Abstract
To extend our understanding of flowering time control in eudicots, we screened for mutants in the model legume Medicago truncatula (Medicago). We identified an early flowering mutant, spring1, in a T-DNA mutant screen, but spring1 was not tagged and was deemed a somaclonal mutant. We backcrossed the mutant to wild type R108. The F1 plants and the majority of F2 plants were early flowering like spring1, strongly indicating that spring1 conferred monogenic, dominant early flowering. We hypothesized that the spring1 phenotype resulted from over expression of an activator of flowering. Previously, a major QTL for flowering time in different Medicago accessions was located to an interval on chromosome 7 with six candidate flowering- time activators, including a CONSTANS gene, MtCO, and three FLOWERING LOCUS T (FT) genes. Hence we embarked upon linkage mapping using 29 markers from the MtCO/FT region on chromosome 7 on two populations developed by crossing spring1 with Jester. Spring1 mapped to an interval of ∼0.5 Mb on chromosome 7 that excluded MtCO, but contained 78 genes, including the three FT genes. Of these FT genes, only FTa1 was up-regulated in spring1 plants. We then investigated global gene expression in spring1 and R108 by microarray analysis. Overall, they had highly similar gene expression and apart from FTa1, no genes in the mapping interval were differentially expressed. Two MADS transcription factor genes, FRUITFULLb (FULb) and SUPPRESSOR OF OVER EXPRESSION OF CONSTANS1a (SOC1a), that were up-regulated in spring1, were also up-regulated in transgenic Medicago over-expressing FTa1. This suggested that their differential expression in spring1 resulted from the increased abundance of FTa1. A 6255 bp genomic FTa1 fragment, including the complete 5′ region, was sequenced, but no changes were observed indicating that the spring1 mutation is not a DNA sequence difference in the FTa1 promoter or introns.
Introduction
Flowering is a critical step in the life cycle of plants as it heralds the onset of sexual reproduction and the formation of seeds and fruits. The timing of flowering is controlled by environmental cues such as photoperiod and temperature as well as internal signals including developmental age [1]. In eudicots, the genetic regulation of the timing of flowering is best understood in Arabidopsis. In monocots, great progress has been made in rice and the temperate cereals, barley and wheat [2].
In Arabidopsis, a flowering time gene network is involved in perception and response to the signals which are integrated by a set of floral integrator genes [2]. These integrators include FLOWERING LOCUS T (FT) which encodes a major florigen, the long sought-after universal mobile flowering hormone that in combination with a b-ZIP transcription factor FD, activates pathways leading to the development of flowers [3], [4]. FT genes are widespread in plants and many activate flowering, indicating that this function is highly conserved in monocots and eudicots [3]. However, some FT genes have other functions, including more general roles in growth [5].
CONSTANS (CO) plays a key role in promoting flowering in Arabidopsis in long daylength conditions by up-regulating FT and over expression of CO accelerates flowering [6]–[7]. Rice CO also regulates flowering time, but in this plant it has a more complex dual function depending on the daylength [8]. Other genes, not found in Arabidopsis, also strongly influence flowering in the cereals [9], [10]. On the other hand, a key repressor of Arabidopsis flowering FLOWERING LOCUS C (FLC) that targets FT and other floral integrators appears to be missing from non-Brassicaceous plants [2].
The eudicot legume (Fabaceae) family are the third largest group of plants with commercially significant crop and forage plants such as soybean and alfalfa, and in developing countries providing major staples such as cowpeas, chickpeas and peanuts [11]. It is thus important to understand legume flowering control mechanisms as it is one of the determinants of their performance as a crop in particular geographic locations and climate. Study of flowering in legumes also promises to reveal novel mechanisms of flowering control, because genes such as FLC, that are key to Arabidopsis flowering time control, are not found in legume genomes [12], [13], but a similar role might be carried out by another gene. Ultimately, this work should yield new tools for manipulating and customising flowering time using modern plant breeding strategies.
Medicago has a number of attractive features for genetic analysis of flowering time control. There is natural variation in flowering time amongst different Medicago accessions being investigated by Quantitative Trait Locus (QTL) analysis [14]–[16]. For example, a major QTL for flowering time in Medicago has been mapped to an interval on chromosome 7 that contains several candidate flowering regulators, including a CO-like gene, MtCO, three FT genes and an FD-like gene [15], [16]. Extensive Medicago mutant resources are available such as Tnt1 transposon-tagged mutant populations, tilling and fast neutron lines which may be used for forward genetics via screening for flowering time mutants [17]. These mutant populations also provide a powerful opportunity to use reverse genetics to analyse the function of candidate genes mined from the genome sequence and have recently resulted in the identification of a Medicago FT gene, FTa1, as a regulator of flowering time [18]. In combination with synteny and gene mining in the Medicago genome sequence, work in the related temperate legume, garden pea (Pisum sativum) has focused successfully on mutants, many known from classical genetic studies [19]. This work has led to the molecular identification of several flowering time regulators [20], [21], including GIGAS, which encodes a pea florigen FTa1 [22].
To extend our understanding of flowering time control in eudicots, we aim to carry out forward screens for Medicago flowering time mutants as a prelude to functional gene characterization. Here we report that we screened a Medicago T-DNA mutant population [23] and identified an early flowering mutant that we named spring1. Spring1 is not T-DNA tagged and thus can be classed as a somaclonal mutant. However, we show that spring1 behaves as a single dominant Mendelian gene that confers early flowering. We mapped spring1 to an interval of ∼0.5 Mb on chromosome 7. This interval contains 78 predicted genes, including the three FT genes, but not MtCO or other known candidate flowering time genes. Only one of the FT genes, the floral regulator FTa1, was up-regulated in spring1 mutants, and no changes, either up or down, were observed to expression of the other genes in the interval by microarray analysis. These results strongly indicate that it is the increased abundance of FTa1 that is causing the spring1 early flowering phenotype.
Materials and Methods
Plant Material and Growth Conditions
Jester [24] and R108_C3 (R108) [25] are two genotypes belonging to two subspecies of Medicago truncatula Gaertn (barrel medic), ssp. truncatula and ssp. tricycla respectively. Jester is an aphid-resistant line closely related to Jemalong/A17. The spring1 mutant was identified during a glasshouse mutant screen (Institut des Sciences du Végétal, CNRS, Gif sur Yvette, France) of a R108 T-DNA tagged population [23]. The 35S::FTa1 transgenic Medicago lines in the R108 genotype were previously reported [18].
Plants for all subsequent flowering time experiments and gene expression experiments (with the exception of the diurnal timecourse where plants were grown in sterile conditions in plant growth cabinets as described previously [18]) were grown under long-day conditions (LD, 16 h light/8 h dark) in a growth room with ∼200 µM m−2 s−1 cool white fluorescent light at ∼22°C. For germination, seeds were scarified by gently rubbing them between two pieces of sand paper (grade P160) until small signs of abrasion appeared. Scarified seeds were incubated at 4°C on 0.8% water agar in the dark for 3 days to overcome embryonic dormancy, and then left at 22°C in the dark for another 4 to 5 hours to complete germination. Germinated seedlings were grown in a soil mix consisting of 9 parts of Black Magic® seed raising mix (Yates, Orica New Zealand Ltd.), 3 parts of coarse graded vermiculite (Pacific Growers Supplies Ltd.) and 1 part of No. 2 Propagating Sand (Daltons Ltd.). They were watered with tap water and a complete liquid nutrient media [26].
Plant Crosses and Scoring Flowering Time
Plant crosses were carried out to investigate the genetics and inheritance of spring1 on the one hand and to develop populations for mapping spring1 by linkage analysis with DNA markers on the other. Multiple crosses between each genotype were done, in both directions, using spring1 as a male or as a female, under a binocular microscope by emasculating the female plants, dusting pollen from the male plant over the stigma and then wrapping the pollinated flower in plastic film for three days [27]. Four types of crosses were made; Backcrosses between spring1 and R108, Control crosses between the two wild type genotypes, R108 and Jester, Mapping crosses between spring1 and Jester and Test crosses between the F1 plants from spring1 x Jester and Jester. These crosses are described in more detail below and flowering time results are presented in Table 1.
Table 1. Flowering time of plant populations derived from crosses with the spring1 mutant.
| Genotype | Average Number of Days after Germination to Flowering | Day Range | Average Nodes on PrimaryAxis at Flowering | Node Range | Flowering Time | Total No. Plants | |
| No. Early Plants | No. Late Plants | ||||||
| Parental lines | |||||||
| R108 | 49.8+/− SE 0.13 | 49–50 | 12.9+/− SE 0.31 | 12–16 | 0 | 12 | 12 |
| Jester | 37.5+/− SE 0.92 | 35–41 | 12.2+/− SE 0.31 | 11–13 | 0 | 6 | 6 |
| spring1 | 25.3+/− SE 0.25 | 25–28 | 6.6+/− SE 0.26 | 6–9 | 12 | 0 | 12 |
| Backcross “ spring1 x R108” | |||||||
| F1 (♂spring1 x ♀R108) | 29+/− SE 0 | 29 | 7.4+/− SE 0.21 | 6–8 | 27 | 0 | 27 |
| Early F2 (♂spring1 x ♀R108) | 24.7+/− SE 0.21 | 24–31 | 7.3+/− SE 0.17 | 6–10 | 62 | 0 | 78 |
| Late F2 (♂spring1 x ♀R108) | 54.1+/− SE 0.72 | 49–59 | nd | nd | 0 | 16 | |
| Mapping Cross “ spring1 x Jester” | |||||||
| F1 (♂spring1 x ♀Jester) | 32.2+/− SE 0.64 | 31–39 | 7.7+/− SE 0.37 | 6–9 | 32 | 0 | 32 |
| Early F2 (♂spring1 x ♀Jester) | 25.3+/− SE 0.27 | 16–57 | 6.4+/− SE 0.04 | 4–9 | 421 | 0 | 747 |
| Late F2 (♂spring1 x ♀Jester)a | – | >39– >87 | – | 11– >25 | 0 | 57 | |
| Unclassified F2 (♂spring1 x ♀Jester)b | – | 28– >73 | – | 8– >10 | 94 | ||
| Dead F2 (♂spring1 x ♀Jester) | – | – | – | – | 175 | ||
| Control Cross “R108 x Jester” | |||||||
| F1 (♂R108 x ♀Jester) | 60.2+/− SE 4.83 | 44–78 | 13.6+/− SE 0.60 | 11–16 | 0 | 12 | 12 |
| F2 (♂R108 x ♀Jester)c | – | 52– >65 | – | 11–>19 | 0 | 12 | 18 |
| Unclassified F2 (♂R108 x ♀Jester) | 53.7+/− SE 2.03 | 50–57 | 8.3+/− SE 0.67 | 7–9 | 3 | ||
| Dead F2 (♂spring1 x ♀R108) | – | – | – | – | 3 | ||
| Mapping “Test Cross” | |||||||
| Early Test Cross | 22.4+/− SE 0.34 | 18–35 | 7.2+/− SE 0.08 | 5–9 | 83 | 0 | 275 |
| Late Test Crossd | – | 38– >69 | – | 11– >21 | 0 | 95 | |
| Unclassified Test Crosse | – | 30– >69 | – | 8– ≥11 | 30 | ||
| Dead Test Cross | – | – | – | – | 67 | ||
Flowering time was scored and plants were classified for flowering time. Plants were classified as early flowering if they had ≤7 nodes at flowering or had flowered rapidly (≤28 days after germination), similar to spring1. They were classified as late flowering if they had ≥11 nodes at flowering or had not yet flowered but had ≥11 nodes, similar to Jester. They were scored as unclassified due to not falling into our two classes, or being difficult to score due to their tiny size or altered aerial architecture.
