Abstract
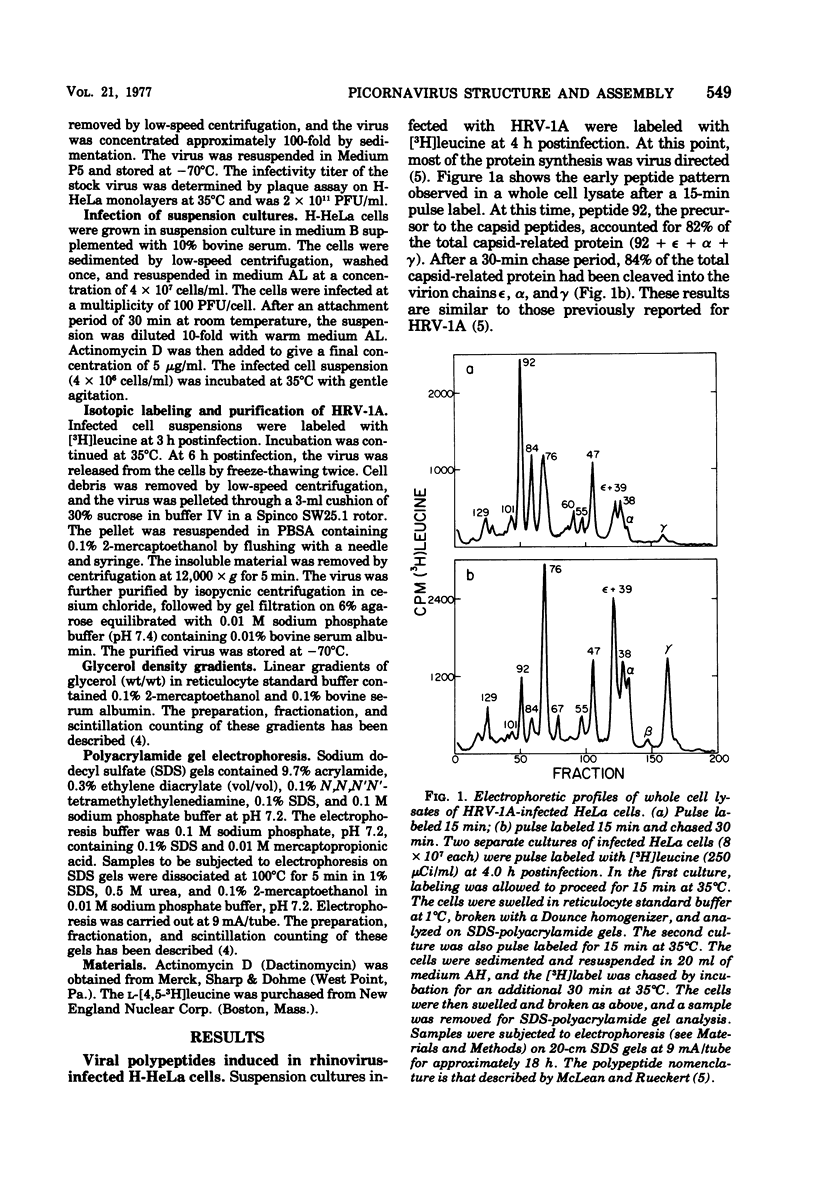
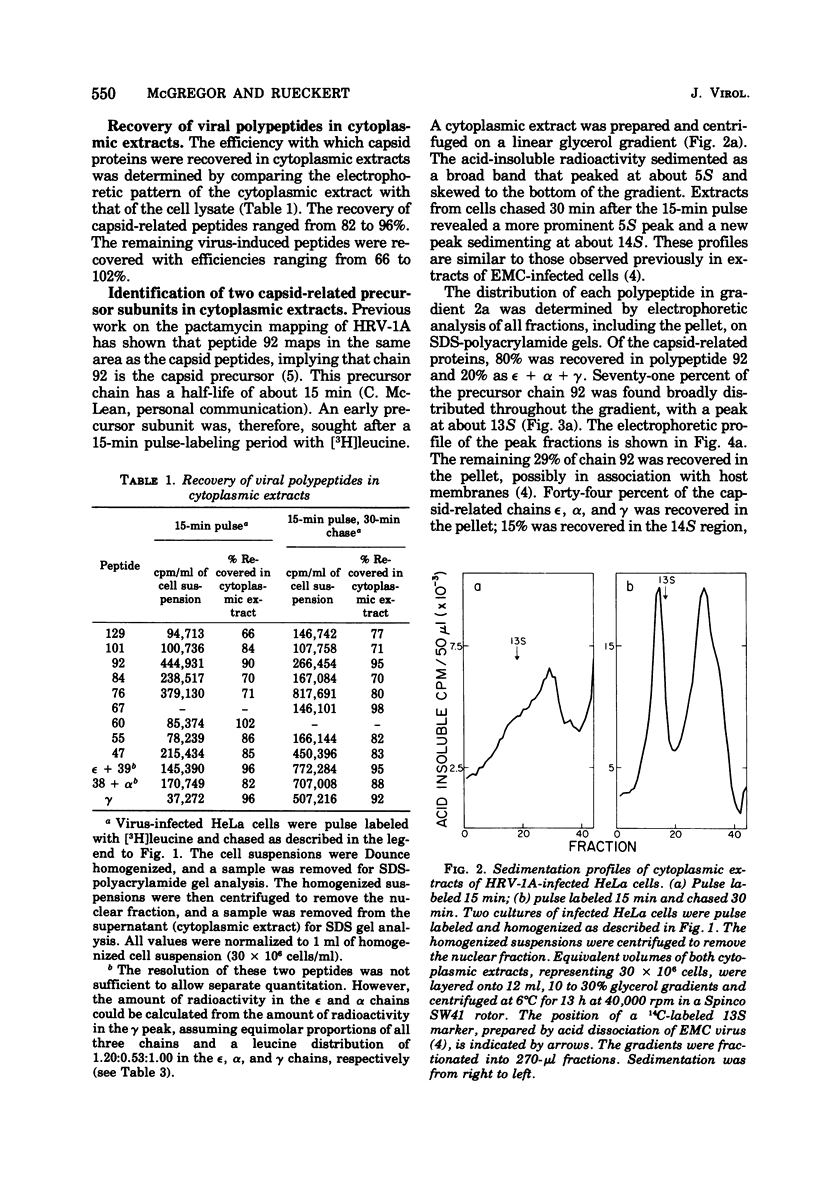
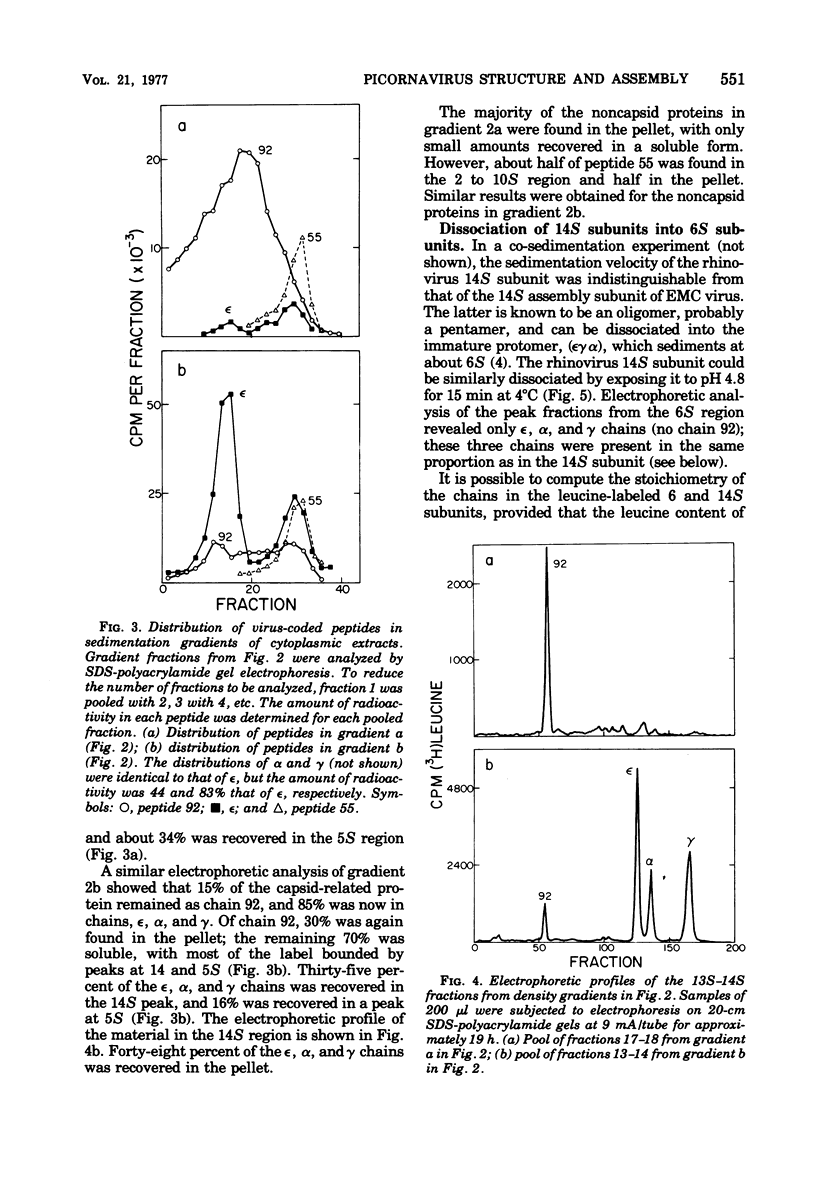
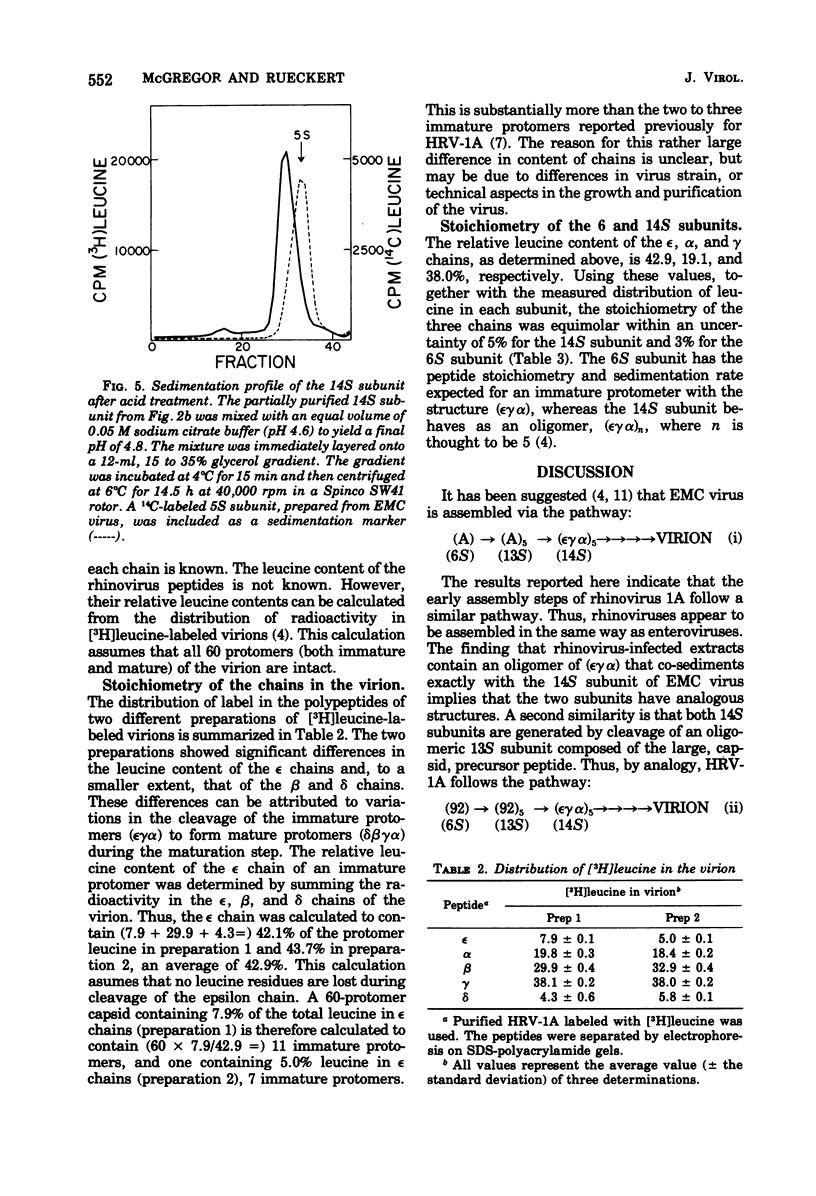
Cytoplasmic extracts of rhinovirus 1A-infected HeLa cells, pulsed 15 min with [3H]leucine, contained a 13S subunit which was rich in the capsid precursor, peptide 92. After a 30-min chase, most of the capsid-related protein sedimented in a 14S peak that contained equimolar amounts of the capsid peptides epsilon, alpha, and gamma, and some residual chain 92. The 14S subunit could be dissociated at pH 4.8 into 6S subunits containing only epsilon, alpha, and gamma chains in equal proportions, indicating that the 14S subunit is an oligomer of (epsilon gamma alpha) protomers. These subunits resemble subunits previously identified in the assembly of enteroviruses. These