Abstract
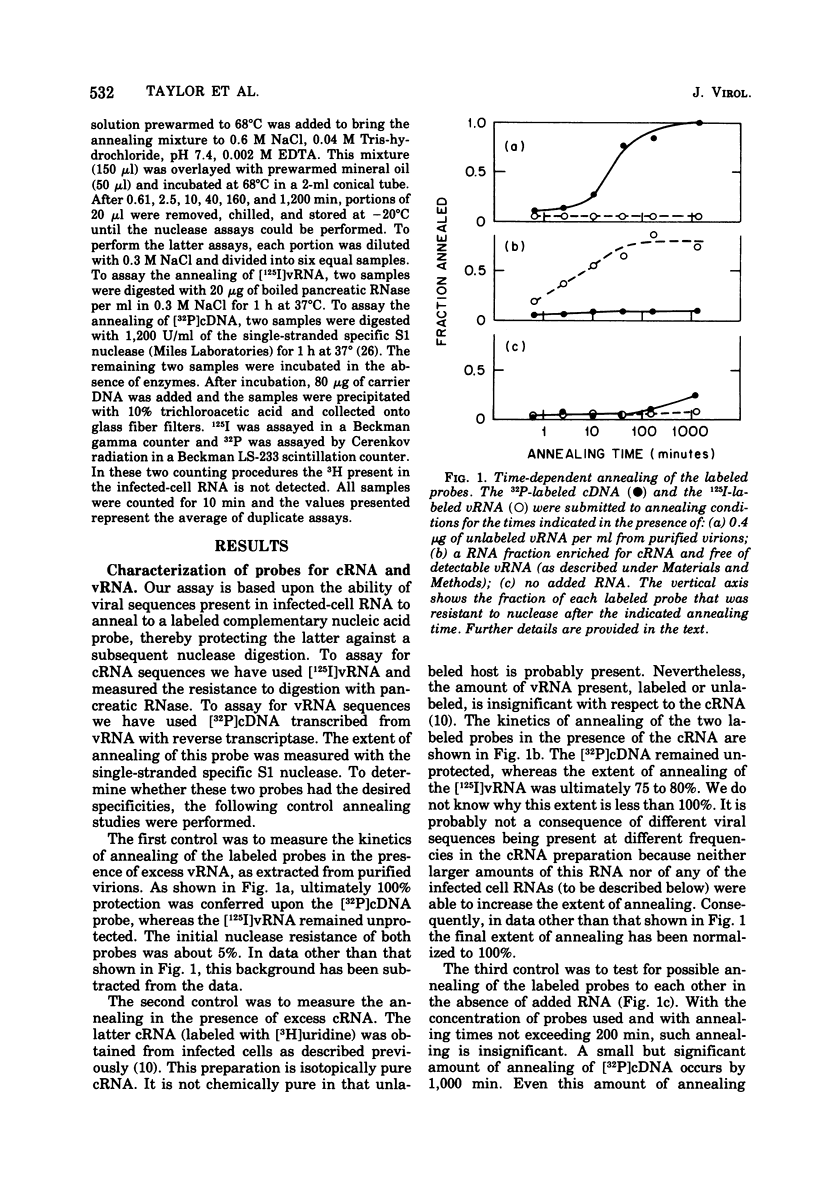
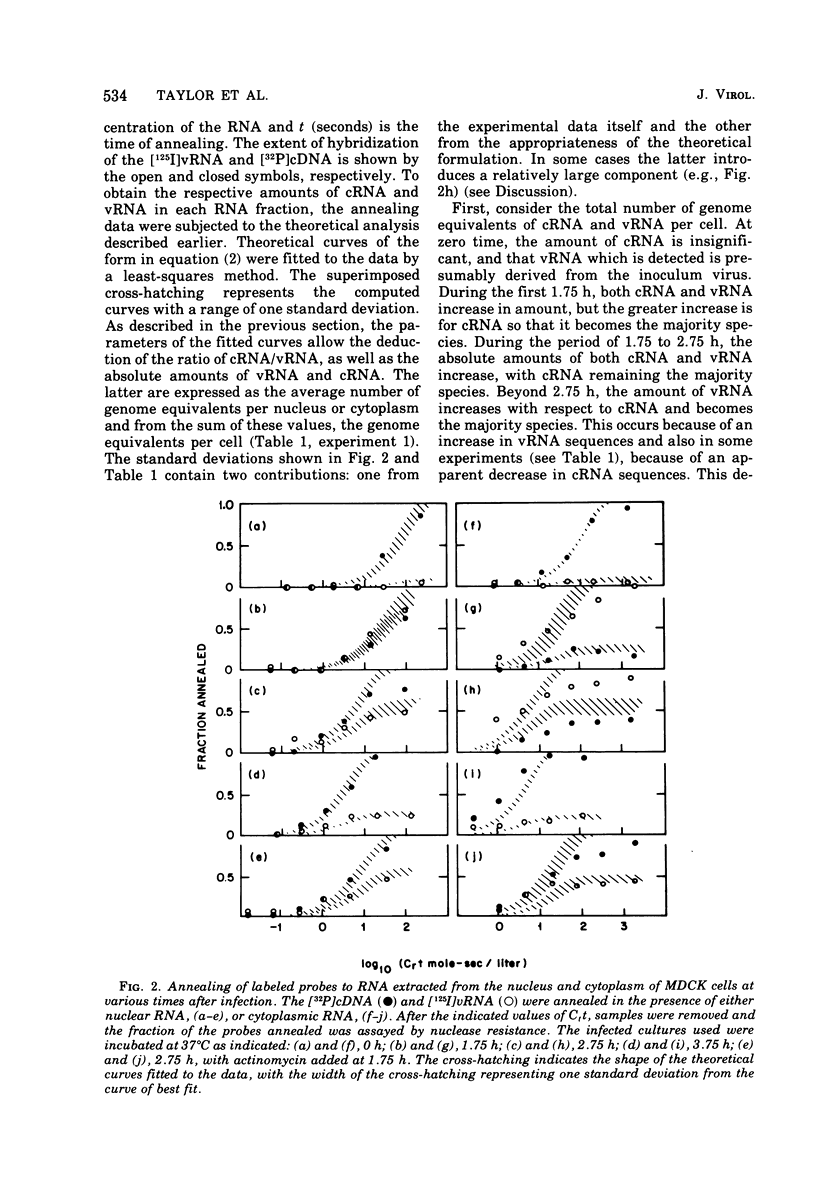
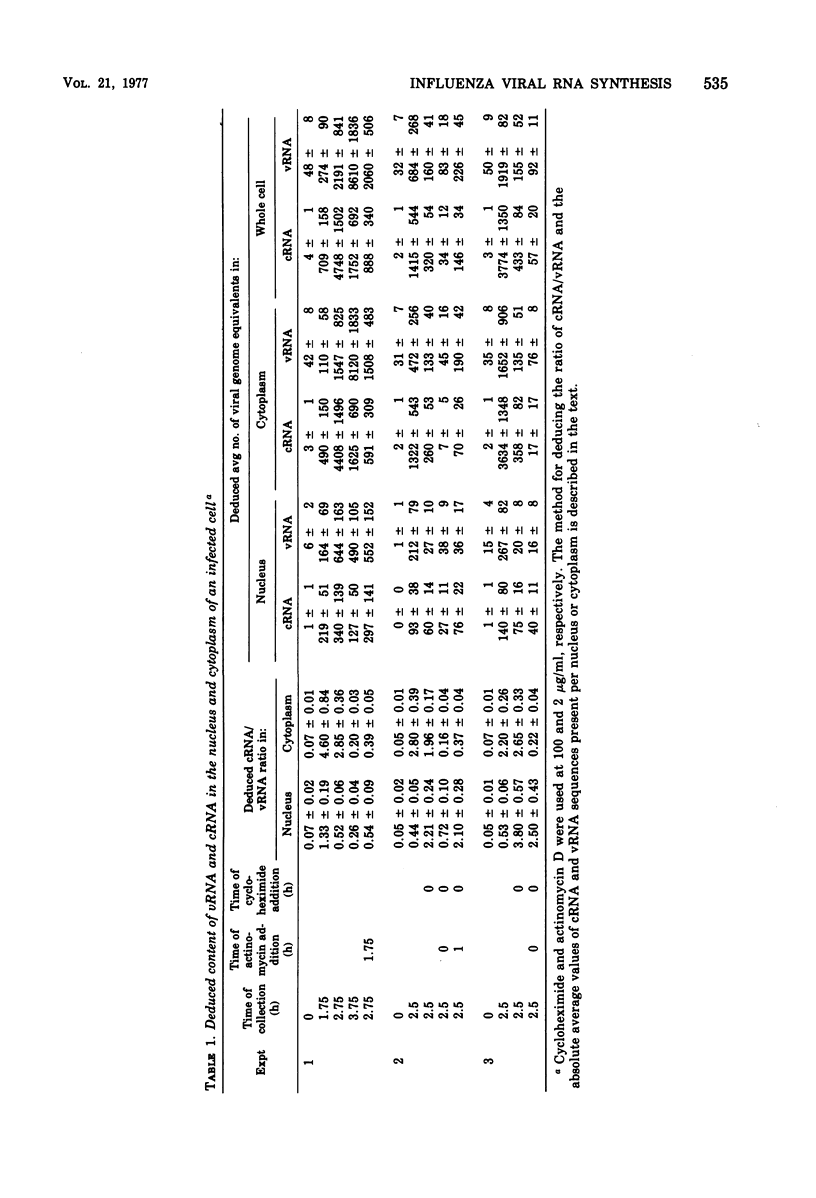
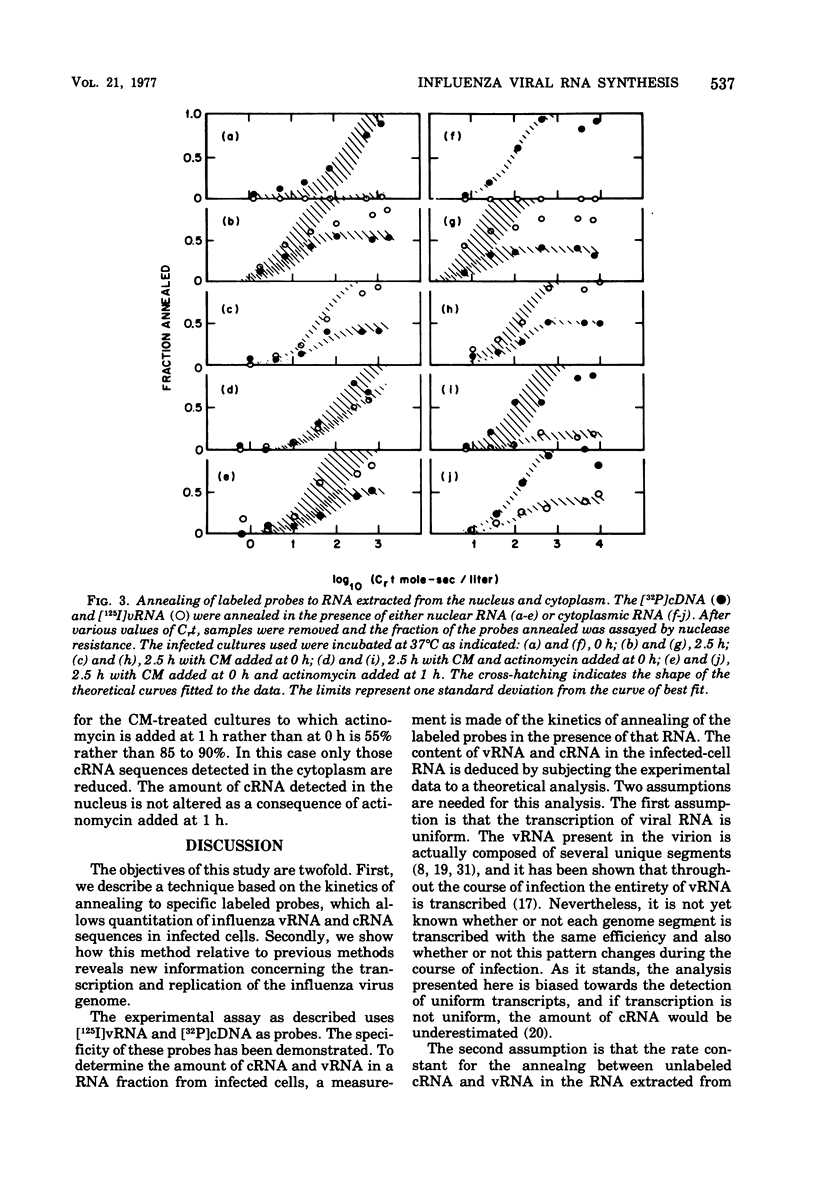
Specific radioactive probes have been obtained for both influenza virion RNA (vRNA) and for its complement (complementary RNA or cRNA): 32P-labeled complementary DNA (cDNA) synthesized with the avian sarcoma virus reverse transcriptase, and [125I]vRNA, respectively. From the kinetics of annealing of these two probes to RNA from canine kidney cells infected with the WSN strain of influenza virus, we have determined the average number of cRNA and vRNA sequences in the nucleus and cytoplasm as a function of time after infection. Immediately after infection, a small amount of vRNA is detected, presumably from the inoculum virus. As expected, the amount of cRNA is insignificant. During the first 1.75 h of infection, the most significant increase observed is in cRNA sequences. Most of these cRNA sequences are found in the cytoplasm, but a significant amount (30%) is found in the nucleus. During this time, a small but significant increase in vRNA is also detected in the nucleus and cytoplasm. From 1.75 to 2.75 h, the absolute amounts of both cRNA and vRNA increase, predominantly in the cytoplasm, with cRNA remaining as the majority species. Subsequently, the amount of vRNA increases with respect to cRNA and becomes the majority species. At 3.75 h, 95% of both cRNA and vRNA are found in the cytoplasm. Addition of actinomycin D at 1.75 h completely suppresses the subsequent ninefold increase in cRNA and does not have a significant effect on the subsequent 14-fold increase in