate the advantage of initial biologic therapy, in this case with infliximab, further supporting our approach of not using a biologic agent initially. To date, no studies have evaluated a more intermediate starting dose (ie, 20 mg/d).
Considering the known association of higher dosages with increased risk of opportunistic infections, as well as problems with tolerability in some patients, our approach is to individualize the initial prednisone dosage on the basis of disease activity, metabolic factors (ie, diabetes mellitus), and patient risk factors for infection and osteoporosis. Our approach to the use of prednisone is supported by evidence that the disease-modifying and erosion-inhibiting benefits of low-dose oral prednisone therapy (5-10 mg/d) are sustained for at least 2 years, with minimal corticosteroid-related adverse effects.70,71 Because most of the benefit is in the first year, our recommendation is to continue prednisone at 5 mg/d for 6 months to 1 full year before gradually tapering in decrements of 1 mg every 2 to 4 weeks. It is likely that treatment using low-dose prednisone may increase the probability of a successful outcome of MTX therapy.
Critical Time Point
Low disease activity, as defined using various composite measures, is established as an important therapeutic target in early rheumatoid arthritis on the basis of findings of the TICORA (Tight Control of Rheumatoid Arthritis) and BeSt trials.36,60 The absolute disease activity state, either low disease activity or remission, as defined by the SDAI, has been found in a meta-analysis of biologic therapy to be strongly predictive of the probability of remission at 1 year irrespective of initial MTX monotherapy or combination therapy with TNF inhibitors.72 Not many more patients achieve remission at 6 months than at 3 months. Overall, more than 75% of patients with low disease activity or remission at 3 months are in remission at 1 year. It is our view that 3 months after initiation of therapy is the most useful time to assess the probability of attaining clinical remission at 1 year (Figure 1).
Patients in whom initial MTX therapy, optimized to 20 to 25 mg/wk or more (or a maximally tolerated oral or subcutaneous dosage), along with prednisone therapy (starting with an initial moderate dose and tapered to 5 mg/d by week 8), does not result in low to moderate disease activity by 3 months are unlikely to achieve long-term remission of disease by 6 to 12 months without treatment modification. These patients are at substantial risk of continued radiographic joint destruction. The importance of treating to this defined target at 3 months is supported by the finding that therapy driven by disease activity score vs routine care leads to higher rates of remission and lower rates of radiographic progression of disease.24,73
We make separate treatment recommendations for patients with moderate vs high disease activity at 3 months (Figure 1). The results from the BeSt and SWEFOT (Swedish Farmacotherapy) trials indicate that step-up therapy using sulfasalazine (SSZ) and hydroxychloroquine (HCQ) in these patients is inferior to the addition of a TNF inhibitor.60,74,75 In contrast, the results of the TEAR trial suggest that step-up treatment using SSZ and HCQ is comparably effective to step-up etanercept therapy in both clinical response and radiographic outcomes.65,66
To mitigate this apparent contradiction, we have considered data regarding the probability of remission at 1 year on the basis of absolute disease activity at 3 months. Patients in whom moderate, but not low, disease activity (SDAI >11 but ≤26) has been achieved at 3 months have an indeterminate probability of low disease activity or remission at 1 year.72 Thus, we advocate more conservative treatment modifications in this group at 3 months. Options include increasing the MTX dose to 25 mg/wk orally, switching to subcutaneous MTX, adding SSZ and HCQ (triple therapy), and less commonly, adding leflunomide.
In patients with high disease activity (SDAI >26 or CDAI >22) at 3 months that is refractory to treatment with optimized MTX and prednisone, the probability of attaining remission at 1 year is low without addition of combination therapy or a biologic response modifier.36,60,72,74 In this setting, both TNF inhibitors59,62,76-78 and abatacept79 are approved and are recommended biologic response modifiers for step-up treatment. Although interleukin 1 receptor antagonist therapy (anakinra) is approved for treatment in this context, we regard this medication as generally less effective than the above-mentioned agents, and, thus, cannot recommend its use at this disease stage.
The results of the TEAR study suggest that step-up therapy with SSZ and HCQ in addition to MTX as late as 6 months is similarly effective as step-up therapy with a biologic agent in this group at high risk; thus, on the basis of available evidence, triple-DMARD therapy may be a reasonable option in some patients. A caveat is the need to reevaluate this recommendation after full publication of the TEAR study results.
Six to 12 Months of Disease
Attainment of minimal disease activity is an important goal, and one that is of particular consequence to achieve by 1 year after diagnosis. Patients in the FIN-RACo (Finnish Rheumatoid Arthritis Combination Therapy) study who did not achieve remission by 1 year experienced a substantially higher rate of progression of joint erosions over the ensuing decade than did patients who did achieve remission.80 Combination regimens are markedly more likely than monotherapy regimens to induce remission.81 The window of 6 to 12 months is chosen because in patients in whom biologic therapy was initiated at 3 months, up to 6 months may justifiably be required before the treatment response can be definitively assessed.
In our practice, treatment is intensified in patients with an SDAI score higher than 11 (CDAI >10) at 6 to 12 months (Figure 2). In patients receiving MTX monotherapy, treatment should be escalated either with the addition of SSZ and HCQ for triple-DMARD therapy or the addition of TNF inhibition or T-cell costimulation blockade (abatacept). In patients already receiving combined MTX plus biologic therapy (either a TNF inhibitor or abatacept), treatment with an alternative biologic response modifier should be initiated. Abatacept is more effective than placebo in patients with inadequate response to TNF inhibitors82 and has a good safety record.83
FIGURE 2.
