Abstract
Mayo Clinic has been involved in an ongoing effort to prevent the diversion of controlled substances from the workplace and to rapidly identify and respond when such diversion is detected. These efforts have found that diversion of controlled substances is not uncommon and can result in substantial risk not only to the individual who is diverting the drugs but also to patients, co-workers, and employers. We believe that all health care facilities should have systems in place to deter controlled substance diversion and to promptly identify diversion and intervene when it is occurring. Such systems are multifaceted and require close cooperation between multiple stakeholders including, but not limited to, departments of pharmacy, safety and security, anesthesiology, nursing, legal counsel, and human resources. Ideally, there should be a broad-based appreciation of the dangers that diversion creates not only for patients but also for all employees of health care facilities, because diversion can occur at any point along a long supply chain. All health care workers must be vigilant for signs of possible diversion and must be aware of how to engage a preexisting group with expertise in investigating possible diversions. In addition, clear policies and procedures should be in place for dealing with such investigations and for managing the many possible outcomes of a confirmed diversion. This article provides an overview of the multiple types of risk that result from drug diversion from health care facilities. Further, we describe a system developed at Mayo Clinic for evaluating episodes of potential drug diversion and for taking action once diversion is confirmed.
Abbreviations and Acronyms: ADM, automated distribution machine; CS, controlled substance; DEA, Drug Enforcement Administration; DDiRT, drug diversion response team; HCW, health care worker; MDPC, medication diversion prevention coordinator; OR, operating room; PCA, patient-controlled analgesia
“Diversion” means the transfer of a controlled substance from a lawful to an unlawful channel of distribution or use.
Uniform Controlled Substances Act (1994)1
“Diversion” means “Any criminal act involving a prescription drug.”
National Association of Drug Diversion Investigators2
In the United States in 2010, nearly 4 billion retail prescriptions were filled, with sales totaling $307 billion. The medication most often prescribed, 131.2 million times, was the opioid hydrocodone combined with acetaminophen.3 The opioid oxycodone combined with acetaminophen was prescribed 31.9 million times. Although most of these sales resulted in the legitimate, targeted administration of pharmaceutical agents to patients, a fraction of the drugs manufactured and prescribed for patients are diverted for illicit purposes. Most drug prescriptions are for use in the outpatient setting, and, thus, most diversions of drugs occur there. This problem has been well documented in multiple publications and will not be further addressed here.4 Although a relatively small fraction of the nation's drug supply is administered in a health care facility such as a hospital or outpatient surgery center, the nature of these practices provides ample opportunity for drug diversion. This less appreciated form of drug diversion is associated with adverse consequences, the scope of which is incalculable, with harm to the drug diverter and others that is at times horrific. There are no available data that precisely define the extent of drug diversion from the health care facility workplace. However, it is well recognized that anesthesiologists, perhaps more than any other class of physician, have ready access to highly addictive psychotropic medications and have a higher rate of addiction to opioid drugs than physicians in other specialties.5 Furthermore, the drugs most commonly abused by anesthesiologists are obtained through diversion.6 Such data suggest that ready access is a critical component of drug diversion from the health care facility workplace.
The most common drugs diverted from the health care facility setting are opioids. Although other high-value drugs such as antiretroviral drugs, athletic performance–enhancing drugs (eg, erythropoietin and anabolic steroids), and nonopioid psychotropic drugs have been diverted from the health care facility workplace,7 the ensuing discussion focuses on the theft of controlled substances (CSs), defined as medications classified as Schedules II (ie, substances with high potential for abuse) through V (ie, substances with lower potential for abuse than substances in Schedules II, III, and IV), as defined by the federal Drug Enforcement Administration (DEA) and state statutes.8 We do not discuss the topic of theft within the pharmacy setting, which is largely accomplished by other means. Typically, drugs stolen from health care facilities are used to support an addiction of either the health care worker (HCW) or an associate and, less commonly, for sale for financial gain. This theft can be of unopened vials; vials or syringes that have been tampered with, resulting in either substituted or diluted dosages being administered to the patient; or residual drug left in a syringe or vial after only a fraction of the drug that has been signed out was actually administered to the patient. In addition, this theft can be of discarded syringes or ampules that have been properly disposed of in a “sharps” safety container.