cytoplasmic vRNA. This assay is also able to detect the cRNA produced as a result of primary transcription, operationally defined as the cRNA produced in the presence of 100 mug of cycloheximide per ml added at zero time of infection. Increases in cRNA in the presence of cycloheximide are detectable in both the nucleus and the cytoplasm. Addition of actinomycin D as well as cycloheximide at zero time completely suppresses the appearance of cRNA in the cytoplasm, whereas a large fraction (50%) of the increase in nuclear cRNA still occurs.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Rhodes D. P., Banerjee A. K. The 5' terminal structure of the methylated mRNA synthesized in vitro by vesicular stomatitis virus. Cell. 1975 May;5(1):51–58. doi: 10.1016/0092-8674(75)90091-4. [DOI] [PubMed] [Google Scholar]
- BARRY R. D. THE EFFECTS OF ACTINOMYCIN D AND ULTRAVIOLET IRRADIATION ON THE PRODUCTION OF FOWL PLAGUE VIRUS. Virology. 1964 Dec;24:563–569. doi: 10.1016/0042-6822(64)90208-9. [DOI] [PubMed] [Google Scholar]
- Bean W. J., Jr, Simpson R. W. Primary transcription of the influenza virus genome in permissive cells. Virology. 1973 Dec;56(2):646–651. doi: 10.1016/0042-6822(73)90067-6. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Jackson N., Levinson W. E., Medeiros E., Quintrell N., Varmus H. E. The presence and expression of RNA tumor virus genes in normal and infected cells: detection by molecular hybridization. Am J Clin Pathol. 1973 Jul;60(1):31–43. doi: 10.1093/ajcp/60.1.31. [DOI] [PubMed] [Google Scholar]