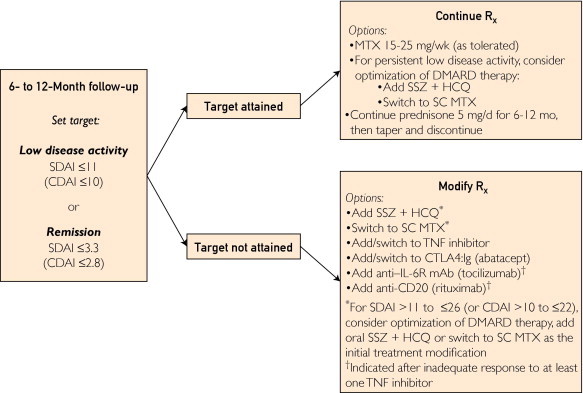
Our treatment approach to early rheumatoid arthritis (RA) from 6 months to 1 year. anti–IL-6R = anti-interleukin 6 receptor; CDAI = Clinical Disease Activity Index; CTLA4:Ig = cytotoxic T lymphocyte–associated antigen 4:immunoglobulin fusion protein; DMARD = disease-modifying antirheumatic drug; HCQ = hydroxychloroquine; mAb = monoclonal antibody; MTX = methotrexate; Rx = prescription; SC = subcutaneous; SDAI = Simplified Disease Activity Index; SSZ = sulfasalazine; TNF = tumor necrosis factor.
A single trial of switching to another TNF inhibitor is appropriate and can be effective; however, additional trials of anti-TNF agents are not likely to be so beneficial as switching to a drug with an alternative mechanism of action.84,85 At this early stage of disease, we favor TNF inhibitors or abatacept over other biologic agents, although anti-CD20 (rituximab)86,87 and anti-interleukin 6 receptor monoclonal antibody (tocilizumab)88,89 therapies are effective and safe for treatment of rheumatoid arthritis with inadequate response to one or more TNF inhibitors. These agents can also be considered under individual circumstances, depending on comorbidities and previous treatment responses.
Beyond the First 6 to 12 Months of Disease
Beyond the first year, patients with persistently moderate to high disease activity despite treatment according to the recommended algorithm are at substantial risk of disease progression. Although we consider low disease activity an acceptable goal, we strongly recommend ongoing efforts to tailor the treatment regimen toward complete abrogation of joint inflammation. At this point, the MTX dose should be increased to 20 to 25 mg/wk or the maximal tolerated dosage, then switched to subcutaneous parenteral administration as needed. Assessment of MTX metabolites may offer some insight as to bioavailability. Patients with inflammatory activity predominantly in single isolated joints should receive local intra-articular glucocorticoid injection.
In our recommendations for patients with active disease at this point, we consider patients receiving single-drug or combination DMARD therapy separately from patients receiving biologic response modifiers (Figure 3). In patients receiving DMARD-only therapy, either triple-DMARD therapy should be initiated, adding SSZ and HCQ to optimize MTX therapy, or a TNF inhibitor or CTLA-4:Ig (abatacept) should be added to MTX therapy. In patients receiving a biologic agent, either the particular biologic agent should be discontinued on initiation of triple-DMARD therapy or treatment using an alternative biologic agent, preferably one with an alternative mechanism of action, should be started.
FIGURE 3.
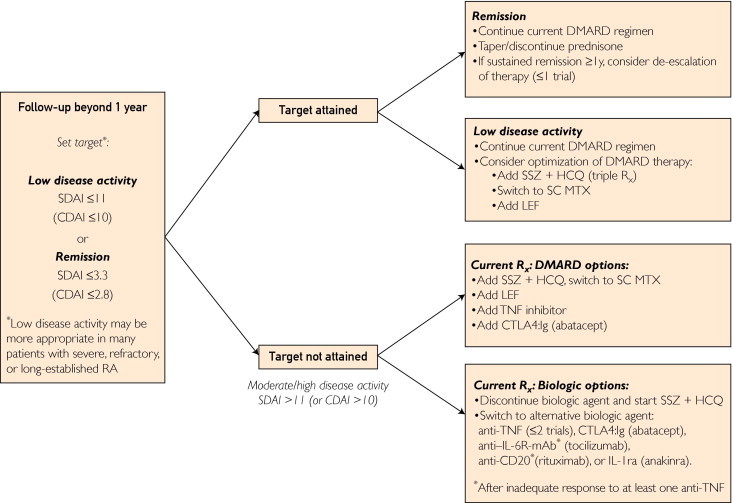
Our approach to treatment of rheumatoid arthritis (RA) beyond the first year of disease. DMARD therapy refers to use of methotrexate (MTX) and other conventional disease-modifying antirheumatic drugs. Biologic therapy refers to treatment with tumor necrosis factor (TNF) inhibitors, abatacept, rituximab, tocilizumab, or anakinra. anti–IL-6R = anti-interleukin 6 receptor; CDAI = Clinical Disease Activity Index; CTLA4:Ig = cytotoxic T lymphocyte–associated antigen 4:immunoglobulin fusion protein; HCQ = hydroxychloroquine; IL-1ra = interleukin 1 receptor antagonist; LEF = leflunomide; mAb = monoclonal antibody; Rx = prescription; SDAI = Simplified Disease Activity Index; SSZ = sulfasalazine.
In our view, any new treatment usually should be tried for at least 3 to 6 months to fully assess its efficacy. No studies to date have compared the efficacy of various therapeutic strategies one-on-one using randomized, double-blind, placebo-controlled designs. In addition, few biomarkers are yet available to guide management of individual patients. An exception is the presence of rheumatoid factor, antibodies to citrullinated protein, or increased serum IgG concentration, all of which are generally predictive of a favorable response to rituximab.90 A biomarker of usefulness in our practice is to recommend abatacept or tocilizumab rather than rituximab in patients who test seronegative for rheumatoid factor and with inadequate response to one or more anti-TNF drugs.
