Abstract
Essential tremor is often markedly reduced during deep brain stimulation simply by implanting the stimulating electrode before activating neurostimulation. Referred to as the microthalamotomy effect, the mechanisms of this unexpected consequence are thought to be related to microlesioning targeted brain tissue, that is, a microscopic version of tissue ablation in thalamotomy. An alternate possibility is that implanting the electrode induces immediate neurochemical release. Herein, we report the experiment performing with real-time fast-scan cyclic voltammetry to quantify neurotransmitter concentrations in human subjects with essential tremor during deep brain stimulation. The results show that the microthalamotomy effect is accompanied by local neurochemical changes, including adenosine release.
Abbreviations and Acronyms: CFM, carbon fiber microelectrode; DBS, deep brain stimulation; ET, essential tremor; FSCV, fast-scan cyclic voltammetry; MRI, magnetic resonance imaging; VIM, ventral intermediate nucleus of the thalamus
Thalamotomy and deep brain stimulation (DBS) are 2 effective neurosurgical treatments for essential tremor (ET), the most frequent form of pathologic tremor and one of the most common adult-onset neurologic impairments.1,2 Thalamotomy is a surgical ablation technique in which targeted areas of the thalamus are electrolytically lesioned. In DBS, neural activity is modulated through implanted stimulating electrodes that are activated by an external pulse generator. Surprisingly, during DBS, the act of implanting an electrode in targeted thalamic brain tissue can reduce tremor before the pulse generator is activated. Referred to as the microthalamotomy effect, symptom relief before neurostimulation has been observed in as many as 53% of patients undergoing DBS.3
The mechanisms of this unexpected consequence are unknown. Microthalmotomy is generally thought to be a microscopic version of the tissue ablation. The effect, however, is temporary, usually lasting 1 to 10 days.3-5 Its limited duration suggests that the microthalamotomy effect may be induced by neurochemical release. By its nature, the impact of neurochemical diffusion into surrounding tissue is of limited duration, weakening over time. The fact that the affected area is microscopic imposes additional effect limits.
Of note, Bekar et al6 found that the local efflux of adenosine triphosphate and adenosine in mice is markedly increased with thalamic DBS. They also demonstrated that intrathalamic infusions of adenosine A1 receptor agonists ameliorated tremor, implicating a neurochemical influence, specifically adenosinergic mechanisms, in tremor control. Additionally, adenosine has been reported to hyperpolarize the membrane potential by an increase in potassium conductance and decrease hyperpolarization-activated cation current in guinea pig geniculocortical neurons.7 Based on this converging evidence, we hypothesized that neurochemical change, particularly adenosine release, is a factor in inducing the microthalamotomy effect during DBS for ET.
To investigate this possibility, we performed fast-scan cyclic voltammetry (FSCV) to detect real-time neurochemical changes in human subjects undergoing DBS. Fast-scan cyclic voltammetry in combination with microsensing probes not only provides subsecond temporal and submillimeter spatial resolutions but also identifies detected chemicals by showing faradaic currents at unique potentials.8,9 If successful, FSCV recordings in humans undergoing DBS might be considered a means of monitoring neurochemical changes and could add important information to understand the therapeutic effects of this form of neurostimulation and the underlying pathology of ET.
Patients and Methods
The complete details of the methods are described in the Supplemental Appendix, available online with the full text of this article at http://www.mayoclinicproceedings.org. All studies were approved by the Mayo Clinic Institutional Review Board, and all participants provided written informed consent.
Fast-scan cyclic voltammetric recordings were obtained from 8 adults (3 men and 5 women ranging in age from 62 to 84 years, with a mean age of 72 years) undergoing DBS for intractable ET. Seven patients were given local anesthesia while a magnetic resonance imaging (MRI)–compatible stereotactic head frame was fixed to the patient's head. One patient, who was unable to tolerate awake surgery, underwent the procedure under general anesthesia. Magnetic resonance imaging was conducted using a 1.5-T MRI clinical system operated by GE EchoSpeed LX version 9.1 (GE Healthcare, Waukesha, WI). The MRI data were merged with the Schaltenbrand and Wahren human atlas, and stereotactic coordinates were identified (Figure 1, A). A microelectrode for electrophysiologic recording was implanted in a single pass in each patient to identify the targeted ventral intermediate nucleus of the thalamus (VIM) area.