F2 plants were grown in six groups and scoring was terminated after 39, 46, 73, 77, 83 or 87 days.
A total of 27 plants had not yet flowered when scoring was terminated. None of the plants classified as late had flowered by 39 days. Four plants had not flowered by 87 days and had up to 25 nodes on the primary axis.
In total 10 plants had not flowered by the time scoring was terminated.
F2 plants were grown up and scoring was terminated after 65 days. Two plants had not yet flowered and had up to 19 nodes on the primary axis.
Scoring was terminated at 69 days.
A total of 33 plants had not yet flowered and had up to 21 nodes on the primary axis.
Two plants had not yet flowered, one had 10 nodes and one was unscoreable due to its small size and architecture.“Test Cross” is (♂(♂spring1 x ♀Jester) x ♀Jester). Plants classified as Dead, usually died as very young seedlings. nd is not done.
The backcross
We backcrossed spring1 with wild type R108 plants to investigate the genetics and inheritance of spring1. The seed from the crosses were collected and we planted out F1 plants, scored their flowering time which was early, similar to spring1, and then allowed these plants to self-fertilise to produce the F2 generation. The F2 generation was then sown out and scored for flowering time. No differences in phenotype were observed when spring1 was used as a male or a female plant in the backcross to wild type. Data from the cross of spring1 as a male and R108 as a female is presented. Flowering time measurements were carried out by recording the days after germination to the first floral bud, and/or by counting the node number on the primary axis of each plant at flowering. Plants segregating for flowering time were classified as early (spring1-like) or late flowering (R108-like) in order to determine the segregation ratio.
The control cross
In order to examine the effect on flowering time and other plant phenotypes of crossing the two genotypes, R108 and Jester, we crossed R108 with Jester. The seed from the crosses were collected and we planted out the F1 plants, scored their flowering time, which was late, and then allowed these plants to self-fertilise to produce the F2 generation. The F2 seed was sown out and scored for flowering time. No differences in phenotype were observed when R108 was used as a male or a female plant in the cross to Jester. However, a feature of the crosses of R108 to Jester, was that the parental and progeny plants grew at different rates. Hence, the number of nodes on the primary axis at flowering, rather than days after germination to flowering, was selected as the most accurate way of scoring the flowering time in the control cross. All of the F1 progeny were classified as late flowering as they had ≥11 nodes at flowering, similar to Jester and R108. The F2 progeny were classified as late flowering as they had ≥11 nodes at flowering, similar to Jester and R108, or unclassified due to being difficult to score due to their tiny size or altered aerial architecture.
The mapping cross
To develop a population for mapping spring1 by linkage analysis with DNA markers, spring1 was crossed to Jester. We used spring1 either as the male or as the female plant. The seed from the crosses was collected and we planted out the F1 plants, scored their flowering time, which was early, similar to spring1, and then allowed these plants to self-fertilise to produce the F2 generation. The F2 seed from five different F1 plants was then sown out and scored for flowering time. No differences in phenotype were observed when spring1 was used as a male or a female plant in the backcross to Jester. Due to lack of space, we grew the F2 plants in batches with spring1, Jester or R108, which confirmed that the control plants flowered at a similar time in each experiment. Data from the cross of spring1 as a male and Jester as a female is presented. F2 plants were classified as early flowering if they had ≤7 nodes at flowering or had flowered rapidly (≤28 days after germination), similar to spring1. The F2 progeny were classified as late flowering if they had ≥11 nodes at flowering, similar to Jester. A third group F2 plants were categorised as unclassified, due to not falling into our two classes, or being difficult to score due to their tiny size or altered aerial architecture.
The test cross
In order to further analyse the inheritance of spring1 and to generate more plants for linkage analysis, we carried out a Test cross between F1 plants from “spring1 x Jester” as the male and Jester as female. Eighty crosses were carried out. Barrels from the crosses were collected and progeny plants were grown up and classified as early flowering if they had ≤7 nodes at flowering or had flowered rapidly (≤28 days after germination) similar to spring1, or late flowering if they had ≥11 nodes at flowering, similar to Jester. A third group of progeny plants were categorised as unclassified, due to not falling into our two classes, or being difficult to score due to their tiny size or altered aerial architecture.
Spring1 Linkage Mapping with DNA Markers
We tested if spring1 co-segregated with DNA markers from BACs in the region of chromosome 7 that had been previously shown to contain a major QTL for flowering and carried six candidate activators of flowering [15]. The candidate activators were MtCO, Medtr7g083540; FTa1, Medtr7g084970; FTa2, Medtr7g085020 and Medtr7g085030; FTc, Medtr7g085040; FD, Medtr7g088090 and PHYTOCHROME KINASE SUBSTRATE I (PKS), Medtr7g088200. We carried out the linkage analysis on the F2 plants from the Mapping cross of spring1 x Jester and on the progeny of the Test Cross. For example, a marker that was closely linked to spring1 would be expected to co-segregate close to 100% with flowering time in the following way: The spring1 version of the marker would be present in the early-flowering segregants (homozygous or heterozygous in the F2 plants and heterozygous in the Test cross) and the Jester version homozygous in the late-flowering plants. Recombination events between a closely-linked marker and spring1 were detected as follows: Recombinants in the late flowering F2 class from the “spring1 x Jester” cross were identified by plants that were heterozygous for the Jester allele. The recombinant plants amongst the early flowering F2 plants, that could be distinguished, were homozygous for the Jester allele. In the Test cross, recombinants in the late flowering class were heterozygous for the Jester allele and in the early flowering class they were homozygous for the Jester allele.
The primer sequence of existing markers were obtained from the Integrated Genetic Map of Medicago truncatula http://www.medicago.org/genome/map.php or from Pierre et al [15]. New insertion/deletion (indel) or Simple Sequence Repeat (SSR) markers were also developed. Primers that flanked introns or that encompassed SSRs were tested for the ability to detect polymorphisms. When DNA sequence annotation was not provided for a BAC from Genbank, a Chromosome Visualisation Tool (CViT) BLAST search http://medicagohapmap.org/with the BAC sequence against the Medicago pseudomolecule Mt3.5 genome assembly was carried out. This provided GeneCall Identities of all the genes in the BAC “Mt3.5 BAC Genecall Table”. By searching with a GeneCall ID against the TIGR/JCVI GBrowse “Medicago GBrowse- IMGAG Annotation v3.5” http://gbrowse.jcvi.org/cgi-bin/gbrowse/medicago/#search, a detailed gene model provided annotation of predicted coding sequences, 5′ and 3′ untranslated regions. Additional SSR markers in non-annotated BACs were identified using a Geneious http://www.geneious.com/plug in “Phobos” http://www.ruhr-uni-bochum.de/spezzoo/cm/cm_phobos.htm.
DNA Extraction and PCR Amplification
Genomic DNA from plants was extracted with the Extract-N-Amp™ Plant PCR Kit (Sigma-Aldrich New Zealand Ltd.) on ∼0.5 cm diameter disks of young leaf tissue. The guidelines of the manufacturer were followed but the volume of Extraction Solution and Dilution Solution used per sample was halved. For PCR, 1 µL of the extracted genomic DNA was used in a 10 µL reaction containing 1× Phire® Reaction Buffer, 0.2 mM dNTPs, 0.2 µM of each primer and 0.2 µL of Phire Hot Start II DNA Polymerase. Genomic DNA was omitted for control reactions. The primer sequences are given in Table 2. The PCR reaction was carried out on an Applied Biosystems 96-well GeneAmp® PCR System 9700 machine. The reaction program consisted of an initial denaturation step at 98°C for 30s, followed by 35 cycles of denaturation at 98°C for 10s, annealing at 55°C for 10s and elongation at 72°C for 15 s/kb depending on expected amplicon size, and a final elongation step at 72°C for 1 min. The reaction was then cooled down to 15°C. PCR products were separated on a 3% agarose gel or on a 12.5% polyacrylamide gel.