In the outpatient setting, there is an elaborate system of checks and balances for prescription, procurement, and dosing of a CS. However, in the health care facility environment, vulnerability to diversion exists when a single provider, out of view of others, is free to engage in drug procurement from central stores, drug preparation, drug administration to patients, and/or disposal of drug waste. Given that CSs are often titrated to a desired effect in patients who may have widely varying drug requirements (eg, as a result of baseline individual variability, acquired habituation, or other factors), in the absence of sufficient controls, it is relatively easy for a single HCW, without the knowledge or collusion of others, to divert drugs intended for patients. It is beyond question that HCW diversion of opioids and other CSs from the health care workplace has occurred since time immemorial.
Recent experience at Mayo Clinic and elsewhere have revealed that such health care workplace drug diversion creates numerous potential victims. Specifically, harm can come not only to drug diverters but also to their patients and co-workers and to the reputation of the health care institution that employs them. A recent devastating example is that of an infected HCW in the Denver, Colorado, area who, in the process of diverting narcotics for self-use, passed on hepatitis C virus to approximately 36 patients.9 Such cases provide graphic examples of why it is essential to reduce or eliminate opportunities for diversion of drugs in the health care facility workplace.
Mayo Clinic has recently expanded its efforts to better educate its staff about the risks of drug diversion and to enhance its methods for detection. With encouragement from institutional physician-executives, we provide details of several instances that are instructive in alerting others to the sub-rosa crimes and harms that are likely occurring in health care systems worldwide. In addition, we define the many potential harms and victims associated with drug diversion. We also review strategies that we have developed, and that continue to evolve, in our efforts to combat diversion.
Illustrative Vignettes
There are many patterns of drug diversion in the health care facility workplace environment, involving personnel from diverse backgrounds engaged in diversions in a variety of practice locations. The heterogeneity of drug diversion practices is exemplified in the following vignettes, all based on cases detected at Mayo Clinic medical centers during 2010 and 2011. These vignettes represent only a portion of the instances of diversion that were detected during that time. For reasons of confidentiality, the vignettes have been slightly altered.
-
•
A visitor to the hospital asked to assist in bathing a patient, who was a loved one. After the bath, the patient's nurse noticed that the time-released patches used to transcutaneously administer the potent opioid fentanyl were no longer attached to the patient. An initial search of the patient's room, including a search of the trash cans, yielded nothing. When pressed, the visitor later “found” the missing fentanyl patches in a previously searched trash can.
-
•
A nurse working in a hospital unit discovered that during her work shift she had misplaced her security key, which was required to refill and alter the electronic programming of patient-controlled analgesia (PCA) devices used to administer opioids to patients. Later in that same work shift, a PCA pump on the same hospital unit was found to have “malfunctioned” and delivered the entire syringe of the potent opioid hydromorphone to a single patient over a relatively short time. However, it was astonishing that the patient exhibited no evidence of narcotic overdose. Electronic analysis of the pump revealed that the PCA device had been entered on several occasions, using a security key, and large amounts of opioid were administered during each trespass. On questioning, the patient admitted to having found and hidden the key and to having overridden the pump's safety lockout in order to self-administer large dosages of opioid. It was only by virtue of this patient being chronically habituated to opioids that a potentially fatal overdose was avoided.
-
•
A procedural sedation nurse assigned to administer opioids and sedatives to patients during colonoscopy was found to have a secret pocket sewn inside her uniform top, into which she dropped syringes of the potent opioid fentanyl and substituted them with syringes containing saline solution. During the colonoscopy, the nurse would inject saline solution, rather than the prescribed fentanyl, into the patients and divert the prescribed fentanyl for her own use.
-
•
A radiology technician who was positive for hepatitis C diverted unused fentanyl syringes intended for administration to patients in the interventional radiology area. It is believed that the technician would remove the needle from a syringe, replace it with a smaller gauge needle for self-injection, and then reattach the original needle to the syringe. The technician would then refill the syringe with saline solution and return it to the patient care area. In so doing, the technician infected 5 patients with hepatitis C virus.
