Abstract
Objective
To describe the clinical characteristics, histopathologic features, and outcomes of patients in whom vasculitis developed in association with use of tumor necrosis factor-α (TNF-α) inhibitors.
Patients and Methods
This is a retrospective review of patients evaluated at Mayo Clinic, Rochester, Minnesota, from January 1, 1998, through March 31, 2011, with a diagnosis of vasculitis induced by anti–TNF-α therapy.
Results
Of 8 patients with vasculitis associated with anti–TNF-α therapy (mean age, 48.5 years), 6 (75%) were female. Four (50%) had rheumatoid arthritis, 1 (13%) had Crohn disease, and 3 (38%) had ulcerative colitis. Five (63%) were treated with infliximab, 2 (25%) with etanercept, and 1 (13%) with adalimumab. The mean duration of treatment before development of vasculitis was 34.5 months. The skin was the predominant organ affected (5 patients [63%]), with the most common cutaneous lesion being palpable purpura (4 of 5 [80%]). Two organs involved in systemic vasculitis were the peripheral nervous system (4 patients [50%]) and kidney (1 patient [13%]). All cases of vasculitis were histopathologically confirmed. Seven of 8 patients improved with discontinuation of therapy (mean time to resolution, 6.9 months) and adjuvant treatment (all 8 received prednisone; another agent was also used in 7); rechallenge with anti–TNF-α therapy was not attempted in any patient. At last follow-up, no patients had experienced a recurrence of vasculitis after therapy discontinuation.
Conclusion
Cutaneous small-vessel vasculitis was the most common finding, but systemic vasculitis, including peripheral nerve and renal vasculitis, was also frequently observed.
Abbreviations and Acronyms: IBD, inflammatory bowel disease; RA, rheumatoid arthritis; TNF-α, tumor necrosis factor-α
Inhibitors of tumor necrosis factor α (TNF-α) are effective for the management of rheumatic and systemic autoimmune diseases.1,2 However, along with increasing use of these agents in clinical practice have come secondary autoimmune conditions paradoxically induced by anti–TNF-α therapy.3-7 The induction of vasculitis after the use of TNF-α inhibitors has been reported in the French- and Spanish-language medical literature.8-11 To our knowledge, there is no single-center series in the English-language medical literature describing the development of vasculitis attributable to TNF-α antagonist therapy in patients in the United States. We describe the clinical characteristics, histopathologic features, and outcomes of patients in whom vasculitis developed in association with TNF-α inhibitor use.
Patients and Methods
After approval by the Mayo Clinic Institutional Review Board, we searched the institutional medical index and text retrieval system to retrospectively identify patients who were evaluated at Mayo Clinic, Rochester, Minnesota, from January 1, 1998, through March 31, 2011, and who had a diagnosis of any form of vasculitis induced by anti–TNF-α therapy. We searched coded medical index data to identify patients with a diagnosis containing the terms vasculitis, Wegener's granulomatosis, polyarteritis nodosa, palpable purpura, Henoch-Schönlein purpura, Churg-Strauss vasculitis, temporal arteritis, or urticaria who had also received anti–TNF-α therapy as documented in the medical record (as noted either in the medication data or in the diagnosis information). We then searched the text retrieval system to identify any patients who had both a clinical note containing any of the diagnostic terms listed and documentation of treatment with anti–TNF-α therapy (specific agents or generalized terms). All patients included in this study allowed review of their medical records for research purposes.
The following information was abstracted from the medical records of identified patients by one of the authors (O.S.): characteristics of patients, disease for which anti–TNF-α therapy was prescribed, type of anti–TNF-α agent, organ involvement, type of vasculitis, histopathologic findings, type of cutaneous lesions, treatment of vasculitis, and outcome after discontinuation of use of the medication that induced the vasculitis (with or without initiation of new anti–TNF-α therapy).
Inclusion Criteria of Vasculitis Attributable to Anti–TNF-α Therapy
A diagnosis of vasculitis associated with anti–TNF-α therapy was considered in patients who had the following: (1) 1 or more clinical manifestations of vasculitis (eg, peripheral nerve, skin, kidney, central nervous system, or lung involvement) that occurred while the patient was undergoing anti-TNF-α therapy, (2) histopathologic confirmation from at least 1 site of involvement, (3) quiescence of underlying disease being treated with anti–TNF-α therapy (eg, rheumatoid arthritis [RA] or inflammatory bowel disease [IBD]), and (4) absence of other more likely causes of vasculitis, such as infection, malignant tumor, or a more likely medication.
