Abstract
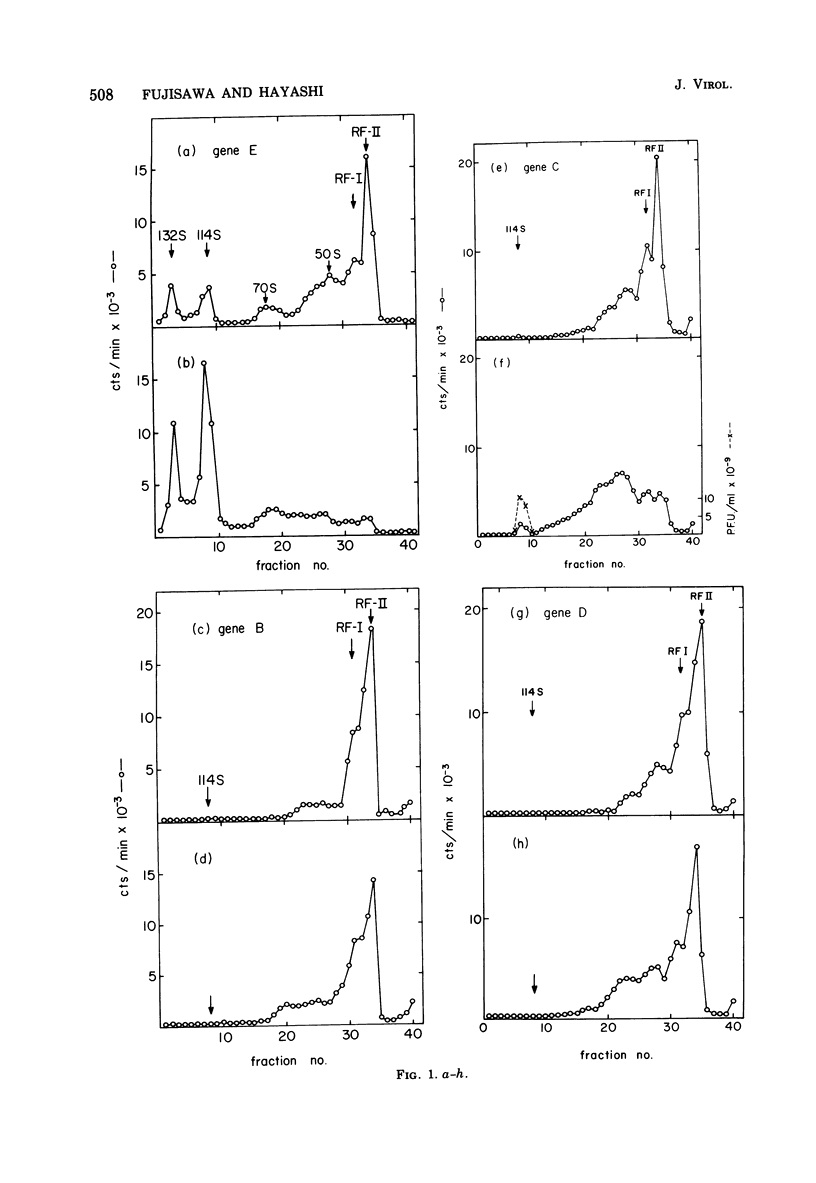
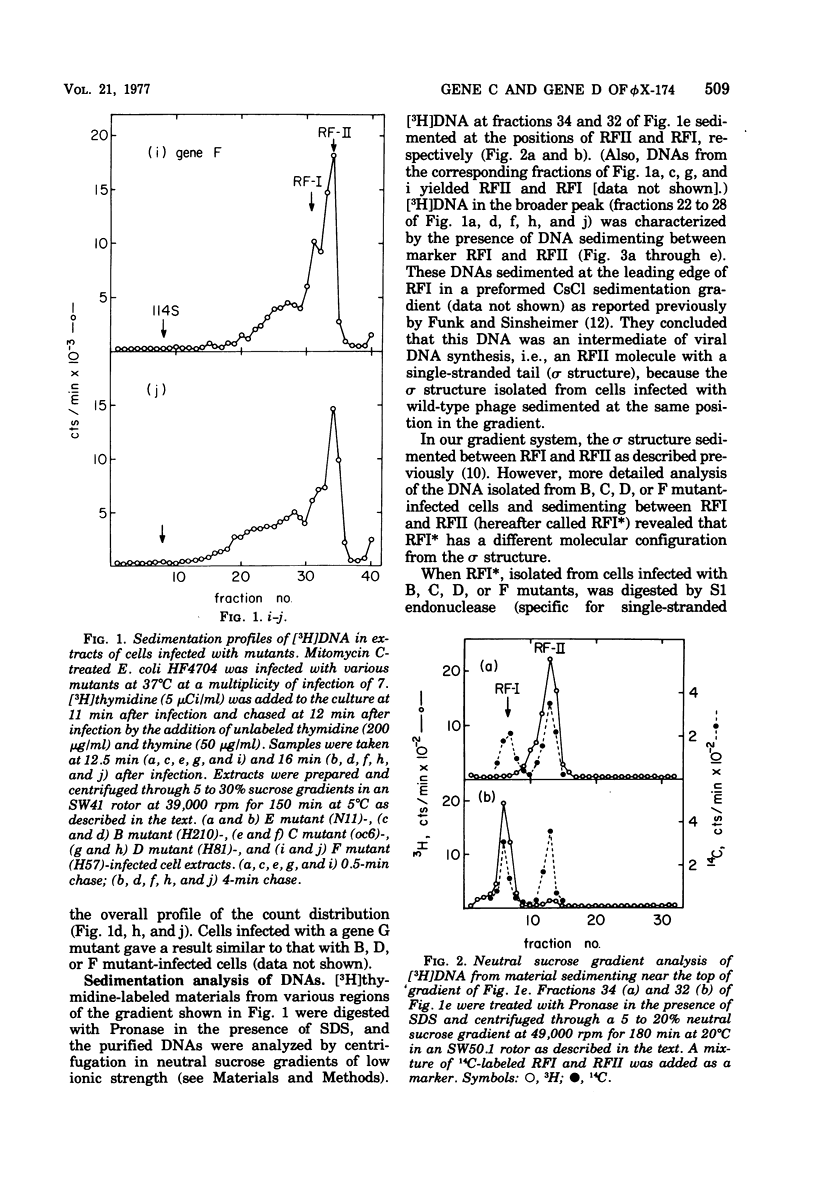
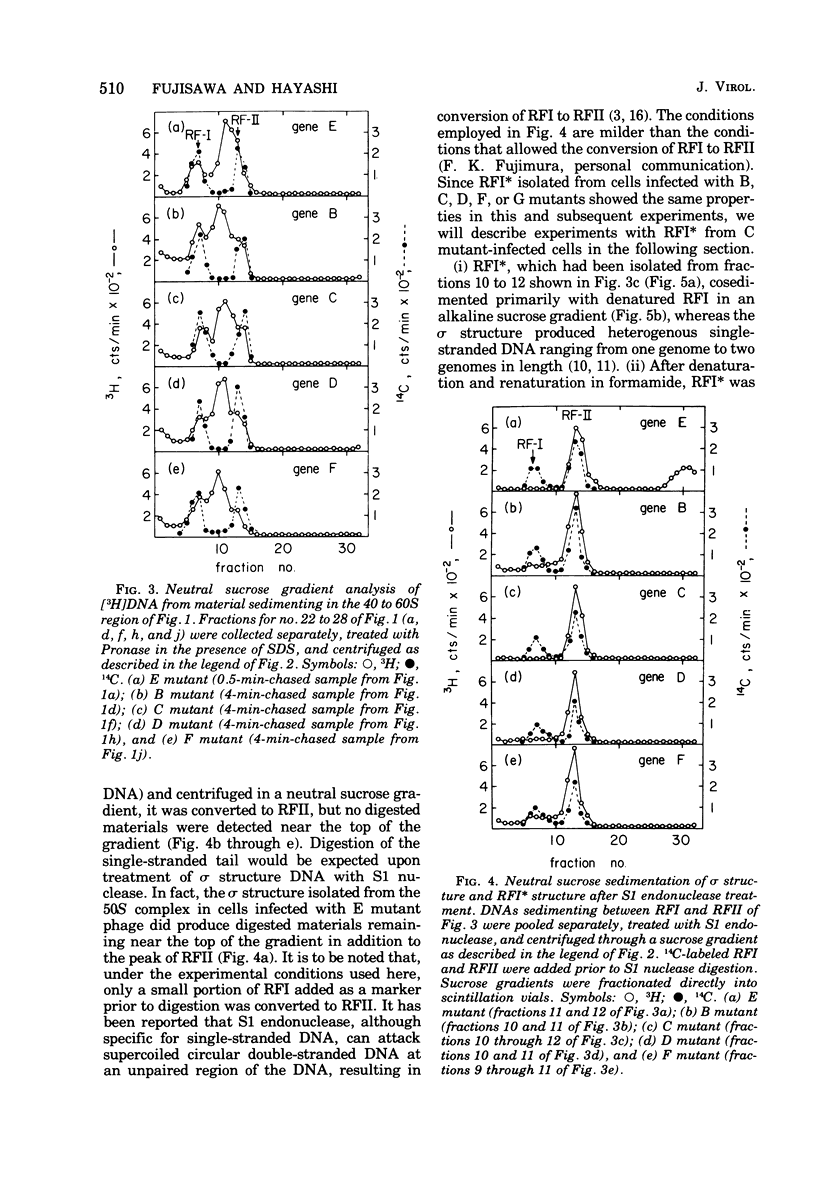
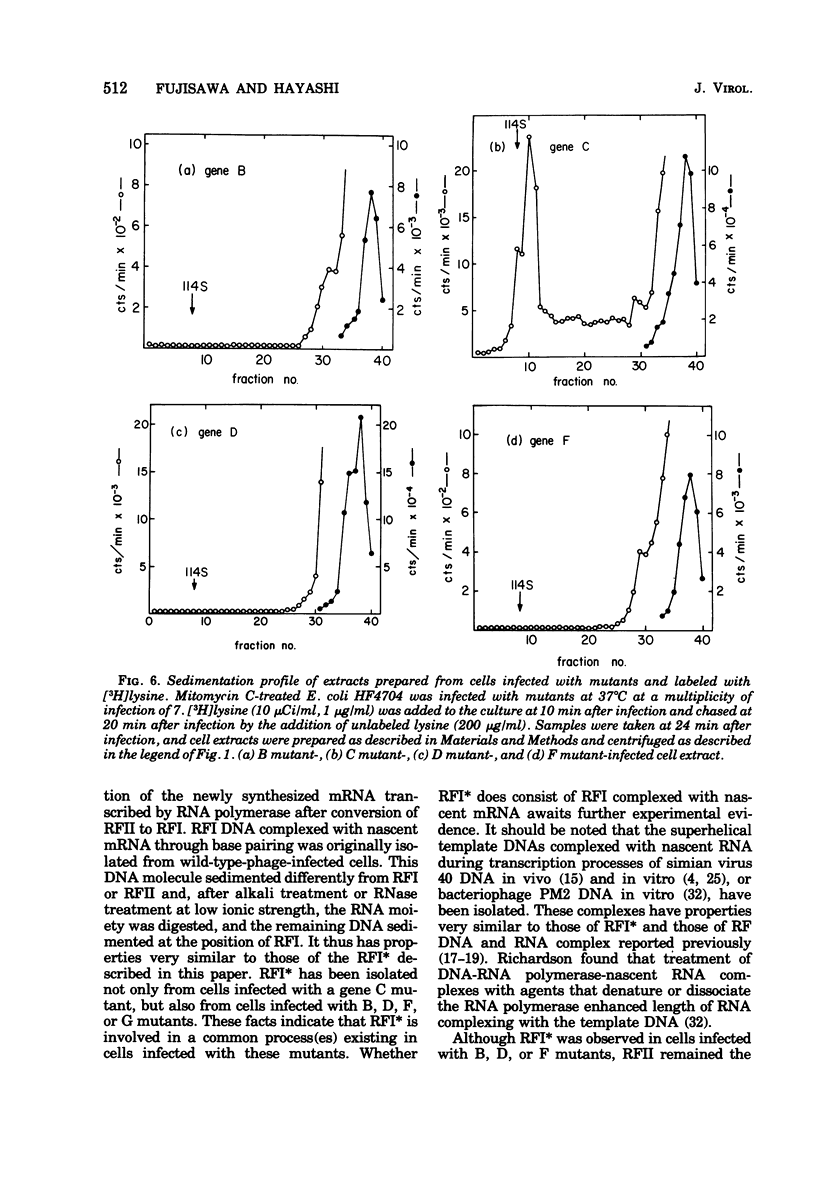
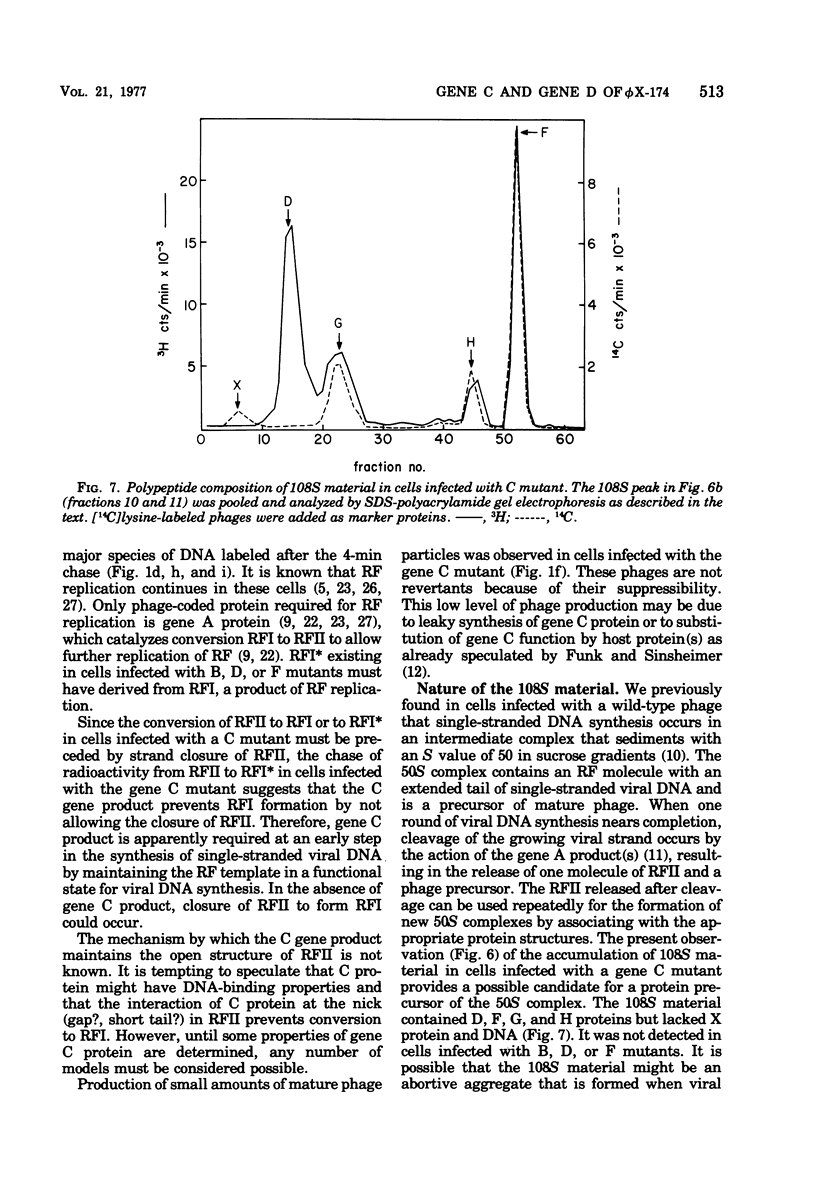
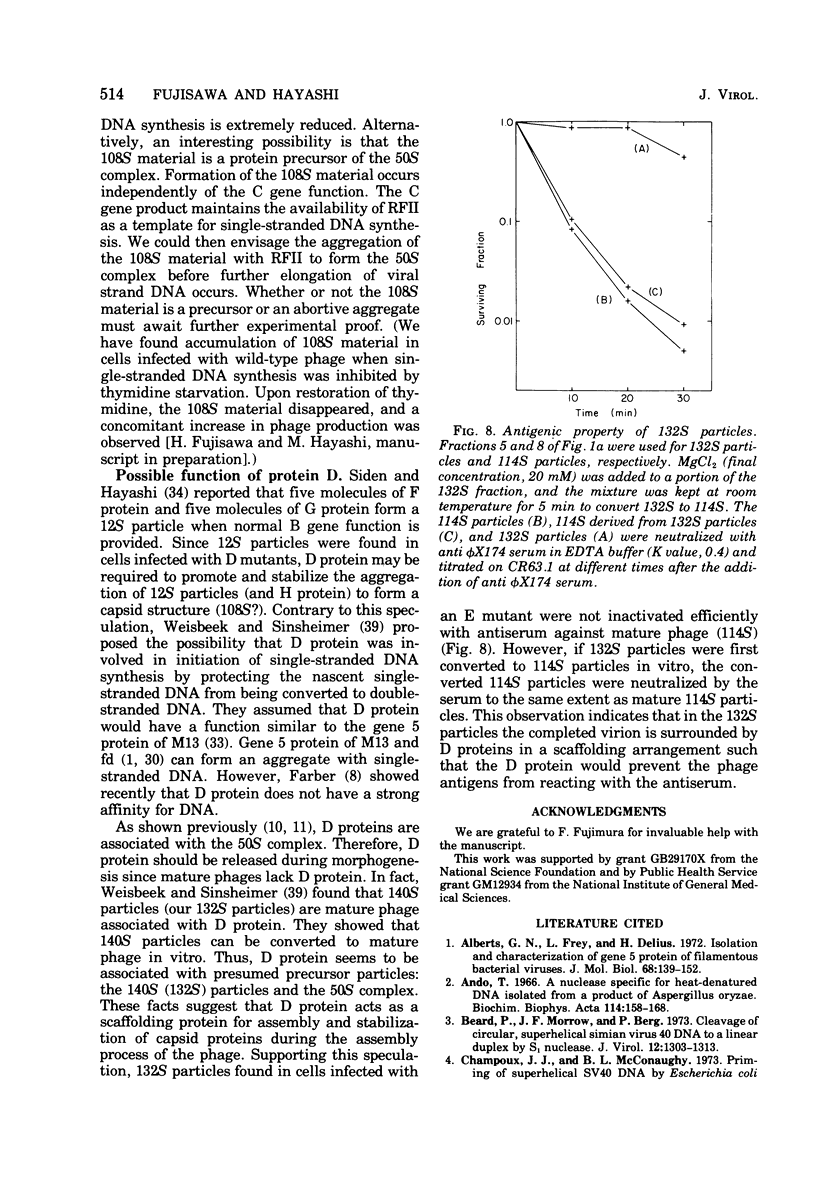
Phage-related materials existing in cells infected with various mutants of bacteriophage phi chi 174 were investigated. A novel species of replicative-form (RF) DNA was found in cells infected with a phage mutant of gene B, C, D, F, or G. This species, called RFI, sedimented at a position between RFI and RFII in a neutral sucrose gradient. It was converted to RFI upon denaturation in alkali, denaturation in formamide and subsequent renaturation, or RNase treatment at low ionic strength. In cells infected with a phage mutant of gene C, RFI was derived from pulse-labeled RFII after a short chase. TLLS INFECTED WITH A MUTANT OF GENE B, D, or F. A possible function of the C gene product of phi chi 174 could be to prevent the conversion of RFII to RFI, thereby maintaining the availability of RFII to act as the template for single-stranded viral DNA synthesis. A protein complex containing no DNA, which sedimented with an S value of 108 in a sucrose gradient and contained virion proteins F, G, and H, and nonvirion protein D, was found in cells infected with the gene C mutant. A possible function of protein D was considered