-
•
Sharps waste containers (used to collect previously used syringes, needles, and vials) filled with uncapped needles and used syringes were on multiple occasions found hidden in hospital areas where they did not belong. Many containers had clearly been broken into, and nearby were plastic bags filled with unprotected used needles protruding from them. Video surveillance ultimately led to discovery of an employee who was stealing waste by transferring the contents of used sharps containers into the bags and taking them home in a search for discarded CSs. Video surveillance also revealed this employee attempting to retrieve narcotics from an intact sharps container by sticking her hand blindly into the container, resulting in her hand being cut and bleeding from contact with needles and glass.
-
•
A night custodian was discovered rummaging through sharps waste containers holding nearly empty vials of fentanyl. On questioning, the custodian revealed that he had been withdrawing and consolidating the miniscule remaining fentanyl from each vial, which he later self-injected to support his addiction.
Potential Harm Arising From Drug Diversion
Addiction is sometimes viewed as a victimless crime. When the addiction is supported by drug diversion within the health care facility workplace environment, it becomes, in most situations, a multiple-victim crime in which patients, health care workers, and employers can be harmed directly or indirectly.
Harm to the Patient
In cases of drug diversion, patients may potentially receive substandard care from an addicted and drug-diverting individual. If one assumes that the patients required the drugs that were prescribed for them, the absence of the drug entirely or dilution of the drug such that they receive a dosage less than that intended will likely result in the patient experiencing undue pain and/or anxiety, at least temporarily. This pain can be excruciating, as evidenced by the recent diversion of fentanyl by a sedation nurse in Minneapolis, Minnesota.10 While the patient was undergoing a surgical procedure, the nurse took most of the prescribed dose of fentanyl for herself, leaving the patient “in agonizing pain.” Immediately before the procedure, this nurse allegedly instructed the patient that he would have to “man up” and tolerate some pain because he could not be given much pain medication. This patient, a law enforcement officer, was so distressed by the care he received that he reported it to the police.10
In vignette 1, the harm was caused by a trusted visitor who volunteered to bathe the patient in order to steal the patient's opioids. In vignette 2, the patient might easily have brought harm or death to herself by overriding the safety mechanism built into the PCA. In vignette 3, multiple patients likely experienced pain as a consequence of insufficient sedation and analgesia because they did not receive the analgesia that was ordered for their procedures. In vignette 4, 5 patients were infected with hepatitis C, and in 1 of these, the infection contributed to the patient's death. Only in vignettes 5 and 6 was there no clear patient harm, yet the reckless actions occurring in vignette 5 exposed many other innocent HCWs, and possibly patients and visitors, to the danger of a needle stick injury with all of the attendant infectious risks of bloodborne pathogens.
Other potential harm to patients includes the possibility that they will receive an adulterated or contaminated drug in place of the diverted drug, such as the substitution of unsterile tap water for the sterile drug that was intended for them, or an alternate drug to which they may be allergic. A contaminated drug could put patients at risk for blood-borne infections, sepsis, wound infections, and infections of implanted foreign bodies such as prosthetic joints and heart valves.11,12
Furthermore, patients are at risk simply by virtue of being under the care of an addicted and potentially impaired HCW. The impairment may be as obvious as the HCW being acutely intoxicated by a drug or as subtle as the risk posed by a distracted HCW whose primary focus is not patient care but where, when, and how to obtain the next needed dose of drug to stave off withdrawal symptoms. In such a state, it is easy to imagine an HCW being susceptible to making cognitive patient-management or medication errors that might directly harm the patient.
Potential Risks Addicted HCWs Pose to Themselves
An individual who diverts drugs within the hospital environment introduces a profound risk of morbidity and mortality to himself or herself. One need look no further than the anesthesiology literature to find numerous examples of presumably unintentional overdose and death associated with the diversion of drugs, typically highly potent sedative, opioid, and anesthetic agents.6,13 Death typically results from severe respiratory depression or arrest, producing anoxic insults to the brain, heart, and other organs. Death or anoxic brain injury to those who otherwise survive the drug overdose are certainly the most feared consequences; however, there are many other important risks to the addicted HCW. Among these are the risk of infection from unsterile drugs and needles, unsanitary injection techniques, or blood-to-blood transmission of pathogens from a patient to an HCW.