Results
The combined searches of the institutional medical index and text retrieval system yielded a preliminary cohort of 345 patients. Patients who did not meet the predefined inclusion criteria for this study were excluded (ie, evaluation was not compatible with vasculitis, patient was not receiving anti–TNF-α agent at the time vasculitis developed, underlying disease [eg, RA or IBD] was active at the time of vasculitis, or another more likely cause of vasculitis was found [eg, infection]), for a final study cohort of 8 patients with a confirmed diagnosis after detailed medical record review of vasculitis induced by anti–TNF-α therapy. The clinical characteristics, treatment, and outcomes of these 8 patients are summarized in Table 1.
TABLE 1.
Clinical Characteristics of 8 Patients With Vasculitis Associated With TNF-α Inhibitorsa
| Patient No. | Age (y)/sex | Conditionb | Anti–TNF-α agent | Duration of treatment (mo)c | Korean algorithm scored | Organ involvement | Cutaneous lesions | Type of vasculitis | Use of anti–TNF-α agent discontinued | Time to resolution (mo)e | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54/F | Rheumatoid arthritis | Etanercept | 12 | 9 | Cutaneous | Ulcerated lesions, erythematous macules, blisters | CSVV | Yes | 1 | Prednisone | Complete response |
| 2 | 70/M | Rheumatoid arthritis | Infliximab | 72 | 9 | Cutaneous | Palpable purpura | CSVV | Yes | 2 | Prednisone, rituximab | Complete response |
| 3 | 43/F | Rheumatoid arthritis | Etanercept | 36 | 9 | Peripheral nerve | None | Neuropathy | Yes | 24 | Prednisone, cyclophosphamide | Partial response |
| 4 | 18/F | Crohn disease | Adalimumab | 60 | 9 | Cutaneous, peripheral nerve | Palpable purpura | CSVV, neuropathy | Yes | 2 | Prednisone, methotrexate | Complete response |
| 5 | 64/F | Rheumatoid arthritis | Infliximab | 60 | 9 | Cutaneous | Palpable purpura, ulcerated lesions | CSVV | Yes | 1 | Prednisone, hydroxychloroquine | Partial response |
| 6 | 52/M | Ulcerative colitis | Infliximab | 24 | 9 | Peripheral nerve | None | Neuropathy | Yes | 6 | Prednisone, azathioprine | Complete response |
| 7 | 21/F | Ulcerative colitis | Infliximab | 6 | 6 | Cutaneous, kidney | Palpable purpura | HSP | Yes | NAf | Prednisone, mycophenolate mofetil | No response |
| 8 | 66/F | Ulcerative colitis | Infliximab | 2 | 9 | Peripheral nerve | None | Neuropathy | Yes | 12 | Prednisone, mycophenolate mofetil | Partial response |
CSVV = cutaneous small-vessel vasculitis; HSP = Henoch-Schönlein purpura; NA = not applicable; TNF-α, = tumor necrosis factor-α.
The underlying condition was quiescent at the time vasculitis developed.
Before development of vasculitis.
Adverse drug reaction scores are as follows: 9 or higher for “certain,” 6 to 8 for “probable/likely,” 3 to 5 for “possible,” 1 to 2 for “unlikely,” and less than 0 for “contradictory.”12
After discontinued use of the anti–TNF-α agent.
Skin lesions persisted.
The most common indication for anti–TNF-α therapy use was RA (4/8; 50%). Other indications included ulcerative colitis (3/8; 38%) and Crohn disease (1/8; 13%). The mean age at diagnosis of vasculitis was 48.5 years (range, 18-70 years), with a female predominance (6/8; 75%). The mean duration of anti–TNF-α therapy before development of vasculitis was 34.5 months (range, 2-72 months). None of the 8 patients had a history of vasculitis.
Clinical Features of Vasculitis Attributable to Anti–TNF-α Therapy
The clinical features of vasculitis attributed to anti–TNF-α therapy are delineated in Table 1 and Table 2. Vasculitis involved the skin in 5 patients (63%), the peripheral nerve in 4 patients (25%), and the kidney in 1 patient (13%). There was no involvement of the central nervous system or the lung in any patient.