as a scaffolding protein for assembly of phage structural proteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B., Frey L., Delius H. Isolation and characterization of gene 5 protein of filamentous bacterial viruses. J Mol Biol. 1972 Jul 14;68(1):139–152. doi: 10.1016/0022-2836(72)90269-0. [DOI] [PubMed] [Google Scholar]
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Beard P., Morrow J. F., Berg P. Cleavage of circular, superhelical simian virus 40 DNA to a linear duplex by S1 nuclease. J Virol. 1973 Dec;12(6):1303–1313. doi: 10.1128/jvi.12.6.1303-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J., McConaughy B. L. Priming of superhelical SV40 DNA by Escherichia coli RNA polymerase for in vitro DNA synthesis. Biochemistry. 1975 Jan 28;14(2):307–316. doi: 10.1021/bi00673a017. [DOI] [PubMed] [Google Scholar]
- Dowell C. E., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. IX. Studies on the physiology of three phi-X174 temperature-sensitive mutants. J Mol Biol. 1966 Apr;16(2):374–386. doi: 10.1016/s0022-2836(66)80180-8. [DOI] [PubMed] [Google Scholar]
- Dressler D. H., Denhardt D. T. Mechanism of replication of phi-X-174 single stranded DNA. Nature. 1968 Jul 27;219(5152):346–351. doi: 10.1038/219346a0. [DOI] [PubMed] [Google Scholar]