FIGURE 1.
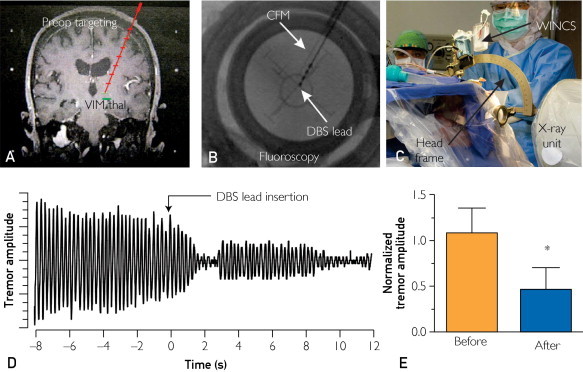
Intraoperative electrochemical and tremor recordings in patients with essential tremor. A, Preoperative (preop) magnetic resonance image of the planned deep brain stimulation (DBS) electrode implantation trajectory into ventral intermediate thalamus (VIM thal), a target for essential tremor. B, Fluoroscopic image confirming the position of the carbon fiber microelectrode (CFM) (arrow) and DBS lead (arrow). C, Instrument setup for detecting electrochemical signals using Wireless Instantaneous Neurochemical Sensing system (WINCS) (secured to an electrode microdrive system and attached to the arc of the stereotaxic head frame). D, A representative tremor recording. Gravity force was measured relatively. E, Normalized tremor amplitude from awake patients before and after DBS electrode implantation. Averaged tremor recording (mean ± SEM in 7 patients) during DBS showing a 61.2%±33.7% decrease in tremor amplitude on DBS electrode insertion into the VIM of the thalamus (asterisk indicates a significant difference as determined by paired 2-tailed t test; P<.05).
The electrochemical sensing probe consisted of an in-house designed carbon fiber microelectrode (CFM) that was either disk-shaped (3 patients) or columnar-shaped (5 patients) (Supplemental Figure 1, available online at http://www.mayoclinicproceedings.org). The CFM was implanted in a single pass simultaneously with the electrophysiologic recording electrode and was located 2 mm anterior to it. Once the target area was identified by the electrophysiologic recording electrode, it was replaced with the DBS electrode (Figure 1, B).
During the DBS electrode insertion, arm and hand tremor were measured using an in-house–designed 3-axis accelerometer, which patients held during the procedure in the hand opposite the implantation hemisphere. Simultaneously, FSCV recordings were made from the CFM. Accelerometer recordings were made for at least 20 and 30 seconds before and after DBS electrode implantation, respectively (see Table 1). There were no complications following DBS surgery and the concurrent electrochemical recording.
TABLE 1.
Changes in the Amplitude and Frequency of Hand Tremor Before and Immediately Following DBS Electrode Implantationa,b
| Patient No./age (y)/sex | CFM type | Tremor amplitude |
Tremor frequency (Hz) |
||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| 1/62/F | Disk | 0.56±0.11 | NA | 4.3 | NA |
| 2/72/M | Disk | 0.32±0.05 | 0.19±0.06 | 5.6 | 5.4 |
| 3/68/M | Disk | 1.09±0.17 | 0.39±0.04 | 4.9 | 4.0 |
| 4/84/F | Column | 0.37±0.14 | 0.24±0.09 | 5.5 | 5.6 |
| 5/75/F | Column | NA | NA | NA | NA |
| 6/75/M | Column | 2.19±0.90 | 1.92±1.32 | 5.1 | 5.0 |
| 7/64/F | Column | 1.02±0.18 | Nonvisible | 4.1 | Nonvisible |
| 8/74/F | Column | 2.00±0.26 | 0.46±0.16 | 3.5 | 4.2 |
CFM = carbon fiber microelectrode; DBS = deep brain stimulation.
Numeric values not available (NA) for patient 1 after the procedure (measurement not quantified) and for patient 5 before and after the procedure (surgery performed under general anesthesia). In patient 7, tremor was completely arrested by the insertion of the DBS electrode.