Findings of recent studies suggest that in a small number of patients managed using the treat-to-target strategy, therapy can be tapered successfully, and even sustained drug-free remission can be achieved in as many as 15% to 25%.91,92 Although these patients may experience a flare-up of disease after observation for 3 to 6 months, in most, clinical remission is achieved again with resumption of therapy and there is no radiologic progression of disease.91 Shorter symptom duration, absence of rheumatoid factor or ACPA, lower mean disease activity before remission, and less baseline disability are associated with attainment of sustained drug-free remission.92,93
After the first 1 to 2 years, the benefits of long-term corticosteroid therapy are often outweighed by the risks, including cataracts, osteoporosis and fractures, and potentially, cardiovascular disease. Beyond the early disease period, prednisone therapy primarily provides symptom relief and does not modify the course of disease progression. The most recent EULAR guidelines for cardiovascular risk management in patients with rheumatoid arthritis recommend using the lowest possible prednisone dosage.94 From the recent 2010 recommendations for prevention of glucocorticoid-induced osteoporosis, it is clear that there is no “safe” dosage of prednisone insofar as bone density and structure are concerned.95 Thus, beyond the first 1 to 2 years of disease, the use of prednisone should be primarily as bridge therapy for flare-ups while awaiting the efficacy of nonbiologic and biologic DMARD therapy. Attempts should be made in all patients to gradually taper and eventually discontinue glucocorticoid therapy before accepting long-term low-dose prednisone therapy.
Potential Pitfalls and Alternative Approaches
No treatment approach or guidelines can ever encompass all scenarios relevant to clinical practice adequately; we firmly acknowledge this truth. The judgment of the primary rheumatologist is of paramount importance in cost-effective, appropriate, and safe implementation of our treatment recommendations. Biomarkers such as CRP and composite disease activity measures such as the SDAI and CDAI augment clinical judgment. Experienced clinicians recognize the limitations of composite measures at both the high and low ends of the scale. For example, patients with fibromyalgia can have high disease activity scores because of high tender joint counts and patient global assessment in the absence of swollen joint counts or elevated acute phase reactant levels.96 In patients with disease remission according to composite measures, active joint inflammation can, nonetheless, be detected with high-resolution imaging.97-99
The art of medicine is alive and well in rheumatology. Patients with rheumatoid arthritis can experience central pain amplification yet have active inflammation. When joint tenderness, fatigue, and subjective disease activity are disproportionate to the provider assessments of disease activity, the response should not be to follow our approach according to the calculated SDAI and initiate therapy with, for example, biologic agents. Rather, the provider should investigate the cause of the symptoms. For example, noninflammatory causes of pain such as regional musculoskeletal pain syndromes or osteoarthritis should be identified and treated accordingly.
Magnetic resonance imaging or high-resolution ultrasonography with power Doppler examination could be considered to identify evidence of subclinical inflammation that might be clinically relevant, for example, in predicting progression of erosive disease.97,100 Discrepancy between the provider and patient in assessment of disease activity should be addressed, because this discordance is associated with increased symptoms of depression and reduced quality of life.101 Widespread chronic pain due to central pain amplification in the absence of active inflammatory disease might be treated using pharmacologic and nonpharmacologic approaches. Comorbid depressive or anxiety disorders should be treated in conjunction with our colleagues in psychiatry and pain management.
Recommendations
Contemporary management of rheumatoid arthritis emphasizes early diagnosis, quantitative monitoring of disease activity, and intensive goal-directed therapy to achieve the best possible outcomes for patients. The major goal of treatment is to abrogate the inflammatory disease activity and achieve long-term remission, which in the future ideally will mean absence of disease and minimal need for medications. Application of the 2010 ACR/EULAR classification criteria for rheumatoid arthritis facilitates early diagnosis, which is critical to the highest probability of clinical remission with disease-modifying therapy. Our treatment approach is to use MTX plus prednisone as the initial treatment. Three months is the critical time at which we assess the response to MTX and consider step-up to combination treatment strategies.
Our treatment approach reflects several gaps remaining in our understanding of the best practices for the management of rheumatoid arthritis. We do not know how best to measure disease activity and to assess the clinical response to treatment. This can create uncertainty in decision making about treatment. The benefits and limitations of targeting low disease activity vs remission are still to some extent uncertain. The relative benefits and harms of emphasizing initial prednisone use vs initial biologic therapy remain largely unexplored. The effects of treatment on the risk of developing comorbidities such as cardiovascular disease remain unclear. Current methods for predicting the efficacy and toxicity of specific treatments are imperfect, hindering us from achieving truly personalized therapy. We hope resolution of these issues will be forthcoming from clinical research studies, enabling us to improve our management approach to patients with rheumatoid arthritis.
Footnotes
Potential Competing Interests: Dr Davis is an investigator on studies with Centocor Inc, Malvern, PA; VA Cooperative Studies Program, US Department of Veterans Affairs, Washington, DC; Genentech, Inc, South San Francisco, CA; Novartis Corp, East Hanover, NJ; Rheumatoid Arthritis Investigational Network/University of Nebraska Medical Center, Omaha; and UCB Pharma, Inc, Smyrna, GA. He has received grant support or contracts from the Arthritis Foundation, Indianapolis, IN; Centocor; Genentech; Mayo Foundation, Rochester, MN; Myriad RBM, Inc, Austin, TX; National Center for Research Resources and National Institutes of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD; Novartis Corp; UCB Pharma, Inc; and University of Nebraska Medical Center Cooperative Studies Program.