In addition to the biologic risks, drug diversion is associated with profound professional risks for HCWs. Stealing CSs from the supply chain exposes them to felony criminal prosecution and civil malpractice actions, as well as actions against their professional licenses. Other potential professional hazards include the risk of prosecution for fraudulent charting if they chart that the patient received medication that they know they did not give and the attendant risk of prosecution for billing fraud resulting from submitting charges to the patient or insurance provider for drugs and services they know the patient did not receive.
Potential Risk to Other HCWs
Vignette 5 graphically demonstrates a potential source of risk to other HCWs from the irresponsible actions of an HCW who was diverting drugs. Unexpectedly finding uncapped contaminated needles and broken glass vials in areas where they should not be puts fellow HCWs at risk for mechanical injury and infection by bloodborne pathogens. In addition, although co-workers may be unaware of the colleague's impairment, shared patient-care responsibilities might result in adverse patient outcomes, thereby placing all members of the care team at risk for medicolegal liability.
Many addicted HCWs become masters at manipulating both the drug control systems in the areas where they work and at manipulating their colleagues so that the addict can divert drugs undetected. Often this involves breaches of policies and procedures on the part of the addicted HCW's colleagues, whereby they unwittingly aid their addicted colleague by “witnessing” the disposal of leftover CSs that they did not witness. This puts the otherwise innocent HCW at risk of disciplinary action for being found in violation of such routine policies and procedures.
Potential Risk to Employer
Health care workers who divert CSs from the workplace introduce potential risk to their employer. Harms include the loss of revenue from diverted drugs and the potentially poor work quality or absenteeism of the addicted HCW. Drug-diverting HCWs also place their employer at risk of civil liability for failure to prevent, recognize, or address signs of drug diversion or of an impaired or addicted employee. Should harm befall a patient while under the care of an addicted HCW, both the HCW and the employer are vulnerable to civil litigation.
When there is suspected diversion of CSs in the workplace, the employer typically invests considerable resources to conduct investigations and forensic testing required to confirm or refute diversion. The objectives are to halt any ongoing or future harm from a drug-diverting HCW and to assess any past harm to patients, the care environment, and other vulnerable areas. The exhaustive nature of these investigations has been effectively reported by Thompson et al,14 who described early efforts to unravel the cases of unexplained hepatitis C in vignette 4. That the investigations in vignette 4 did not end with the activities described in the report by Thompson et al is astounding. Other critical events were discovered subsequent to that report, and the source of the transmission was ultimately confirmed (ie, more than 1 year after the publication by Thompson et al, during which time 3 additional patients were infected by the diverting HCW).15 The sense that the illicit activities by this HCW could have possibly harmed scores of patients resulted in a massive “look back” evaluation of the practices and timelines involving the suspected HCW. Free testing was offered to nearly 4,000 patients who were believed to have possibly been at risk of exposure. As a result of these tests, 5 patients were ultimately proved to have the same genetic subtype of hepatitis C as the infected HCW, presumably because the infections emanated from a common source.
Investigations such as this are expensive in terms of human and nonhuman resources, time, and finances; however, they are a necessary part of health care systems acting responsibly. Such efforts first and foremost fulfill an ethical obligation to past, present, and future patients. They also are a critical component of long-term risk management for the employer.
Mandatory reporting requirements may cause diversion incidents to become public knowledge and potentially highly publicized in the media. In the long term, this can have a devastating effect on the morale of the employees of the health care institution. It also can have an even more profound effect on the patients and their families who have entrusted their care to the institution, whether or not they were directly affected by an episode of drug diversion. Disappointed or mistrustful patients may seek medical care elsewhere, resulting in financial losses to the institution that, in turn, harm its ability to sustain a mission of providing high-quality patient-centered care.
Because of these cumulative risks, it is essential that all health care institutions establish surveillance programs in an effort to detect potential diversion of CSs and implement mechanisms, policies, and procedures that enable quick intervention when diversion is identified, ideally before harm to patients and HCWs can ensue. This is true not only because it is an ethical imperative but also because it is the most fiscally responsible plan for the survival and prosperity of the mission of the health care institution.