TABLE 2.
Comparison of Clinical Characteristics of Patients With Vasculitis Associated With TNF-α–Targeted Therapya,b
| Characteristic | Current study | Study by Ramos-Casals et al9c |
|---|---|---|
| Demographic characteristics | ||
| No. of patients | 8 | 118 |
| Female-male ratio | 6:2 (75:25) | 82:21 (80:20)d |
| Age at diagnosis (y), mean (range) | 48.5 (18-70) | 51.7 (NA) |
| Duration of anti-TNF-α therapy (mo), mean (range) | 34.5 (2-72) | 9.5 (NA) |
| Previous vasculitis | 0 (0) | 10 (8) |
| Underlying diseasee | ||
| No. of patients | 8 | 118 |
| Rheumatoid arthritis | 4 (50) | 99 (84) |
| Crohn disease | 1 (13) | 8 (7) |
| Ulcerative colitis | 3 (38) | 0 (0) |
| Other/unknown | 0 (0) | 11 (9) |
| Anti–TNF-α agente | ||
| No. of patients | 8 | 118 |
| Etanercept | 2 (25) | 60 (51) |
| Infliximab | 5 (63) | 51 (43) |
| Adalimumab | 1 (13) | 5 (4) |
| Other/unknown | 0 (0) | 2 (2) |
| Cutaneous involvement | ||
| No. (%) of patients | 5 (63)f | 102 (86)e |
| Palpable purpura | 4 (80) | 24 (24) |
| Ulcerated lesions | 2 (40) | 9 (9) |
| Blisters | 1 (20) | 0 (0) |
| Erythematous macules | 1 (20) | 4 (4) |
| Other/unknown | 0 (0) | 65 (64) |
| Visceral involvement | ||
| No. (%) of patients | 8e | 118 |
| Peripheral nerve | 4 (50) | 18 (15) |
| Kidney | 1 (13) | 16 (14) |
| Central nervous system | 0 (0) | 5 (4) |
| Lung | 0 (0) | 3 (3) |
| Other/unknown | 3 (38) | 76 (64)g |
| Histologic biopsy | ||
| No. (%) of patients | 8 (100)h | 87 (74)i |
| Skin | 5 (63) | 64 (74) |
| Kidney | 1 (13) | 10 (11) |
| Neuromuscular | 3 (38) | 6 (7) |
| Other/unknown | 0 (0) | 7 (8) |
| Type of vasculitis | ||
| No. of patients | 8j | 118 |
| Cutaneous small-vessel vasculitis | 4 (50) | 44 (37) |
| Henoch-Schönlein purpura | 1 (13) | 2 (2) |
| Neuropathy | 4 (50) | 5 (4) |
| Other/unknown | 0 (0) | 67 (57) |
| (continued) | ||
| Rechallenge with an alternate anti–TNF-α agent | ||
| No. of patients | 0 | NA |
| Recurrence of vasculitis | 0 (0) | NA |
| No recurrence of vasculitis | 0 (0)i | NA |
| Outcome | ||
| No. of patients | 8e | 104k |
| Resolution (ratio of complete to partial) | 4:3 (50:38) | 71:25 (68:24) |
| Time to resolution, mean (range), mo | 6.9 (1-24) | NA |
| Death | 1 (13) | 2 (2) |
| Unknown | 0 (0) | 20 (18) |
NA = not available; TNF-α = tumor necrosis factor-α.
Data are presented as No. (percentage) unless indicated otherwise.
Inclusion criteria were not clearly defined.
Sex was not reported for 15 patients.
Percentages total more than 100 because of rounding.
Numbers total more than 5 and percentages total more than 100 because some patients had more than 1 type of cutaneous involvement.
Authors noted “infrequent vasculitic presentations involved the gallbladder, the temporal arteries, and the heart.”
Numbers total more than 8 and percentages total more than 100 because some patients had more than 1 type of biopsy.
Percentages total less than 100 because of rounding.
Numbers total more than 8 and percentages total more than 100 because some patients had more than 1 type of vasculitis.
Numbers total more than 104 and percentages total less than 100 because outcome was not reported in 14 patients.
