Abstract
Background
Currently, 68Ga-labeled 1,4,7,10-tetraazacyclododecane-tetraacetic acid (DOTA)-peptides are the most widely used class of 68Ga radiotracers for PET, although DOTA is not optimal for 68Ga complexation. More recently, 1,4,7-triazacyclononane-triacetic acid (NOTA) and particularly triazacyclononane-phosphinate (TRAP) chelators have been shown to possess superior 68Ga binding ability. Here, we report on the efficiency, reproducibility, and achievable specific activity for fully automated 68Ga labeling of DOTA-, NOTA-, and TRAP-peptide conjugates.
Findings
Compared to NOTA- and DOTA-peptides, achievable specific activity (AS) for TRAP-peptide is approximately 10 and 20 times higher, respectively. AS values in the range of 5,000 GBq/μmol were routinely obtained using 1 GBq of 68Ga, equivalent to 0.11 μg of cold mass for a 185-MBq patient dose of a 3-kDa conjugate. The TRAP-peptide could be 68Ga-labeled with excellent reproducibility and > 95% radiochemical yield for precursor amounts as low as 1 nmol.
Conclusions
High 68Ga labeling efficiency of TRAP-peptides could facilitate realization of kit labeling procedures. The good reproducibility of the automated synthesis is of relevance for GMP production, and the possibility to provide very high specific activities offers a high degree of safety in first clinical trials, due to reduction of cold mass content in tracer formulations.
Keywords: macrocyclic ligands, gallium-68, positron-emission tomography, peptides, bioconjugates, radiolabeling
Findings
Background
With the commercial availability of 68Ge/68Ga generators, cyclotron-independent on-site production of tracers for positron-emission tomography (PET) has become widely feasible [1,2]. Thus, in the near future, a ubiquitous implementation of PET and PET/CT even in regions with less well-developed infrastructure can be expected, similar to the global story of success of 99mTc-based scintigraphy which started half a century ago with the introduction of 99Mo/99mTc generators [1,3]. In the long run, a partial substitution of single photon emission computed tomography (SPECT) by PET (and SPECT/CT by PET/CT, respectively) appears to be a realistic scenario in view of the advantages of PET, such as superior spatial resolution and sensitivity. Besides, in contrast to reactor-produced 99Mo, 68Ge is cyclotron-produced. This can be considered advantageous with regard to the recent insufficiency of global reactor capacity for reliable 99Mo supply [4], and independence of 68Ga-PET from nuclear reactors might positively influence the bias of its public perception.
Generally, 68Ga labeling is done by complexation of the 68Ga3+ ion. For this purpose, dedicated chelators usually have to be introduced into precursor molecules by bioconjugation, wherein they readily determine the labeling chemistry. To facilitate global implementation of 68Ga-PET, production of 68Ga radiopharmaceuticals must be simple, robust, and reliable; this demands highly efficient labeling chemistry and, therefore, highly efficient chelators. Recently, we have shown that the bifunctional triazacyclononane-phosphinate (TRAP) ligand [5-8] possesses markedly improved 68Ga labeling properties [6]. This applies also to TRAP-based peptide conjugates, the practical consequences of which we further elucidate in this study.
Methods
TRAP(RGD)3 was prepared as described before [6]. NODAGA-cyclo(RGDyK) (‘NODAGA-RGD’) was purchased from ABX GmbH (Radeberg, Germany). DOTATOC was obtained from Bachem (Bubendorf, Switzerland). Fully automated 68Ga labeling was performed using unpurified eluate fractions of a 68Ge/68Ga generator with SnO2 matrix (iThemba LABS, Somerset West, South Africa), as described previously [6,9] (5 min reaction at 95°C, pH adjusted with HEPES, pH 3.2 for DOTATOC and NODAGA-RGD, pH 2 for TRAP(RGD)3, purification using C8 SPE cartridge). Radiochemical yield was calculated from decay-corrected product activity in relation to the sum of significant decay-corrected residual activities contained elsewhere, that is, in reaction vial, SPE cartridge, and non-product cartridge purging liquids.
Calculation of specific activities
Product activities (AP) were measured after the end of preparation (approximately 15 min after the start of syntheses) and decay corrected to a typical injection time, 30 min after the start of synthesis (AP,30). In order to be able to calculate corresponding specific activity values that are representative for the respective precursor amounts and independent from small deviations in the starting activity A0 (in our experiments, ranging from 800 to 1,050 MBq, depending on the regeneration state of the 68Ga generator), product activities were normalized to a representative starting activity AN = 1 GBq, according to . Specific activity (AS) values were calculated by the division of AP,30,N by the precursor amount used. It is assumed that all precursor peptide is actually retained on and subsequently eluted from the cartridge, and thus transferred into the formulation. This means that both retention and elution efficiency are considered 100%, both of which can be somewhat lower in practice. As a result, all given AS represent the lower bounds and will never overestimate actual values.
