Abstract
Thirty years into the human immunodeficiency virus (HIV) epidemic in the United States, an estimated 50,000 persons become infected each year: highest rates are in black and Hispanic populations and in men who have sex with men. Testing for HIV has become more widespread over time, with the highest rates of HIV testing in populations most affected by HIV. However, approximately 55% of adults in the United States have never received an HIV test. Because of the individual and community benefits of treatment for HIV, in 2006 the Centers for Disease Control and Prevention recommended routine screening for HIV infection in clinical settings. The adoption of this recommendation has been gradual owing to a variety of issues: lack of awareness and misconceptions related to HIV screening by physicians and patients, barriers at the facility and legislative levels, costs associated with testing, and conflicting recommendations concerning the value of routine screening. Reducing or eliminating these barriers is needed to increase the implementation of routine screening in clinical settings so that more people with unrecognized infection can be identified, linked to care, and provided treatment to improve their health and prevent new cases of HIV infection in the United States.
Abbreviations and Acronyms: CDC, Centers for Disease Control and Prevention; HIV, human immunodeficiency virus; HPTN 052, HIV Prevention Trials Network 052; IDU, injection drug use; MSM, men who have sex with men; NHIS, National Health Interview Survey; QALY, quality-adjusted life-year; USPSTF, US Preventive Services Task Force
CME Activity.
Target Audience: The target audience for Mayo Clinic Proceedings is primarily internal medicine physicians and other clinicians who wish to advance their current knowledge of clinical medicine and who wish to stay abreast of advances in medical research.
Statement of Need: General internists and primary care providers must maintain an extensive knowledge base on a wide variety of topics covering all body systems as well as common and uncommon disorders. Mayo Clinic Proceedings aims to leverage the expertise of its authors to help physicians understand best practices in diagnosis and management of conditions encountered in the clinical setting.
Accreditation: College of Medicine, Mayo Clinic is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Statement: College of Medicine, Mayo Clinic designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s).™ Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Learning Objectives: Educational objectives. On completion of this article, you should be able to (1) describe briefly the current epidemiology of human immunodeficiency virus (HIV) in the United States, (2) summarize current status of testing for HIV in the United States, (3) explain the Centers for Disease Control and Prevention's revised recommendations for HIV testing in health care settings, (4) discuss potential barriers and solutions to the implementation of the Centers for Disease Control and Prevention's revised recommendations for HIV testing in health care settings, and (5) articulate the potential benefits of antiretroviral therapy.
Disclosures: As a provider accredited by ACCME, College of Medicine, Mayo Clinic (Mayo School of Continuous Professional Development) must ensure balance, independence, objectivity, and scientific rigor in its educational activities. Course Director(s), Planning Committee members, Faculty, and all others who are in a position to control the content of this educational activity are required to disclose all relevant financial relationships with any commercial interest related to the subject matter of the educational activity. Safeguards against commercial bias have been put in place. Faculty also will disclose any off-label and/or investigational use of pharmaceuticals or instruments discussed in their presentation. Disclosure of this information will be published in course materials so that those participants in the activity may formulate their own judgments regarding the presentation.
In their editorial and administrative roles, William L. Lanier, MD, Scott C. Litin, MD, Terry L. Jopke, Kimberly D. Sankey, and Nicki M. Smith, MPA, have control of the content of this program but have no relevant financial relationship(s) with industry.
Drs Rizza, MacGowan, Purcell, and Branson have no potential competing interests to declare. Dr. Temesgen has educational grants from Merck, Gilead Sciences, Inc, and Janssen Pharmaceuticals, Inc, and a research grant from Pfizer.
Method of Participation: In order to claim credit, participants must complete the following:
-
1
Read the activity.
-
2
Complete the online CME Test and Evaluation. Participants must achieve a score of 80% on the CME Test. One retake is allowed.
Participants should locate the link to the activity desired at http://bit.ly/MiTZ8G. Upon successful completion of the online test and evaluation, you can instantly download and print your certificate of credit.
Estimated Time: The estimated time to complete each article is approximately 1 hour.
Hardware/Software: PC or MAC with Internet access.
Date of Release: 9/1/2012
Expiration date: 8/31/2014 (Credit can no longer be offered after it has passed the expiration date.)
Privacy Policy: http://www.mayoclinic.org/global/privacy.html
Questions? contact dletcsupport@mayo.edu.
The Centers for Disease Control and Prevention (CDC) recently estimated that each year approximately 50,000 Americans are infected with human immunodeficiency virus (HIV) and that 18,000 people with AIDS die.1 The number of people living with HIV in the United States is estimated to be almost 1.2 million, and it continues to grow annually, thereby providing more opportunities for transmission.2 From 2007 through 2010, the number of diagnoses of HIV infection in adults and adolescents remained stable in the 46 states and 5 US-dependent areas with long-term confidential name-based HIV infection reporting.3 In 2010 specifically, an estimated 48,079 adults and adolescents were diagnosed as having HIV infection; of these, 79% of diagnoses were in males and 21% were in females, a ratio that was stable from 2007 through 2010.3
Some populations are particularly burdened by HIV and account for a disproportionate number of cases owing to social, economic, and demographic factors, such as stigma, discrimination, income, education, and geographic region.4 The percentage of HIV infection diagnoses in adults and adolescents exposed through male-to-male sexual contact increased from 55% in 2007 to 61% in 2010, and this was the only group to experience an increase during those years. The percentages of diagnosed HIV infections attributed to injection drug use (IDU) (8%), male-to-male sexual contact and IDU (3%), and heterosexual contact (28%) remained relatively stable from 2007 through 2010. Gay and bisexual men and other men who have sex with men (MSM) are the most severely and disproportionately affected by HIV. Although composing only an estimated 4% of men,5 MSM accounted for 77% of new HIV diagnoses in men in 2010.3
Black individuals are the most affected racial/ethnic group, comprising 14% of the population and 44% of estimated reported cases in 2010 in the 46 states and 5 US-dependent areas with long-term confidential name-based HIV infection reporting.3 Hispanic adults and adolescents are also disproportionately affected by HIV. Hispanics/Latinos represent approximately 16% of the population but accounted for 22% of new HIV diagnoses in 2010.3 In 2010, the estimated rate (per 100,000 population) of HIV infection diagnosis for black males (116.0) was more than 7.5 times higher than the rate for white males (15.3) and more than 2.5 times higher than the rate for Hispanic/Latino males (44.7). For female adults and adolescents, the estimated rate of HIV infection diagnosis for blacks (41.7) was approximately 20 times higher than the rate for white females (2.1) and approximately 4.5 times higher than the rate for Hispanic/Latino females (9.2).3
HIV Testing in the United States
In 1985, when the US Food and Drug Administration approved the first tests for the detection of antibodies to HIV, the primary purpose was to screen blood donations to prevent HIV transmission from blood transfusion.6 To dissuade persons from using blood donation centers to obtain an HIV test, HIV counseling and testing programs based at “alternative testing sites” were established to provide these services. During the past 25 years, HIV testing has become more widely available and acceptable.