Of the 5 patients with cutaneous involvement, 4 had palpable purpura (Figure), 2 had ulcerated lesions, and 1 had erythematous macules or blisters. Of the 4 patients with peripheral nerve involvement, 2 had mononeuritis multiplex, and 1 each had asymmetric polyneuropathy or dysesthesia. Only 1 patient had renal involvement, with microscopic hematuria and proteinuria.
FIGURE.
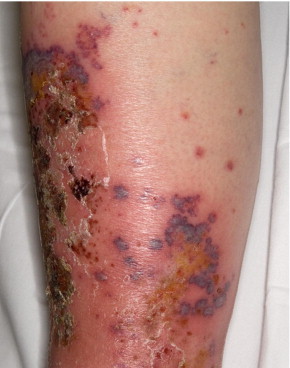
Cutaneous small-vessel vasculitis associated with tumor necrosis factor-α inhibitor. Necrotic ulcerated lesions and palpable purpura involving the left leg of a patient (patient 5 in Table 1). Histopathologic examination revealed leukocytoclastic vasculitis.
Histologic Features of Vasculitis Attributable to Anti–TNF-α Therapy
Biopsy specimens (5 skin, 3 nerve, and 1 kidney) were obtained in 8 patients (100%) for confirmation of diagnosis. All 5 skin biopsy specimens revealed leukocytoclastic vasculitis on histologic examination. The kidney biopsy was consistent with mild IgA nephropathy (Henoch-Schönlein purpura) on the basis of mesangial staining of IgA on immunofluorescent studies. Two of the 3 nerve biopsy specimens revealed perivascular epineural inflammation with evidence of multifocal neuropathy; the third nerve biopsy specimen revealed perivascular inflammation and an increased rate of axonal degeneration and perineural thickening.
Treatment of Vasculitis
Anti–TNF-α therapy was discontinued in all 8 patients. All 8 patients received adjuvant systemic treatment for vasculitis that consisted of prednisone; another agent was also used in 7 patients (88%): 2 received mycophenolate mofetil, and 1 each received hydroxychloroquine, methotrexate, azathioprine, cyclophosphamide, or rituximab. Four of the 8 patients (50%) experienced complete resolution of the vasculitis, whereas 3 patients (38%) had a partial response to treatment. The mean time to partial or complete resolution of vasculitis was 6.9 months.
Use of Additional Anti–TNF-α Therapy After Vasculitis
None of the 8 patients were rechallenged with anti–TNF-α therapy after its discontinuation.
Follow-up
The mean follow-up after diagnosis was 27.1 months (median, 12.5 months; range, 1-108 months). None of the 8 patients had recurrent vasculitis after discontinuation of anti–TNF-α therapy. One patient died of unknown causes 7 years after the diagnosis of vasculitis associated with anti–TNF-α therapy.
Discussion
Anti–TNF-α therapy has been increasingly associated with drug-induced autoimmune diseases, such as cutaneous vasculitis, lupus-like syndrome, systemic lupus erythematosus, and interstitial lung disease.10,13-16 Vasculitis is the most common autoimmune disease that results from anti–TNF-α therapy.8,9 To date, 213 cases of TNF-induced vasculitis have been reported in the medical literature: 39 in a nationwide series in France, 139 in Spain, and 35 in the United States that were derived from the Adverse Events Reporting System of the US Food and Drug Administration.8-11,14 To our knowledge, the current study is the first single-center series describing vasculitis associated with anti–TNF-α therapy in a US cohort. Table 2 presents a comparative analysis of 8 patients in the current study with 118 previously reported cases.
