Abstract
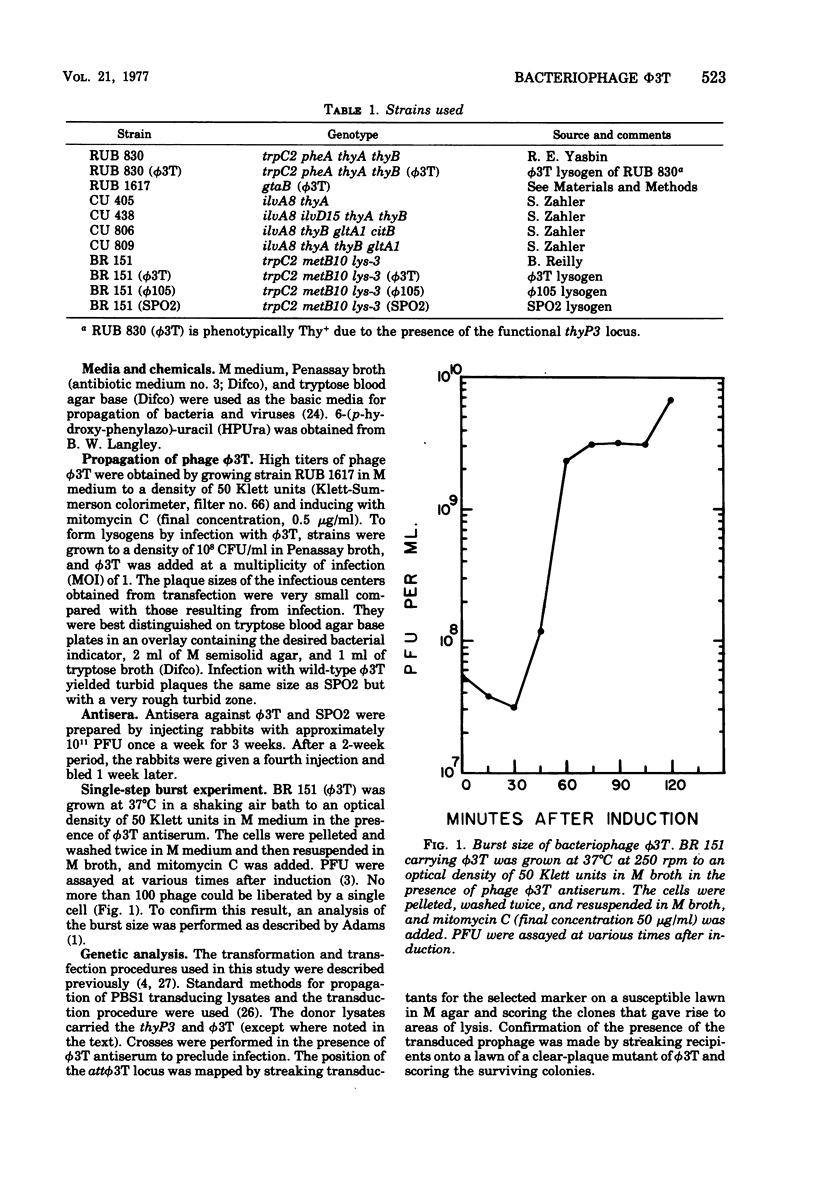
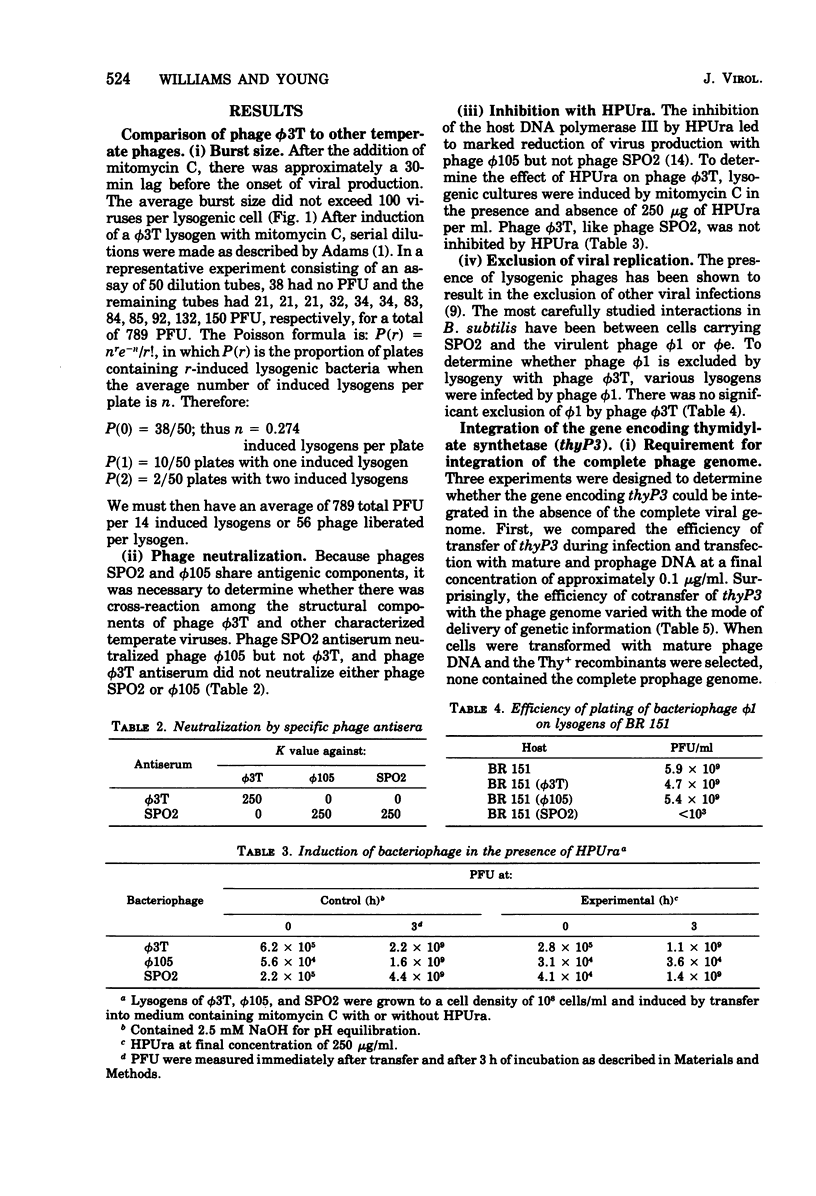
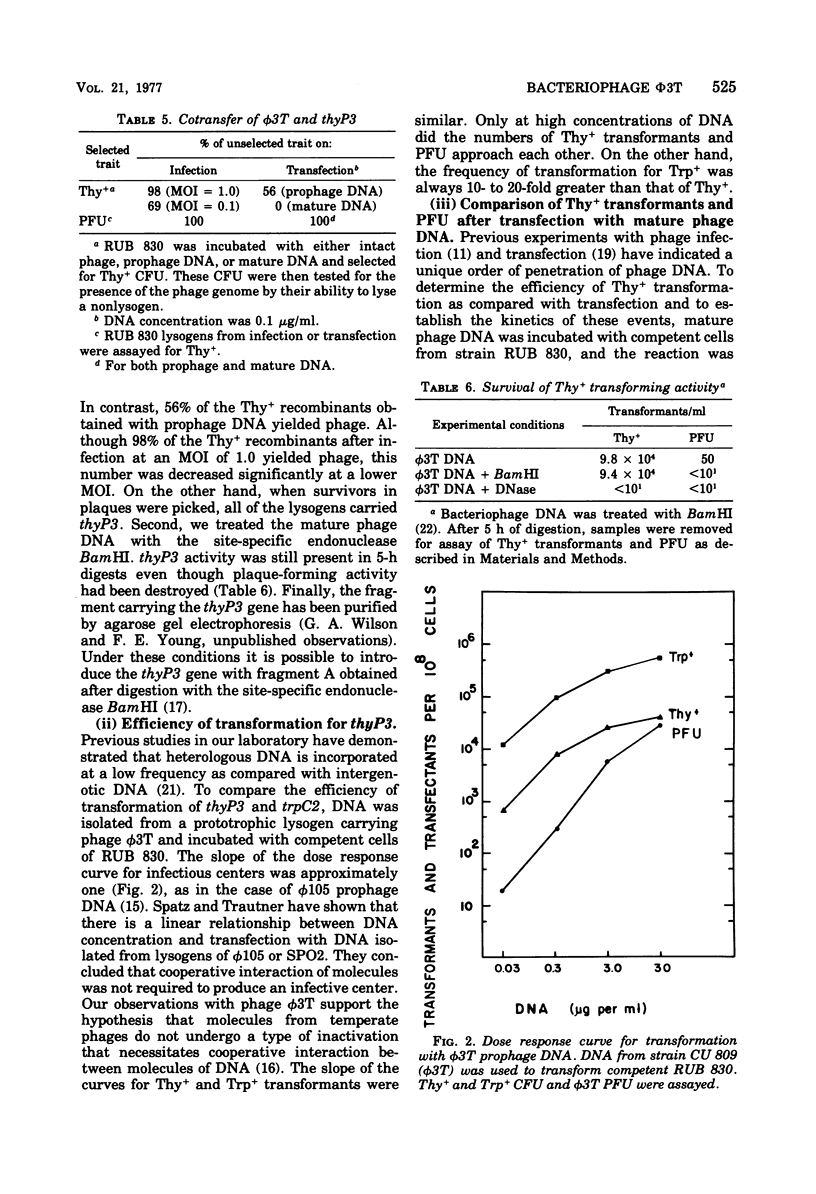
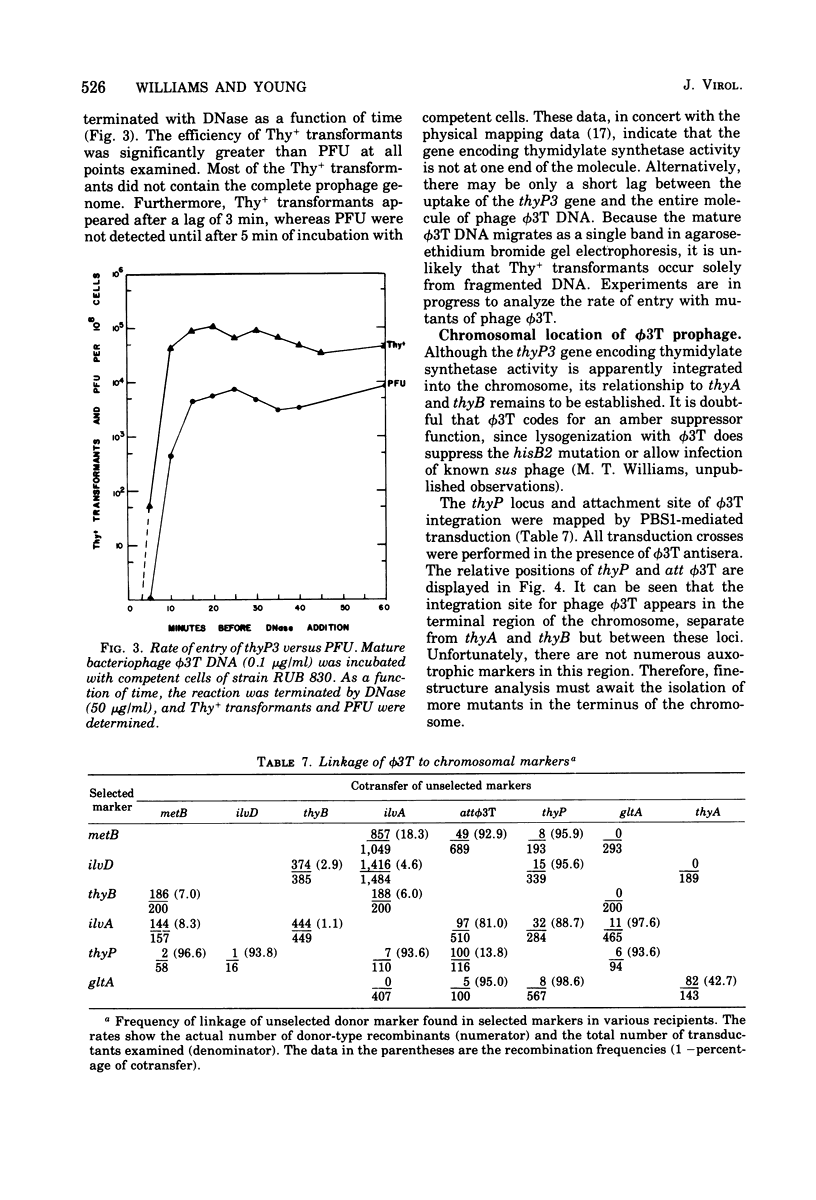
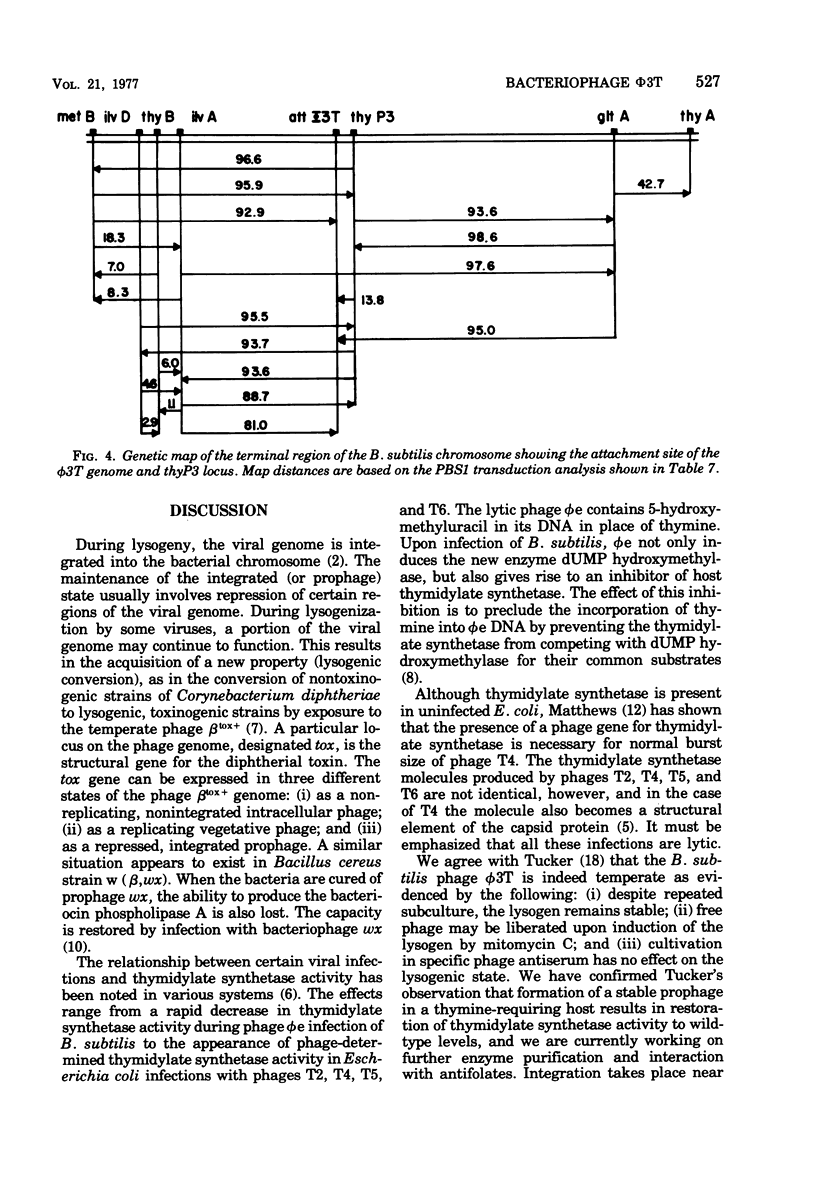
The temperate Bacillus subtilis bacteriophage phi 3T contains within its genome a locus, designated thyP3, that encodes for a protein with thymidylate synthetase activity. Bacteriophage phi 3T is different from the two previously characterized temperate phages, phi 105 and SPO2, in: heteroimmunity, response to bacteriophage antisera, endonuclease digestion pattern, induction in the presence of 6-(p-hydroxyphenylazo)-uracil, and effect on the lytic cycle of bacteriophage phi 1. The mean burst size of phi 3T is 56. The dose response curve with bacteriophage phi 3T DNA is linear for transfection and transformation to the Thy+ phenotype. The inserted prophage has been mapped by PBS1 transduction; it is between chromosomal markers ilvA8 and gltA in the terminus of the chromosome. Thus thyP3 maps at a site separate from, but between, the bacterial markers thyA and thyB when thyP3 is in the prophage state.