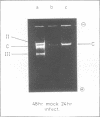
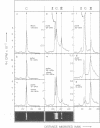
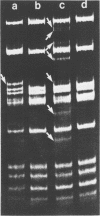
Abstract
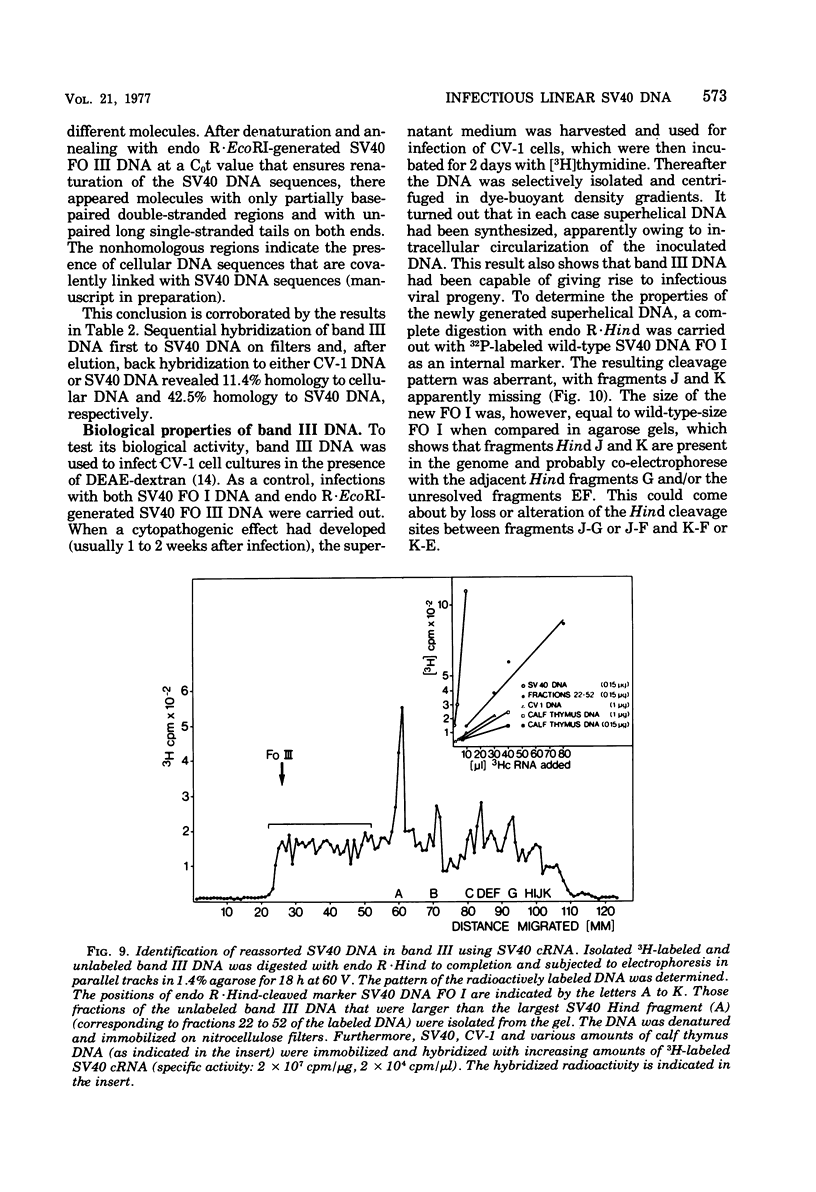
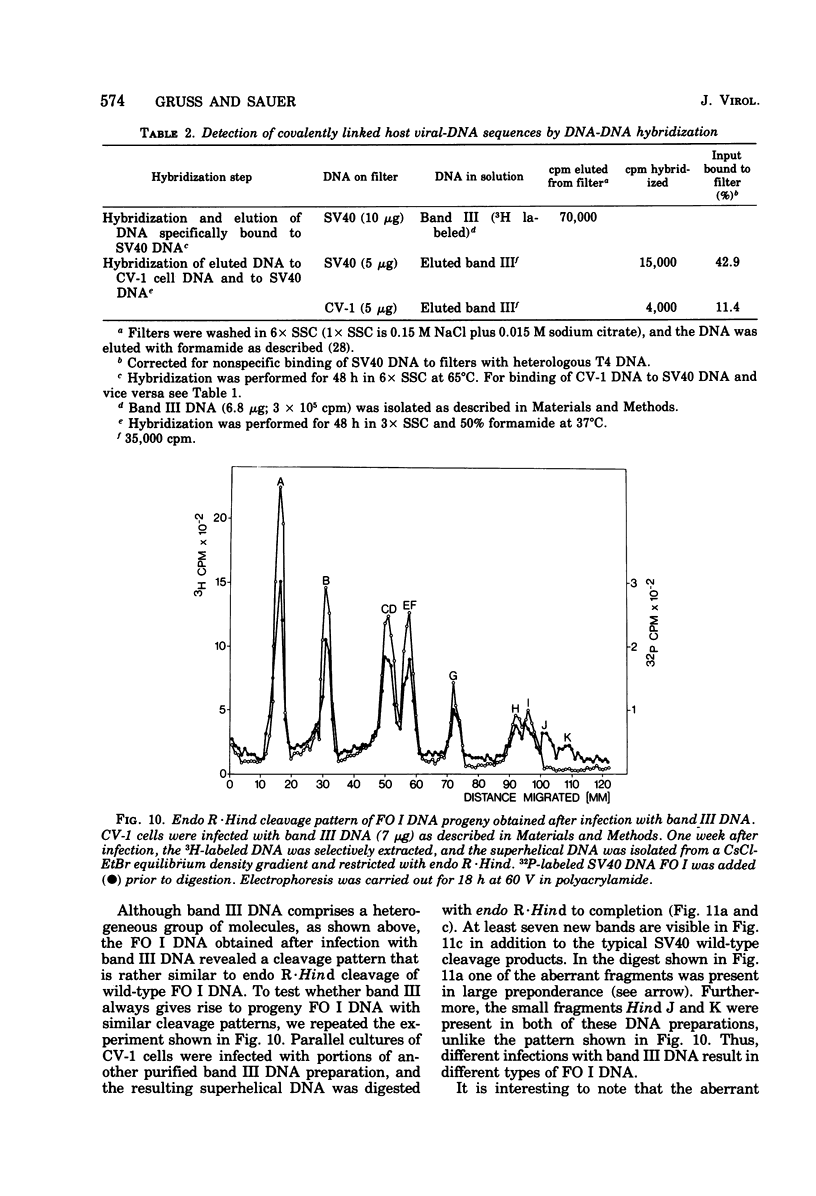
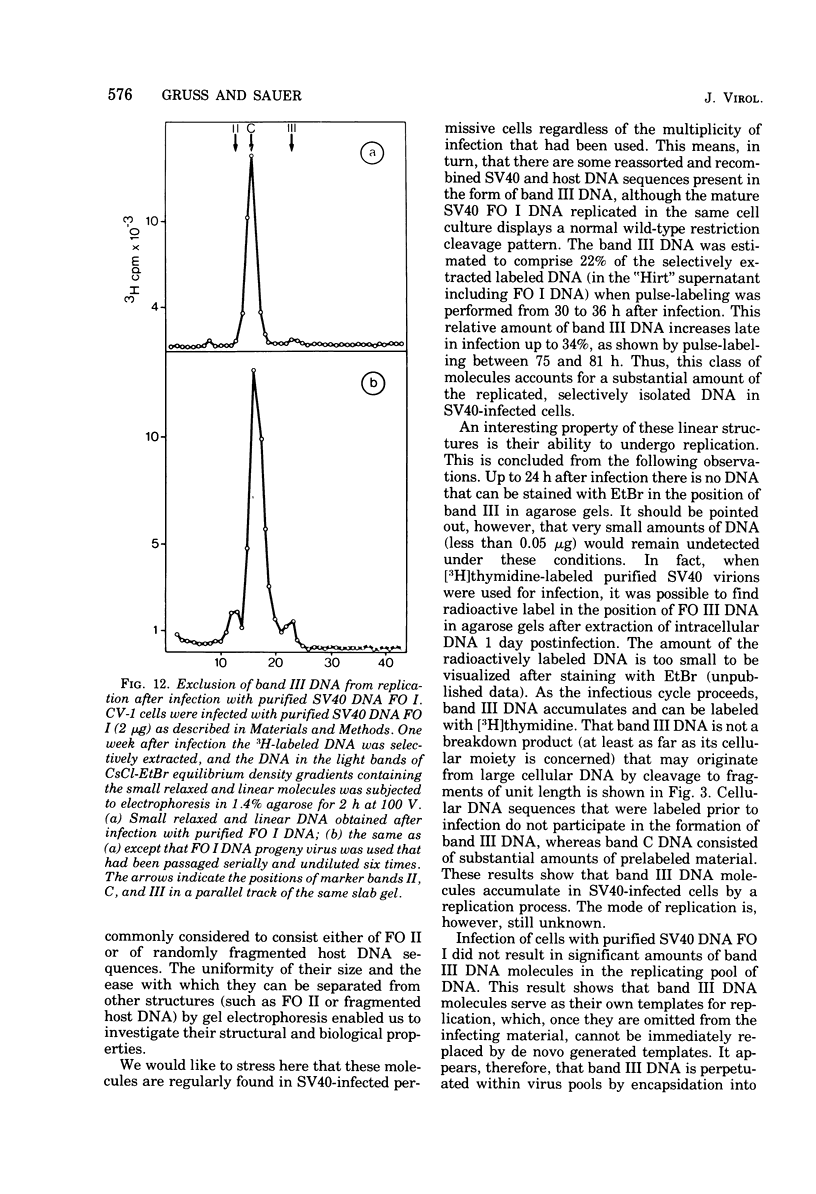
A new class of linear duplex DNA structures that contain simian virus 40 (SV40) DNA sequences and that are replicated during productive infection of cells with SV40 is described. These structures comprise up to 35% of the radioactively labeled DNA molecules that can be isolated by selective extraction. These molecules represent a unique size class corresponding to the length of an open SV40 DNA molecule (FO III), and they contain a heterogeneous population of DNA sequences either of host or of viral origin, as shown by restriction endonuclease analysis and nucleic acid hybridization. Part of the FO III DNA molecules contain viral-host DNA sequences covalently linked with each other. They start to replicate with the onset of SV40 superhelix replication 1 day after infection. Their rate of synthesis is most pronounced 3 days after infection when superhelix replication is already declining. Furthermore, they cannot be chased into other structures. At least a fraction of these molecules is infectious when administered together with DEAE-dextran to permissive cells. After intracellular circularization, superhelical DNA FO I with an aberrant cleavage pattern accumulates. In addition, tumor and viral capsid antigen are induced, and infectious viral progeny is obtained. Infection of cells with purified SV40 FO I DNA does not result in FO III DNA molecules in the infected cells or in the viral progeny. It is suggested, therefore, that these FO III DNA molecules are perpetuated within SV40 virus pools by encapsidation into pseudovirions.