Since 1987, numerous national surveys have been conducted to estimate the proportion of US adults who have ever received an HIV test. Although the sample populations and methods have varied, the results for various periods have been consistent across surveys. By the late 1980s, 1 in 6 adults in the United States had been tested for HIV.7,8 As access to HIV testing services increased, by the mid-1990s estimates of the percentage of adults in the United States who had been tested for HIV, excluding via blood donations, ranged from 31% to 40%.9 Between 2000 and 2006, the percentage of adults who had been tested for HIV remained at approximately 40%, and since then, there has been a gradual increase to approximately 45%, leaving 55% of adults in the United States who have never been tested for HIV (with considerable variation by demographic groups).10
Although most HIV/AIDS cases in the United States continue to be in males, a higher percentage of women report HIV testing. In 1985, 92% of AIDS cases were in men,11 and HIV/AIDS was viewed as a disease affecting primarily MSM and IDUs; by 1988, HIV testing was higher in men than in women.8 As HIV infection became more prevalent in the heterosexual population, HIV testing significantly increased in women. A variety of factors may have contributed to this increase, including women accessing medical services more frequently than men, clinicians being more comfortable offering an HIV test to women, and the CDC recommending HIV screening for all pregnant women in 1995.12,13 In the 2002 National Survey of Family Growth, a higher percentage of females (57%) reported HIV testing than males (47%),7 and in the 2008 National Health Interview Survey (NHIS), 48% of women and 41% of men reported ever being tested for HIV.10
Testing for HIV also has differed significantly by race and ethnicity during the epidemic. In 1988, a greater proportion of white adults (17%) reported having been tested for HIV compared with black (14%) and Hispanic (14%) adults.8 By 1999, percentages of HIV testing were higher in black (46%) and Hispanic (33%) individuals than in white individuals (29%), and these patterns have persisted.14 In 2008, 62% of black individuals, 48% of Hispanic individuals, and 41% of white individuals reported ever being tested for HIV,10 with comparable percentages reported in other surveys.15,16
A key driver of testing during the past 25 years has been recommendation by the CDC regarding who should be tested for HIV. Early in the epidemic, HIV testing in the United States was predominantly recommended for persons considered at risk for HIV infection, and HIV testing programs primarily targeted persons who regarded themselves to be at risk. In 1988, 1 in 3 adults who acknowledged at least one risk behavior (from a list of behaviors) for HIV infection had received an HIV test,8 a percentage twice that in adults overall. In the 1995 NHIS and the 1996 National Household Survey on Drug Abuse, 70% of adults at increased risk (eg, those who have sex with an at-risk partner, IDUs, and MSM) reported that they had ever been tested for HIV.9 In 1995, 48% of females aged 15 to 44 years at increased risk for infection reported being tested for HIV, and by 2002, this percentage had increased to 68%.7 The NHISs conducted in 1999 and 2008 showed little change in the percentage of persons aged 18 to 64 years who reported an HIV risk factor and had been tested for HIV, approximately 72% in both surveys.10,14 A high percentage of the populations at greatest risk for HIV infection, MSM and IDUs, report that they have been tested for HIV. Recent data from the CDC's National HIV Behavioral Surveillance surveys indicate that 90% of each of these populations report ever being tested for HIV.17,18 Given the continued transmission of HIV in these populations, particularly MSM, testing more frequently than once a year may be needed to identify undiagnosed cases earlier in the course of infection and to link infected persons to treatment services.19 Rates of HIV testing are higher in regions where disproportionately affected populations are greatest. Data from the Behavioral Risk Factor Surveillance System in 2001 and 2008 have documented that the percentages ever tested and recently tested (within the past 12 months) were typically higher in states with high AIDS case rates.10,20
The Critical Role of HIV Testing in Curbing the HIV Epidemic
Testing for HIV plays a prominent role in the National HIV/AIDS Strategy released by the White House in July 2010.21 As shown in the Figure, of the estimated 1.2 million persons living with HIV in the United States, 80% are aware of their infection, 62% have been linked to HIV care, 41% stay in HIV care, 36% are receiving antiretroviral therapy, and only 28% have a suppressed viral load.19 Transmission rate modeling estimates that the 20% of persons living with HIV who are unaware of their infection account for 49% of HIV transmissions.22
FIGURE.
