Abstract
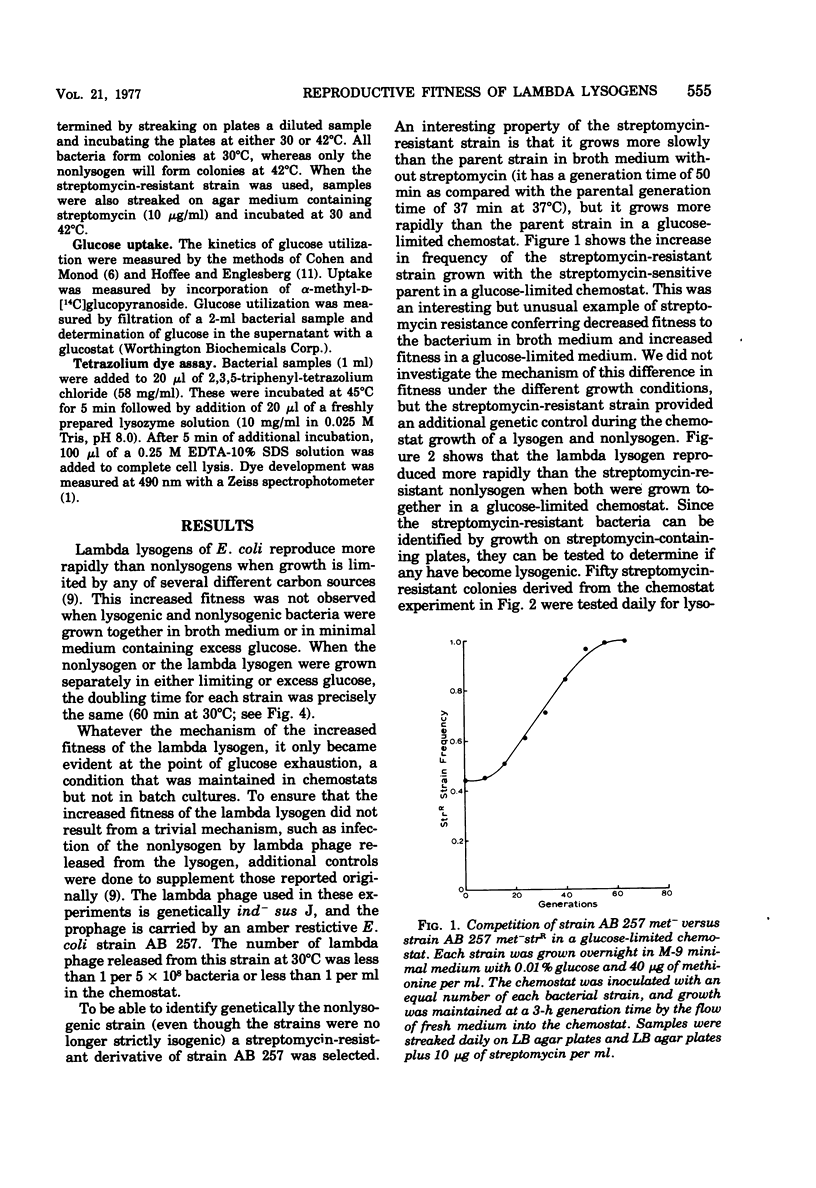
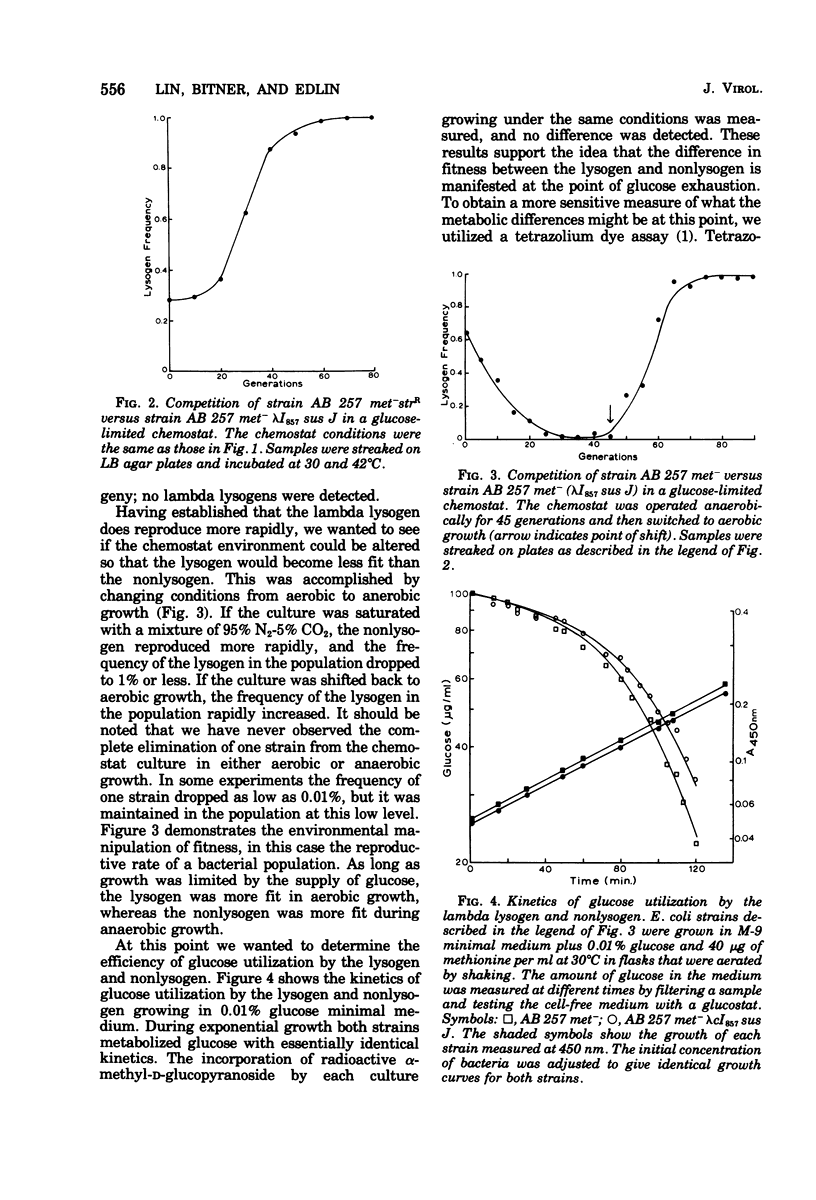
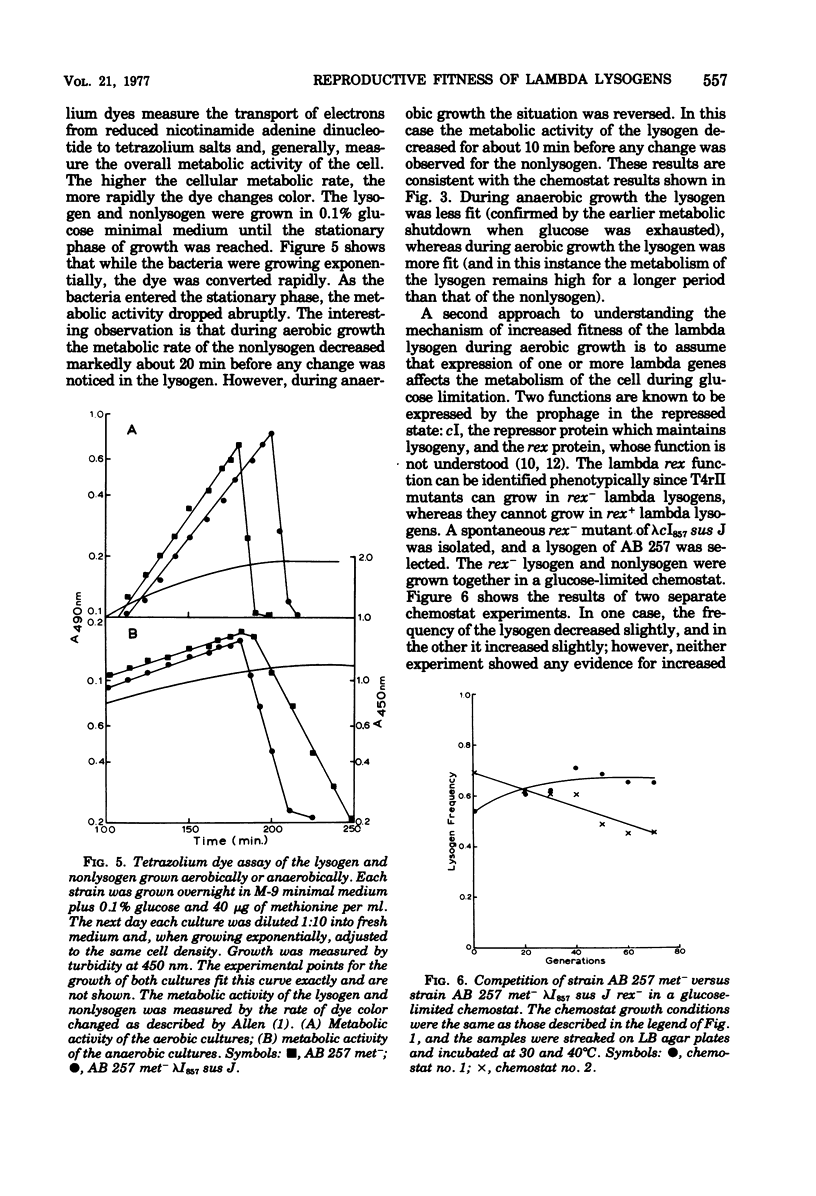
Lambda lysogens of Escherichia coli reproduce more rapidly than nonlysogens during aerobic growth in glucose-limited chemostats. If the environment is changed to anaerobic growth, the situation is reversed, and the lysogen reproduces more slowly than the nonlysogen. Based on a tetrazolium dye assay, the increased fitness of the lambda lysogen during aerobic growth seems to result from a continued high metabolic rate as glucose becomes limiting, whereas the metabolic rate of the nonlysogen declines. The lambda rex gene is required for the growth advantage of lysogens since lack of rex function causes lambda lysogens to lose their reproductive advantage over nonlysogens.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. G. Use of tetrazolium salts for electron transport studies in meningopneumonitis. I. Reduced nicotinamide adenine dinucleotide system. J Bacteriol. 