Full text
PDF


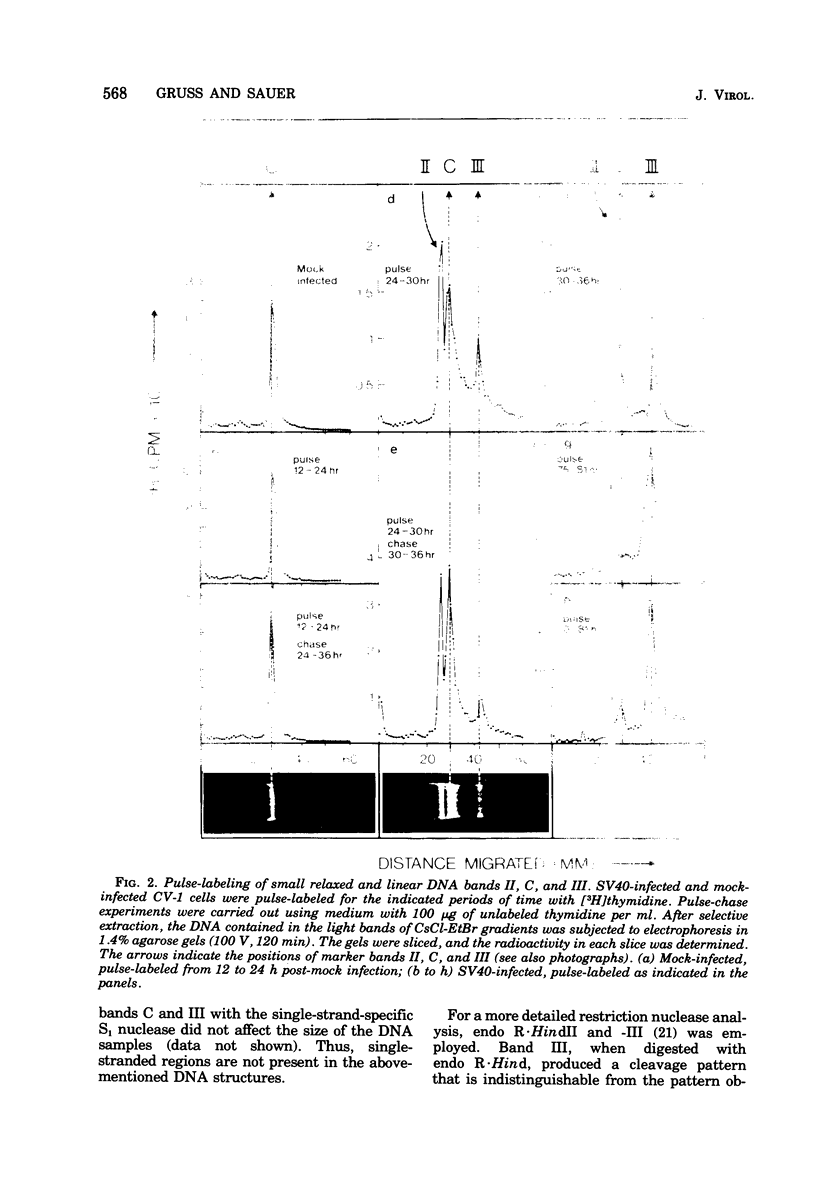



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard P., Morrow J. F., Berg P. Cleavage of circular, superhelical simian virus 40 DNA to a linear duplex by S1 nuclease. J Virol. 1973 Dec;12(6):1303–1313. doi: 10.1128/jvi.12.6.1303-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury K., Gruss P., Waldeck W., Sauer G. Action of S1 nuclease on nicked circular simian virus 40 DNA. Biochem Biophys Res Commun. 1975 May 19;64(2):709–716. doi: 10.1016/0006-291x(75)90378-2. [DOI] [PubMed] [Google Scholar]
- Danna K., Nathans D. Specific cleavage of simian virus 40 DNA by restriction endonuclease of Hemophilus influenzae. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2913–2917. doi: 10.1073/pnas.68.12.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried A. H. Temperature dependence of strand separation of the DNA molecules containing integrated SV40 DNA in transformed cells. Nucleic Acids Res. 1975 Sep;2(9):1591–1608. doi: 10.1093/nar/2.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi A. J. Prevention of SV40 virus oncogenesis in hamsters. I. Tumor resistance induced by human cells transformed by SV40. Proc Natl Acad Sci U S A. 1965 Aug;54(2):445–451. doi: 10.1073/pnas.54.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady L., Axelrod D., Trilling D. The SV40 pseudovirus: its potential for general transduction in animal cells. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1886–1893. doi: 10.1073/pnas.67.4.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P., Sauer G. Repetitive primate DNA containing the recognition sequences for two restriction endonucleases which generate cohesive ends. FEBS Lett. 1975 Dec 1;60(1):85–88. doi: 10.1016/0014-5793(75)80424-8. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- JENSEN F. C., GIRARDI A. J., GILDEN R. V., KOPROWSKI H. INFECTION OF HUMAN AND SIMIAN TISSUE CULTURES WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jul;52:53–59. doi: 10.1073/pnas.52.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashmiri S. V., Aposhian H. V. Degradation of pseudoviral DNA after infection of mouse cells with polyoma pseudovirions. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3834–3838. doi: 10.1073/pnas.71.10.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M., Melnick J. L. Enzyme induction in SV40-infected green monkey kidney cultures. Virology. 1966 May;29(1):69–83. doi: 10.1016/0042-6822(66)90197-8. [DOI] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Teresky A. K. Deoxyribonucleic acid replication in simian virus 40-infected cells. II. Detection and characterization of simian virus 40 pseudovirions. J Virol. 1970 Apr;5(4):451–457. doi: 10.1128/jvi.5.4.451-457.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Michel M. R., Hirt B., Weil R. Mouse cellular DNA enclosed in polyoma viral capsids (pseudovirions). Proc Natl Acad Sci U S A. 1967 Oct;58(4):1381–1388. doi: 10.1073/pnas.58.