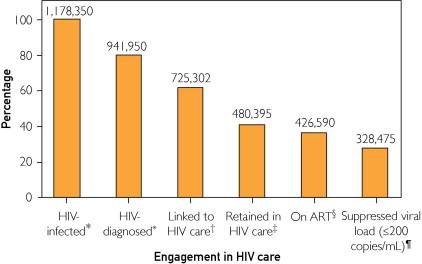
The figure shows the number and percentage of HIV-infected persons engaged in selected stages of the continuum of HIV care in the United States. CDC synthesized these findings to determine the number of persons in selected categories of the continuum of HIV care, and estimated that 328,475 (35%) of 941,950 persons diagnosed with HIV (or 28% of all 1,178,350 persons with HIV) in the United States are virally suppressed.19 *Source: Centers for Disease Control and Prevention.2 †Calculated as estimated number diagnosed times estimated percentage linked to care (77%). ‡Calculated as estimated number diagnosed times estimated percentage retained in care (51%). §Calculated as estimated number retained in HIV care times percentage prescribed antiretroviral therapy (ART) in the Medical Monitoring Project (88.8%). ¶Calculated as estimated number receiving ART times percentage with suppressed viral load in the Medical Monitoring Project (77.0%). This value is 28% of the estimated 1,178,350 persons in the United States who are infected with HIV.
To prevent further HIV transmission, improve the quality of life of persons with HIV, and reduce disparities associated with HIV infection, intensified effort is needed to increase the percentage of persons at each stage of this continuum of care. Increasing HIV diagnoses is the first step in this critical process.
After testing, it is important to immediately link newly identified HIV-positive persons to care so that their disease status and need for treatment can be evaluated. Between 2005 and 2007, 41.4% of persons with a new HIV diagnosis from the 37 states with name-based reporting systems had no CD4 cell count reported within 12 months, indicating that they were likely not receiving care for their HIV infection.23 In addition, 33.8% of those with a new diagnosis had a CD4 cell count of less than 200/μL, which since 1993 has indicated an immunologic diagnosis of AIDS.24 Such a low CD4 cell count at the time of HIV diagnosis indicates that HIV was first identified late in the course of infection.23
Despite the increase in HIV testing during the first 30 years of the epidemic in the United States, an estimated 236,000 persons with HIV are unaware of their infection.2,25 The CDC has continued to promote HIV testing in the US populations and specifically in persons disproportionately affected by HIV. In 2003, the CDC launched an initiative to increase access to early diagnosis and to services for persons with HIV.26 In 2006, the CDC revised the recommendations for HIV testing for adults, adolescents, and pregnant women in health care settings to reduce barriers to providing HIV testing. The major modifications included the following recommendations: (1) opt-out testing should be provided unless the patient declined, (2) persons at high risk for HIV infection should be screened at least annually, (3) informed consent for medical care should cover HIV testing on the same basis as other diagnostic or screening tests, (4) prevention counseling should not be required in conjunction with HIV testing for diagnostic or screening purposes, and (5) HIV screening should be a routine component of prenatal screening.27 These guidelines encourage physicians to incorporate HIV screening into clinical practice for all adults, regardless of risk, when the prevalence of undiagnosed HIV infection is 0.1% or greater and to offer HIV screening to high-risk persons more frequently. The recommendations also seek to reduce barriers associated with determining risk status and the requirement for prevention counseling to the extent that this was a barrier to testing. In 2007, the CDC funded the Expanded HIV Testing Initiative, under which 25 health departments were funded to facilitate HIV testing and increase diagnosis of infection in disproportionately affected populations, especially non-Hispanic black individuals.28
The increase since 2006 in the percentage of adults tested has coincided with CDC efforts to promote HIV screening and earlier diagnosis of infection. The use of point-of-care rapid HIV tests and reduced barriers to HIV testing have enhanced the ability of providers to conduct testing in settings in which time can be a limiting factor, such as jails29 and clinical and acute care settings.30-32 Through the Expanded HIV Testing Initiative, nearly 2.8 million tests were performed, many of them in clinical settings, and more than 18,000 people were newly diagnosed as having HIV infection.28
Barriers to Routine HIV Testing in the Health Care Setting and Potential Solutions
Health care professionals in the United States have been slow to implement the 2006 CDC recommendations for HIV screening of individuals aged 13 to 64 years. For example, only 33% of community health care personnel from Massachusetts incorporated HIV screening into their practices.33 In another study, only one-quarter of eligible patients in an emergency department were offered HIV screening,34 and less than 5% of adults seen in an emergency or urgent care setting were tested for HIV.35 These studies demonstrate that significant barriers to implementation of universal HIV testing in health care settings in the United States still exist. These barriers are multifactorial and complex and require a multipronged approach and strategy to overcome.