Results and discussion
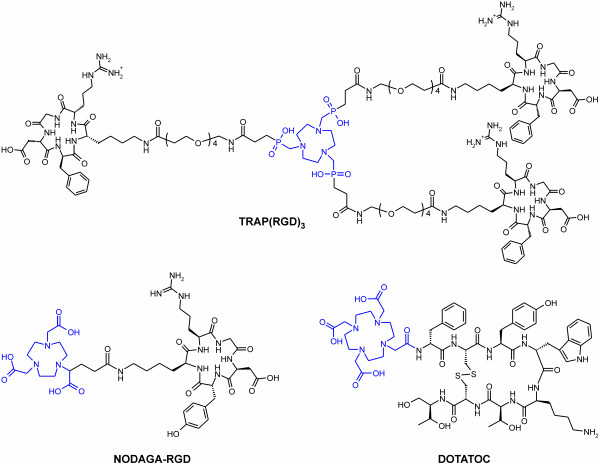
Although previous comparative studies focusing on the basic chelator structures TRAP, 1,4,7-triazacyclononane-triacetic acid (NOTA), and 1,4,7,10-tetraazacyclododecane-tetraacetic acid (DOTA) already proved superior Ga3+ complexation/68Ga labeling properties of TRAP [6,7], these data are not sufficient to quantify the behavior of respective peptide conjugates, for two reasons: Firstly, functionalization of chelators with peptides, resulting in conjugates with a multiple of the molecular weight of the neat chelators, is definitely prone to change overall complexation properties with potentially unpredictable outcome. Secondly, the chelating moiety in compounds commonly dubbed ‘DOTA-peptides’ is actually not DOTA, but DOTA-monoamide (see Figure 1), which exhibits different Ga3+ complexation behavior [10]. In contrast, for TRAP and the bifunctional NOTA-derivative NODAGA, the structure of the chelating site is not affected by conjugation. To assess the impact of these effects, representative peptide conjugates (TRAP(RGD)3[6] and the commercially available ‘NOTA’- and ‘DOTA’-peptides NODAGA-RGD and DOTATOC, respectively, see Figure 1) were labeled under similar conditions using our standard automated procedure [6,9].
Figure 1.
Structures of peptide conjugates used in this study. The complexation sites of TRAP, NOTA, and DOTA-monoamide, featured in TRAP(RGD)3, NODAGA-RGD, and DOTATOC, respectively, are highlighted in blue color.
Figure 2 shows that TRAP(RGD)3 allows to use much lower precursor concentrations for labeling than required for NODAGA-RGD and particularly DOTATOC, which is why 68Ga-TRAP(RGD)3 can be prepared with much higher AS (see also Table 1). Using 0.1 nmol of TRAP(RGD)3, almost 5,000 GBq/μmol was reached with a satisfying decay-corrected yield of 66 ± 6%. The use of even lower amounts of TRAP(RGD)3 (17 pmol) frequently resulted in preparations with extremely high AS of >10,000 GBq/μmol, although not reliably reproducible. The highest AS value observed during these experiments was 14,900 GBq/μmol (actual value, not normalized to starting activity), which is approximately 1/7 of the theoretically possible maximum value, that of carrier-free 68Ga. Although such high specific activities are not usually needed for clinical applications, we nevertheless, deem this feature of high practical value for the following reasons:
Figure 2.
Radiochemical yields and corresponding calculated minimal AS of radiopharmaceutical formulations. Radiochemical yields (solid lines, %, mean ± SD, n ≥ 4) and corresponding calculated minimal AS (dashed lines, GBq/μmol, mean ± SD) of the formulations at typical time of injection (30 min after the start of synthesis) as functions of precursor amount for automated 68Ga labeling of TRAP(RGD)3 (T), NODAGA-RGD (N), and DOTATOC (D). AS for TRAP(RGD)3 concentrations > 1 nmol are not shown for clarity of presentation.
Table 1.
