Abstract
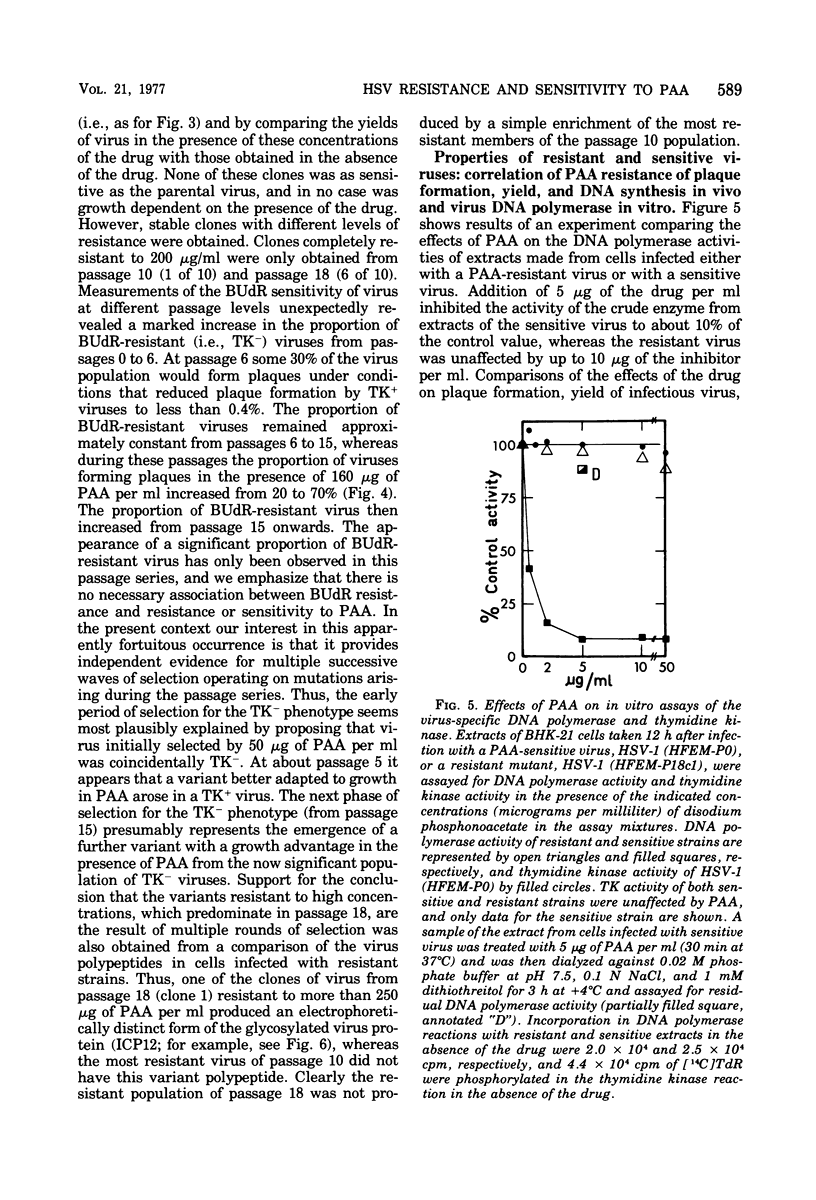