Real-time FSCV recordings were performed by the Wireless Instantaneous Neurotransmitter Concentration Sensing system (WINCS) (Figure 1, C and Supplemental Figure 2, available online at http://www.mayoclinicproceedings.org),10,11 which records neurochemical changes from the CFM (Supplemental Figure 1, available online at http://www.mayoclinicproceedings.org). In preparatory in vitro studies, an FSCV acquired during the exogenic application of adenosine shows 2 distinct oxidation peaks. The oxidation peak currents remained consistent during the bolus injection of adenosine (0.5 μM) (Supplemental Figure 3, available online at http://www.mayoclinicproceedings.org). The oxidation peak current measured by the CFM was linearly correlated with the concentration of adenosine (Supplemental Figure 3, D, available online at http://www.mayoclinicproceedings.org).
Results
The microthalamotomy effect for the 7 conscious patients was observed in reduced tremor amplitude (61.2%±33.7%; P<.05; Figure 1, D and E). The mean ± SEM tremor frequency was 4.7±0.8 Hz, which is within a range typical for ET.12,13 Tremor frequency was not affected by DBS electrode implantation (3.4±2.4 Hz; P=.12) (Supplemental Figure 4, A, available online at http://www.mayoclinicproceedings.org), nor was it expected, consistent with previous reports that neither drugs nor surgical interventions alter tremor frequency.14 A representative handwriting sample before and after DBS electrode implantation and before neurostimulation demonstrates marked tremor reduction (Supplemental Figure 4, B, available online at http://www.mayoclinicproceedings.org).
Implantation of the DBS electrode evoked a large increase in the FSCV oxidation peak current at +1.45±0.03 V (Figure 2, A and E), which in 4 of the 7 awake patients was followed by a second significantly smaller oxidation current peak at +1.19±0.06 V (blue arrow in Figure 2, A, B, and C). Postcalibration of the CFMs using flow cell analyses demonstrated that these 2 oxidation current peaks matched those for authentic adenosine (Supplemental Figure 5, available online at http://www.mayoclinicproceedings.org). For the patient who underwent general anesthesia, 2 significant oxidation current peaks, similar to the peaks obtained in the conscious patients, appeared at +1.41 and +1.11 V (Supplemental Figure 6, B and D, available online at http://www.mayoclinicproceedings.org). Adenosine release induced by electrode implantation was verified using an in vitro pharmacological test in rat brain slices (Supplemental Figure 7, available online at http://www.mayoclinicproceedings.org).
FIGURE 2.
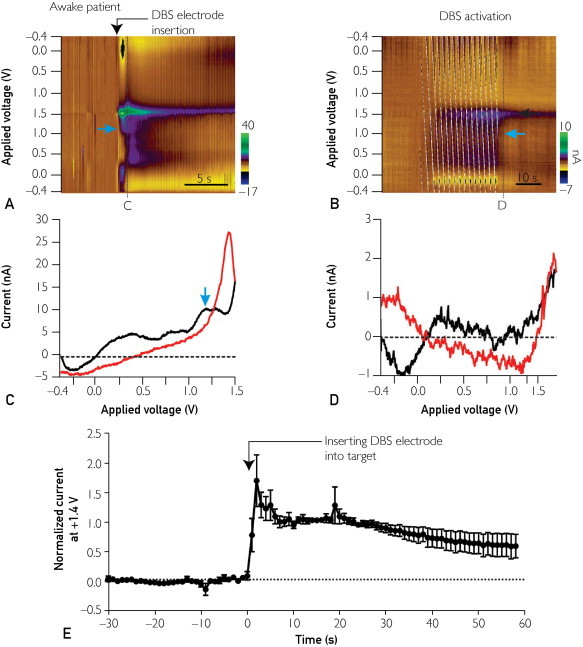
Electrochemical changes induced by DBS electrode implantation into the VIM of the thalamus. A, Pseudocolor plot obtained from an awake patient shows the appearance of oxidation currents immediately on DBS electrode implantation. Blue arrow highlights a second significantly smaller oxidation current peak. C = timing of cyclic voltammograms shown in C. B, Pseudocolor plot depicting the appearance of oxidation currents immediately on electrical stimulation through DBS electrode (130 Hz, 60-μsec pulse width, 2 V) (in 4 of 7 awake patients; in the other 3 patients, significant oxidation currents were not detected). Black arrow highlights significant oxidation current peak at +1.4 V. Blue arrow highlights a second significantly smaller oxidation current peak. D = timing of cyclic voltammograms shown in D. C, Representative cyclic voltammogram for the pseudocolor plots shown in A, highlighting 2 significant oxidation peak currents at +1.4 and +1.1 V. The solid black tracing indicates the current detected by forward-going voltage protocol and the red tracing by reverse-going voltage protocol. D, Representative cyclic voltammogram obtained during DBS application at black arrow in B, showing a significant oxidation peak current at +1.4 V but not at +1.1 V. The solid black tracing indicates the current detected by forward-going voltage protocol and the red tracing by reverse-going voltage protocol. E, Averaged oxidation current at +1.4 V induced by DBS electrode implantation in which each current point was normalized to the amplitude of oxidation current detected at 10 seconds after DBS electrode implantation in 7 awake patients.