Physician Awareness and Perception
Inconsistent levels of awareness of the 2006 CDC recommendations for universal HIV screening among health care professionals in the United States have created perceived barriers to implementing the recommendations. Among medical directors and administrators from non–Ryan White–supported community health centers in Massachusetts, only 27% were aware of the CDC recommendations compared with 60% in Ryan White–supported centers.36 Most health care professionals surveyed also believed that they needed to obtain written consent and provide counseling before obtaining an HIV test. Physicians also expressed concerns that patients would not have time to reflect on the significance of an HIV test and make an informed decision about whether to accept the testing.37
Health care professionals also report feeling insecure about broaching the topic of HIV testing with their patients, particularly those from low-risk backgrounds, citing that discussing HIV testing would be uncomfortable for the patient and might damage the patient-physician bond.36 Physician were also concerned that they would not receive support from the administration at their health care facility to initiate HIV screening, believing that it would be regarded as more of a burden than a help. Moreover, many physicians did not feel equipped to answer all the patient's questions regarding HIV testing and did not feel that they were capable of convincing the patient that the test should be performed.38
The primary solution to overcome this particular barrier to HIV testing is education. Physicians should be familiar with the CDC's revised recommendations for HIV Testing of Adults, Adolescents, and Pregnant Women in Health-Care Settings and the rationale behind them. Providers should understand that despite the availability of effective treatment, HIV infection remains a leading cause of death and illness in the United States and that substantial numbers of previously unrecognized infections are diagnosed each year. Also, people who are aware of their infection can not only receive treatment that is effective for improving their health and reducing transmission but also adopt behaviors to avoid transmitting their infection to others. Physicians should be informed that the processes and procedures that have previously impeded HIV testing, such as pretest and posttest counseling and separate written consent, are no longer required and that the currently recommended screening for HIV uses an opt-out strategy. This information should be accompanied by an explanation of what an opt-out strategy is together with simulation of how to use it for HIV screening in various clinical settings. The CDC has developed free materials for physicians on these topics.39
An important component of physician education is correcting misperceptions regarding HIV screening in the clinical setting. Physicians may believe that their patients are at low risk for HIV based on patients' own lack of reported risky behavior but not uncommonly also because of inherent biases related to race, ethnicity, age, sex, and socioeconomic status and their associations with risk of HIV. Physicians may not realize that some patients may simply choose not to disclose high-risk behaviors and that as many as 10% to 25% of people testing positive for HIV had reported no high-risk behaviors before their diagnosis.40
Public Awareness and Perception
The HIV epidemic is now more than 30 years old. Although perceptions regarding HIV have improved during the past 3 decades, HIV still carries a stigma that concerns many patients. In a poll conducted by the Kaiser Family Foundation in 2003, more than one-third of people stated that they feared people would think less of them if they were infected with HIV. They expressed additional worry that they would be discriminated against or be seen as morally inferior if others knew they were HIV positive.41 In a more recent study, patients presenting to an emergency department without a life-threatening illness or psychiatric diagnosis cited fear and denial as the most significant barrier to HIV screening. However, this was reported in less than 5% of those surveyed, with participants expressing overwhelming (>85%) support for the CDC recommendations to perform HIV screening.42
Another contributing factor for suboptimal HIV testing in the United States is that many individuals feel that they do not require HIV testing because they have no HIV risks. In fact, when HIV-infected patients were asked why they had not been tested earlier for HIV, the most common answer (69%) was that they did not feel that they were at risk for HIV infection.43 Patients also expressed concerns about the confidentiality, access, and anonymity of HIV testing in a health care setting. Furthermore, a recent survey of patients in a health care setting demonstrated that most were unaware that 20% of people infected with HIV in the United States are unaware of their diagnosis and that the CDC recommends HIV screening without regard to risk, and they assumed that consent for HIV testing would be overwhelming or intimidating.44
Education plays a primary role in raising awareness and removing negative perceptions regarding HIV testing among the public. This education can be provided in various forms and at various levels of society. The CDC leads the national effort to promote HIV awareness and prevention in collaboration with other public health organizations and organizations that represent the populations hardest hit by the HIV epidemic. On a regional and local level, community-based agencies, hospitals, clinics, physician groups, and managed care organizations should participate in this national effort and educate their patients and clients about HIV. Schools also have the responsibility of teaching their students about HIV and sexually transmitted infections. Finally, physicians should have the appropriate knowledge and education to be able to discuss behavioral risk factors for HIV and motivate their patients to be tested for HIV.
The public should be made aware of the magnitude of the HIV epidemic in the United States, the persistent substantial numbers of new infections, and the contribution of those who are not aware of their infection to new transmissions. In addition, the public should be made to realize that effective treatment, although not curative, exists for HIV infection and that this treatment is associated with substantial improvement in survival and quality of life. It is now clear that treatment has an additional public health role as it provides protection for sex partners of HIV-infected persons who themselves are not HIV infected.45 Education should also involve making the public aware of how HIV is diagnosed and reported to state health departments, the confidentiality of testing, and the implications of a positive result on health insurance coverage and employment.
Systemic Barriers
A variety of systemic issues at the facility, state, and national levels have been identified as barriers to universal HIV screening in health care settings.
Systemic Barriers at the Facility Level
The CDC recommendations to perform HIV screening asks most health care providers to change their traditional way of thinking. Conventionally, a patient's presenting illness has always been the focus of the limited time and resources available. Asking the physician to consider, offer, and order what may be a completely unrelated laboratory test can be seen as intrusive and unwelcome. In fact, physicians cite that lack of time is the leading reason they have been reticent to implement HIV screening in their practices.33,36 Health care professionals' concerns about adequate time relates to obtaining written consent before testing each patient for HIV and time to counsel and discuss the implications of a positive or equivocal HIV test result with their patients. This was a particularly important issue to physicians in emergency departments and urgent care settings, where many physicians averaged 10 minutes per patient visit.46 Further concerns arose that time and resources would be needed to train staff and develop protocols in each health care setting to initiate HIV testing.47