Dr Matteson is an investigator on studies with the American College of Rheumatology, Atlanta, GA; Amgen Inc, Thousand Oaks, CA; Ardea Biosciences, San Diego, CA; AstraZeneca, London, UK; Biogen Idec, Weston, MA; Centocor Inc, Malvern, PA; Eli Lily and Co, Indianapolis, IN; Genentech, Inc, South San Francisco, CA; Hoffman-LaRoche Inc, Nutley, NJ; Human Genome Sciences, Inc, Rockville, MD; Janssen Biotech, Inc, Horsham, PA; Pfizer Inc, New York, NY; Novartis Corp, East Hanover, NJ; Roche, Indianapolis, IN; Schering-Plough/Merck & Co, Inc, Whitehouse Station, NJ; and UCB Group; UCB, Inc, Atlanta, GA. He has received grant support or contracts from Amgen, Bristol-Myers Squibb, New York, NY; Centocor/Johnson & Johnson; Genentech; Mayo Foundation, Rochester, MN; Novartis; National Institutes of Health, Bethesda, MD; and Veteran's Administration, US Department of Veterans Affairs, Washington, DC. He participates as consultant/scientific advisory board member for Abbott Laboratories, Abbott Park, IL; Amgen, Biogen Idec; Centocor; Crescendo Bioscience, South San Francisco, CA; West Lafayette, IN; Horizon Pharma USA, Inc, Deerfield, IL; and Novartis.
Supplemental Online Material
Author Interview Video
References
- 1.Helmick C.G., Felson D.T., Lawrence R.C., National Arthritis Data Workgroup Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Crowson C.S., Matteson E.L., Myasoedova E. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011;63(3):633–639. doi: 10.1002/art.30155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allaire S., Wolfe F., Niu J., Lavalley M.P. Contemporary prevalence and incidence of work disability associated with rheumatoid arthritis in the US. Arthritis Rheum. 2008;59(4):474–480. doi: 10.1002/art.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokka T., Kautiainen H., Pincus T., QUEST-RA Work disability remains a major problem in rheumatoid arthritis in the 2000s: data from 32 countries in the QUEST-RA study. Arthritis Res Ther. 2010;12(2):R42. doi: 10.1186/ar2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turesson C., O'Fallon W.M., Crowson C.S., Gabriel S.E., Matteson E.L. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29(1):62–67. [PubMed] [Google Scholar]
- 6.Gabriel S.E., Crowson C.S., Kremers H.M. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum. 2003;48(1):54–58. doi: 10.1002/art.10705. [DOI] [PubMed] [Google Scholar]
- 7.Lindhardsen J., Ahlehoff O., Gislason G.H. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis. 2011;70(6):929–934. doi: 10.1136/ard.2010.143396. [DOI] [PubMed] [Google Scholar]
- 8.Semb A.G., Kvien T.K., Aastveit A.H. Lipids, myocardial infarction and ischaemic stroke in patients with rheumatoid arthritis in the Apolipoprotein-related Mortality RISk (AMORIS) Study [published online ahead of print June 15, 2010] Ann Rheum Dis. 2010;69(11):1996–2001. doi: 10.1136/ard.2009.126128. [DOI] [PubMed] [Google Scholar]
- 9.Maradit-Kremers H., Crowson C.S., Nicola P.J. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52(2):402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 10.Nicola P.J., Crowson C.S., Maradit-Kremers H. Contribution of congestive heart failure and ischemic heart disease to excess mortality in rheumatoid arthritis. Arthritis Rheum. 2006;54(1):60–67. doi: 10.1002/art.21560. [DOI] [PubMed] [Google Scholar]
- 11.Nicola P.J., Maradit-Kremers H., Roger V.L. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005;52(2):412–420. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 12.Aletaha D., Neogi T., Silman A.J. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 13.Saag K.G., Teng G.G., Patkar N.M., American College of Rheumatology American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 14.Smolen J.S., Aletaha D., Bijlsma J.W., T2T Expert Committee Treating rheumatoid arthritis to target: recommendations of an international task force [published correction appears in Ann Rheum Dis. 2011;70(8):1519] [published online ahead of print March 9, 2010] Ann Rheum Dis. 2010;69(4):631–637. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neogi T., Aletaha D., Silman A.J., American College of Rheumatology; European League Against Rheumatism The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: Phase 2 methodological report. Arthritis Rheum. 2010;62(9):2582–2591. doi: 10.1002/art.27580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duer A., Østergaard M., Horslev-Petersen K., Vallø J. Magnetic resonance imaging and bone scintigraphy in the differential diagnosis of unclassified arthritis. Ann Rheum Dis. 2008;67(1):48–51. doi: 10.1136/ard.2006.063792. [DOI] [PubMed] [Google Scholar]
- 17.Narváez J., Sirvent E., Narváez J.A. Usefulness of magnetic resonance imaging of the hand versus anticyclic citrullinated peptide antibody testing to confirm the diagnosis of clinically suspected early rheumatoid arthritis in the absence of rheumatoid factor and radiographic erosions [published online ahead of print January 28, 2008] Semin Arthritis Rheum. 2008;38(2):101–109. doi: 10.1016/j.semarthrit.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Solau-Gervais E., Legrand J.L., Cortet B., Duquesnoy B., Flipo R.M. Magnetic resonance imaging of the hand for the diagnosis of rheumatoid arthritis in the absence of anti-cyclic citrullinated peptide antibodies: a prospective study. J Rheumatol. 2006;33(9):1760–1765. [PubMed] [Google Scholar]