Table 2. DNA markers used for fine mapping spring1 on chromosome 7.
| Marker | BAC | Mt3.5 Genome assembly | UMN (cM) | Type | Forward Primer | Reverse Primer | cBand Size (bp) | dLate F2 | dEarly F2 | dLate Test Cross | dEarly Test Cross |
| ah2_9|7d | CT967302 | chr7:17913549..17913763 | 42.4 | SSR | AAGGGCCACAAAGAGAAGGT | GGTGGAATCAAAGCACCAAT | 215 | 7/57 | NT | NT | NT |
| ah2_172c4a | AC146784 | chr7:19580059..19580346 | 47.0 | SSR | GCCGAATATGCGAGCTTTT | TGGCTCACCTTCCACTTCA | 288 | 6/57 | NT | NT | NT |
| a003B12 | AC159114 | chr7:21245026..21245168 | 47.7 | SSR | GCTTTTGAGGTCAAAGTACAA | CAGGGAGTAGCTTGTAAATCA | 143 | 4/57 | NT | NT | NT |
| ah4_42i13a | AC161750 | chr7:21396080..21396350 | 47.7 | SSR | TGTATGCCAAGCATCGGTTA | TGCCAAGAGGAAACTTGGTT | 271 | 4/57 | NT | NT | NT |
| aH2_142N19_fr1 | AC147179 | chr7:22856921..22857163 | 50.4 | SSR | CCAATTTTGACACCCTACAT | GAGATTAAATCCACCCCTTC | 243 | 1/57 | NT | NT | NT |
| Medtr7g080490 | AC147179 | chr7:22879847..22880323 | – | indel | GCCAGCTCCAGCTGACATTGTGG | CCCTTGAGGTCAGTCTCAACCGT | 477 | 1/57 | 3/419 | 1/95 | 3/83 |
| ah2_14c17b | AC142095 | chr7:23179073..23179192 | 50.4 | SSR | AGCTGCTCTCAGTGCCATTT | TGCGTGTGACAAGTTCCTCT | 120 | 0/57 | NT | 1/95 | 2/83 |
| a003A09 | AC133780 | chr7:24672851..24672966 | 50.4 | SSR | TTGTGGTGACTAGTGATTGG | ATGTGAAGTAAATCCCTTGC | 116 | 0/57 | 3/419 | 0/95 | 0/83 |
| MtCO | AC133780 | chr7:24688717..24688738 | – | indel | GAATAGAGGGGATGCCATGTT | CAAATCGCCCTCTCACTCTC | 381 | NT | 3/419 | NT | NT |
| TF_B3 | AC149131 | chr7:24783230..24783575 | – | indel | TCCGACTGTTATTGTTAGGCCA | TGCAGAAAGCAGCAGAGGCT | 346 | NT | 2/419 | NT | NT |
| eIF-4gamma | AC149131 | chr7:24840562..24840940 | – | indel | TCTGGCTATTGTGAGATGGGTCT | ACGGTTGTATGTTTTGCTTACTGC | 379 | NT | 1/419 | NT | NT |
| Medtr7g084090.1_SUVH4 | AC155894 | chr7:24973864..24974348 | – | indel | TCCAGGACGCTATCAAGCGCA | CGAGTGGTGCTGTTTGCCGC | 485 | NT | 1/419 | NT | NT |
| Medtr7g084170.1 | AC186135 | chr7:25016694..25017139 | – | indel | AGGAAATTCTACCAAAGATTCTGAG | TCATCCAAGTTTGACCGTTTTCT | 446 | NT | 1/419 | NT | NT |
| Medtr7g084560.1 | AC157890 | chr7:25212756..25213142 | – | indel | AGCATTGACAGGATTTCGTGATGC | TGGCCACTACGATCCAGCTCA | 387 | NT | 0/419 | NT | NT |
| FTa1 | AC123593 | chr7:25433850..25434142 | – | indel | CCTTCAAAATATGAAAAAGGGCTA | AAATTTAAAATGTCTTCCTTGCTC | 293 | 0/57 | 0/419 | 0/95 | 0/83 |
| Medtr7g085120 | AC123593 | chr7:25499863..25500262 | – | indel | GCTGGTAGCACTGGTAGCCGTA | GCAGCTTGTTCCGGATCATTTGCAT | 400 | 0/57 | NT | 0/95 | 0/83 |
| Medtr7g085190.1 | AC145753 | chr7:25527032..25527377 | – | indel | TGAGGCTTATTGTTCAAGTCAAGGA | TGGTAAACCTTATGCTGAGAGGA | 346 | 1/57 | 0/419 | 0/95 | 0/83 |
| Medtr7g085200 | AC145753 | chr7:25537421..25538020 | – | indel | CCCAAGTTTCCCAAAACATAGT | GGTTTTGGTTTCATGAGAGATGGT | 600 | 1/57 | NT | 0/95 | 4/83 |
| AC145753_RR1 | AC145753 | chr7:25593027..25593223 | – | SSR | ACGAGGAACGGTGGCAGAAGGA | TGGTTGGTCTTGTAATATTGGC | 197 | 2/57 | NT | NT | NT |
| AC167711_RR8 | AC167711 | chr7:25677751..25677868 | – | SSR | TGCTGAGGCACGTCCCAGTG | ACCCCTGAAACCAGACAAGCCA | 118 | 3/57 | NT | NT | NT |
| AC167711_RR5 | AC167711 | chr7:25695231..25695594 | – | SSR | GTGAAGAGCAAGGGGTCGCGT | TGATACAACAAAGACAAACCACAGGCT | 364 | 3/57 | NT | NT | NT |
| AC148816_RR1 | AC148816 | chr7:25943053..25943342 | – | SSR | TCAGCTTGGGAACGTTGGTCGT | GGAGGCGCATCATCACCCCG | 290 | 4/57 | NT | NT | NT |
| AC148816_RR4 | AC148816 | chr7:25952355..25952590 | – | SSR | GGGTCATGGCCTTGGACCACA | ACCTCCTCCAACAAAAGCCACCT | 236 | 4/57 | NT | NT | NT |
| AC148816_RR5 | AC148816 | chr7:25957056..25957337 | – | SSR | AGACGATGATGAAGGTGAGGATGGA | TCAGCCTTCTTTTCCAAGAGTGCTTC | 282 | 4/57 | NT | NT | NT |
| AC148816_RR12 | AC148816 | chr7:26039826..26040091 | – | SSR | TGGCTGAATAACTGTTGTGCAAGGCT | TCAGTGGACGATCGTTGATACTTGTG | 266 | 4/57 | NT | NT | NT |
| a002E05 | AC126009 | chr7:26164550..26164669 | 53.3 | SSR | ATGGAAGGTGGAACCTATCT | GGTGTCGACTGATCCTAGC | 120 | 6/57 | NT | NT | NT |
| a005G08 | AC141107 | chr7:27227337..27227598 | 53.3 | SSR | GGTTTACTGGCCCTCAACAA | CTCCGTATGCCTTTCTTCCA | 262 | 13/57 | NT | NT | NT |
| ah2_81g19a | AC153128 | chr7:28185897..28186094 | 56.1 | SSR | GTTCCAAAAACGCACCAAGT | CATGACAGCAGTACATTGCC | 198 | 20/57 | NT | NT | NT |
| bMTIC714 | AC126016 | chr7:34518760..34518883 | 57.0 | SSR | TAGAAAAGCACAACAAGCTG | TGCTACGTATCAAATCAACAA | 124 | 29/57 | NT | NT | NT |
DNA markers from the MtCO region of chromosome 7 were analysed for linkage to spring1 in the mapping crosses (see Text). The interval containing spring1 is flanked by Medtr7g084170.1 and Medtr7g085190.1 shown in bold; each marker is separated from spring1 by one recombination event.
DNA marker taken from the University of Minnesota (UMN) Integrated Genetic Map of Medicago truncatula http://www.medicago.org/genome/map.php.
DNA marker from Pierre et al (2010).
Size of PCR fragment predicted from Mt3.5 Genome assembly from http://medicagohapmap.org/.
Numbers of recombinants detected by the marker in the late or early flowering classes from the F2 of the “spring1 x Jester” cross, or from the Test cross (see Table 1 and Text). “Test Cross” is (♂(♂spring1 x ♀Jester) x ♀Jester). NT is not tested. Indel is insertion/deletion; SSR is Simple Sequence Repeat. The column entitled Mt3.5 Genome assembly gives the position of the marker on the pseudomolecule from Mt3.5 Genome assembly from http://medicagohapmap.org/after a Chromosome Visualisation Tool (CViT) BLAST search http://medicagohapmap.org/was carried out.
Analysis of Gene Expression by qRT-PCR
RNA extraction, cDNA synthesis using an oligo dT primer and qRT-PCR on an Applied Biosystems 7900HT Sequence Detection System was carried out as previously described [18]. Each data point presented is derived from two or three biological replicates harvested in parallel, with each replicate consisting of a pool of tissues from at least three independent plants. All qRT-PCR results were replicated in one or more experiments on independently grown plants. Whole aerial parts of plants with three fully-expanded true leaves were analysed, or leaf and shoot apical samples were harvested separately, as described in the text. The PCR primer sequences used were as previously described for FTa1, FTa2, FTc, Tubulin (TUB) and PROTODERMAL FACTOR 2 (PDF2) [18]. The other primer sequences used for qRT-PCR are listed in Table S3. The identity of PCR amplicons were confirmed by DNA sequencing.
Microarray Analysis of Global Gene Expression
Total RNA was extracted from R108 and spring1 grown in long daylength conditions from the first trifoliate leaf when seedlings were 12–14 days old and at the three true-leaf stage (one monofoliate leaf and two trifoliate leaves). Three biological replicates were harvested in parallel, each consisting of a pool of leaves from three independent plants. RNA quality was checked using RNA 6000 Nano Chip using the Agilent 2100 Bioanalyzer instrument. cDNA synthesis from each of the biological replicates, labelling and hybridisation to the Affymetrix Medicago GeneChip arrays were performed according to the manufacturer’s instructions (Affymetrix, Santa Clara, CA, USA).
Statistical analysis of microarray data was performed using Bioconductor in the R statistical computing environment (http://www.R-project.org). Briefly, normalization was performed using the Robust Multichip Algorithm (RMA) with background correction. Normalized data were then analyzed using the limma package [28] to identify differences in expression levels between the genotypes. Differentially-expressed genes were selected based on statistical significance (false discovery-rate (FDR), corrected p-values of ≤0.05 and fold-change magnitude (2-fold or greater up or down).