- Chow N. L., Simpson R. W. RNA-dependent RNA polymerase activity associated with virions and subviral particles of myxoviruses. Proc Natl Acad Sci U S A. 1971 Apr;68(4):752–756. doi: 10.1073/pnas.68.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Content J., Duesberg P. H. Base sequence differences among the ribonucleic acids of influenza virus. J Mol Biol. 1971 Dec 14;62(2):273–285. doi: 10.1016/0022-2836(71)90427-x. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Friderici K. H., Rottman F. M. Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5' terminus. Biochemistry. 1975 Oct 7;14(20):4367–4374. doi: 10.1021/bi00691a004. [DOI] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Purification of influenza viral complementary RNA: its genetic content and activity in wheat germ cell-free extracts. J Virol. 1975 Dec;16(6):1464–1475. doi: 10.1128/jvi.16.6.1464-1475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., McDonnell J. P., Levinson W. E., Bishop J. M. Purification and characterization of the deoxyribonucleic acid polymerase associated with Rous sarcoma virus. Biochemistry. 1972 Jun 6;11(12):2334–2342. doi: 10.1021/bi00762a020. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A. J. Reovirus messenger RNA contains a methylated, blocked 5'-terminal structure: m-7G(5')ppp(5')G-MpCp-. Proc Natl Acad Sci U S A. 1975 Jan;72(1):362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Shatkin A. J., Jelinek W., Salditt-Georgieff M., Darnell J. E. Methylated, blocked 5 termini in HeLa cell mRNA. Proc Natl Acad Sci U S A. 1975 May;72(5):1904–1908. doi: 10.1073/pnas.72.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapin A. C., Leong J., Fanshier L., Levinson W. E., Bishop J. M. Identification of virus-specific RNA in cells infected with Rous sarcoma virus. Biochem Biophys Res Commun. 1971 Mar 5;42(5):919–925. doi: 10.1016/0006-291x(71)90518-3. [DOI] [PubMed] [Google Scholar]