General Concepts of Prevention and Detection
Many HCWs are unaware that drug diversion is a serious problem in the workplace. Thus, broad-based educational efforts must be instituted that focus on the nature and scope of the problem, signs and symptoms of possible diversion and addiction, and proper ways to respond if diversion is suspected. The entire workforce, not only those with ready access to CSs, should be informed of the threats to life and career presented by drug diversion. Orientation of new employees should include such education, and ongoing education should occur throughout an HCW's career. In an effort to discourage diversion, all employees should be made aware that procedures are in place to facilitate detection, with the objective of preventing patient harm, diversion-related addictive illness, and drug-related deaths. Employees also should be taught how to access available resources if they suspect diversion is occurring. A Web-based teaching module on the topic of diversion is currently being created at Mayo Clinic to facilitate these educational goals, with consideration being given to making the satisfactory completion of this module a required part of annual employee competency assessments.
The ideal drug-diversion and prevention program is highly sophisticated, drawing from the knowledge bases of behavioral and biologic sciences, information technology, law enforcement, pharmacy sciences, credentialing and licensure experts, and industry loss-prevention sciences. There is tremendous diversity among the prevention and detection efforts of various medical centers, ranging from haphazard reactionary programs to highly sophisticated proactive programs. This is an evolving field, and even the best efforts have not yet achieved the ideal situation in which the numbers of drug diversions and their harmful consequences approach zero. Mayo Clinic has, for the past several years, been developing and fine-tuning a uniform response to suspected diversion. The following is a brief timeline of the evolution of such diversion prevention efforts by Mayo Clinic.
Operationalization of Drug Diversion Prevention and Detection Efforts at Mayo Clinic
Department of Anesthesiology
Anesthesiologists and nurse anesthetists are health care providers in whom psychotropic drug use and addiction is considered an occupational hazard.6,13,16 Mayo Clinic is no exception in this regard; thus, it is not surprising that concerted efforts to detect drug diversion began in the Department of Anesthesiology. Detection has often been precipitated by personnel underperformance or behavioral changes at work, frequent unexplained work absences, patients who seem to be undermedicated in the period after surgery despite documentation of administration of seemingly adequate narcotic dosages, and, rarely, HCW death related to a CS.
In the early 1990s, in response to several episodes of fentanyl diversion from anesthesia supplies in the operating room (OR) environment, an improvement in CS handling was sought. After a review of procedures used by multiple health care institutions was conducted by members of the departments of anesthesiology and pharmacy, departmental leadership decided to purchase automated distribution machines (ADMs) for the OR environment. Secure return bins were also installed so that all unused portions of CS doses could be submitted for subsequent random quantitative drug assays before destruction of the CS. In addition, close cooperation between the departments of anesthesiology and pharmacy created a system whereby returned waste volumes were reconciled with ADM dispensing records and patient anesthesia records, and any discrepancies were aggressively investigated until resolved. Since initiation of these improved control systems, there has been a dramatic decrease in the frequency of CS diversions (unpublished data, Keith Berge, MD, Department of Anesthesiology, Mayo Clinic, June 7, 2012). Frequent educational sessions about the risk of addiction and diversion by Department of Anesthesiology personnel were also undertaken for all workers in the OR environment, not only anesthesia personnel but also surgical nurses and surgical technicians, among others. In these sessions, the signs and symptoms of drug diversion and use, as we have previously described,15 are discussed in an effort to promote rapid identification of aberrant behavior.
Institution-Wide Reforms at Mayo Clinic
Mayo Clinic is a large and complex organization with 55,000 employees in multiple states. After several diversion incidents in multiple Mayo Clinic facilities, a unified policy has been evolving to combat the problem. As part of this effort, a new position called the Medication Diversion Prevention Coordinator (MDPC) was created within the Department of Pharmacy in the larger facilities. This role calls for an individual familiar with the CS supply chain and management policies who is, therefore, able to assess areas of vulnerability for potential diversion. This individual must also be skilled in the use of systems available for tracking, trending, and evaluating the dispensing patterns of health care professionals and be willing to participate in educational efforts about the dangers of, and recognition of, drug diversion.