DNA Sequence Analysis of the FTa1 Genomic Region
PCR was used to amplify 6346 bp of the FTa1 genomic region from spring1 and R108 from the nearest upstream gene Medtr7g084960 to 511 bp downstream of the translation termination codon. The primers used were: 5′- TGCAAACATAGAAAGGCCATC -3′ and 5′- TGTTTGTGGTTTGCAGCAGT -3′. The resulting PCR fragments were directly sequenced, DNA sequence contigs assembled using Geneious software http://www.geneious.com/ and compared with each other and with the Jemalong/A17 sequence. Alignment of the 5′ region was done using the MultAlin program [29]. The R108 and spring1 sequences were identical, but differed from A17. The accession number of the R108 sequence is GenBank KC108841.
Results and Discussion
Spring1 Confers Dominant Early Flowering
In order to identify genes that regulate flowering in Medicago, we carried out a glasshouse screen of a T-DNA tagged population in the R108 accession [23]. An early flowering mutant was identified that we named spring1 (Fig. 1a). However, the mutant was not T-DNA tagged as determined by PCR genotyping or Southern blot analysis with T-DNA sequence probes (data not shown). Thus spring1 can be deemed a somaclonal mutant and is likely to have arisen during plant regeneration [30]. In Medicago, two genes that were tagged with an endogenous MERE1-1 retroelement were identified in somaclonal mutants [31], [32]. MERE1-1 is a low copy copia-type retroelement that is active during regeneration of Medicago in tissue culture [31]. Thus, we carried out transposon display experiments to identify MERE1-1 insertions in spring1, but none of the six insertions obtained were linked to the spring1 early flowering phenotype (data not shown).
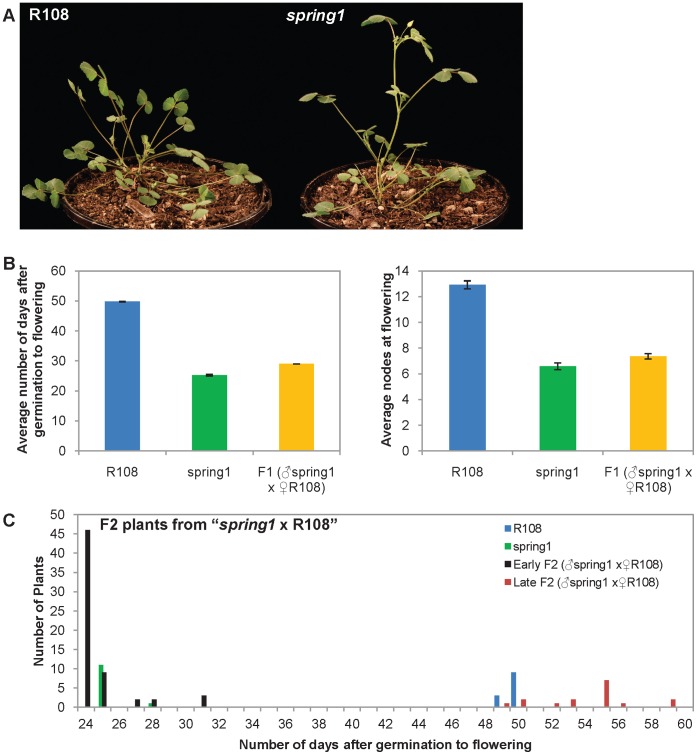
Figure 1. Flowering time of plants from the Backcross “spring1 x R108”.
Spring1, an early flowering mutant, was backcrossed with wild type R108 plants and the F1 and F2 progeny were grown in long day conditions and scored for flowering time. a) Photographs of R108 wild type plant and the spring1 mutant plants. Both plants were photographed 30 days after germination. b) Flowering time of the F1 progeny (n = 27) compared to spring1 (n = 12) and R108 (n = 12). The F1 plants flowered much more rapidly than R108 and at a similar time to spring1. Similar results were obtained when flowering time was scored using either of two methods; the number of days after germination to flowering, or the number of nodes on the primary axis at flowering. c) Distribution of the flowering time of the F2 progeny compared to spring1 and R108. The F2 population segregated 62 early flowering and 16 late flowering plants, as scored by days after germination to flowering, and by comparison to the parental lines, indicating that spring1 was a monogenic dominant mutation.
In order to investigate the genetics and inheritance of spring1, we backcrossed it to wild type R108 plants and grew the plants under long daylength conditions (Fig. 1, Table 1). All the F1 plants flowered significantly earlier than R108, at a similar time to spring1, when flowering time was measured both as the number of days after germination to flowering, or as the number of nodes on the primary axis at the time of flowering (Fig. 1b; Table 1). Next, we allowed the F1 plants to self fertilise, planted out the resulting 78 F2 plants and scored the number of days after germination to flowering. The F2 plants segregated into two groups, with the majority flowering early like spring1 (Fig. 1c; Table 1). The flowering time of the F1 and F2 plants strongly indicate that spring1 confers dominant early flowering time. The experimental hypothesis of a single dominant gene is further supported by an approximate 3∶1 segregation ratio observed in the F2, as 62 F2 plants flowered early while 16 were late (χ2 = 0.84; 0.1<p<0.5).
Crosses of spring1 and R108 to Jester
We carried out two crosses to develop populations for mapping spring1. These were the Mapping cross and the Test cross, both of which involved crossing spring1 with Jester. We also carried out a Control cross between R108 and Jester. Jester is closely related to Jemalong/A17 (ssp. truncatula), the subject of the Medicago genome sequencing project [13], [24]. R108 is the background genotype of spring1, but is a different subspecies from Jester, ssp. tricycla [33]. While the cross between the two Medicago subspecies provides abundant polymorphisms for mapping, a disadvantage is that it also affects plant growth. For example, previously, chlorosis in F1 plants was observed in a cross of R108 × A17 [33]. Therefore, the Control cross was also done between wild type R108 and Jester to enable us to test the hypothesis that a seedling’s reduced growth was not due to spring1 and that flowering time was not affected in progeny of crosses between Jester and R108 genotypes. Developmental difficulties were not reported in the mapping populations previously used to identify Medicago flowering time QTLs, but these were carried between Jemalong and other accessions of the same sub species (ssp. truncatula) [16].
The F1 plants from the crosses between spring1 and Jester and the cross between R108 and Jester grew more slowly and were smaller and paler than the parents. Because of the variation in growth rates between the parents and the F1 progeny, we elected to score the flowering time of progeny from these crosses, primarily based on the number of nodes to flowering. We reasoned that using the alternative method to score flowering time, in days after germination to flowering, could be misleading as slow growth might result in a plant being classified erroneously as late flowering. The “spring1 x Jester” F1 plants flowered early and at a similar node number as spring1 plants and much earlier than plants from the control cross “R108 x Jester” which flowered late like Jester (Fig. 2a, Table 1). This confirms that spring1 confers dominant early flowering in the cross to Jester, as it does to R108.
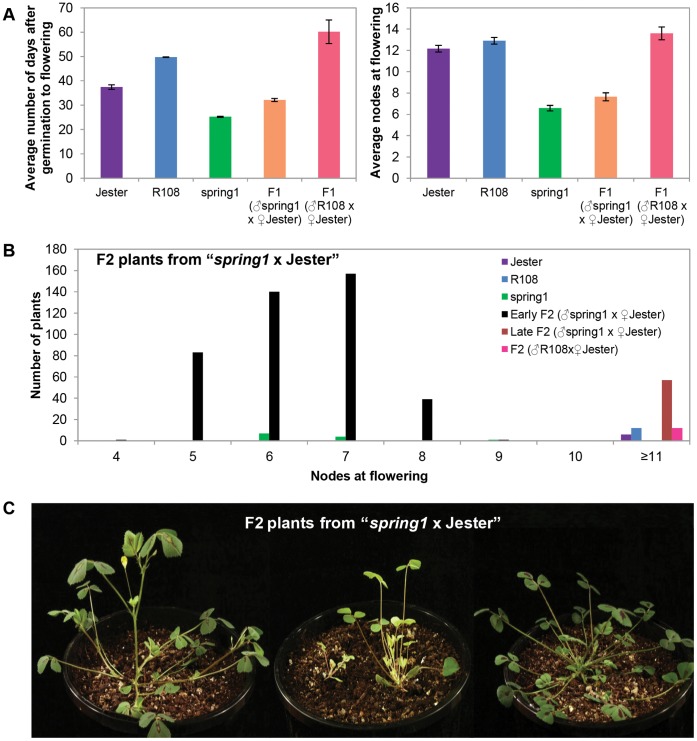
Figure 2. Flowering time of plants from “spring1 x Jester” and from “R108 x Jester”.
Spring1, an early flowering mutant in the R108 accession was crossed with Jester plants and the F1 and F2 progeny were grown in long day conditions and scored for flowering time (Table 1). A Control cross “R108 x Jester” was also performed. a) Flowering time of the F1 progeny from the Backcross “spring1 x Jester” (n = 32) and from the Control cross “R108 x Jester” (n = 12) was compared to spring1 (n = 12), Jester (n = 6) and R108 (n = 12). Flowering time was scored using two methods; the number of days after germination to flowering, or the number of nodes on the primary axis at flowering. The F1 plants from the Mapping cross flowered much more rapidly than the F1 plants from the Control cross by either measure, indicating that spring1 confers dominant early flowering in crosses to Jester. b) Distribution of the flowering time of the F2 progeny from the Mapping cross and the Control cross compared to parental lines. Plants that were scored as “unclassified” or died young are not included. The F2 population from “spring1 x Jester” segregated 421 early flowering and 57 late flowering plants as scored by nodes at flowering. The class with ≥11 nodes includes plants that had up to 25 nodes, but had not flowered by the time scoring was terminated at 87 days. The Control cross produced only late flowering F2 plants, with some having up to 19 nodes, but not having flowered by the time scoring was terminated at 65 days. c) Photographs of F2 plants from the “spring1 x Jester” Mapping cross; a typical early flowering plant with flowers (left), plants that have not flowered that are either very small, pale and slow growing, or small with an altered morphology (middle), and a typical late flowering plant (right). All plants were photographed at 26 days old.