- Dressler D. The rolling circle for phiX DNA replication. II. Synthesis of single-stranded circles. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1934–1942. doi: 10.1073/pnas.67.4.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber M. B. Purification and properties of bacteriophage phi X 174 gene D product. J Virol. 1976 Mar;17(3):1027–1037. doi: 10.1128/jvi.17.3.1027-1037.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Cis-limited action of the gene-A product of bacteriophage phiX174 and the essential bacterial site (E. coli-electron microscopy-cis-acting protein-specifically-nicked RF). Proc Natl Acad Sci U S A. 1972 Feb;69(2):475–479. doi: 10.1073/pnas.69.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H., Hayashi M. Gene A product of phi X174 is required for site-specific endonucleolytic cleavage during single-stranded DNA synthesis in vivo. J Virol. 1976 Aug;19(2):416–424. doi: 10.1128/jvi.19.2.416-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H., Hayashi M. Viral DNA-synthesizing intermediate complex isolated during assembly of bacteriophage phi X174. J Virol. 1976 Aug;19(2):409–415. doi: 10.1128/jvi.19.2.409-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk F. D., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XXXV. Cistron 8. J Virol. 1970 Jul;6(1):12–19. doi: 10.1128/jvi.6.1.12-19.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand D. H., Hayashi M. Electrophoretic characterization of phiX174-specific proteins. J Mol Biol. 1969 Sep 28;44(3):501–516. doi: 10.1016/0022-2836(69)90376-3. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Girard M., Marty L., Manteuil S. Viral DNA-RNA hybrids in cells infected with simian virus: the simian virus 40 transcriptional intermediates. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1267–1271. doi: 10.1073/pnas.71.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N. Action of the single-stranded DNA specific nuclease S1 on double-stranded DNA. Biochim Biophys Acta. 1973 Apr 21;308(7):59–67. doi: 10.1016/0005-2787(73)90122-6. [DOI] [PubMed] [Google Scholar]
- Hayashi M. N., Hayashi M. Fragment maps of phiX-174 replicative DNA produced by restriction enzymes from haemophilus aphirophilus and haemophilus influenzae H-I. J Virol. 1974 Nov;14(5):1142–1151. doi: 10.1128/jvi.14.5.1142-1151.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M. N., Hayashi M. Participation of a DNA-RNA hybrid complex in in vivo genetic transcription. Proc Natl Acad Sci U S A. 1966 Mar;55(3):635–641. doi: 10.1073/pnas.55.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M. N., Hayashi M. The stability of native DNA-RNA complexes during in vivo phiX-174 transcription. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1107–1114. doi: 10.1073/pnas.61.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M. A DNA-RNA complex as an intermediate of in vitro genetic transcription. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1736–1743. doi: 10.1073/pnas.54.6.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Hayashi M. Template activities of the phi X-174 replicative allomorphic deoxyribonucleic acids. Biochemistry. 1971 Nov;10(23):4212–4218. doi: 10.1021/bi00799a009. [DOI] [PubMed] [Google Scholar]
- Henry T. J., Knippers R. Isolation and function of the gene A initiator of bacteriophage phi-chi 174, a highly specific DNA endonuclease. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1549–1553. doi: 10.1073/pnas.71.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya M., Denhardt D. T. The mechanism of replication of phi X174 single-stranded DNA. II. The role of viral proteins. J Mol Biol. 1971 Apr 28;57(2):159–175. doi: 10.1016/0022-2836(71)90339-1. [DOI] [PubMed] [Google Scholar]
- Knippers R., Razin A., Davis R., Sinsheimer R. L. The process of infection with Bacteriophage phi-X174. XXIX. In vivo studies on the synthesis of the single-stranded DNA of progeny phi-X174 bacteriophage. J Mol Biol. 1969 Oct 28;45(2):237–263. doi: 10.1016/0022-2836(69)90103-x. [DOI] [PubMed] [Google Scholar]
- Lebowitz P., Bloodgood R. Transcription of simian virus 40 DNA by Escherichia coli RNA polymerase: synthesis of a DNA-RNA hybrid and discrete RNAs under restrictive transcription conditions. J Mol Biol. 1975 May 15;94(2):183–201. doi: 10.1016/0022-2836(75)90077-7. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XIV. Studies on macromolecular synthesis during infection with a lysis-defective mutant. J Mol Biol. 1967 Aug 28;28(1):87–94. doi: 10.1016/s0022-2836(67)80079-2. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XV. Bacteriophage DNA synthesis in abortive infections with a set of conditional lethal mutants. J Mol Biol. 1967 Nov 28;30(1):69–80. doi: 10.1016/0022-2836(67)90244-6. [DOI] [PubMed] [Google Scholar]
- Linney E., Hayashi M. Two proteins of gene A of psiX174. Nat New Biol. 1973 Sep 5;245(140):6–8. doi: 10.1038/newbio245006a0. [DOI] [PubMed] [Google Scholar]
- Oey J. L., Knippers R. Properties of the isolated gene 5 protein of bacteriophage fd. J Mol Biol. 1972 Jul 14;68(1):125–138. doi: 10.1016/0022-2836(72)90268-9. [DOI] [PubMed] [Google Scholar]
- Puga A., Tessman I. Mechanism of transcription of bacteriophage S13. I. Dependence of messengerRNA synthesis on amount and configuration of DNA. J Mol Biol. 1973 Mar 25;75(1):83–97. doi: 10.1016/0022-2836(73)90530-5. [DOI] [PubMed] [Google Scholar]
- Richardson J. P. Attachment of nascent RNA molecules to superhelical DNA. J Mol Biol. 1975 Nov 5;98(3):565–579. doi: 10.1016/s0022-2836(75)80087-8. [DOI] [PubMed] [Google Scholar]