- 19.Szkudlarek M., Klarlund M., Narvestad E. Ultrasonography of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis: a comparison with magnetic resonance imaging, conventional radiography and clinical examination [published online ahead of print March 6, 2008] Arthritis Res Ther. 2006;8(2):R52. doi: 10.1186/ar1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prevoo M.L., van't Hof M.A., Kuper H.H., van Leeuwen M.A., van de Putte L.B., van Riel P.L. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 21.Smolen J.S., Aletaha D., Grisar J., Redlich K., Steiner G., Wagner O. The need for prognosticators in rheumatoid arthritis: Biological and clinical markers: where are we now? Arthritis Res Ther. 2008;10(3):208. doi: 10.1186/ar2418. [published online ahead of print May 29, 2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowson C.S., Rahman M.U., Matteson E.L. Which measure of inflammation to use?: A comparison of erythrocyte sedimentation rate and C-reactive protein measurements from randomized clinical trials of golimumab in rheumatoid arthritis [published correction appears in J Rheumatol. 2009;36(11):2625] J Rheumatol. 2009;36(8):1606–1610. doi: 10.3899/jrheum.081188. [DOI] [PubMed] [Google Scholar]
- 23.Haavardsholm E.A., Østergaard M., Hammer H.B. Monitoring anti-TNFalpha treatment in rheumatoid arthritis: responsiveness of magnetic resonance imaging and ultrasonography of the dominant wrist joint compared with conventional measures of disease activity and structural damage. Ann Rheum Dis. 2009;68(10):1572–1579. doi: 10.1136/ard.2008.091801. [DOI] [PubMed] [Google Scholar]
- 24.Goekoop-Ruiterman Y.P., de Vries-Bouwstra J.K., Kerstens P.J. DAS-driven therapy versus routine care in patients with recent-onset active rheumatoid arthritis. Ann Rheum Dis. 2010;69(1):65–69. doi: 10.1136/ard.2008.097683. [DOI] [PubMed] [Google Scholar]
- 25.Schipper L.G., Vermeer M., Kuper H.H. A tight control treatment strategy aiming for remission in early rheumatoid arthritis is more effective than usual care treatment in daily clinical practice: a study of two cohorts in the Dutch Rheumatoid Arthritis Monitoring registry. Ann Rheum Dis. 2012;71(6):845–850. doi: 10.1136/annrheumdis-2011-200274. [DOI] [PubMed] [Google Scholar]
- 26.van Gestel A.M., Haagsma C.J., van Riel P.L. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41(10):1845–1850. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 27.Wells G., Becker J.C., Teng J. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68(6):954–960. doi: 10.1136/ard.2007.084459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aletaha D., Smolen J.S. The Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI) to monitor patients in standard clinical care. Best Pract Res Clin Rheumatol. 2007;21(4):663–675. doi: 10.1016/j.berh.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Smolen J.S., Breedveld F.C., Schiff M.H. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003;42(2):244–257. doi: 10.1093/rheumatology/keg072. [DOI] [PubMed] [Google Scholar]
- 30.Aletaha D., Smolen J.S. The Simplified Disease Activity Index and Clinical Disease Activity Index to monitor patients in standard clinical care. Rheum Dis Clin North Am. 2009;35(4):759–772. doi: 10.1016/j.rdc.2009.10.006. viii. [DOI] [PubMed] [Google Scholar]
- 31.Sokka T., Hetland M.L., Mäkinen H., Questionnaires in Standard Monitoring of Patients with Rheumatoid Arthritis Group Remission and rheumatoid arthritis: data on patients receiving usual care in twenty-four countries. Arthritis Rheum. 2008;58(9):2642–2651. doi: 10.1002/art.23794. [DOI] [PubMed] [Google Scholar]
- 32.Burmester G.R., Ferraccioli G., Flipo R.M. Clinical remission and/or minimal disease activity in patients receiving adalimumab treatment in a multinational, open-label, twelve-week study. Arthritis Rheum. 2008;59(1):32–41. doi: 10.1002/art.23247. [DOI] [PubMed] [Google Scholar]
- 33.Balsa A., de Miguel E., Castillo C., Peiteado D., Martín-Mola E. Superiority of SDAI over DAS-28 in assessment of remission in rheumatoid arthritis patients using power Doppler ultrasonography as a gold standard. Rheumatology (Oxford) 2010;49(4):683–690. doi: 10.1093/rheumatology/kep442. [DOI] [PubMed] [Google Scholar]
- 34.Schoels M., Knevel R., Aletaha D. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search [published correction appears in Ann Rheum Dis. 2011;70(8):1519] Ann Rheum Dis. 2010;69(4):638–643. doi: 10.1136/ard.2009.123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Möttönen T., Hannonen P., Leirisalo-Repo M., FIN-RACo trial group Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. Lancet. 1999;353(9164):1568–1573. doi: 10.1016/s0140-6736(98)08513-4. [DOI] [PubMed] [Google Scholar]
- 36.Grigor C., Capell H., Stirling A. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364(9430):263–269. doi: 10.1016/S0140-6736(04)16676-2. [DOI] [PubMed] [Google Scholar]
- 37.Emery P., Breedveld F.C., Hall S. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372(9636):375–382. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- 38.Verstappen S.M., Jacobs J.W., van der Veen M.J., Utrecht Rheumatoid Arthritis Cohort study group Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial) Ann Rheum Dis. 2007;66(11):1443–1449. doi: 10.1136/ard.2007.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felson D.T., Smolen J.S., Wells G., American College of Rheumatology; European League Against Rheumatism American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63(3):573–586. doi: 10.1002/art.30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smolen J.S., Han C., van der Heijde D.M., Active-Controlled Study of Patients Receiving Infliximab for the Treatment of Rheumatoid Arthritis of Early Onset (ASPIRE) Study Group Radiographic changes in rheumatoid arthritis patients attaining different disease activity states with methotrexate monotherapy and infliximab plus methotrexate: the impacts of remission and tumour necrosis factor blockade [published correction appears in Ann Rheum Dis. 2011;70(8):1519] Ann Rheum Dis. 2009;68(6):823–827. doi: 10.1136/ard.2008.090019. [DOI] [PubMed] [Google Scholar]