Next, groups of F2 plants from “spring1 x Jester” (747 total plants), and a small F2 population of 18 plants from the control cross “R108 x Jester”, were grown up. A total of 175 (23.4%) “spring1 x Jester” and 3 (16.6%) “R108 x Jester” F2 plants died (Table 1). Such effects on growth and seedling mortality were observed before in crosses between plants in R108 and Jemalong backgrounds [33]. F2 plants were classified as early flowering if they had ≤7 nodes at flowering or had flowered rapidly (≤28 days after germination), similar to spring1. They were classified as late flowering if they had ≥11 nodes at flowering, similar to Jester (Fig. 2b, Table 1). We scored 421 and 57 plants as early and late flowering, respectively. A third group of 94 F2 plants remained unclassified (Table 1), due to not falling into our two classes, or being difficult to score due to their tiny size or altered aerial architecture (Fig. 2c). The phenotypic distribution of flowering time was broader amongst the late flowering class in the F2 than seen in the parental Jester plants (Table 1). For example, the late class of F2 plants from “R108 x Jester” flowered with 11->19 nodes, while Jester flowered with 10–14 nodes. This may stem from interactions between the two genotypes. From a total of 18 F2 plants grown from the control cross “R108 x Jester”, 12 flowered late and 3 were unclassified (Table 1).
In total, 421 of the 572 surviving plants were classified as early flowering, which is consistent with spring1 conferring dominant early flowering (ie. expected 3∶1 ratio for early:late flowering). However, of the remaining plants, we could only confidently score 57 as late flowering. This gives a ratio of early-flowering to late-flowering plants highly skewed toward early flowering, with a ∼7∶1 ratio (χ2 = 43.6; p<0.001). This observed ratio does not support the experimental hypothesis of a monogenic dominant gene using this F2 progeny. Results of complementary genotyping experiments indicate that there is segregation distortion in the F2 (Table S1) and this was also observed in the Recombinant Inbred Line populations used to map the major QTL for flowering on chromosome 7 [14]–[16].
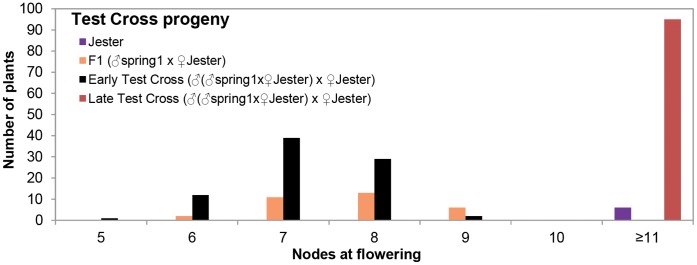
In order to further analyse the inheritance of spring1, we carried out a Test cross between F1 plants from “spring1 x Jester” and Jester [(“spring1 x Jester”) x Jester]. Out of 275 progeny plants (Table 1), 83 and 95 plants were scored as early and late flowering, respectively (Fig. 3, Table 1). Thirty plants were unclassified and 67 died, confirming the lethality for a quarter of the progeny as in the previous crosses with Jester. Similar to the F2 population, a broader distribution of flowering time amongst the late class (11 - >21 nodes, Table 1) was observed in the Test cross. Nevertheless, a 1∶1 segregation ratio (χ2 = 0.81; 0.1<p<0.5) of early and late flowering plants was observed in the Test cross, which supports the hypothesis of a single dominant gene.
Figure 3. Flowering time of plants from the Test cross.
Spring1, an early flowering mutant, was crossed with Jester plants and the resulting F1 plants were then crossed with Jester in the Testcross (♂(♂“spring1 x ♀Jester”) x ♀Jester). The Testcross progeny were grown in long day conditions and scored for flowering time. Graph showing the distribution of flowering time of plants that were classified as early flowering (n = 83) and late flowering (n = 95) compared with Jester (n = 6) and F1 plants (n = 32). The class with ≥11 nodes includes plants that had up to 21 nodes, but had not flowered by the time scoring was terminated at 69 days after germination. Plants that were “unclassified” or died young are not included. As parental and progeny plants grew at different rates, flowering was measured as the node number on the main axis at flowering.
Fine Mapping Excludes MtCO from the ∼0.5 Mb Interval Containing spring1
We reasoned that the dominance of spring1 was unlikely to result from loss of a repressor of flowering, as this would probably confer recessive early flowering, as seen for FLC loss of function plants in Arabidopsis [34]. Instead, we wondered if spring1 was a gain-of-function mutation in an activator of flowering and hypothesized that a highly active CO or FT gene conferred the spring1 dominant early flowering phenotype. This led us to turn to a candidate gene approach for spring1 using candidate activators as mapping markers in linkage analysis.
Previously, a major Medicago flowering time QTL in three mapping populations from different Medicago accessions was positioned on chromosome 7 and fine mapping identified a 2.4 cM confidence interval containing the QTL [15], [16], [35]. A CO-like gene, MtCO, was proposed to underly the QTL, as the gene was differentially expressed in two of the parental lines. However, the other genes in the interval (572 annotated genes) were not definitively excluded [15]. Apart from MtCO, five other candidate flowering time activators were located in the interval; three FT genes, FTa1, FTa2 and FTc (Table S2), and two genes encoding proteins related to FD and PHYTOCHROME KINASE SUBSTRATE I (PKS) [15], [16].
The three FT genes are clustered together within a 33.5 kb region in the QTL interval and present on the BAC AC123593 (Table S2, Fig. S1). These all encode the key residues needed for FT function [18], [36]. Recently, we reported on a study of the Medicago FT genes and demonstrated that fta1 mutants flower late and over expression of FTa1 accelerates flowering in both Medicago and Arabidopsis, indicating that this gene is important for Medicago flowering time [18], [36]. FTc over expression accelerated Arabidopsis flowering, but ftc mutants did not have altered flowering time and over expression was not tested in Medicago. Therefore, FTc also is capable of promoting flowering, but may be redundant in Medicago [18]. Over expression of the third FT gene from the cluster, FTa2, did not promote Arabidopsis flowering and was not tested in Medicago, thus its role in flowering time is uncertain [18].
BLASTp analysis indicated that the FD-like gene on chromosome 7 is not likely to encode the orthologue of FD, as it is less similar to Arabidopsis FD, than are six other Medicago b-ZIP genes, but nevertheless it may still play a role in flowering time control. Similarly, the MtCO gene on chromosome 7 is more related to a CO-LIKE gene, COL14, which has not been shown to regulate Arabidopsis flowering, than to CO [15].
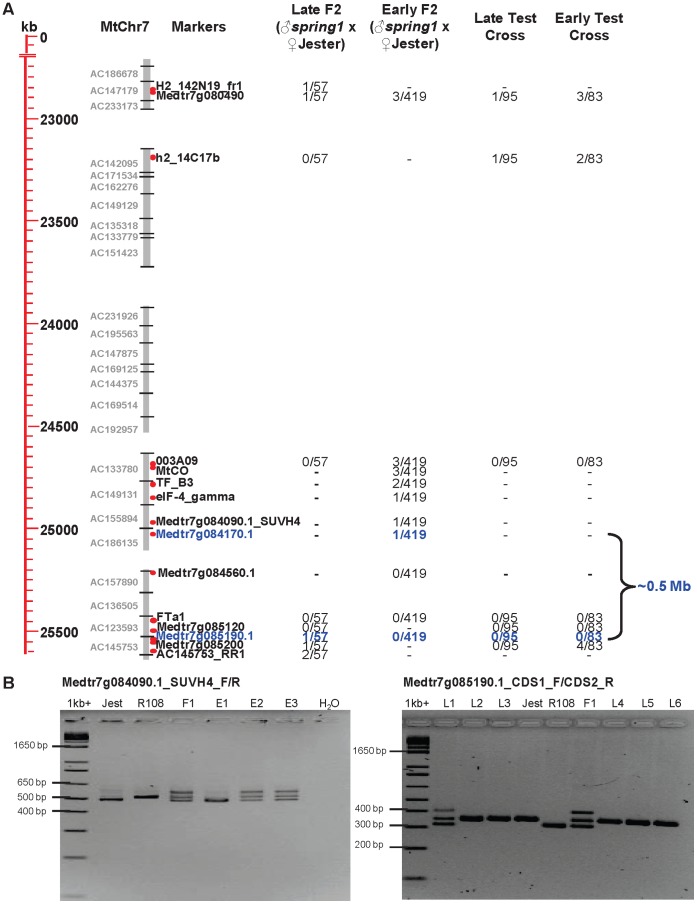
In order to test if the candidate activators from chromosome 7 co-segregated with the spring1 early flowering phenotype, we chose 29 DNA markers from the QTL interval comprised of 18 new and 11 existing markers, [15], [37] http://www.medicago.org/genome/map.php and genotyped 654 early and late flowering plants from the Mapping cross and the Test cross (Table 1). The results are summarized in Table 2 and Figure 4. The MtCO marker that was previously reported [15], was not able to detect DNA sequence polymorphisms between R108 and Jester, but a nearby marker 003A09 did. The marker 003A09 was very closely linked to spring1 as no recombinants were detected with 003A09 in the 57 late flowering F2 plants or in the 178 Test cross progeny. However, our subsequent mapping with 003A09 and a newly developed MtCO indel marker on the 419 early flowering F2 plants, indicated that both these markers were separated by 3 recombination events from spring1. These 3 recombinant plants were also confirmed to be recombinant with a more distant marker Medtr7g080490. Therefore, while MtCO is closely linked to spring1, mapping excludes this gene and thus we rejected the hypothesis that a lesion in MtCO is causative for the spring1 early flowering phenotype.