In our series, the patients had similar demographic characteristics (age and sex) compared with previously reported patient characteristics.9-11 In addition, most (4/8; 50%) of the patients had RA, which is also in keeping with previous reports.9 Although the spectrum of vasculitis was broad, there was a notable predominance of cutaneous involvement (5/8; 63%), with palpable purpura being the most common cutaneous lesion. Although less common, other cutaneous features included ulcerations, blisters, and erythematous macules. An important finding was the presence of systemic vasculitis in an equally large number (5/8; 63%) of patients. Peripheral neuropathy and renal vasculitis were the most frequent types of systemic vasculitis present in these patients. This clinically significant finding was consistent with those reported in both the Spanish- and French-language medical literature.9,10
Histologic examination was performed for all confirmed cases of vasculitis in our study. The histologic confirmation of diagnosis differs from findings reported for the Spanish and French studies, neither of which required confirmatory histologic evidence for all reported cases of vasculitis.9,10 The most common biopsy performed in our study was a skin biopsy (5/8; 63%). Other biopsies were nerve (3/8; 38%) and kidney (1/8; 13%). In our study, patients with cutaneous small-vessel vasculitis had histologic features of leukocytoclastic vasculitis (5/5; 100%). The predominance of this histologic feature on cutaneous biopsy specimens is similar to that in findings reported in other series.9,10,14 However, in addition to reporting cases of leukocytoclastic vasculitis, Ramos-Casals et al9 and Saint Marcoux and De Bandt10 reported cases of necrotizing vasculitis on histologic examination of cutaneous lesions (although the term necrotizing vasculitis was not defined in these reports). Our histologic finding of mononeuritis multiplex and IgA nephropathy on nerve and kidney biopsy specimens, respectively, was consistent with similar findings in the French study.10
An important clinical question is whether patients can subsequently be treated with alternative anti–TNF-α agents after development of vasculitis induced by anti–TNF-α therapy. In our group of 8 patients, none was rechallenged with another anti–TNF-α agent. However, in another study that analyzed data from the US Food and Drug Administration database, relapses occurred in 6 of 9 patients (67%) who were rechallenged with the same anti–TNF-α agent.14 This rate of recurrence differs substantially from the 33% recurrence rate found in the French study.10 The lower relapse rate reported in the French study may be attributable to the fact that patients were rechallenged with an alternative anti–TNF-α agent. In our study, we were unable to elicit why none of the 8 patients were rechallenged with an alternative biologic agent.
It can be challenging to determine causality in cases of vasculitis associated with anti–TNF-α therapy. However, the resolution of vasculitis after drug therapy discontinuation and adjuvant treatment is often helpful in supporting the etiologic role of anti–TNF-α therapy in the development of vasculitis. In our study, 7 of 8 patients (88%) had at least partial resolution of their vasculitis after drug therapy discontinuation. The mean time to resolution was 6.9 months. Unfortunately, we found no comparative data in the medical literature. To provide an objective assessment of causality, we used the Korean algorithm12 to further support our data (Table 1). The Korean algorithm consists of 8 questions on adverse drug reactions, with scores of 9 or higher being “certain” for a reaction, 6 to 8 “probable/likely,” and 3 to 5 “possible.”12 Seven of 8 patients in our study had “certain” reactions (all 7 had scores of 9), and 1 patient had a “probable/likely” reaction (score of 6).
The confounding bias of vasculitis occurring in patients with severe RA and the treatment of severe RA with anti–TNF-α therapy make it challenging to decipher the phenomenon responsible for vasculitis in these cases.17 Both RA and IBD can be associated with vasculitis, and therefore we cannot exclude the possibility that vasculitis was associated with these underlying conditions. However, in our study, the absence of clinical features such as articular flare, which one would expect in active RA,17 led to a favored diagnosis of anti–TNF-α therapy–induced vasculitis. In addition, the underlying disease (ie, RA) was medically managed and under control as determined by the rheumatologist. Similarly, the absence of diarrhea or abdominal pain in patients treated for IBD, as well as evaluation by a gastroenterologist who deemed the IBD to be quiescent at the time of vasculitis, supported our diagnosis in those cases.
The development of autoantibodies, such as antinuclear antibodies and anti–double-stranded DNA antibodies, in patients treated with anti–TNF-α agents is well recognized in the medical literature.2,18 Both antinuclear antibodies and anti–double-stranded DNA antibodies can be induced in patients with RA and Crohn disease treated with infliximab. With the anti–TNF-α agents, induction of antinuclear antibodies occurs in 23% to 57% of patients, whereas induction of anti–double-stranded DNA antibodies occurs in 9% to 33% of patients.4 Although the occurrence of these autoantibodies is not infrequent, a well-defined association has not been characterized between the induction of autoantibodies and the subsequent development of vasculitis associated with anti–TNF-α therapy. Our study did not address the development of autoantibodies in patients in whom vasculitis developed after anti–TNF-α therapy.