Radiochemical yields of automated 68Ga labeling, and corresponding calculated minimal AS of radiopharmaceutical formulations
| Precursor amount (nmol) | Product yield (%) | Specific activity (GBq/μmol) |
|---|---|---|
|
68Ga-TRAP(RGD)3 | ||
| 0.017 |
27.9 ± 16.0 |
12059 ± 6922 |
| 0.04 |
42.9 ± 14.0 |
7889 ± 2567 |
| 0.1 |
65.8 ± 5.6 |
4848 ± 414 |
| 0.33 |
90.0 ± 2.7 |
2006 ± 61 |
| 1 |
95.2 ± 1.7 |
701 ± 12 |
| 10 |
97.8 ± 0.5 |
73 ± 0.4 |
|
68Ga-DOTATOC | ||
| 0.6 |
34.4 ± 21.3 |
422 ± 261 |
| 2.2 |
63.7 ± 13.5 |
213 ± 45 |
| 7 |
82.8 ± 8.7 |
87 ± 9 |
| 14 |
88.0 ± 2.1 |
46 ± 1.1 |
|
68Ga-NODAGA-RGD | ||
| 0.22 |
31.7 ± 28.4 |
1059 ± 951 |
| 0.4 |
51.9 ± 22.8 |
954 ± 419 |
| 1 |
74.1 ± 15.2 |
545 ± 112 |
| 2 |
85.0 ± 7.4 |
313 ± 28 |
| 6 |
94.7 ± 1.1 |
116 ± 1.3 |
| 20.5 | 93.7 ± 1.9 | 34 ± 0.7 |
At typical time of injection (30 min after start of synthesis), values given as mean ± SD.
1. A hypothetical patient dose of 185 MBq (5 mCi) of a 5,000-GBq/μmol preparation contains only 37 pmol of peptide; for a compound like TRAP(RGD)3 with a molecular weight of ≈ 3 kDa, this calculates to a total of 0.11 μg of cold mass, or less than 2 ng/kg body weight for an average patient. Such tiny amounts are extremely unlikely to cause any pharmacological effects. Therefore, TRAP could facilitate the use of such biomolecules for imaging that possess very high pharmacological potential, and 68Ga-labeled TRAP conjugates could generally offer high safety when tested in first clinical trials.
2. As a 15-MBq dose of said preparation is equivalent to 3 pmol or 9 ng of our exemplary 3-kDa peptide, it can always directly be used for evaluation studies in rodents without having to separate off unlabeled precursor or, unfavorably, reduce the administered dose. High receptor occupancy or even saturation effects, which otherwise are frequently encountered in small animal imaging due to the necessity of applying much higher activity doses per kilogram body weight than in humans, can be practically ruled out.
3. Several studies have outlined that the amount of co-injected cold mass can have a significant influence on biodistribution and imaging results [11-14]. In clinical routine, it is therefore highly recommended to utilize radiopharmaceutical formulations with constant, optimized specific activity (i.e., well-defined cold mass content). Such productions could be done most conveniently and reliably by adding the desired amount of active compound to a pre-conditioned vial containing a fixed amount of cold standard. This approach, however, requires radiolabeled tracers with very high specific activity in order not to change the overall contained amount of cold mass significantly. 68Ga-labeled TRAP conjugates appear ideally suited for this purpose.
Furthermore, regarding Figure 2, one notices that variation of radiochemical yields, reflected by the size of error bars, is the larger the lower precursor amounts are. This is because the generator eluate usually contains traces of ionic contaminants, such as Zn2+, Sn4+, Al3+, and Fe3+, the concentration of which in the individual eluates is varying. These can compete with 68Ga3+ at the chelating site of the precursor, which is naturally the more impacting on labeling yield the lower the stoichiometric excess of precursor over 68Ga3+ ion is. The error bars in Figure 2 show that except for precursor amounts exceeding 20 nmol, use of a TRAP peptide will result in a more reliable radiosynthesis, being less prone to be perturbed by variation of other parameters (reaction pH, eluate volume, trace metal contaminations, etc.). Differences in radiochemical yield and reproducibility are very pronounced for peptide amounts in the range of 1 to 10 nmol (e.g., equivalent to 1.4 to 14 μg of DOTATOC) which are frequently used in routine 68Ga labeling procedures; near-quantitative yields and excellent reproducibility can be expected here for a TRAP peptide. This is of high relevance for routine GMP tracer production, where reproducibility and robustness of procedures is crucial. In addition, we assume that due to higher labeling efficiency, realization of kit labeling procedures will be much simpler using TRAP conjugates, which we deem of importance for the aforementioned possibility of global implementation of 68Ga-PET. Finally, the recent introduction of NOPO, a TRAP variety designed specifically for monoconjugation, expands the portfolio of P-functionalized triazacyclononane-triphosphinate chelators, thus offering even more synthetic possibilities for development of 68Ga tracers [15].
Abbreviations
DOTA: 1,4,7,10-tetraazacyclododecane-tetraacetic acid; NOTA: 1,4,7-triazacyclononane-triacetic acid; PET: Positron-emission tomography; SPECT: Single photon emission computed tomography; TRAP: Triazacyclononane-phosphinate.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JN developed the study concept, performed the radiolabeling of TRAP(RGD)3 and DOTATOC, and wrote the manuscript. KP performed the radiolabeling of NODAGA-RGD and critically reviewed the manuscript. HJW gave advice in the interpretation of the data and critically reviewed the manuscript. All authors approved the final manuscript.
Contributor Information
Johannes Notni, Email: johannes.notni@tum.de.