Figure 4. Markers for fine mapping spring1 on chromosome 7 and defining the ∼0.5 Mb interval that contains spring1.
a) Physical map of the spring1 region on chromosome 7 with DNA sequence in kilobases (kb), Bacterial Artificial Chromosome (BAC) clone contigs (grey bars) and mapping marker position (red dots). Markers defining the spring1 interval are blue. Four columns show the numbers of recombinants detected with the markers in the early and late flowering plants from the two Jester mapping populations; the F2 plants from cross “spring1 x Jester” and the progeny of the Test cross. b) Examples of PCR genotyping using two indel DNA markers flanking the spring1 interval. Control PCR reactions from Jester (Jest), R108 and F1 from the control cross (“R108 x Jester”) are shown. R108 and spring1 gave the same PCR products in all cases. PCR genotyping with marker Medtr7g084090.1 (left). Products from genotyping of three early flowering F2 plants (E1 to E3) from the Mapping cross “spring1xJester”. Plant E1 is homozygous for the Jester band, thus Medtr7g084090.1 is separated from spring1 by a recombination event. Genotyping with marker Medtr7g085190.1 on six late flowering F2 plants (L1 to L6) from the Mapping cross “spring1xJester” (right). Plant L1 is heterozygous, thus Medtr7g085190.1 is separated from spring1 by a recombination event. A feature of both indel markers is the F1 plants and the heterozygous plants give three bands after PCR. These are the expected Jester and R108 bands and a third larger band which is likely to be a heteroduplex of the two PCR differently-sized fragments that is slightly retarded during gel electrophoresis compared to the other bands. PCR products were separated by electrophoresis on a 3% agarose gel and photographed. The Invitrogen 1 kb+ ladder provided molecular size standards. Physical maps were redrawn from a Chromosome Visualisation Tool (CViT) BLAST search http://medicagohapmap.org/with the marker sequences against the current Medicago pseudomolecule Mt3.5 genome assembly http://blast.jcvi.org/er-blast/index.cgi?project=mtbe.
We also developed an indel marker for FTa1 based on a small deletion in intron 3 that was present in both R108 and spring1 plants, but not in Jester (Table 2). This marker showed 100% co segregation with spring1 in the F2 and Test cross plants. FTa1 is thus very closely linked (<0.6 cM) to spring1. To confirm linkage and delimit the physical interval containing spring1, we proceeded with linkage analysis with other markers from the FTa1 region of chromosome 7. Two other markers from genes near FTa1, Medtr7g084560.1 and Medtr7g085120, also did not detect recombinants (Table 2, Fig. 4). Finally, our fine mapping located spring1 to a physical interval on chromosome 7 of ∼0.5 Mb defined by markers Medtr7g084170.1 and Medtr7g085190.1 (Table 2, Fig. 4). Each was separated from spring1 by a single cross over event. This region contains 78 annotated genes on two non-overlapping BAC contigs (Fig. 4, Table S2). Along with FTa1, the interval contains the two other FT genes, FTa2 and FTc, but not the other candidate activators, MtCO, the FD-like and the PKS gene from the QTL interval [15].
FTa1 Transcript is more Abundant in spring1 than R108
To test if any of these FT genes were differentially expressed in spring1, we harvested total aerial tissues from 12–14 day old seedlings at the two trifoliate leaf stage and analysed gene expression by qRT-PCR. The FTa1 transcript was much more abundant in spring1 compared to R108 at all time-points over a diurnal time course (Fig. 5a). In contrast, the FTa2 gene was expressed at similarly very low levels in spring1 and R108 in the diurnal time course (Fig. 5b). The expression of the third FT gene in the spring1 mapping interval, FTc, was not detectable either in leaf or shoot apices from 12–14 day old seedlings (data not shown).
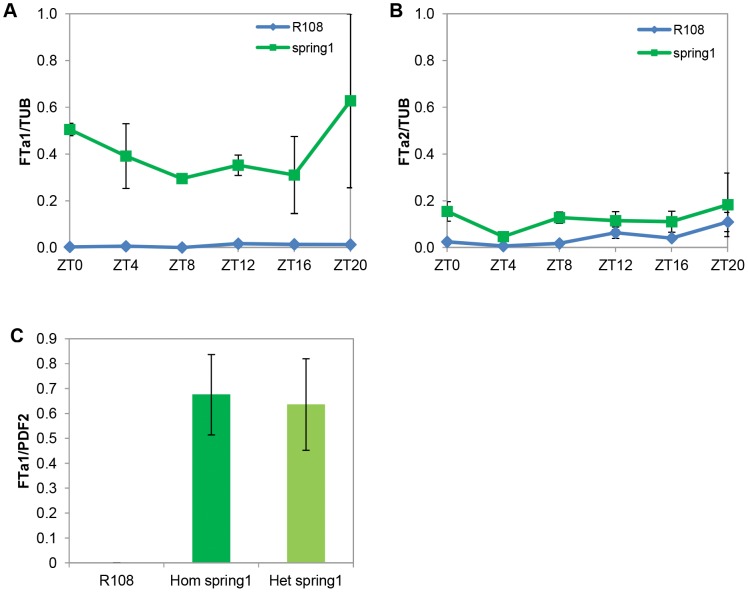
Figure 5. FTa1 is up-regulated in spring1 plants.
Accumulation of FTa1 and FTa2 transcript in spring1 and R108 in long day conditions was measured using qRT-PCR on 12–14 day old seedlings with two trifoliate leaves.Relative transcript abundance of FTa1 (a) and FTa2 (b), over a diurnal timecourse in the aerial parts of seedlings. Levels were normalised to TUBULIN (TUB) and calibrated relative to the expression of FTa1 (second biological rep) at Zeitgeber 20 (ZT0 is the time of lights on). The mean +/− SE of 2 biological replicates is shown for the spring1 samples. For R108, the two cDNA samples from each biological replicate were pooled and the mean +/− SE of the 3 technical replicates are presented. c) Accumulation of FTa1 transcript in the first trifoliate leaf of homozygous (after two backcrosses to R108) and heterozygous spring1 plants (F1 plants from a backcross to R108) with levels normalised to PROTODERMAL FACTOR 2 (PDF2). The mean +/− SE of 3 biological replicates is shown.
We also analysed FTa1 expression in leaves of heterozygous spring1 plants and saw strong up-regulation compared to R108, to the level seen in homozygous spring1 mutants (Fig. 5c). This increased accumulation of the transcript of the flowering-time regulator FTa1 in heterozygous plants correlated very well with the dominant early flowering phenotype of spring1.
Microarray Analysis of Global Gene Expression in spring1 and R108
To test if genes other than FTa1 were mis-expressed in spring1, we compared global gene expression in spring1 and R108 by microarray analysis. The first trifoliate leaf from 12–14 day old plants at the two trifoliate leaf stage was harvested and analysed.
The results indicated that the two genotypes had highly similar gene expression as only 13 genes were differentially expressed; 8 genes were up-regulated in spring1 and 5 genes were down regulated (Table S3). Apart from FTa1, none of these genes was located in the spring1 mapping interval. We carried out qRT-PCR on 12 of these genes which confirmed the microarray results (Fig. 6a, c and e, Table S3, data not shown). We also used qRT-PCR to further confirm that 7 other genes in the immediate vicinity of FTa1, including FTa2 and FTc (Fig. S1a), were not differentially expressed in spring1 (data not shown).
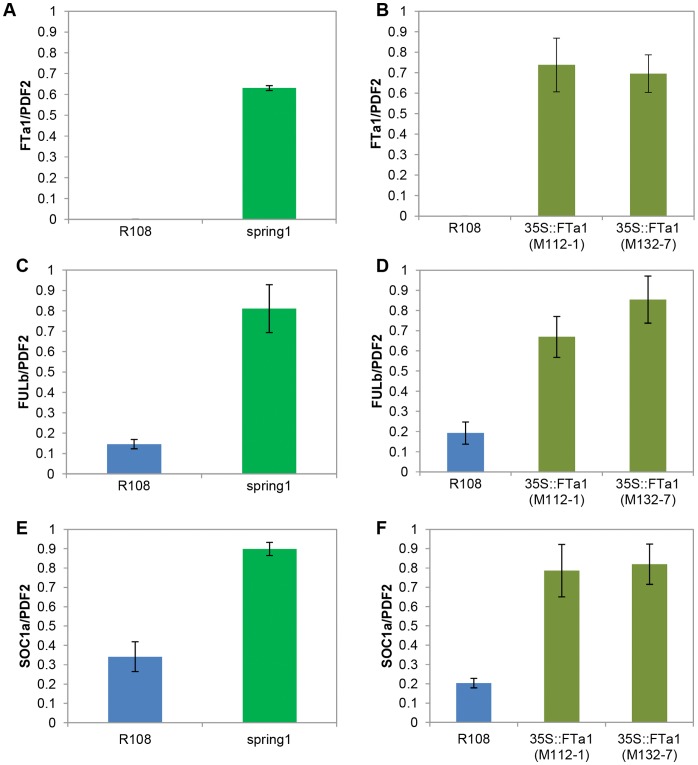
Figure 6. FULb and SOC1a are up-regulated in spring1 and in transgenic Medicago plants over expressing FTa1.
Accumulation of FTa1, FULb and SOC1a in spring1, 35S::FTa1 transgenic Medicago plants and R108 in long daylength conditions was measured using qRT-PCR on the first trifoliate leaf from 12–14 day old seedlings. The mean +/− SE of 3 biological replicates is shown relative to PDF2. Relative transcript abundance of FTa1 (a), FULb (c) and SOC1a (e) in spring1. Relative transcript abundance of FTa1 (b), FULb (d) and SOC1a (f) in 35S::FTa1 lines.
Next, we tested if the genes identified in the microarray still retained their differential expression after spring1 had been backcrossed to R108 by carrying out qRT-PCR on homozygous spring1 plants selected after two backcrosses (Table S3, data not shown). Eight of the 12 genes analysed, were no longer differentially expressed as before; either they were now expressed at the same level as R108 (5 genes) or had the opposite pattern of expression to that previously determined (3 genes). The ninth gene (probeset Mtr.51129.1.S1_s_at) showed very variable expression; it was undetectable by qRT-PCR in the spring1 RNA samples that were used in the microarray, but after two backcrosses it was expressed at much higher levels than before in spring1 (>500× higher), but still less than in R108. Three genes, FTa1, FRUITFULLb (FULb) and SUPPRESSOR OF OVER EXPRESSION OF CONSTANS1a (SOC1a), robustly retained their differential expression in spring1 after the backcrosses to R108. Of these, FTa1 was the only gene that showed 100% co-segregation with spring1 in linkage analysis.