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman J. V., Waddell A., Aposhian H. V. DNA and gene therapy: uncoating of polyoma pseudovirus in mouse embryo cells. Proc Natl Acad Sci U S A. 1970 Sep;67(1):37–40. doi: 10.1073/pnas.67.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasba P. K., Aposhian H. V. DNA and gene therapy: transfer of mouse DNA to human and mouse embryonic cells by polyoma pseudovirions. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2345–2349. doi: 10.1073/pnas.68.10.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer G., Kidwai J. R. The transcription of the SV40 genome in productively infected and transformed cells. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1256–1263. doi: 10.1073/pnas.61.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Emrich J., Stahl M. M. Chromosome structure in phage t4, iii. Terminal redundancy and length determination. Proc Natl Acad Sci U S A. 1967 Feb;57(2):292–295. doi: 10.1073/pnas.57.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Macasaet F. Simian virus 40 deoxyribonucleic acid synthesis: analysis by gel electrophoresis. J Virol. 1972 Oct;10(4):599–604. doi: 10.1128/jvi.10.4.599-604.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Habel K., Green H. Antigenic and cultural properties of cells doubly transformed by polyoma virus and SV40. Virology. 1965 Oct;27(2):179–185. doi: 10.1016/0042-6822(65)90157-1. [DOI] [PubMed] [Google Scholar]
- Trilling D. M., Axelrod D. Encapsidation of free host DNA by simian virus 40: a simian virus 40 pseudovirus. Science. 1970 Apr 10;168(3928):268–271. doi: 10.1126/science.168.3928.268. [DOI] [PubMed] [Google Scholar]
- Trilling D., Axelrod D. Analysis of the three components of the simian virus 40: pseudo-, mature, and defective viruses. Virology. 1972 Feb;47(2):360–369. doi: 10.1016/0042-6822(72)90271-1. [DOI] [PubMed] [Google Scholar]
- Waldeck W., Chowdhury K., Gruss P., Sauer G. Random cleavage of superhelical SV 40 DNA S1 nuclease. Biochim Biophys Acta. 1976 Mar 4;425(2):157–167. doi: 10.1016/0005-2787(76)90021-6. [DOI] [PubMed] [Google Scholar]
- Waldeck W., Kammer K., Sauer G. Preferential integration of simian virus 40 deoxyribonucleic acid into a particular size class of CV-1 cell deoxyribonucleic acid. Virology. 1973 Aug;54(2):452–464. doi: 10.1016/0042-6822(73)90156-6. [DOI] [PubMed] [Google Scholar]
- Westphal H. SV40 DNA strand selection by Escherichia coli RNA polymerase. J Mol Biol. 1970 Jun 14;50(2):407–420. doi: 10.1016/0022-2836(70)90201-9. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Isolation of a sequence-specific endonuclease (BamI) from Bacillus amyloliquefaciens H. J Mol Biol. 1975 Sep 5;97(1):123–125. doi: 10.1016/s0022-2836(75)80028-3. [DOI] [PubMed] [Google Scholar]
- Winocour E. Further studies on the incorporation of cell DNA into polyoma-related particles. Virology. 1968 Apr;34(4):571–582. doi: 10.1016/0042-6822(68)90078-0. [DOI] [PubMed] [Google Scholar]