- 41.O'Dell J.R., Mikuls T.R. To improve outcomes we must define and measure them: toward defining remission in rheumatoid arthritis. Arthritis Rheum. 2011;63(3):587–589. doi: 10.1002/art.30199. [DOI] [PubMed] [Google Scholar]
- 42.de Vries-Bouwstra J.K., Goekoop-Ruiterman Y.P., Verpoort K.N. Progression of joint damage in early rheumatoid arthritis: association with HLA-DRB1, rheumatoid factor, and anti-citrullinated protein antibodies in relation to different treatment strategies. Arthritis Rheum. 2008;58(5):1293–1298. doi: 10.1002/art.23439. [DOI] [PubMed] [Google Scholar]
- 43.Saevarsdottir S., Wedrén S., Seddighzadeh M. Patients with early rheumatoid arthritis who smoke are less likely to respond to treatment with methotrexate and tumor necrosis factor inhibitors: observations from the Epidemiological Investigation of Rheumatoid Arthritis and the Swedish Rheumatology Register cohorts. Arthritis Rheum. 2011;63(1):26–36. doi: 10.1002/art.27758. [DOI] [PubMed] [Google Scholar]
- 44.Nyhäll-Wåhlin B.M., Jacobsson L.T., Petersson I.F., Turesson C., BARFOT study group Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):601–606. doi: 10.1136/ard.2005.039172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manfredsdottir V.F., Vikingsdottir T., Jonsson T. The effects of tobacco smoking and rheumatoid factor seropositivity on disease activity and joint damage in early rheumatoid arthritis. Rheumatology (Oxford) 2006;45(6):734–740. doi: 10.1093/rheumatology/kei240. [DOI] [PubMed] [Google Scholar]
- 46.Papadopoulos N.G., Alamanos Y., Voulgari P.V., Epagelis E.K., Tsifetaki N., Drosos A.A. Does cigarette smoking influence disease expression, activity and severity in early rheumatoid arthritis patients? Clin Exp Rheumatol. 2005;23(6):861–866. [PubMed] [Google Scholar]
- 47.Visser K., Goekoop-Ruiterman Y.P., de Vries-Bouwstra J.K. A matrix risk model for the prediction of rapid radiographic progression in patients with rheumatoid arthritis receiving different dynamic treatment strategies: post hoc analyses from the BeSt study. Ann Rheum Dis. 2010;69(7):1333–1337. doi: 10.1136/ard.2009.121160. [DOI] [PubMed] [Google Scholar]
- 48.Vastesaeger N., Xu S., Aletaha D., St Clair E.W., Smolen J.S. A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rheumatology (Oxford) 2009;48(9):1114–1121. doi: 10.1093/rheumatology/kep155. [DOI] [PubMed] [Google Scholar]
- 49.Masiero S., Boniolo A., Wassermann L., Machiedo H., Volante D., Punzi L. Effects of an educational-behavioral joint protection program on people with moderate to severe rheumatoid arthritis: a randomized controlled trial. Clin Rheumatol. 2007;26(12):2043–2050. doi: 10.1007/s10067-007-0615-0. [DOI] [PubMed] [Google Scholar]
- 50.Barsky A.J., Ahern D.K., Orav E.J. A randomized trial of three psychosocial treatments for the symptoms of rheumatoid arthritis. Semin Arthritis Rheum. 2010;40(3):222–232. doi: 10.1016/j.semarthrit.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathieux R., Marotte H., Battistini L., Sarrazin A., Berthier M., Miossec P. Early occupational therapy programme increases hand grip strength at 3 months: results from a randomised, blind, controlled study in early rheumatoid arthritis. Ann Rheum Dis. 2009;68(3):400–403. doi: 10.1136/ard.2008.094532. [DOI] [PubMed] [Google Scholar]
- 52.Niedermann K., de Bie R.A., Kubli R. Effectiveness of individual resource-oriented joint protection education in people with rheumatoid arthritis: a randomized controlled trial. Patient Educ Couns. 2011;82(1):42–48. doi: 10.1016/j.pec.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Steultjens E.M., Dekker J., Bouter L.M., van Schaardenburg D., van Kuyk M.A., Van den Ende C.H. Occupational therapy for rheumatoid arthritis. Cochrane Database Syst Rev. 2008;(1) doi: 10.1002/14651858.CD003114.pub2. CD003114.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hewlett S., Ambler N., Almeida C. Self-management of fatigue in rheumatoid arthritis: a randomised controlled trial of group cognitive-behavioural therapy. Ann Rheum Dis. 2011;70(6):1060–1067. doi: 10.1136/ard.2010.144691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hurkmans E., van der Giesen F.J., Vliet Vlieland T.P.M., Schoones J., Van den Ende E.C.H.M. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst Rev. 2009;(4) doi: 10.1002/14651858.CD006853.pub2. CD006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lemmey A.B., Marcora S.M., Chester K., Wilson S., Casanova F., Maddison P.J. Effects of high-intensity resistance training in patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum. 2009;61(12):1726–1734. doi: 10.1002/art.24891. [DOI] [PubMed] [Google Scholar]