FULb [12] and a SOC1-like protein with 66% amino acid identity with Arabidopsis SOC1 [7], designated SOC1a, are MADs transcription factors. In Arabidopsis, SOC1 and FUL function in flowering control, as a floral integrator and a floral meristem identity gene respectively, and over expression of FT results in an increase in the abundance of FUL transcripts in leaves and SOC1 in seedlings [38]–[40]. Therefore, we reasoned that the up-regulation of FULb and SOC1a in spring1 might result from the increased levels of FTa1. In order to test this, we analysed transgenic Medicago plants that were over expressing FTa1 from the CaMV 35S promoter [18] (Figure 6b, d and f). Both FULb and SOC1a were up-regulated in the transgenic lines compared to R108. This strongly indicated that their differential expression in spring1 resulted from the increased abundance of FTa1, rather than being the cause of it.
DNA Sequence Analysis of the FTa1 Gene in spring1 and R108
Since FTa1 transcript accumulation was higher in spring1 plants and linkage mapping showed that FTa1 co-segregated with the dominant early flowering spring1 phenotype, we hypothesised that a change to the FTa1 promoter in spring1 might lead to transcriptional up-regulation of the FTa1 gene. Therefore, to test if the spring1 FTa1 genomic region differed in sequence from R108, we used PCR to amplify a segment of chromosomal DNA from both genotypes spanning the complete 5′ FTa1 region from the nearest upstream gene (Medtr7g084960), to just beyond the end of the 3′UTR of FTa1 (Fig. S1b). We obtained a 6346 bp DNA fragment from both genotypes, including the 4062 bp 5′ intergenic region, 1682 bp of the FTa1 gene (including the three introns), the 3′UTR (349 bp) and 162 bp of the 3′ intergenic region. However, after comparing these genomic sequences we found that they were identical, showing that the difference in FTa1 expression is not due to promoter or intron sequence changes in spring1.
Conclusions
We were able to carry out a forward screen of a Medicago mutant population, identify the flowering time mutant spring1 and fine map spring1 to a small interval on chromosome 7. Thus we demonstrated the feasibility of mapping mutations in hybrids between Medicago truncatula sub species, R108 and Jemalong/A17, despite the difficulties with growth that were encountered. The spring1 mutation confers dominant early flowering. Based on the paradigm of CO activating flowering in Arabidopsis [1], and the proposal that a CO-like gene, MtCO, might underly a major QTL for Medicago flowering [15], one idea at the outset of this work, was that a highly active CO might lead to rapid flowering in Medicago. However, fine mapping has excluded MtCO from the interval containing spring1. Nevertheless, our candidate gene mapping approach was successful as we demonstrated that another candidate activator we selected, FTa1, co-segregated 100% with spring1. We delimited a ∼0.5 Mb spring1 interval, raising the possibility that one of the three clustered FT genes was responsible for the spring1 phenotype.
Analysis of the expression of the three FT genes showed that one of them, the known activator of Medicago flowering, FTa1, was strongly up-regulated in both heterozygous and homozygous spring1 plants. The increased abundance of FTa1 in heterozygous spring1 plants is consistent with the dominant early flowering conferred by the spring1 mutation. Global analysis of gene expression in spring1 and R108 further reinforced the linkage of FTa1 with the spring1 phenotype as it was the only gene mis-expressed from the mapping interval. Two of the genes that showed consistent mis-expression, encode the MADs transcription factors and candidate flowering regulators, SOC1a and FULb, both of which are also upregulated in transgenic plants over expressing FTa1, suggesting that their mis-expression in spring1 results from up-regulation of FTa1. However, as there is no DNA sequence change in the spring1 FTa1 promoter or introns, further work is underway to identify the genetic, or epigenetic, basis of the up-regulation of FTa1 in spring1.
Supporting Information
The DNA sequence of R108 and spring1 is identical in the FTa1 genomic region, but differs from the reference genome A17. a) Diagram showing the predicted gene annotation in the spring1 mapping interval in the vicinity of the three FT genes. b) Diagram comparing the DNA sequences of the FTa1 region between R108 and A17. PCR was used to amplify the region of DNA from the nearest upstream gene (Medtr7g084960) to just downstream of the 3′UTR of FTa1 in spring1 and R108. Both fragments were directly sequenced and their sequences compared to each other and to A17. The spring1 and R108 sequences were identical. The predicted FTa1 protein encoded by A17 and R108 was identical and the three intron sequences were highly conserved, ranging from 100% nucleotide identity in the first two introns to 96% identity in the longer, third intron which has an indel of 23 bp. The 3′ UTR sequences were also highly conserved (98% identical). However, there was a striking difference in the length of the FTa1 5′ region, with the A17 sequence being 1347 bp shorter than the R108 sequence. This resulted from a series of indels in this region, the largest of which was a 1442 bp solo Long Terminal Repeat (LTR) from the Angela family of the Ty1/copia super family retrotransposons [41] in R108 and spring1, that was missing in A17. There are over 40 of this type of solo LTR in the genome [41]. There was a 5 bp repeated sequence flanking the solo LTR in R108 and spring1. Apart from the indels, there were blocks of high sequence conservation (94–99% nucleotide identity) shared between the 5′ region in R108 and A17.
(TIF)
Genotyping shows segregation distortion of a DNA marker in the spring1 interval in F2 plants of the cross of “spring1 x Jester”. PCR genotyping using a DNA marker (FTa1) from the interval containing spring1 was carried out on plant DNA samples from the two mapping crosses. These included the early and late flowering plants, but also additional samples comprising most of the “unclassified” plants and a few of the dead plants. a) In the Mapping cross “spring1 x Jester”, our experimental hypothesis was that we expected ¼ of the plants to be homozygous for the Jester marker genotyped. However, we scored only 62 plants (1/9) as homozygous Jester out of 578 genotyped. This gives a χ2 value of ∼63, a value of p<0.001 leading us to reject the experimental hypothesis. b) In the Testcross, our experimental hypothesis was that we expected 1/2 of the plants to be homozygous for the Jester marker genotyped. We scored 101 plants as homozygous Jester out of 218 genotyped. This gives a χ2 value of ∼1.2, a value of 0.5<p<0.1 leading us to accept the experimental hypothesis.
(DOCX)
List of annotated genes predicted within the ∼0.5 Mb interval containing spring1. The gene annotations were obtained from the BAC sequences in Medicago pseudomolecule Mt3.5 genome assembly http://medicagohapmap.org/. The three FT genes are in BAC AC123593 and shown in bold.
(DOCX)
Microarray identification of genes that are differentially expressed in leaves of spring1 compared to R108. The log fold change in gene expression with a p value of ≤0.05 was calculated from 3 biological repeats of each genotype grown in long day conditions. The first trifoliate leaf at the three-leaf stage was harvested. Each biological replicate was a pool of three leaves. Genes with a two-fold or greater change in gene expression are listed. aAll of these genes were confirmed to be differentially expressed by qRT-PCR on the RNA used in the microarray, except Mtr.21428.1.S1_at which was not done. bAfter two backcrosses to R108, gene expression in homozygous spring1 plants was again compared to R108, but only 3 genes, FTa1, FUlb and SOC1a, retained similar differential expression as first observed in spring1. The fourth gene Mtr.51129.1.S1_s_at showed very variable expression; it was undetectable by qRT-PCR in the original spring1 RNA samples and after two backcrosses was expressed about 16× less than R108, but at much higher levels than before in spring1. After the backcrosses, the remaining genes either were expressed at the same level as R108 (c5 genes) or had opposite pattern of expression to that previously determined (d3 genes).
(DOCX)
Acknowledgments
We thank David Chagne and Robert Schaffer for constructive criticism of the manuscript and David Chagne and Richard Gardner for advice on mapping. We thank Viviane Cosson for the preliminary identification of the spring1 mutant.