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barksdale L., Arden S. B. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu Rev Microbiol. 1974;28(0):265–299. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- Birdsell D. C., Hathaway G. M., Rutberg L. Characterization of Temperate Bacillus Bacteriophage phi105. J Virol. 1969 Sep;4(3):264–270. doi: 10.1128/jvi.4.3.264-270.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H., Brooks D., Young F. E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972 Apr;110(1):281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capco G. R., Mathews C. K. Bacteriophage-coded thymidylate synthetase. Evidence that the T4 enzyme is a capsid protein. Arch Biochem Biophys. 1973 Oct;158(2):736–743. doi: 10.1016/0003-9861(73)90568-7. [DOI] [PubMed] [Google Scholar]
- Friedkin M. Thymidylate synthetase. Adv Enzymol Relat Areas Mol Biol. 1973;38:235–292. doi: 10.1002/9780470122839.ch5. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Uchida T., Singer R. A. Expression of diphtheria toxin genes carried by integrated and nonintegrated phage beta. Virology. 1972 Dec;50(3):664–668. doi: 10.1016/0042-6822(72)90420-5. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975 Sep;39(3):257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivánovics G., Gaál V., Prágai B. Lysogenic conversion to phospholipase A production in Bacillus cereus. J Gen Virol. 1974 Aug;24(2):349–358. doi: 10.1099/0022-1317-24-2-349. [DOI] [PubMed] [Google Scholar]
- Mathews C. K. Phage Growth and Deoxyribonucleic Acid Synthesis in Escherichia coli Infected by a Thymine-Requiring Bacteriophage. J Bacteriol. 1965 Sep;90(3):648–652. doi: 10.1128/jb.90.3.648-652.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister W. T. Bacteriophage infection: which end of the SP82G genome goes in first? J Virol. 1970 Feb;5(2):194–198. doi: 10.1128/jvi.5.2.194-198.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg L., Armentrout R. W., Jonasson J. Unrelatedness of temperate Bacillus subtilis bacteriophages SP02 and phi105. J Virol. 1972 May;9(5):732–737. doi: 10.1128/jvi.9.5.732-737.