With input from multiple participants, a “best practices” list was created that identified 77 specific points that, were all the points implemented, would create what we believe to be the best possible system to date to prevent CS diversion (Supplemental Table, available online at http://www.mayoclinicproceedings.org). Inasmuch as all of the most recently discovered diversion episodes were occurring outside of the tightly controlled OR environment, OR control systems were expanded to enable similar controls in other areas with high CS use. Thus, the ADM/waste retrieval system has been implemented in the Emergency Department and the gastrointestinal endoscopy suite at Mayo Clinic in Rochester. In time, it will also be used in the intensive care unit and other nursing units with high CS use. Educational efforts have been increased to publicize the Medication Diversion Prevention Committee and the active surveillance efforts it brings to the problem. Although institution-wide uniformity in the approach to diversion prevention efforts is the desired goal, at this time, various entities within the Mayo Clinic system are at different points along the continuum of developing and introducing this uniform approach. The ultimate goal is for an MDPC or designee to be available via pager at all times to respond to and rapidly investigate potential diversions, with the number for this pager prominently displayed on every ADM. Some Mayo Clinic sites currently rely on a compliance hotline to service this notification role.
As further best practices are identified, they are distributed to all Mayo Clinic facilities. In addition, details of each incident of confirmed diversion are shared among the MDPCs at each site in an effort to gain insights into and address any potential areas of vulnerability. Efforts are under way to align policies and procedures across all Mayo Clinic facilities for response to suspected drug diversions. Uniform policies will provide a consistent approach to confirming that a diversion has occurred, notifying the appropriate institutional leaders and other parties with a need to know, altering employment status of the drug-diverting personnel, and ensuring that mandatory reporting requirements are met (eg, to governmental agencies). Although some of these efforts are still evolving, many of these policies are now in the final stages of implementation. All policies adhere to core principles but are tailored to both the requirements of the individual health care site and the laws of the state in which each affected Mayo Clinic site is located.
Organizational Features of Institution-Wide Mayo Clinic Effort
At the larger Mayo Clinic facilities, teams have been created to investigate suspected diversion episodes. These multidisciplinary teams, all subgroups of the Medication Diversion Prevention Committees, are called the drug diversion response teams (DDiRT). Each team consists of the MDPC, the Director of Pharmacy, a member of the Department of Safety and Security, and a physician chair of the Medication Diversion Prevention Committee. Other departments represented include human resources, legal, nursing, and administration. The Rochester-based team currently assists with investigation in the Mayo Clinic Health System sites near Mayo Clinic Rochester, although some of the larger sites are in the process of creating their own on-site DDiRTs.
When diversion is suspected, any Mayo Clinic employee can initiate the chain of events that results in a thorough investigation. The Figure provides a flowchart of how these investigations proceed, starting with the diversion either suspected as a result of aberrancies identified through routine surveillance of prescribing patterns or by a witnessed event. If there is concern that the diversion might involve an acutely impaired or intoxicated HCW, his or her supervisor is engaged and the HCW is immediately, but discreetly, removed from patient care duties and escorted to a new location (eg, Employee Health Services) for further evaluation including a “for cause” drug test. However, such drug testing can neither conclusively confirm nor conclusively rule out all forms of CS diversion or HCW self-administration. If the test result is negative, it does not eliminate the possibility that the drug was being diverted for use by another. If the test is positive, it does not eliminate the possibility that the individual under suspicion for diversion might in addition have a legitimate prescription for and use of the drug allegedly diverted. In any event, the MDPC (or designee) is promptly contacted via pager.
FIGURE.