observations support the idea that rhinovirus assembly is basically similar to that of enteroviruses. Comparative studies on the peptide stoichiometry of the virion and the capsid precursor subunits indicate that rhinovirus 1A can contain as many as 11 immature protomers per virion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Fragments generated by pH dissociation of ME-virus and their relation to the structure of the virion. J Mol Biol. 1971 May 28;58(1):217–235. doi: 10.1016/0022-2836(71)90242-7. [DOI] [PubMed] [Google Scholar]
- Hall L., Rueckert R. R. Infection of mouse fibroblasts by cardioviruses: premature uncoating and its prevention by elevated pH and magnesium chloride. Virology. 1971 Jan;43(1):152–165. doi: 10.1016/0042-6822(71)90233-9. [DOI] [PubMed] [Google Scholar]
- Mak T. W., Colter J. S., Scraba D. G. Structure of the Mengo virion. II. Physicochemical and electron microscopic analysis of degraded virus. Virology. 1974 Feb;57(2):543–553. doi: 10.1016/0042-6822(74)90193-7. [DOI] [PubMed] [Google Scholar]
- McGregor S. Evidence for the existence of protomers in the assembly of encephalomyocarditis virus. J Virol. 1975 May;15(5):1107–1120. doi: 10.1128/jvi.15.5.1107-1120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean C., Rueckert R. R. Picornaviral gene order: comparison of a rhinovirus with a cardiovirus. J Virol. 1973 Feb;11(2):341–344. doi: 10.1128/jvi.11.2.341-344.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medappa K. C., McLean C., Rueckert R. R. On the structure of rhinovirus 1A. Virology. 1971 May;44(2):259–270. doi: 10.1016/0042-6822(71)90258-3. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Agol V. I., Bachrach H. L., Brown F., Cooper P. D., Fiers W., Gard S., Gear J. H., Ghendon Y., Kasza L. Picornaviridae. Intervirology. 1974;4(5):303–316. doi: 10.1159/000149863. [DOI] [PubMed] [Google Scholar]
- Perlin M., Phillips B. A. In vitro assembly of polioviruses. 3. Assembly of 14 S particles into empty capsids by poliovirus-infected HeLa cell membranes. Virology. 1973 May;53(1):107–114. doi: 10.1016/0042-6822(73)90469-8. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Fennell R. Polypeptide composition of poliovirions, naturally occurring empty capsids, and 14S precursor particles. J Virol. 1973 Aug;12(2):291–299. doi: 10.1128/jvi.12.2.291-299.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P., Brown F. A model for foot-and-mouth disease virus. J Gen Virol. 1972 May;15(2):163–169. doi: 10.1099/0022-1317-15-2-163. [DOI] [PubMed] [Google Scholar]