Karolin Pohle, Email: karolin.pohle@tum.de.
Hans-Jürgen Wester, Email: h.j.wester@tum.de.
References
- Decristoforo C, Pickett RD, Verbruggen A. Feasibility and availability of 68Ga-labelled peptides. Eur J Nucl Med Mol Imaging. 2012;39:S31–S40. doi: 10.1007/s00259-011-1988-5. [DOI] [PubMed] [Google Scholar]
- Fani M, André JP, Maecke HR. 68Ga-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol Imaging. 2008;3:67–77. doi: 10.1002/cmmi.232. [DOI] [PubMed] [Google Scholar]
- Bartholomä MD, Louie AS, Valliant JF, Zubieta J. Technetium and gallium derived radiopharmaceuticals: comparing and contrasting the chemistry of two important radiometals for the molecular imaging era. Chem Rev. 2010;110:2903–2920. doi: 10.1021/cr1000755. [DOI] [PubMed] [Google Scholar]
- Ballinger JR. 99Mo shortage in nuclear medicine: crisis or challenge? J Label Compd Radiopharm. 2010;53:167–168. [Google Scholar]
- Notni J, Hermann P, Havlíčková J, Kotek J, Kubíček V, Plutnar J, Loktionova N, Riss PJ, Rösch F, Lukeš I. A triazacyclononane-based bifunctional phosphinate ligand for the preparation of multimeric 68Ga tracers for positron emission tomography. Chem Eur J. 2010;16:7174–7185. doi: 10.1002/chem.200903281. [DOI] [PubMed] [Google Scholar]
- Notni J, Šimeček J, Hermann P, Wester HJ. TRAP, a powerful and versatile framework for gallium-68 radiopharmaceuticals. Chem Eur J. 2011;17:14718–14722. doi: 10.1002/chem.201103503. [DOI] [PubMed] [Google Scholar]
- Šimeček J, Schulz M, Notni J, Plutnar J, Kubíček V, Havlíčková J, Hermann P. Complexation of metal ions with TRAP (1,4,7-triazacyclononane phosphinic acid) ligands and NOTA: phosphinate-containing ligands as unique chelators for trivalent gallium. Inorg Chem. 2012;51:577–590. doi: 10.1021/ic202103v. [DOI] [PubMed] [Google Scholar]
- Notni J, Plutnar J, Wester HJ. Bone seeking TRAP conjugates: surprising observations and implications on development of gallium-68-labeled bisphosphonates. EJNMMI Res. 2012;2:13. doi: 10.1186/2191-219X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohle K, Notni J, Bussemer J, Kessler H, Schwaiger M, Beer AJ. 68Ga-NODAGA-RGD is a suitable substitute for 18F-Galacto-RGD and can be produced with high specific activity in a cGMP/GRP compliant automated process . Nucl Med Biol. 2012. [DOI] [PubMed]
- Kubíček V, Havlíčková J, Kotek J, Tircsó G, Hermann P, Tóth E, Lukeš I. Gallium(III) complexes of DOTA and DOTA-monoamide: kinetic and thermodynamic studies. Inorg Chem. 2010;49:10960–10969. doi: 10.1021/ic101378s. [DOI] [PubMed] [Google Scholar]
- Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, Eberle AN. A gallium-labeled DOTA-alpha-melanocyte-stimulating hormone analog for PET imaging of melanoma metastases. J Nucl Med. 2004;45:116–123. [PubMed] [Google Scholar]
- Breeman WAP, Kwekkeboom DK, Kooij PPM, Bakker WH, Hofland LJ, Visser TJ, Ensing GJ, Lamberts SWJ, Krenning EP. Effect of dose and specific activity on tissue distribution of indium-111-pentetreotide in rats. J Nucl Med. 1995;36:623–627. [PubMed] [Google Scholar]
- de Jong M, Breeman WAP, Bernard BF, van Gameren A, de Bruin E, Bakker WH, van der Pluijm ME, Visser TJ, Mäcke HR, Krenning EP. Tumour uptake of the radiolabelled somatostatin analogue [DOTA0, TYR3]octreotide is dependent on the peptide amount. Eur J Nucl Med. 1999;26:693–698. doi: 10.1007/s002590050439. [DOI] [PubMed] [Google Scholar]
- Velikyan I, Sundin A, Eriksson B, Lundqvist H, Sörensen J, Bergström M, Långström B. In vivo binding of [68 Ga]-DOTATOC to somatostatin receptors in neuroendocrine tumours—impact of peptide mass. Nucl Med Biol. 2010;37:265–275. doi: 10.1016/j.nucmedbio.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Simecek J, Zemek O, Hermann P, Wester HJ, Notni J. A monoreactive bifunctional triazacyclononane-phosphinate chelator with high selectivity for gallium-68. Chem Med Chem. 2012. [DOI] [PubMed]