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg L., Hoch J. A., Spizizen J. Mechanism of transfection with deoxyribonucleic acid from the temperate Bacillus bacteriophage phi-105. J Virol. 1969 Jul;4(1):50–57. doi: 10.1128/jvi.4.1.50-57.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz H. C., Trautner T. A. The role of recombination in transfection of B. subtilis. Mol Gen Genet. 1971;113(2):174–190. doi: 10.1007/BF00333191. [DOI] [PubMed] [Google Scholar]
- Tucker R. G. Acquisition of thymidylate synthetase activity by a thymine-requiring mutant of Bacillus subtilis following infection by the temperate phage phi 3. J Gen Virol. 1969 Jun;4(4):489–504. doi: 10.1099/0022-1317-4-4-489. [DOI] [PubMed] [Google Scholar]
- Williams G. L., Green D. M. Early extracellular events in infection of competent Bacillus subtilis by DNA of bacteriophage SP82G. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1545–1549. doi: 10.1073/pnas.69.6.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Williams M. T., Baney H. W., Young F. E. Characterization of temperate bacteriophages of Bacillus subtilis by the restriction endonuclease EcoRI: evidence for three different temperate bacteriophages. J Virol. 1974 Oct;14(4):1013–1016. doi: 10.1128/jvi.14.4.1013-1016.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Intergenotic transformation of the Bacillus subtilis genospecies. J Bacteriol. 1972 Sep;111(3):705–716. doi: 10.1128/jb.111.3.705-716.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Isolation of a sequence-specific endonuclease (BamI) from Bacillus amyloliquefaciens H. J Mol Biol. 1975 Sep 5;97(1):123–125. doi: 10.1016/s0022-2836(75)80028-3. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Ganesan A. T., Young F. E. Bacteriophage interference in Bacillus subtilis 168. J Virol. 1974 Apr;13(4):916–921. doi: 10.1128/jvi.13.4.916-921.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973 Feb;113(2):540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E., Smith C., Reilly B. E. Chromosomal location of genes regulating resistance to bacteriophage in Bacillus subtilis. J Bacteriol. 1969 Jun;98(3):1087–1097. doi: 10.1128/jb.98.3.1087-1097.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