- Garapin A. C., Varmus H. E., Faras A. J., Levinson W. E., Bishop J. M. RNA-directed DNA synthesis by virions of Rous sarcoma virus: further characterization of the templates and the extent of their transcription. Virology. 1973 Mar;52(1):264–274. doi: 10.1016/0042-6822(73)90414-5. [DOI] [PubMed] [Google Scholar]
- Ghendon Y., Blagoveshienskaya O. Polyadenylate sequences of fowl plague virus complementary RNA (cRNA) synthesized in vivo and in vitro. Virology. 1975 Dec;68(2):330–337. doi: 10.1016/0042-6822(75)90276-7. [DOI] [PubMed] [Google Scholar]
- Glass S. E., McGeoch D., Barry R. D. Characterization of the mRNA of influenza virus. J Virol. 1975 Dec;16(6):1435–1443. doi: 10.1128/jvi.16.6.1435-1443.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst J., Content J., Mandeles S., Fraenkel-Conrat H., Duesberg P. Distinct oligonucleotide patterns of distinct influenza virus RNA's. J Mol Biol. 1972 Aug 21;69(2):209–215. doi: 10.1016/0022-2836(72)90226-4. [DOI] [PubMed] [Google Scholar]
- Khoury A. T., Hanafusa H. Synethesis and integration of viral DNA in chicken cells at different time after infection with various multiplicities of avian oncornavirus. J Virol. 1976 May;18(2):383–400. doi: 10.1128/jvi.18.2.383-400.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M. Cytoplasmic and nucleoplasmic viral RNPs in influenza virus-infected MDCK cells. Virology. 1972 Oct;50(1):103–113. doi: 10.1016/0042-6822(72)90350-9. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Influenza viral RNPs newly synthesized during the latent period of viral growth in MDCK cells. Virology. 