1965 Dec;90(6):1505–1512. doi: 10.1128/jb.90.6.1505-1512.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: preservation of ancestral murine type C viral sequences in pig cellular DNA. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4090–4094. doi: 10.1073/pnas.72.10.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H. Developmental pathways for the temperate phage: lysis vs lysogeny,. Annu Rev Genet. 1972;6(0):157–190. doi: 10.1146/annurev.ge.06.120172.001105. [DOI] [PubMed] [Google Scholar]
- Edlin G., Lin L., Kudrna R. Lambda lysogens of E. coli reproduce more rapidly than non-lysogens. Nature. 1975 Jun 26;255(5511):735–737. doi: 10.1038/255735a0. [DOI] [PubMed] [Google Scholar]
- Gussin G. N., Peterson V. Isolation and properties of rex - mutants of bacteriophage lambda. J Virol. 1972 Oct;10(4):760–765. doi: 10.1128/jvi.10.4.760-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E. Effect of metabolic activity on the glucose permease of bacterial cells. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1759–1765. doi: 10.1073/pnas.48.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. D. Phage lambda mutants deficient in r-II exclusion. Science. 1967 Dec 22;158(3808):1588–1589. doi: 10.1126/science.158.3808.1588. [DOI] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LWOFF A. Lysogeny. Bacteriol Rev. 1953 Dec;17(4):269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Smith D., de Salsas M. F. Temperate Bacteriophage Which Causes the Production of a New Major Outer Membrane Protein by Escherichia coli. J Virol. 1975 May;15(5):1121–1130. doi: 10.1128/jvi.15.5.1121-1130.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Mechanism of cell transformation by RNA tumor viruses. Annu Rev Microbiol. 1971;25:609–648. doi: 10.1146/annurev.mi.25.100171.003141. [DOI] [PubMed] [Google Scholar]