Conventional HIV testing requires a blood test that may take 24 to 48 hours for results to return in clinical settings. Therefore, after an HIV screening test is performed in a health care setting, a patient must return or be called for results of the test. A rapid test might alleviate this barrier; however, especially in high-volume settings, rapid tests can be labor intensive and disrupt patient flow. Even if a rapid HIV test is used, a preliminary positive result requires a confirmatory test, typically Western blot analysis, which takes several days for results to be returned. To overcome these barriers, some high-volume emergency departments have used opt-out screening and conventional (nonrapid) testing technology to screen all patients who have blood collected by batching the blood hourly.48
Linkage-to-care concerns are enhanced in the scenario in which a patient is diagnosed as having HIV after being screened in a health care setting but does not seek HIV care.49 In one study, only 48% of patients sought HIV care within 3 months of their HIV diagnosis, with 22% not seeking HIV care at 12 months.50 Possible reasons an HIV-infected individual would not seek follow-up HIV care are complex and may include lack of health care insurance, mental illness, substance abuse, lack of sophistication maneuvering through the health care system, and denial of HIV status.51 This dichotomy between prevention and care remains a significant hurdle for health care workers who do not have the infrastructure needed to follow up on HIV test results and ensure that all HIV-positive patients are referred for HIV care.47,52
The current CDC recommendation for HIV screening in the health care setting does away with many of the facility- and physician-level systemic barriers, such as the need for counseling and separate written informed consent. However, it does not remove all the barriers, perceived or otherwise, associated with the mechanics of ordering tests, as well as interpreting and reporting test results, or informing patients of their test results. Solutions to overcome these barriers include full integration and incorporation of HIV testing into standards of care and standard clinic operating procedures. All relevant staff in the facility must be engaged and participate in this practice. Clinical informatics solutions have been found to be useful in enabling and accelerating integration of HIV testing into the clinic work flow by identifying eligible patients and prompting clinicians to order the test. These informatics solutions can also be used to facilitate linkage for those found to be HIV infected and increase overall program efficiency.34
Legislative and Legal Barriers
The legal implications of HIV screening continue to make health care providers anxious.33,36,52 Although national recommendations influence state law, HIV testing laws remain under state jurisdiction. Given that most health care professionals are not directly involved in providing HIV care, many would not be familiar with state laws pertaining to HIV testing. Physicians have expressed concerns about their legal obligation to document consent for HIV testing, to adequately counsel patients before testing, and to ensure that patients receive the test result and, if positive, are linked to HIV care. There is also a general sense of insecurity regarding partner notification and the physician's legal roles and obligations.52
Because HIV screening programs must comply with state laws about HIV testing, physicians should have a clear and easily accessible source of information about state laws relevant to their practice. The Compendium of State HIV Testing Laws from the National HIV/AIDS Clinicians' Consultation Center provides state profiles of key HIV testing laws and policies and is updated periodically.53 However, revisions depend on input from individuals or knowledge about newly passed legislation, and, as a result, the information may not always be up-to-date. It would facilitate testing substantially if such information were provided by each individual state department of health with clear and unambiguous language and were posted prominently in all relevant clinical facilities. Note that many states have made changes in their laws to make them compatible with the CDC recommendations, and, at present, HIV testing laws no longer present a barrier to routine screening for HIV in the clinical setting in nearly all states.
Cost and Cost-effectiveness of HIV Testing
The cost of HIV testing is another perceived barrier to HIV screening. There is a considerable amount of uncertainty about insurance coverage for HIV screening. Many health care professionals are concerned that HIV testing will not be reimbursed by insurance or that funding is not available to support each patient's test.54 In fact, lack of funding or reimbursement for HIV testing was listed as a major reason by community health care professionals for not performing HIV screening on their patients.33,36,52 There was also a worry that the health care system in the United States would not be able to bear the burden and cost of caring for increased numbers of people being diagnosed as having HIV infection.55 What these physicians may not fully appreciate is the substantially increased cost of care for patients diagnosed late in the course of their HIV disease and the increased societal costs of additional cases of HIV when people with undiagnosed infection spread HIV to their partners.
Medicaid currently allows coverage for routine HIV screening in the clinical setting as recommended by the CDC. However, it is considered an optional service, and each state chooses whether to cover it in their Medicaid program. The US Department of Health and Human Services accepted the recommendation of a recent Institute of Medicine report that private insurers be required to cover annual HIV counseling and screening for sexually active women at no cost.
Some health insurance plans do not cover routine HIV screening. Instead, these plans cover HIV tests for patients with known or perceived risk factors (eg, MSM and IDUs) and for patients who show symptoms of AIDS. In 2008, California became the first state to mandate that private insurers pay for HIV testing even when it's not related to a patient's primary diagnosis during a medical visit. Cost-effective is not the same as inexpensive or cost saving. Cost-effectiveness analysis attempts to provide information on the relationship between resources expended on 2 or more alternative health interventions and health outcomes resulting from these interventions. This relationship or ratio (the difference in costs over the difference in effectiveness) is commonly expressed as cost per quality-adjusted life-year (QALY) saved. Several studies on the cost-effectiveness of HIV screening have been published. These studies have found that the cost-effectiveness of HIV screening compares favorably with that of other health interventions that are accepted as good uses of resources.56 Annual mammography screening of all women in the United States aged 40 to 80 years was associated with a cost of $40,000 per additional QALY,57 whereas the cost-effectiveness of HIV screening was estimated to be $41,736 per QALY.58
Furthermore, HIV screening has been demonstrated to be cost-effective across a variety of risk groups and age ranges, whether conventional or rapid testing was used, and in populations with low HIV prevalence.58-64
Part of the solution to overcoming the perception of cost or cost-effectiveness as a barrier to HIV screening is education. In addition to emphasizing the benefit of testing to the individual and the public, physicians, policy makers, and the public should be made aware that screening for HIV has been shown to be cost-effective, even with a prevalence as low as 0.05% to 0.1%.47,54,63 The issue of insurance coverage for HIV testing needs to be addressed at a national level legislatively, through revision of the US Preventive Services Task Force (USPSTF) recommendations, or both.