- Salstrom J. S., Pratt D. Role of coliphage M13 gene 5 in single-stranded DNA production. J Mol Biol. 1971 Nov 14;61(3):489–501. doi: 10.1016/0022-2836(71)90061-1. [DOI] [PubMed] [Google Scholar]
- Siden E. J., Hayashi M. Role of the gene beta-product in bacteriophage phi-X174 development. J Mol Biol. 1974 Oct 15;89(1):1–16. doi: 10.1016/0022-2836(74)90159-4. [DOI] [PubMed] [Google Scholar]
- Siegel J. E., Hayashi M. Phi-X-174 bacteriophage structural mutants which affect deoxyribonucleic acid synthesis. J Virol. 1969 Oct;4(4):400–407. doi: 10.1128/jvi.4.4.400-407.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsheimer R. L., Knippers R., Komano T. Stages in the replication of bacteriophage phi X174 DNA in vivo. Cold Spring Harb Symp Quant Biol. 1968;33:443–447. doi: 10.1101/sqb.1968.033.01.051. [DOI] [PubMed] [Google Scholar]
- Tessman E. S. Mutants of bacteriophage S13 blocked in infectious DNA synthesis. J Mol Biol. 1966 May;17(1):218–236. doi: 10.1016/s0022-2836(66)80104-3. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Hayashi M. Intermediates in the assembly of phi X174. J Mol Biol. 1970 Mar 14;48(2):219–242. doi: 10.1016/0022-2836(70)90158-0. [DOI] [PubMed] [Google Scholar]
- Weisbeek P. J., Sinsheimer R. L. A DNA-protein complex involved in bacteriophage phi chi 174 particle formation. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3054–3058. doi: 10.1073/pnas.71.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]