Funding Statement
The research was funded by the New Zealand Foundation for Research Science and Technology (www.msi.govt.nz/) contract numbers C10X0816 MeriNET and C10X0704 and by the New Zealand Marsden Fund (www.royalsociety.org.nz/programmes/funds/marsden/) contract 10-UOA-200. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Putterill J, Laurie R, Macknight R (2004) It´s time to flower: the genetic control of flowering time. BioEssays 26: 363–373. [DOI] [PubMed] [Google Scholar]
- 2. Amasino RM, Michaels SD (2010) The Timing of Flowering. Plant Physiology 154: 516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turck F, Fornara F, Coupland G (2008) Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annual Review of Plant Biology 59: 573–594. [DOI] [PubMed] [Google Scholar]
- 4. Turnbull C (2011) Long-distance regulation of flowering time. Journal of Experimental Botany 62: 4399–4413. [DOI] [PubMed] [Google Scholar]
- 5. Shalit A, Rozman A, Goldshmidt A, Alvarez JP, Bowman JL, et al. (2009) The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proceedings of the National Academy of Sciences of the United States of America 106: 8392–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simon R, Coupland G (1996) Arabidopsis genes that regulate flowering time in response to day-length. Seminars in Cell & Developmental Biology 7: 419–425. [Google Scholar]
- 7. Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, et al. (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis . Science 288: 1613–1616. [DOI] [PubMed] [Google Scholar]
- 8. Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, et al. (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS . Plant Cell 12: 2473–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greenup A, Peacock WJ, Dennis ES, Trevaskis B (2009) The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Annals of Botany 103: 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JA, Bailey PC, Laurie DA (2010) Comparative Genomics of Flowering Time Pathways Using Brachypodium distachyon as a Model for the Temperate Grasses. Plos One. pp. e10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graham P, Vance C (2003) Legumes: Importance and Constraints to Greater Use. Plant Physiology 131: 872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hecht V, Foucher F, Ferrandiz C, Macknight R, Navarro C, et al. (2005) Conservation of Arabidopsis flowering genes in model legumes. Plant Physiology 137: 1420–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young ND, Debelle F, Oldroyd GED, Geurts R, Cannon SB, et al. (2011) The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 480: 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Espinoza LDL, Huguet T, Julier B (2012) Multi-population QTL detection for aerial morphogenetic traits in the model legume Medicago truncatula. Theoretical and Applied Genetics 124: 739–754. [DOI] [PubMed] [Google Scholar]
- 15. Pierre JB, Bogard M, Herrmann D, Huyghe C, Julier B (2011) A CONSTANS-like gene candidate that could explain most of the genetic variation for flowering date in Medicago truncatula. Molecular Breeding 28: 25–35. [Google Scholar]
- 16. Pierre JB, Huguet T, Barre P, Huyghe C, Julier B (2008) Detection of QTLs for flowering date in three mapping populations of the model legume species Medicago truncatula. Theoretical and Applied Genetics 117: 609–620. [DOI] [PubMed] [Google Scholar]
- 17. Tadege M, Wang TL, Wen JQ, Ratet P, Mysore KS (2009) Mutagenesis and Beyond! Tools for Understanding Legume Biology. Plant Physiology 151: 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laurie RE, Diwadkar P, Jaudal M, Zhang LL, Hecht V, et al. (2011) The Medicago FLOWERING LOCUS T Homolog, MtFTa1, Is a Key Regulator of Flowering Time. Plant Physiology 156: 2207–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weller JL, Hecht V, Liew LC, Sussmilch FC, Wenden B, et al. (2009) Update on the genetic control of flowering in garden pea. Journal of Experimental Botany 60: 2493–2499. [DOI] [PubMed] [Google Scholar]
- 20. Liew LC, Hecht V, Laurie RE, Knowles CL, Schoor JKV, et al. (2009) DIE NEUTRALIS and LATE BLOOMER 1 Contribute to Regulation of the Pea Circadian Clock. Plant Cell 21: 3198–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hecht V, Knowles CL, Schoor JKV, Liew LC, Jones SE, et al. (2007) Pea LATE BLOOMER1 is a GIGANTEA ortholog with roles in photoperiodic flowering, deetiolation, and transcriptional regulation of circadian clock gene homologs. Plant Physiology 144: 648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hecht V, Laurie RE, Vander Schoor JK, Ridge S, Knowles CL, et al. (2011) The Pea GIGAS Gene Is a FLOWERING LOCUS T Homolog Necessary for Graft-Transmissible Specification of Flowering but Not for Responsiveness to Photoperiod. Plant Cell 23: 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scholte M, d'Erfurth I, Rippa S, Mondy S, Cosson V, et al. (2002) T-DNA tagging in the model legume Medicago truncatula allows efficient gene discovery. Molecular Breeding 10: 203–215. [Google Scholar]
- 24. Hill J (2000 ) Jester. Plant Varieties J 13: 40. [Google Scholar]
- 25. Trinh TH, Ratet P, Kondorosi E, Durand P, Kamate K, et al. (1998) Rapid and efficient transformation of diploid Medicago truncatula and Medicago sativa ssp falcata lines improved in somatic embryogenesis. Plant Cell Reports 17: 345–355. [DOI] [PubMed] [Google Scholar]
- 26. Gibeaut DM, Hulett J, Cramer GR, Seemann JR (1997) Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiology 115: 317–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chabaud M, Lichtenzveig J, Ellwood J, Pfaff T, Journet E-P (2006) Vernalization, crossings and testing for pollen viability. In: Mathesius U, Journet E, Sumner L, editors. The Medicago truncatula handbook. Available: http://www.noble.org/MedicagoHandbook/.
- 28. Wettenhall JM, Smyth GK (2004) limmaGUI: A graphical user interface for linear modeling of microarray data. Bioinformatics 20: 3705–3706. [DOI] [PubMed] [Google Scholar]
- 29.Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Research 16 10881–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brocard L, Schultze M, Kondorosi A, Ratet P (2006) T-DNA mutagenesis in the model plant Medicago truncatula: Is it efficient enough for legume molecular genetics?. CAB Reviews 23. [Google Scholar]
- 31. Rakocevic A, Mondy S, Tirichine L, Cosson V, Brocard L, et al. (2009) MERE1, a Low-Copy-Number Copia-Type Retroelement in Medicago truncatula Active during Tissue Culture. Plant Physiology 151: 1250–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ovchinnikova E, Journet E-P, Chabaud M, Cosson V, Ratet P, et al. (2011) IPD3 Controls the Formation of Nitrogen-Fixing Symbiosomes in Pea and Medicago Spp. MPMI 24: 1333–1344. [DOI] [PubMed] [Google Scholar]
- 33.Chabaud M, Boisson-Dernier A, Journet E-P, de Carvalho-Niebel F, Barker DG (2008) Agrobacterium-mediated transformation of Medicago truncatula. In: Kirti P, editor. Handbook of New Technologies for Genetic Improvement of Legumes. Boca Raton, New York: CRC press. pp. 45–67. [Google Scholar]
- 34. Michaels SD, Amasino RM (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Julier B, Huguet T, Chardon F, Ayadi R, Pierre J, et al. (2007) Identification of quantitative trait loci influencing aerial morphogenesis in the model legume Medicago truncatula. Theoretical and Applied Genetics 114: 1391–1406. [DOI] [PubMed] [Google Scholar]
- 36.Yeoh CC, Balcerowicz M, Laurie R, Macknight R, Putterill J (2011) Developing a method for customized induction of flowering. BMC Biotechnology 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mun JH, Kim DJ, Choi HK, Gish J, Debelle F, et al. (2006) Distribution of microsatellites in the genome of Medicago truncatula: A resource of genetic markers that integrate genetic and physical maps. Genetics 172: 2541–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Teper-Bamnolker P, Samach A (2005) The flowering integrator FT regulates SEPALLATA3 and FRUITFULL accumulation in Arabidopsis leaves. Plant Cell 17: 2661–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, et al. (2005) CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiology 139: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Srikanth A, Schmid M (2011) Regulation of flowering time: all roads lead to Rome. Cellular and Molecular Life Sciences 68: 2013–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smykal P, Kalendar R, Ford R, Macas J, Griga M (2009) Evolutionary conserved lineage of Angela-family retrotransposons as a genome-wide microsatellite repeat dispersal agent. Heredity 103: 157–167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The DNA sequence of R108 and spring1 is identical in the FTa1 genomic region, but differs from the reference genome A17. a) Diagram showing the predicted gene annotation in the spring1 mapping interval in the vicinity of the three FT genes. b) Diagram comparing the DNA sequences of the FTa1 region between R108 and A17. PCR was used to amplify the region of DNA from the nearest upstream gene (Medtr7g084960) to just downstream of the 3′UTR of FTa1 in spring1 and R108. Both fragments were directly sequenced and their sequences compared to each other and to A17. The spring1 and R108 sequences were identical. The predicted FTa1 protein encoded by A17 and R108 was identical and the three intron sequences were highly conserved, ranging from 100% nucleotide identity in the first two introns to 96% identity in the longer, third intron which has an indel of 23 bp. The 3′ UTR sequences were also highly conserved (98% identical). However, there was a striking difference in the length of the FTa1 5′ region, with the A17 sequence being 1347 bp shorter than the R108 sequence. This resulted from a series of indels in this region, the largest of which was a 1442 bp solo Long Terminal Repeat (LTR) from the Angela family of the Ty1/copia super family retrotransposons [41] in R108 and spring1, that was missing in A17. There are over 40 of this type of solo LTR in the genome [41]. There was a 5 bp repeated sequence flanking the solo LTR in R108 and spring1. Apart from the indels, there were blocks of high sequence conservation (94–99% nucleotide identity) shared between the 5′ region in R108 and A17.
(TIF)
Genotyping shows segregation distortion of a DNA marker in the spring1 interval in F2 plants of the cross of “spring1 x Jester”. PCR genotyping using a DNA marker (FTa1) from the interval containing spring1 was carried out on plant DNA samples from the two mapping crosses. These included the early and late flowering plants, but also additional samples comprising most of the “unclassified” plants and a few of the dead plants. a) In the Mapping cross “spring1 x Jester”, our experimental hypothesis was that we expected ¼ of the plants to be homozygous for the Jester marker genotyped. However, we scored only 62 plants (1/9) as homozygous Jester out of 578 genotyped. This gives a χ2 value of ∼63, a value of p<0.001 leading us to reject the experimental hypothesis. b) In the Testcross, our experimental hypothesis was that we expected 1/2 of the plants to be homozygous for the Jester marker genotyped. We scored 101 plants as homozygous Jester out of 218 genotyped. This gives a χ2 value of ∼1.2, a value of 0.5<p<0.1 leading us to accept the experimental hypothesis.
(DOCX)
List of annotated genes predicted within the ∼0.5 Mb interval containing spring1. The gene annotations were obtained from the BAC sequences in Medicago pseudomolecule Mt3.5 genome assembly http://medicagohapmap.org/. The three FT genes are in BAC AC123593 and shown in bold.
(DOCX)
Microarray identification of genes that are differentially expressed in leaves of spring1 compared to R108. The log fold change in gene expression with a p value of ≤0.05 was calculated from 3 biological repeats of each genotype grown in long day conditions. The first trifoliate leaf at the three-leaf stage was harvested. Each biological replicate was a pool of three leaves. Genes with a two-fold or greater change in gene expression are listed. aAll of these genes were confirmed to be differentially expressed by qRT-PCR on the RNA used in the microarray, except Mtr.21428.1.S1_at which was not done. bAfter two backcrosses to R108, gene expression in homozygous spring1 plants was again compared to R108, but only 3 genes, FTa1, FUlb and SOC1a, retained similar differential expression as first observed in spring1. The fourth gene Mtr.51129.1.S1_s_at showed very variable expression; it was undetectable by qRT-PCR in the original spring1 RNA samples and after two backcrosses was expressed about 16× less than R108, but at much higher levels than before in spring1. After the backcrosses, the remaining genes either were expressed at the same level as R108 (c5 genes) or had opposite pattern of expression to that previously determined (d3 genes).
(DOCX)