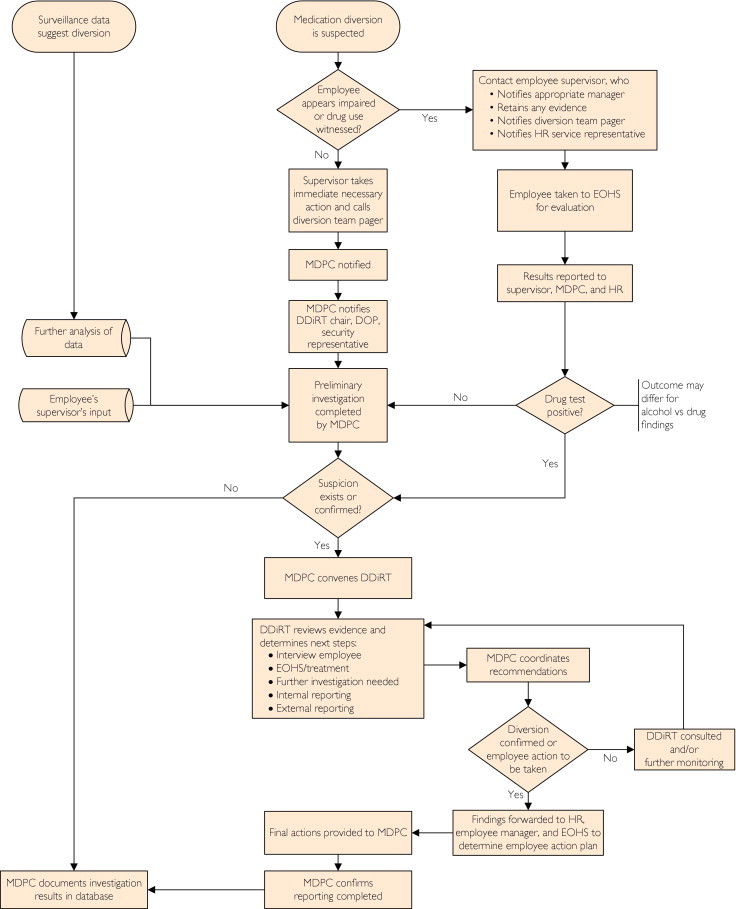
Medication diversion response flowchart. DDiRT = Drug Diversion Response Team; DOP = Director of Pharmacy; EOHS = Employee and Occupational Health Services; HR = Department of Human Resources; MDPC = Medication Diversion Prevention Coordinator.
In the absence of obvious employee impairment, the MDPC will ask the supervisor to secure any evidence that might be inadvertently destroyed but not to initiate an investigation and to refrain from questioning the employee about the event. If possible, the MDPC notifies the chair of the local DDiRT and the Director of Pharmacy and promptly initiates a preliminary investigation consisting of review of the documentary records and a discussion with the immediate supervisor in the area of the suspected diversion. If this inquiry reveals no evidence of diversion, the case is closed and logged into the database. Should the investigation reveal cause for ongoing concern, the MDPC convenes the DDiRT within 24 hours to determine the optimal course of action. This may involve further surveillance, an employee interview by the MDPC and the DDiRT Department of Security representative, for-cause drug testing, consideration of enlisting the assistance of law enforcement agencies (eg, local law enforcement, the DEA, and the US Food and Drug Administration), or other approaches deemed appropriate by the group. As the investigation proceeds, each DDiRT meeting gives consideration to the most appropriate time for reporting on the presence or progress of the investigation to senior institutional leadership.
The investigation ends when enough proof is gathered to determine that a diversion has or has not occurred. If it is determined that there was no diversion, the case is closed. If, however, a diversion has been found, the case is turned over to the Department of Human Resources to determine what action will be taken insofar as the employment status of the drug diverter. At this point, the role of the DDiRT is complete but for the MDPC entering the details into the database and making certain that all mandatory internal and external reporting (eg, to the DEA, the state Pharmacy Board, and local law enforcement) has been accomplished. Then, details of the drug diversion incident are shared among MDPCs in an effort to identify and remedy areas of shared vulnerability for future diversions.
Prompt actions are of utmost importance throughout these deliberations, both to remove those guilty of drug diversion from the patient care environment and to avert harming the well-being of HCWs who are innocent. In actual practice, we estimate that approximately 75% of suspected diversion investigations at the Rochester Mayo Clinic location have been brought to closure by a confession on the part of the drug diverter (personal communication, Karen Sikkink, CPhT, Mayo Clinic Rochester MDPC, August 26, 2011).
Conclusion
Diversion of drugs from legitimate to illicit use is being recognized with increasing frequency in the United States. Although the full extent of diversion from health care facilities is unknown and probably unknowable, our experience makes clear that it is a considerable and ongoing problem. Addicted HCWs who are diverting drugs from the health care facility workplace pose a risk to their patients, their employers, their co-workers, and themselves. It is essential that all health care institutions have a robust system in place to identify and investigate suspected diversion as rapidly and efficiently as possible and that they implement policies and procedures that enable a standardized and effective response to confirmed diversion.
Drug diversion by HCWs violates the core value that the needs of the patient come first. Clearly, if we are to optimize our approach to inpatient drug diversion and its consequences, we must look at such diversion not as a victimless act but as a multiple-victim crime.