It is unclear why benign limited cutaneous vasculitis developed in some patients, whereas systemic involvement developed in others. However, this discrepant course of illness suggests a potential role for individual genetic susceptibility, which would explain why some biologic agents are successful in treating systemic vasculitis, yet these same agents paradoxically trigger vasculitis in other patients being treated for an underlying rheumatic or systemic autoimmune disease. Although the pathogenesis of vasculitis associated with anti–TNF-α therapy remains unknown, the development of antibodies against anti–TNF-α agents could lead to an immune complex–mediated hypersensitivity vasculitis.5 Another hypothesis suggests that inhibition of TNF-α promotes the expression of type 1 interferon by altering the balance between TH1 and TH2 cytokine production, leading to the induction of autoimmune diseases such as lupus erythematosus, dermatomyositis, and vasculitis.19,20 Although injection site reactions after anti-TNF therapy have been implicated as playing a role in direct antigen-mediated hypersensitivity vasculitis,9 none of the 8 patients in our cohort had a documented injection site reaction before the development of vasculitis.
Our study has several novel features that augment previous reports describing vasculitis associated with anti–TNF-α therapy. As mentioned, to our knowledge, the current study is the first single-center series describing vasculitis associated with anti–TNF-α therapy in a US cohort. We required strict inclusion criteria that consisted of confirmed histologic evidence of vasculitis (in contrast, only 87 of the 118 patients [74%] described by Ramos-Casals et al9 had histologic confirmation of vasculitis). We also used an objective assessment algorithm (Korean algorithm12) in our patient cohort to corroborate a diagnosis of vasculitis associated with anti–TNF-α therapy. Whereas most of the patients described by Ramos-Casals et al9 had RA (99 of 118 patients [84%]), 4 of our 8 patients (50%) had IBD; this discrepancy may have been due to the large IBD clinic at our institution. Moreover, we found a higher rate of peripheral nerve involvement by vasculitis (4 of 8 patients [50%]) compared with that reported by Ramos-Casals et al9 (18 of 118 patients [15%]) and by Saint Marcoux and De Bandt10 (10 of 39 patients [26%]). Finally, although the report of Ramos-Casals et al9 does not provide information on the exact time to resolution of vasculitis in their 118 cases, we report a mean time of 6.9 months to resolution of vasculitis after cessation of anti–TNF-α therapy; this information can help health care professionals counsel their patients about the prognosis of vasculitis associated with anti–TNF-α agents.
Our study is limited by its retrospective nature. In addition, confounding factors make it challenging to correctly diagnose vasculitis attributable to anti–TNF-α use (eg, underlying disease that can cause vasculitis, other causes of vasculitis, and clinical mimickers of vasculitis). To limit the effect of such confounders, we excluded patients who lacked histologic confirmation of vasculitis and whose diagnosis of vasculitis was questionable. Similarly, causality was difficult to assess because rechallenge with the same or alternative anti-TNF agents was not performed in our patients. Finally, there is a referral bias inherent in our institution's status as a tertiary care academic medical center because of the complexity of cases referred there. As a result, our cohort is not representative of a general US-based medical practice.
Conclusion
Anti–TNF-α therapy remains a valuable treatment for rheumatic and systemic autoimmune diseases. Although the risk of systemic vasculitis may be minimal, health care professionals should nonetheless be aware of, and should monitor for, this potential complication. If vasculitis associated with anti–TNF-α is suspected, histologic evaluation and extensive investigations for organ involvement must be pursued. The resolution of symptoms may be achieved by discontinuing use of the culprit anti–TNF-α agent and initiating adjuvant treatment. Although the subsequent use of alternative anti–TNF-α agents may be possible, this decision should be approached with caution.