Figure 2, E shows the time course of change in the oxidation current recorded at +1.4 V following DBS electrode implantation in the VIM in the 7 awake patients. Postcalibration was performed on 3 of the 7 electrodes (one of the other 4 electrodes was broken after the surgery, and the background currents of the remaining 3 became unstable). In accordance with the postcalibration of the CFMs, the maximal increase in adenosine at this oxidation peak potential corresponded to an increase of 1.76±0.99 μM (in 3 of the 7 awake patients). The basal level of extracellular adenosine has been estimated to be in the range of 20 to 200 nM.15 In various pathologic conditions, however, extracellular adenosine concentrations can increase as much as 31-fold.16
Following implantation of the DBS electrode, the increase in the oxidation peak current at +1.4 V persisted much longer than did the peak at +1.1 V (Figure 2, A). In addition, during DBS application, the oxidation peak current was detected only at +1.4 V (Figure 2, B and D). The apparent lack of a second peak at +1.1 V across our study patients could be due to the insensitivity of the CFM or to the release of other moieties (eg, hydrogen peroxide, potassium) that oxidize at +1.4 V (Supplemental Figure 8, available online at http://www.mayoclinicproceedings.org).17 DBS electrode implantation also induced several other oxidation peaks at +0.8 V and at approximately +0.5 V (Figure 2, A and B). Further studies are warranted to identify the molecules that may be contributing to these oxidation peaks. Together, these findings suggest that DBS electrode implantation in the VIM evokes local release of adenosine and possibly other biologically active molecules and ions.
Discussion
In this study, real-time in vivo FSCV neurochemical recordings in human subjects demonstrated that implanting a DBS electrode induces adenosine release concurrent with tremor arrest. It has generally been accepted that the microthalamotomy effect is caused by a microscopic version of thalamotomy. However, our findings suggest that changes in neurochemical concentration may be a contributing factor and that the transient nature of the microthalamotomy effect could be explained in part by the limited temporal duration of neurochemical release and diffusion into surrounding tissue.
In some cases, the microthalamotomy effect has been sustained for as long as a year.3,4 This sustained effect may be mediated by different mechanisms, such as a wider area of cell death than is normally encountered in observations of tremor arrest and reversal before DBS. Of note, adenosine can also mediate long-term effects via G protein–coupled receptors through which adenosine can modulate cellular activities in the central nervous system.18,19
All 7 of the awake patients in this study had the microthalamotomy effect. It is not possible to predict the effect before insertion of the DBS electrode. Reports of it occurring in 50% of patients are based on observations made over several days between DBS electrode insertion and stimulation onset.3 Because the microthalamotomy effect is known to weaken over time, it may be that the percentage of patients with an immediate effect, as found in our study, is much greater than 50%. In the future, a comparison of adenosine release in subjects with and without the microthalamotomy effect would be of value.
In 3 of our patients, a second oxidation peak was not detected (Supplemental Figure 4, C-E, available online at http://www.mayoclinicproceedings.org). In the other 5 patients, both the first and second oxidation peaks of adenosine were significant (Figure 2, A). The in-house columnar CFM was designed to be a more sensitive and reliable probe, which it has proved to be in this study and others in our laboratory. In vivo adenosine has been detected by the first oxidation peak,20 but given the nature of this experiment, we felt it important that both oxidation peaks reach significance.