Footnotes
For editorial comment, seepage 607
Supplemental Online Material
Supplemental material can be found online at http://www.mayoclinicproceedings.org.
Supplemental Online Material
Author Interview Video
References
- 1.Drug Abuse Prevention and Control 21 USC Chapter 13 (2011) http://uscode.house.gov/download/pls/21C13.txt Accessed March 19, 2012.
- 2.National Association of Drug Diversion Investigators (NADDI) Web site http://naddi.associationdatabase.com/aws/NADDI/pt/sp/Home_Page Accessed March 19, 2012.
- 3.U.S. Prescription Drug Sales Grow Slowly; Hydrocodone Most Prescribed Seeking Alpha Web site. http://seekingalpha.com/article/128003-u-s-prescription-drug-sales-grow-slowly-hydrocodone-most-prescribed Accessed March 19, 2012.
- 4.National Institute on Drug Abuse (NIDA) Prescription medications: brief description. http://www.nida.nih.gov/drugpages/prescription.html Accessed March 19, 2012.
- 5.Alexander B.H., Checkoway H., Nagahama B.A., Domino K.B. Cause-specific mortality risks of anesthesiologists. Anesthesiology. 2000;93(4):922–930. doi: 10.1097/00000542-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Bryson E.O., Silverstein J.H. Addiction and substance abuse in anesthesiology. Anesthesiology. 2008;109(5):905–917. doi: 10.1097/ALN.0b013e3181895bc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inciardi J.A., Surratt H.L., Kurtz S.P., Cicero T.J. Mechanisms of prescription drug diversion among drug-involved club- and street-based populations. Pain Med. 2007;8(2):171–183. doi: 10.1111/j.1526-4637.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Information and legal resources US Department of Justice; Drug Enforcement Administration (DEA), Office of Diversion Control. http://www.deadiversion.usdoj.gov/schedules/index.html Accessed March 19, 2012.
- 9.AP Daily News Staff Writer Heroin-addicted technician Kristen Diane Parker infected 3 dozen with hepatitis C. http://articles.nydailynews.com/2010-01-18/news/17945085_1_kristen-diane-parker-heroin-addiction-infected New York Daily News. Accessed March 19, 2012.
- 10.CITYPAGES Blogs. Van Denburg H., Larry V. King. Dakota County Deputy, was Sarah May Casareto's O.R. –bound victim. http://blogs.citypages.com/blotter/2011/02/larry_v_king_sarah_may_casareto_victim.php Accessed March 19, 2012.
- 11.Maki D.G., Klein B.S., McCormick R.D. Nosocomial Pseudomonas pickettii bacteremias traced to narcotic tampering: a case for selective drug screening of health care personnel. JAMA. 1991;265(8):981–986. [PubMed] [Google Scholar]
- 12.Ostrowsky B.E., Whitener C., Bredenberg H.K. Serratia marcescens bacteremia traced to an infused narcotic. N Engl J Med. 2002;346(20):1529–1537. doi: 10.1056/NEJMoa012370. [DOI] [PubMed] [Google Scholar]
- 13.Berge K.H., Seppala M.D., Lanier W.L. The anesthesiology communities approach to opioid- and anesthetic-abusing personnel: time to change course. Anesthesiology. 2008;109(5):762–764. doi: 10.1097/ALN.0b013e31818a3814. [DOI] [PubMed] [Google Scholar]
- 14.Thompson N.D., Hellinger W.C., Kay R.S. Healthcare-associated hepatitis C virus transmission among patients in an abdominal organ transplant center [published online ahead of print May 26, 2009] Transpl Infect Dis. 2009;11(4):324–329. doi: 10.1111/j.1399-3062.2009.00406.x. [DOI] [PubMed] [Google Scholar]
- 15.Hellinger W.C., Bacalis L.P., Kay R.S. Health care-associated hepatitis C virus infections attributed to narcotic diversion. Ann Intern Med. 2012;156:477–482. doi: 10.7326/0003-4819-156-7-201204030-00002. [DOI] [PubMed] [Google Scholar]
- 16.Berge K.H., Seppala M.D., Schipper A.M. Chemical dependency and the physician. Mayo Clin Proc. 2009;84(7):625–631. doi: 10.1016/S0025-6196(11)60751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview Video