- 57.Visser K., Katchamart W., Loza E. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis. 2009;68(7):1086–1093. doi: 10.1136/ard.2008.094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katchamart W., Trudeau J., Phumethum V., Bombardier C. Methotrexate monotherapy versus methotrexate combination therapy with non-biologic disease modifying anti-rheumatic drugs for rheumatoid arthritis. Cochrane Database Syst Rev. 2010;(4) doi: 10.1002/14651858.CD008495. CD008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Breedveld F.C., Weisman M.H., Kavanaugh A.F. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 60.Goekoop-Ruiterman Y.P., de Vries-Bouwstra J.K., Allaart C.F. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2007;146(6):406–415. doi: 10.7326/0003-4819-146-6-200703200-00005. [DOI] [PubMed] [Google Scholar]
- 61.Goekoop-Ruiterman Y.P., de Vries-Bouwstra J.K., Allaart C.F. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52(11):3381–3390. doi: 10.1002/art.21405. [DOI] [PubMed] [Google Scholar]
- 62.St Clair E.W., van der Heijde D.M., Smolen J.S., Active-Controlled Study of Patients Receiving Infliximab for the Treatment of Rheumatoid Arthritis of Early Onset Study Group Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50(11):3432–3443. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- 63.Bathon J., Robles M., Ximenes A.C. Sustained disease remission and inhibition of radiographic progression in methotrexate-naive patients with rheumatoid arthritis and poor prognostic factors treated with abatacept: 2-year outcomes. Ann Rheum Dis. 2011;70(11):1949–1956. doi: 10.1136/ard.2010.145268. [DOI] [PubMed] [Google Scholar]
- 64.Westhovens R., Robles M., Ximenes A.C. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68(12):1870–1877. doi: 10.1136/ard.2008.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moreland L.W., O'Dell J.R., Paulus H.E. Two-year radiographic results from the TEAR trial [abstract] Arthritis Rheum. 2010;62(suppl 10):S568–S569. [Google Scholar]
- 66.Moreland L.W., O'Dell J.R., Paulus H.E. TEAR: Treatment of Early Aggressive Rheumatoid Arthritis: a randomized, double-blind, 2-year trial comparing immediate triple DMARD versus MTX plus etanercept to step-up from initial MTX monotherapy [abstract] Arthritis Rheum. 2009;60(suppl 10):S1895. [Google Scholar]
- 67.Drouin J., Haraoui B., 3e Initiative Group Predictors of clinical response and radiographic progression in patients with rheumatoid arthritis treated with methotrexate monotherapy. J Rheumatol. 2010;37(7):1405–1410. doi: 10.3899/jrheum.090838. [DOI] [PubMed] [Google Scholar]
- 68.Ortiz Z., Shea B., Suarez-Almazor M.E., Moher D., Wells G.A., Tugwell P. The efficacy of folic acid and folinic acid in reducing methotrexate gastrointestinal toxicity in rheumatoid arthritis: a metaanalysis of randomized controlled trials. J Rheumatol. 1998;25(1):36–43. [PubMed] [Google Scholar]
- 69.Landewé R.B., Boers M., Verhoeven A.C. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 2002;46(2):347–356. doi: 10.1002/art.10083. [DOI] [PubMed] [Google Scholar]
- 70.Svensson B., Boonen A., Albertsson K., van der Heijde D., Keller C., Hafström I. Low-dose prednisolone in addition to the initial disease-modifying antirheumatic drug in patients with early active rheumatoid arthritis reduces joint destruction and increases the remission rate: a two-year randomized trial. Arthritis Rheum. 2005;52(11):3360–3370. doi: 10.1002/art.21298. [DOI] [PubMed] [Google Scholar]
- 71.Bakker M.F., Jacobs J.W., Welsing P.M., Utrecht Rheumatoid Arthritis Cohort Study Group Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2012;156(5):329–339. doi: 10.7326/0003-4819-156-5-201203060-00004. [DOI] [PubMed] [Google Scholar]
- 72.Aletaha D., Funovits J., Keystone E.C., Smolen J.S. Disease activity early in the course of treatment predicts response to therapy after one year in rheumatoid arthritis patients. Arthritis Rheum. 2007;56(10):3226–3235. doi: 10.1002/art.22943. [DOI] [PubMed] [Google Scholar]
- 73.Soubrier M., Lukas C., Sibilia J. Disease activity score-driven therapy versus routine care in patients with recent-onset active rheumatoid arthritis: data from the GUEPARD trial and ESPOIR cohort. Ann Rheum Dis. 2011;70(4):611–616. doi: 10.1136/ard.2010.137695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Kooij S.M., de Vries-Bouwstra J.K., Goekoop-Ruiterman Y.P. Limited efficacy of conventional DMARDs after initial methotrexate failure in patients with recent onset rheumatoid arthritis treated according to the disease activity score. Ann Rheum Dis. 2007;66(10):1356–1362. doi: 10.1136/ard.2006.066662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Vollenhoven R.F., Ernestam S., Geborek P. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet. 2009;374(9688):459–466. doi: 10.1016/S0140-6736(09)60944-2. [DOI] [PubMed] [Google Scholar]
- 76.Genovese M.C., Bathon J.M., Martin R.W. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46(6):1443–1450. doi: 10.1002/art.10308. [DOI] [PubMed] [Google Scholar]
- 77.Klareskog L., van der Heijde D., de Jager J.P., TEMPO (Trial of Etanercept and Methotrexate With Radiographic Patient Outcomes) study investigators Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363(9410):675–681. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 78.Maini R., St Clair E.W., Breedveld F., ATTRACT Study Group Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet. 1999;354(9194):1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 79.Kremer J.M., Genant H.K., Moreland L.W. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144(12):865–876. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 80.Rantalaiho V., Korpela M., Laasonen L., FIN-RACo Trial Group Early combination disease-modifying antirheumatic drug therapy and tight disease control improve long-term radiologic outcome in patients with early rheumatoid arthritis: the 11-year results of the Finnish Rheumatoid Arthritis Combination Therapy trial. Arthritis Res Ther. 2010;12(3):R122. doi: 10.1186/ar3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma M.H., Scott I.C., Kingsley G.H., Scott D.L. Remission in early rheumatoid arthritis. J Rheumatol. 2010;37(7):1444–1453. doi: 10.3899/jrheum.091131. [DOI] [PubMed] [Google Scholar]