Conflicting Recommendation From the USPSTF
Although the revised CDC recommendations were endorsed by many professional societies and organizations, such as the American College of Physicians, the American Academy of HIV Medicine, and the HIV Medicine Association, endorsement has not been universal. Critically, the USPSTF reiterated in 2007 its 2005 recommendation on HIV testing declaring that the USPSTF did not find enough evidence to recommend for or against routine HIV screening in the general population (a “C” recommendation).65 This USPSTF recommendation is important because coverage and reimbursement for preventive services under Medicare, and most private insurance depends on the level of USPSTF endorsement. Currently, an “A-” or “B-” level USPSTF recommendation is required for coverage and reimbursement for preventive services under Medicare. The discrepancy between the CDC and USPSTF recommendations has also confused physicians and the general public. What many do not realize is that both agencies, despite the differing recommendations, actually agree on most issues that pertain to HIV testing; both acknowledge that targeted screening would miss many infected persons and that identification and treatment of asymptomatic HIV infection can result in marked reduction in clinical progression and mortality. The primary difference seems to be differing conclusions about the strength of the data regarding the effect of routine testing on HIV transmission. In recent years, evidence documenting the impact of antiretroviral therapy on reducing HIV transmission, so-called treatment as prevention, has been accumulating. A landmark study, the HIV Prevention Trials Network 052 (HPTN 052) clinical trial, reported that antiretroviral therapy reduced the risk of heterosexual transmission by 96%.30 However, the results of the HPTN 052 cannot be applied unless HIV-infected individuals are identified and linked to care and treatment.66 Evidence supporting earlier initiation of antiretroviral therapy has also emerged, leading to updated treatment recommendations from the Department of Health and Human Services and others. This potentially invalidates one assumption in the evidence model used by the USPSTF in 2005 as a basis for their recommendation: antiretroviral treatment would be initiated only at CD4 T-cell counts of less than 200/μL. The USPSTF is currently reconsidering its recommendation on routine HIV screening.67
Conclusion
The benefits of antiretroviral therapy are undisputed; it substantially reduces illness and death attributed to HIV infection. In addition, the HPTN 052 clinical trial showed that antiretroviral therapy prevents the transmission of HIV to uninfected sexual partners from HIV-infected persons receiving treatment. There is also emerging data supporting that earlier initiation of antiretroviral therapy results in improved outcomes for the individual and communities. These benefits of antiretroviral therapy are not available for HIV-infected individuals who have not yet been diagnosed as having HIV infection. A fifth of the 1.2 million people estimated to be living with HIV in the United States remain unaware of their infection and contribute disproportionately to the overall transmission of HIV. The CDC's revised recommendations for HIV testing for adults, adolescents, and pregnant women in health care settings are intended to facilitate the reduction of HIV transmission in the United States by removing barriers to providing HIV testing. Although implementation of these revised recommendations has been limited by a variety of real and perceived barriers, these barriers are not insurmountable. With appropriate education of the public and physicians, and with the involvement of all stakeholders at national, regional, state, and community levels, it is hoped that one of the primary goals of the National HIV/AIDS Strategy, reducing the number of people who become infected with HIV, will soon be achieved.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Footnotes
Individual reprints of this article and a bound reprint of the entire Symposium on Antimicrobial Therapy will be available for purchase from our website www.mayoclinicproceedings.org.
The End of the Symposium on Antimicrobial Therapy.
Supplemental Online Material
Author Interview Video
References
- 1.Prejean J., Song R., Hernandez A. Estimated HIV incidence in the United States, 2006-2009. PLoS One. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) HIV surveillance—United States, 1981-2008. MMWR Morb Mortal Wkly Rep. 2011;60(21):689–693. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) HIV surveillance Report, 2010. March 2012. http://www.cdc.gov/hiv/topics/surveillance/resources/reports/ vol. 22. Accessed July 31, 2012.
- 4.Centers for Disease Control and Prevention (CDC) Highimpact HIV prevention: CDC's approach to reducing HIV infections in the United States. http://www.cdc.gov/hiv/strategy/dhap/pdf/nhas_booklet.pdf Accessed July 31, 2012.
- 5.Purcell DW, Johnson C, Lansky A, et al. Estimating the population size of men who have sex with men in the United States to obtain HIV and syphilis rates. Open AIDS. In press. [DOI] [PMC free article] [PubMed]
- 6.Rugg D.L., MacGowan R.J., Stark K.A., Swanson N.M. Evaluating the CDC program for HIV counseling and testing. Public Health Rep. 1991;106(6):708–713. [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson J.E., Chandra A., Mosher W.D. HIV testing in the United States, 2002. Adv Data. 2005;363:1–32. [PubMed] [Google Scholar]
- 8.Hardy A.M., Dawson D.A. HIV antibody testing among adults in the United States: data from 1988 NHIS. Am J Public Health. 1990;80(5):586–589. doi: 10.2105/ajph.80.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson J.E., Carey J.W., Taveras S. HIV testing among the general US population and persons at increased risk: information from national surveys, 1987-1996. Am J Public Health. 2000;90(7):1089–1095. doi: 10.2105/ajph.90.7.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Vital signs: HIV testing and diagnosis among adults—United States, 2001-2009. MMWR Morb Mortal Wkly Rep. 2010;59(47):1550–1555. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) HIV and AIDS—United States, 1981-2000. MMWR Morb Mortal Wkly Rep. 2001;50(21):430–434. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) Revised guidelines for HIV counseling, testing, and referral and revised recommendations for HIV screening of pregnant women. MMWR Recomm Rep. 2001;50(RR-19):1–81. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) U.S. Public Health Service recommendations for human immunodeficiency virus counseling and voluntary testing for pregnant women. MMWR Recomm Rep. 1995;44(RR-7):1–15. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) HIV testing among racial/ethnic minorities—United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50(47):1054–1058. [PubMed] [Google Scholar]
- 15.Henry J. Kaiser Family Foundation: HIV/AIDS policy: fact sheet: HIV testing in the United States. June 2011. http://www.kff.org/hivaids/upload/6094-11.pdf Accessed July 31, 2012.