It is not possible from this study to infer a causal relationship between adenosine release and tremor reduction. As noted earlier, intrathalamic infusions of adenosine A1 receptor agonists directly ameliorated harmaline-induced tremor in a mouse model.6 However, Pape7 showed that exogenic adenosine promotes a burst firing mode rather than a tonic firing mode in thalamic neurons. Thalamic neurons set to the burst firing mode will readily oscillate together and thus increase tremor behavior. These contradictory findings suggest that any conclusions about a causal relationship require further investigation.
Because this was an attempt to record neurochemical changes that occur with behavioral effects in patients undergoing DBS, patient safety was our first priority. Introducing the experimental procedure into the human operating theater imposed certain limitations, one of which was time. Having conducted the experiment without adverse effects, it may be possible in future studies to investigate the relationship between magnitude and stability of tremor reduction and adenosine release over time.
In addition to the implications for the microthalamotomy effect, the results of our study suggest that the therapeutic effect of DBS itself may be mediated in part by neurochemical changes. Given our FSCV results, we propose 3 possible candidate molecules: adenosine, potassium, and hydrogen peroxide. The voltammetric signatures of these 3 molecules are similar to those we obtained during our experiment. There is evidence in the literature that these 3 molecules directly modulate neuronal activity. The influence of potassium on neural networks has been extensively studied in epilepsy. For example, Durand et al21,22 found that electrical stimulation induced an increase in extracellular potassium concentrations, which decreased epileptic activity. It is thus possible that DBS also increases potassium efflux. Hydrogen peroxide, a well-known signal molecule in the nervous system, has also been found to modify neuronal activity.23 These findings, together with our results, suggest that neurochemical factors influencing the clinical effects of DBS warrant further investigation.
Conclusion
This study represents the successful application of an in vivo real-time wireless electrochemical sensing system to record changes in extracellular concentrations of biologically important chemicals in the brains of patients undergoing DBS. Neurochemicals released on insertion of the DBS electrode included adenosine and other chemicals yet to be identified. Future applications of this in vivo chemical recording technique might provide important insights into the neurobiological mechanisms of ET and help elucidate the therapeutic effects of neuromodulation.24, 25
Acknowledgments
We thank Penelope S. Duffy, PhD, for her substantive contributions to the writing of this paper.
Footnotes
For editorial comment, seepage 705
Grant Support: This work was supported in part by National Institutes of Health grants K08 NS 52232, R01 NS 70872, and R01 NS 75013 (K.H.L.) and The Grainger Foundation.
Supplemental Online Material
Author Interview Video
References
- 1.Louis E.D., Ottman R., Hauser W.A. How common is the most common adult movement disorder?: Estimates of the prevalence of essential tremor throughout the world. Mov Disord. 1998;13(1):5–10. doi: 10.1002/mds.870130105. [DOI] [PubMed] [Google Scholar]
- 2.Zesiewicz T.A., Elble R., Louis E.D., Quality Standards Subcommittee of the American Academy of Neurology Practice parameter: therapies for essential tremor; report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2005;64(12):2008–2020. doi: 10.1212/01.WNL.0000163769.28552.CD. [DOI] [PubMed] [Google Scholar]
- 3.Tasker R.R. Deep brain stimulation is preferable to thalamotomy for tremor suppression. Surg Neurol. 1998;49(2):145–153. doi: 10.1016/s0090-3019(97)00459-x. discussion 153-154. [DOI] [PubMed] [Google Scholar]
- 4.Kondziolka D., Lee J.Y. Long-lasting microthalamotomy effect after temporary placement of a thalamic stimulating electrode. Stereotact Funct Neurosurg. 2004;82(2-3):127–130. doi: 10.1159/000079844. [DOI] [PubMed] [Google Scholar]