1971 Apr;44(1):125–136. doi: 10.1016/0042-6822(71)90159-0. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Morgan M. A., Shatkin A. J. Influenza viral mRNA contains internal N6-methyladenosine and 5'-terminal 7-methylguanosine in cap structures. J Virol. 1976 Oct;20(1):45–53. doi: 10.1128/jvi.20.1.45-53.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M., Soeiro R. Studies on the intranuclear localization of influenza virus-specific proteins. Virology. 1975 Apr;64(2):378–387. doi: 10.1016/0042-6822(75)90114-2. [DOI] [PubMed] [Google Scholar]
- Lavi S., Shatkin A. J. Methylated simian virus 40-specific RNA from nuclei and cytoplasm of infected BSC-1 cells. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2012–2016. doi: 10.1073/pnas.72.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhoet E., Miller H., Doyle M., Blatti S. RNA-dependent RNA polymerase activity in influenza virions. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1369–1371. doi: 10.1073/pnas.68.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Influenza virion transcriptase: synthesis in vitro of large, polyadenylic acid-containing complementary RNA. J Virol. 1977 Jan;21(1):24–34. doi: 10.1128/jvi.21.1.24-34.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W. A reexamination of influenza single-and double-stranded RNAs by gel electrophoresis. Virology. 1976 Feb;69(2):789–792. doi: 10.1016/0042-6822(76)90508-0. [DOI] [PubMed] [Google Scholar]
- Pons M. W. The inhibition of influenza virus RNA synthesis by actinomycin D and cycloheximide. Virology. 1973 Jan;51(1):120–128. doi: 10.1016/0042-6822(73)90372-3. [DOI] [PubMed] [Google Scholar]