- 82.Genovese M.C., Becker J.C., Schiff M. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition [published correction appears in N Engl J Med. 2005;353(21):2311] N Engl J Med. 2005;353(11):1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 83.Schiff M., Keiserman M., Codding C. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67(8):1096–1103. doi: 10.1136/ard.2007.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smolen J.S., Kay J., Doyle M.K., GO-AFTER study investigators Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial [published correction appears in Lancet. 2009;374(9699):1422] Lancet. 2009;374(9685):210–221. doi: 10.1016/S0140-6736(09)60506-7. [DOI] [PubMed] [Google Scholar]
- 85.Furst D.E., Gaylis N., Bray V. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the OPPOSITE study. Ann Rheum Dis. 2007;66(7):893–899. doi: 10.1136/ard.2006.068304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Emery P., Fleischmann R., Filipowicz-Sosnowska A., DANCER Study Group The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54(5):1390–1400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 87.Cohen S.B., Emery P., Greenwald M.W., REFLEX Trial Group Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54(9):2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 88.Smolen J.S., Beaulieu A., Rubbert-Roth A., OPTION Investigators Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371(9617):987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 89.Emery P., Keystone E., Tony H.P. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial [published correction appears in Ann Rheum Dis. 2009;68(2):296] Ann Rheum Dis. 2008;67(11):1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sellam J., Hendel-Chavez H., Rouanet S. B cell activation biomarkers as predictive factors for the response to rituximab in rheumatoid arthritis: a six-month, national, multicenter, open-label study. Arthritis Rheum. 2011;63(4):933–938. doi: 10.1002/art.30233. [DOI] [PubMed] [Google Scholar]
- 91.Klarenbeek N.B., van der Kooij S.M., Güler-Yüksel M. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: exploratory analyses from the BeSt study. Ann Rheum Dis. 2011;70(2):315–319. doi: 10.1136/ard.2010.136556. [DOI] [PubMed] [Google Scholar]
- 92.van der Woude D., Young A., Jayakumar K. Prevalence of and predictive factors for sustained disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: results from two large early arthritis cohorts. Arthritis Rheum. 2009;60(8):2262–2271. doi: 10.1002/art.24661. [DOI] [PubMed] [Google Scholar]
- 93.Saleem B., Keen H., Goeb V. Patients with RA in remission on TNF blockers: when and in whom can TNF blocker therapy be stopped? Ann Rheum Dis. 2010;69(9):1636–1642. doi: 10.1136/ard.2009.117341. [published correction appears in Ann Rheum Dis. 2011;70(8):1520. [DOI] [PubMed] [Google Scholar]
- 94.Peters M.J., Symmons D.P., McCarey D. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69(2):325–331. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 95.Grossman J.M., Gordon R., Ranganath V.K. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis [published correction appears in Arthritis Care Res (Hoboken). 2012;64(1):157; author reply 157-158] Arthritis Care Res (Hoboken) 2010;62(11):1515–1526. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 96.Leeb B.F., Andel I., Sautner J., Nothnagl T., Rintelen B. The DAS28 in rheumatoid arthritis and fibromyalgia patients. Rheumatology (Oxford) 2004;43(12):1504–1507. doi: 10.1093/rheumatology/keh322. [DOI] [PubMed] [Google Scholar]
- 97.Brown A.K., Conaghan P.G., Karim Z. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58(10):2958–2967. doi: 10.1002/art.23945. [DOI] [PubMed] [Google Scholar]
- 98.Brown A.K., Quinn M.A., Karim Z. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54(12):3761–3773. doi: 10.1002/art.22190. [DOI] [PubMed] [Google Scholar]
- 99.Hetland M.L., Stengaard-Pedersen K., Junker P., CIMESTRA study group Radiographic progression and remission rates in early rheumatoid arthritis: MRI bone oedema and anti-CCP predicted radiographic progression in the 5-year extension of the double-blind randomised CIMESTRA trial. Ann Rheum Dis. 2010;69(10):1789–1795. doi: 10.1136/ard.2009.125534. [DOI] [PubMed] [Google Scholar]
- 100.Hetland M.L., Ejbjerg B.J., Hørslev-Petersen K., CIMESTRA study group MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis: results from a 2 year randomized controlled trial (CIMESTRA) Ann Rheum Dis. 2009;68(3):384–390. doi: 10.1136/ard.2008.088245. [DOI] [PubMed] [Google Scholar]
- 101.Barton J.L., Imboden J., Graf J., Glidden D., Yelin E.H., Schillinger D. Patient-physician discordance in assessments of global disease severity in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62(6):857–864. doi: 10.1002/acr.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview Video