- 16.Du P., Camacho F., Zurlo J., Lengerich E.J. Human immunodeficiency virus testing behaviors among US adults: the roles of individual factors, legislative status, and public health resources. Sex Transm Dis. 2011;38(9):858–864. doi: 10.1097/OLQ.0b013e31821a0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) HIV testing among men who have sex with men—21 cities, United States, 2008. MMWR Morb Mortal Wkly Rep. 2011;60(21):694–699. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC) HIV infection and HIV-associated behaviors among injection drug users—20 cities, United States, 2009. MMWR Morb Mortal Wkly Rep. 2012;61:133–138. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Vital signs: HIV prevention through care and treatment—United States. MMWR Morb Mortal Wkly Rep. 2011;60(47):1618–1623. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) HIV testing—United States, 2001. MMWR Morb Mortal Wkly Rep. 2003;52(23):540–545. [PubMed] [Google Scholar]
- 21.White House Office of National AIDS Policy . White House Office of National AIDS Policy; Washington, DC: 2010. National HIV/AIDS Strategy for the United States; pp. 1–60. [Google Scholar]
- 22.Hall H.I., Holtgrave D.R., Maulsby C. HIV transmission rates from persons living with HIV who are aware and unaware of their infection. AIDS. 2012;26(7):893–896. doi: 10.1097/QAD.0b013e328351f73f. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) Reported CD4+ T-lymphocyte and viral load results for adults and adolescents with HIV infection—37 states, 2005-2007. HIV Surveillance Supplemental Report. 2010;March 2011;16(1) http://ww.cdc.gov/hiv/topoics/surveillance/resources/reports/index.htm#supplemental Accessed July 31, 2012. [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 25.Campsmith M.L., Rhodes P.H., Hall H.I., Green T.A. Undiagnosed HIV prevalence among adults and adolescents in the United States at the end of 2006. J Acquir Immune Defic Syndr. 2010;53(5):619–624. doi: 10.1097/QAI.0b013e3181bf1c45. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC) Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(15):329–332. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC) Revised recommendations for HIV testing in adults, adolescents, and pregnant women in health-care settings. MMWR Morb Mortal Wkly Rep. 2006;55(RR-14):1–13. [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC) Results of the Expanded Testing Initiative—25 jurisdictions, United States, 2007-2010. MMWR Morb Mortal Wkly Rep. 2011;60(24):805–810. [PubMed] [Google Scholar]
- 29.MacGowan R., Margolis A., Richardson-Moore A. Voluntary rapid human immunodeficiency virus (HIV) testing in jails. Sex Transm Dis. 2009;36(2, suppl):S9–S13. doi: 10.1097/OLQ.0b013e318148b6b1. [DOI] [PubMed] [Google Scholar]
- 30.Zetola N.M., Grijalva C.G., Gertler S. Simplifying consent for HIV testing is associated with an increase in HIV testing and case detection in highest risk groups, San Francisco January 2003-June 2007. PLoS One. 2008;3(7):e2591. doi: 10.1371/journal.pone.0002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freeman A.E., Sattin R.W., Miller K.M., Dias J.K., Wilde J.A. Acceptance of rapid HIV screening in a southeastern emergency department. Acad Emerg Med. 2009;16(11):1156–1164. doi: 10.1111/j.1553-2712.2009.00508.x. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) HIV screening of male inmates during prison intake medical evaluation—Washington, 2006-2010. MMWR Morb Mortal Wkly Rep. 2011;60(24):811–813. [PubMed] [Google Scholar]
- 33.Mimiaga M.J., Johnson C.V., Reisner S.L., Vanderwarker R., Mayer K.H. Barriers to routine HIV testing among Massachusetts community health center personnel. Public Health Rep. 2011;126(5):643–652. doi: 10.1177/003335491112600506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilbur L., Huffman G., Lofton S., Finnell J.T. The use of a computer reminder system in an emergency department universal HIV screening program. Ann Emerg Med. 2011;58(1, suppl 1):S71–S73.e1. doi: 10.1016/j.annemergmed.2011.03.028. S71-S73.e1. [DOI] [PubMed] [Google Scholar]
- 35.Torres G.W., Heffelfinger J.D., Pollack H.A., Barrera S.G., Rothman R.E. HIV screening programs in US emergency departments: a cross-site comparison of structure, process, and outcomes. Ann Emerg Med. 2011;58(1, suppl 1):S104–S113. doi: 10.1016/j.annemergmed.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 36.Johnson C.V., Mimiaga M.J., Reisner S.L., VanDerwarker R., Mayer K.H. Barriers and facilitators to routine HIV testing: perceptions from Massachusetts Community Health Center personnel. AIDS Patient Care STDs. 2011;25(11):647–655. doi: 10.1089/apc.2011.0180. [DOI] [PubMed] [Google Scholar]
- 37.National Institutes of Health Reducing new infections: behavioral and social science trans-NIH plan for HIV-related research. http://www.oar.nih.gov/strategicplan/fy2010/pdf/ Accessed July 31, 2012.
- 38.Sullivan L.E., Fiellin D.A. The case for routine HIV screening and impact of managed care. Am J Manag Care. 2010;16(13(suppl)):S345–S351. [PubMed] [Google Scholar]
- 39.HIV screening: standard care Act Against AIDS Web site. http://www.actagainstaids.org/provider/hssc/index.html Accessed July 31, 2012.