- 5.Benabid A.L., Pollak P., Gao D. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84(2):203–214. doi: 10.3171/jns.1996.84.2.0203. [DOI] [PubMed] [Google Scholar]
- 6.Bekar L., Libionka W., Tian G.F. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med. 2008;14(1):75–80. doi: 10.1038/nm1693. [DOI] [PubMed] [Google Scholar]
- 7.Pape H.C. Adenosine promotes burst activity in guinea-pig geniculocortical neurones through two different ionic mechanisms. J Physiol. 1992;447:729–753. doi: 10.1113/jphysiol.1992.sp019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swamy B.E., Venton B.J. Subsecond detection of physiological adenosine concentrations using fast-scan cyclic voltammetry. Anal Chem. 2007;79(2):744–750. doi: 10.1021/ac061820i. [DOI] [PubMed] [Google Scholar]
- 9.Robinson D.L., Hermans A., Seipel A.T., Wightman R.M. Monitoring rapid chemical communication in the brain. Chem Rev. 2008;108(7):2554–2584. doi: 10.1021/cr068081q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimble C.J., Johnson D.M., Winter B.A. Wireless Instantaneous Neurotransmitter Concentration Sensing System (WINCS) for intraoperative neurochemical monitoring. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4856–4859. doi: 10.1109/IEMBS.2009.5332773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shon Y.M., Chang S.Y., Tye S.J. Comonitoring of adenosine and dopamine using the Wireless Instantaneous Neurotransmitter Concentration System: proof of principle. J Neurosurg. 2010;112(3):539–548. doi: 10.3171/2009.7.JNS09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elble R.J. Central mechanisms of tremor. J Clin Neurophysiol. 1996;13(2):133–144. doi: 10.1097/00004691-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Deuschl G., Raethjen J., Lindemann M., Krack P. The pathophysiology of tremor. Muscle Nerve. 2001;24(6):716–735. doi: 10.1002/mus.1063. [DOI] [PubMed] [Google Scholar]
- 14.Lakie M., Arblaster L.A., Roberts R.C., Varma T.R. Effect of stereotactic thalamic lesion on essential tremor. Lancet. 1992;340(8813):206–207. doi: 10.1016/0140-6736(92)90470-n. [DOI] [PubMed] [Google Scholar]
- 15.Latini S., Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79(3):463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 16.During M.J., Spencer D.D. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32(5):618–624. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- 17.Sanford A.L., Morton S.W., Whitehouse K.L. Voltammetric detection of hydrogen peroxide at carbon fiber microelectrodes. Anal Chem. 2010;82(12):5205–5210. doi: 10.1021/ac100536s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basheer R., Strecker R.E., Thakkar M.M., McCarley R.W. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73(6):379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Fredholm B.B., Chen J.F., Masino S.A., Vaugeois J.M. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- 20.Cechova S., Venton B.J. Transient adenosine efflux in the rat caudate-putamen. J Neurochem. 2008;105(4):1253–1263. doi: 10.1111/j.1471-4159.2008.05223.x. [DOI] [PubMed] [Google Scholar]
- 21.Bikson M., Lian J., Hahn P.J., Stacey W.C., Sciortino C., Durand D.M. Suppression of epileptiform activity by high frequency sinusoidal fields in rat hippocampal slices. J Physiol. 2001;531(pt 1):181–191. doi: 10.1111/j.1469-7793.2001.0181j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durand D.M., Park E.H., Jensen A.L. Potassium diffusive coupling in neural networks. Philos Trans R Soc Lond B Biol Sci. 2010;365(1551):2347–2362. doi: 10.1098/rstb.2010.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamsler A., Segal M. Hydrogen peroxide as a diffusible signal molecule in synaptic plasticity. Mol Neurobiol. 2004;29(2):167–178. doi: 10.1385/MN:29:2:167. [DOI] [PubMed] [Google Scholar]
- 24.Clark J.J., Sandberg S.G., Wanat M.J. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat Methods. 2010;7(2):126–129. doi: 10.1038/nmeth.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griessenauer C.J., Chang S.Y., Tye S.J. Wireless Instantaneous Neurotransmitter Concentration System: electrochemical monitoring of serotonin using fast-scan cyclic voltammetry—a proof-of-principle study. J Neurosurg. 2010;113(3):656–665. doi: 10.3171/2010.3.JNS091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview Video