Supplemental Online Material
Author Interview Video
References
- 1.Atzeni F., Turiel M., Capsoni F., Doria A., Meroni P., Sarzi-Puttini P. Autoimmunity and anti-TNF-α agents. Ann N Y Acad Sci. 2005;1051:559–569. doi: 10.1196/annals.1361.100. [DOI] [PubMed] [Google Scholar]
- 2.Keystone E.C., Ware C.F. Tumor necrosis factor and anti-tumor necrosis factor therapies. J Rheumatol Suppl. 2010;85:27–39. doi: 10.3899/jrheum.091463. [DOI] [PubMed] [Google Scholar]
- 3.Callen J.P. Complications and adverse reactions in the use of newer biologic agents. Semin Cutan Med Surg. 2007;26(1):6–14. doi: 10.1016/j.sder.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Mongey A.B., Hess E.V. Drug insight: autoimmune effects of medications: what's new? Nat Clin Pract Rheumatol. 2008;4(3):136–144. doi: 10.1038/ncprheum0708. [DOI] [PubMed] [Google Scholar]
- 5.Moustou A.E., Matekovits A., Dessinioti C., Antoniou C., Sfikakis P.P., Stratigos A.J. Cutaneous side effects of anti-tumor necrosis factor biologic therapy: a clinical review. J Am Acad Dermatol. 2009;61(3):486–504. doi: 10.1016/j.jaad.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 6.Scheinfeld N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. J Dermatolog Treat. 2004;15(5):280–294. doi: 10.1080/09546630410017275. [DOI] [PubMed] [Google Scholar]
- 7.Viguier M., Richette P., Bachelez H., Wendling D., Aubin F. Paradoxical cutaneous manifestations during anti-TNF-alpha therapy [in French] Ann Dermatol Venereol. 2010;137(1):64–71. doi: 10.1016/j.annder.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Casals M., Brito-Zeron P., Munoz S. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007;86(4):242–251. doi: 10.1097/MD.0b013e3181441a68. [DOI] [PubMed] [Google Scholar]
- 9.Ramos-Casals M., Brito-Zeron P., Soto M.J., Cuadrado M.J., Khamashta M.A. Autoimmune diseases induced by TNF-targeted therapies. Best Pract Res Clin Rheumatol. 2008;22(5):847–861. doi: 10.1016/j.berh.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Saint Marcoux B., De Bandt M., CRI (Club Rhumatismes et Inflammation) Vasculitides induced by TNFα antagonists: a study in 39 patients in France. Joint Bone Spine. 2006;73(6):710–713. doi: 10.1016/j.jbspin.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Ramos-Casals M., Perez-Alvarez R., Diaz-Lagares C., Cuadrado M.J., Khamashta M.A., BIOGEAS Study Group Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010;9(3):188–193. doi: 10.1016/j.autrev.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Son Y.M., Lee J.R., Roh J.Y. Causality assessment of cutaneous adverse drug reactions. Ann Dermatol. 2011;23(4):432–438. doi: 10.5021/ad.2011.23.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Bandt M., Sibilia J., Le Loet X., Club Rhumatismes et Inflammation Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7(3):R545–R551. doi: 10.1186/ar1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohan N., Edwards E.T., Cupps T.R. Leukocytoclastic vasculitis associated with tumor necrosis factor-alpha blocking agents. J Rheumatol. 2004;31(10):1955–1958. [PubMed] [Google Scholar]
- 15.Perez-Alvarez R., Perez-de-Lis M., Diaz-Lagares C. Interstitial lung disease induced or exacerbated by TNF-targeted therapies: analysis of 122 cases. Semin Arthritis Rheum. 2011;41(2) doi: 10.1016/j.semarthrit.2010.11.002. 256–w64. [DOI] [PubMed] [Google Scholar]
- 16.Wetter D.A., Davis M.D. Lupus-like syndrome attributable to anti-tumor necrosis factor alpha therapy in 14 patients during an 8-year period at Mayo Clinic. Mayo Clin Proc. 2009;84(11):979–984. doi: 10.4065/84.11.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guignard S., Gossec L., Bandinelli F., Dougados M. Comparison of the clinical characteristics of vasculitis occurring during anti-tumor necrosis factor treatment or not in rheumatoid arthritis patients: a systematic review of 2707 patients, 18 vasculitis. Clin Exp Rheumatol. 2008;26(3, suppl 49):S23–S29. [PubMed] [Google Scholar]
- 18.Aringer M., Smolen J.S. The role of tumor necrosis factor-alpha in systemic lupus erythematosus. Arthritis Res Ther. 2008;10(1):202. doi: 10.1186/ar2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein R., Rosenbach M., Kim E.J., Kim B., Werth V.P., Dunham J. Tumor necrosis factor inhibitor-associated dermatomyositis. Arch Dermatol. 2010;146(7):780–784. doi: 10.1001/archdermatol.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorentino D.F. The yin and yang of TNF-α inhibition. Arch Dermatol. 2007;143(2):233–236. doi: 10.1001/archderm.143.2.233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview Video