- 40.Chou R., Huffman L.H., Fu R., Smits A.K., Korthuis P.T. US Preventive Services Task Force. Screening for HIV: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;143(1):55–73. doi: 10.7326/0003-4819-143-1-200507050-00010. [DOI] [PubMed] [Google Scholar]
- 41.Brimlow D.L., Cook J.S., Seaton R. US Department of Health and Human Services, Health Resources and Services Administration; Washington, DC: 2003. Stigma & HIV/AIDS: A Review of the Literature. [Google Scholar]
- 42.Batey D.S., Hogan V.L., Cantor R. Short communication routine HIV testing in the emergency department: assessment of patient perceptions. AIDS Res Hum Retroviruses. 2012;28(4):352–356. doi: 10.1089/aid.2011.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mills C.W., Sabharwal C.J., Udeagu C.C. Barriers to HIV testing among HIV/AIDS concurrently diagnosed persons in New York City. Sex Transm Dis. 2011;38(8):715–721. doi: 10.1097/OLQ.0b013e31820ead73. [DOI] [PubMed] [Google Scholar]
- 44.Society of General Internal Medicine AIDS Task Force . Society of General Internal Medicine; Washington, DC: 2006. HIV Prevention With National Organizations. [Google Scholar]
- 45.Cohen M.S., Chen Y.Q., McCauley M. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothman R.E. Current Centers for Disease Control and Prevention guidelines for HIV counseling, testing, and referral: critical role of and a call to action for emergency physicians. Ann Emerg Med. 2004;44(1):31–42. doi: 10.1016/j.annemergmed.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Bokhour B.G., Solomon J.L., Knapp H., Asch S.M., Gifford A.L. Barriers and facilitators to routine HIV testing in VA primary care. J Gen Intern Med. 2009;24(10):1109–1114. doi: 10.1007/s11606-009-1078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoxhaj S., Davila J.A., Modi P. Using nonrapid HIV technology for routine, opt-out HIV screening in a high-volume urban emergency department. Ann Emerg Med. 2011;58(1, suppl 1):S79–S84. doi: 10.1016/j.annemergmed.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 49.Anaya H.D., Bokhour B., Feld J., Golden J.F., Asch S.M., Knapp H. Implementation of routine rapid HIV testing within the U.S: Department of Veterans Affairs Healthcare System. J Healthc Quality. 2011 doi: 10.1111/j.1945-1474.2011.00151.x. [DOI] [PubMed] [Google Scholar]
- 50.Tripathi A., Gardner L.I., Ogbuanu I. Predictors of time to enter medical care after a new HIV diagnosis: a statewide population-based study. AIDS Care. 2011;23(11):1366–1373. doi: 10.1080/09540121.2011.565032. [DOI] [PubMed] [Google Scholar]
- 51.Jenness SM, Myers JE, Neaigus A, Lulek J, Navejas M, Raj-Singh S. Delayed entry into HIV medical care after HIV diagnosis: risk factors and research methods [published online ahead of print February 9, 2012]. AIDS Care. [DOI] [PubMed]
- 52.Waxman M.J., Popick R.S., Merchant R.C., Rothman R.E., Shahan J.B., Almond G. Ethical, financial, and legal considerations to implementing emergency department HIV screening: a report from the 2007 conference of the National Emergency Department HIV Testing Consortium. Ann Emerg Med. 2011;58(1, suppl 1):S33–S43. doi: 10.1016/j.annemergmed.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 53.2012 compendium of state HIV testing laws National HIV/ AIDS Clinicians' Consultation Center Web site. http://www.nccc.ucsf.edu/consultation_library/state_hiv_testing_laws Accessed July 31, 2012.
- 54.Bozzette S.A. Routine screening for HIV infection: timely and cost-effective. N Engl J Med. 2005;352(6):620–621. doi: 10.1056/NEJMe048347. [DOI] [PubMed] [Google Scholar]
- 55.Clark H.A., Bowles K.E., Song B., Heffelfinger J.D. Implementation of rapid HIV testing programs in community and outreach settings: perspectives from staff at eight community-based organizations in seven U.S. cities. Public Health Rep. 2008;123(suppl 3):86–93. doi: 10.1177/00333549081230S311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graham J.D., Corso P.S., Morris J.M., Segui-Gomez M., Weinstein M.C. Evaluating the cost-effectiveness of clinical and public health measures. Annu Rev Public Health. 1998;19:125–152. doi: 10.1146/annurev.publhealth.19.1.125. [DOI] [PubMed] [Google Scholar]
- 57.Stout N.K., Rosenberg M.A., Trentham-Dietz A., Smith M.A., Robinson S.M., Fryback D.G. Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst. 2006;98(11):774–782. doi: 10.1093/jnci/djj210. [DOI] [PubMed] [Google Scholar]
- 58.Sanders G.D., Bayoumi A.M., Sundaram V. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352(6):570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 59.Sanders G.D., Bayoumi A.M., Holodniy M., Owens D.K. Cost-effectiveness of HIV screening in patients older than 55 years of age. Ann Intern Med. 2008;148(12):889–903. doi: 10.7326/0003-4819-148-12-200806170-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Owens D.K., Nease R.F., Jr, Harris R.A. Cost-effectiveness of HIV screening in acute care settings. Arch Intern Med. 1996;156(4):394–404. [PubMed] [Google Scholar]
- 61.Paltiel A.D., Weinstein M.C., Kimmel A.D. Expanded screening for HIV in the United States: an analysis of cost-effectiveness. N Engl J Med. 2005;352(6):586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 62.Walensky R.P., Freedberg K.A., Weinstein M.C., Paltiel A.D. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis. 2007;45(suppl 4):S248–S254. doi: 10.1086/522546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paltiel A.D., Walensky R.P., Schackman B.R. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006;145(11):797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 64.Owens D.K., Sundaram V., Lazzeroni L.C. Prevalence of HIV infection among inpatients and outpatients in Department of Veterans Affairs health care systems: implications for screening programs for HIV. Am J Public Health. 2007;97(12):2173–2178. doi: 10.2105/AJPH.2007.110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chou R., Huffman L. Screening for Human Immunodeficiency Virus: Focused Update of a 2005 Systematic Evidence Review for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality; Rockville, MD: 2007. [PubMed] [Google Scholar]
- 66.Johnstone-Robertson S.P., Hargrove J., Williams B.G. Antiretroviral therapy initiated soon after HIV diagnosis as standard care: potential to save lives? HIV AIDS (Auckl) 2011;3:9–17. doi: 10.2147/HIV.S7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.U.S. Preventive Services Task Force Topics in Progress. http://www.uspreventiveservicestaskforce.org/uspstf/topicsprog.htm Accessed July 31, 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview Video