- Pons M. Effect of actinomycin D on the replication of influenza virus and influenza virus RNA. Virology. 1967 Sep;33(1):150–154. doi: 10.1016/0042-6822(67)90104-3. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Kaluza G., Rott R. Stability and precursor relationships of virus RNA. J Gen Virol. 1972 Nov;17(2):213–219. doi: 10.1099/0022-1317-17-2-213. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rott R. Synthesis in vivo of influenza virus plus and minus strand RNA and its preferential inhibition by antibiotics. Virology. 1970 Apr;40(4):989–996. doi: 10.1016/0042-6822(70)90145-5. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Both G. W. Reovirus mRNA: transcription and translation. Cell. 1976 Mar;7(3):305–313. doi: 10.1016/0092-8674(76)90159-8. [DOI] [PubMed] [Google Scholar]
- Skehel J. J. RNA-dependent RNA polymerase activity of the influenza virus. Virology. 1971 Sep;45(3):793–796. doi: 10.1016/0042-6822(71)90197-8. [DOI] [PubMed] [Google Scholar]
- Straus N. A., Bonner T. I. Temperature dependence of RNA-DNA hybridization kinetics. Biochim Biophys Acta. 1972 Aug 16;277(1):87–95. doi: 10.1016/0005-2787(72)90355-3. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Tereba A., McCarthy B. J. Hybridization of 125I-labeled ribonucleic acid. Biochemistry. 1973 Nov 6;12(23):4675–4679. doi: 10.1021/bi00747a020. [DOI] [PubMed] [Google Scholar]
- WHITE D. O., DAY H. M., BATCHELDER E. J., CHEYNE I. M., WANSBROUGH A. J. DELAY IN THE MULTIPLICATION OF INFLUENZA VIRUS. Virology. 1965 Feb;25:289–302. doi: 10.1016/0042-6822(65)90207-2. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]