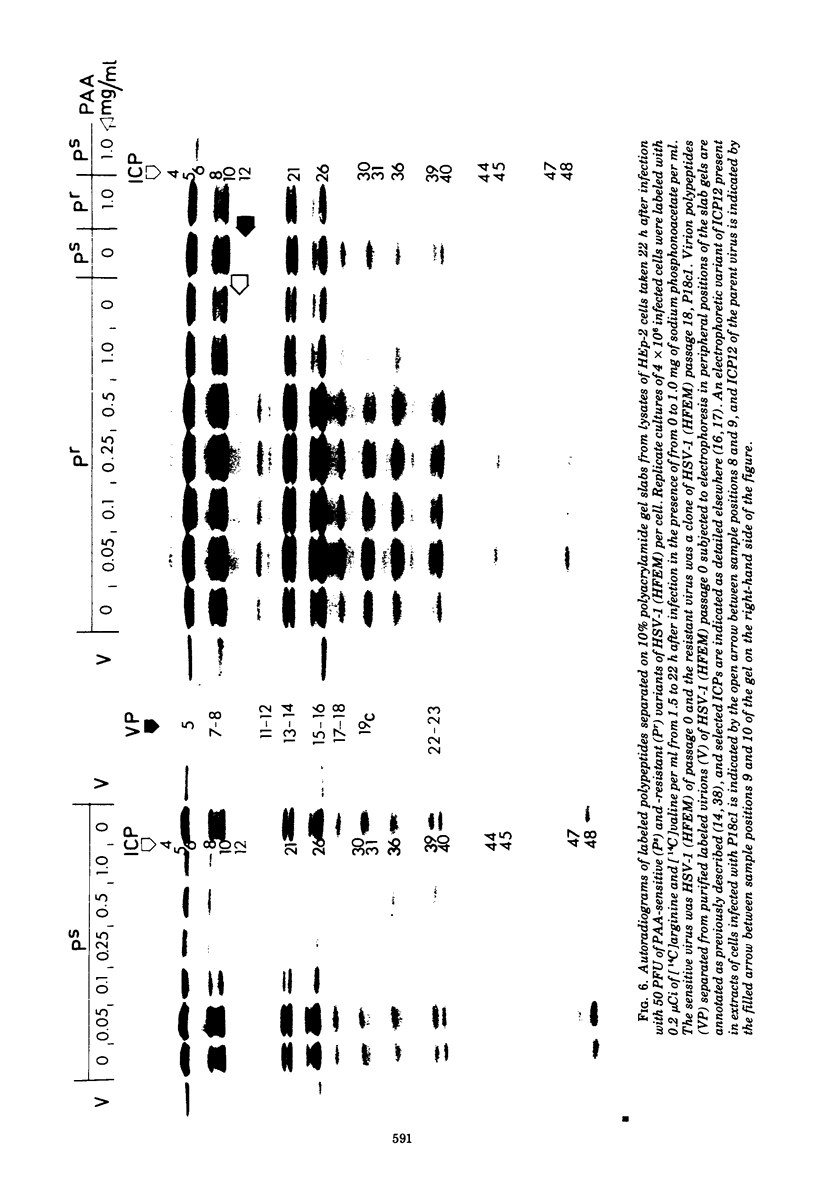
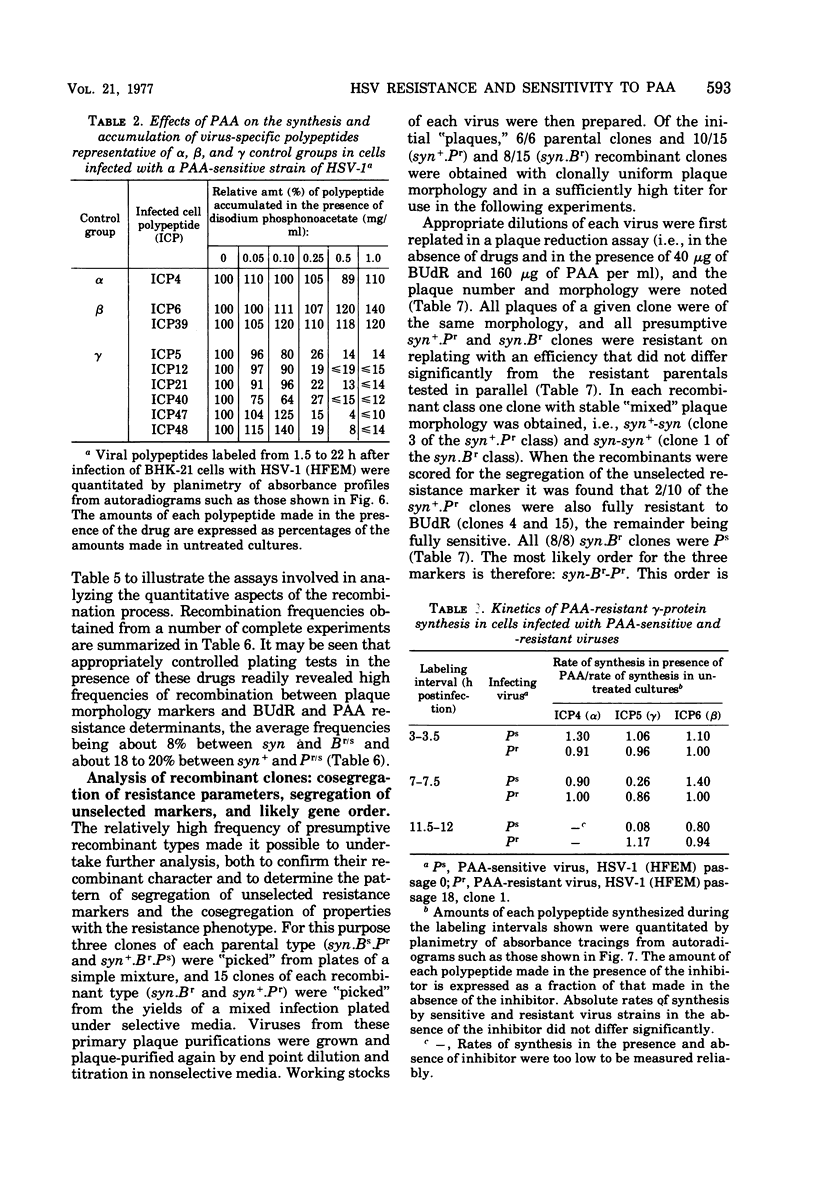
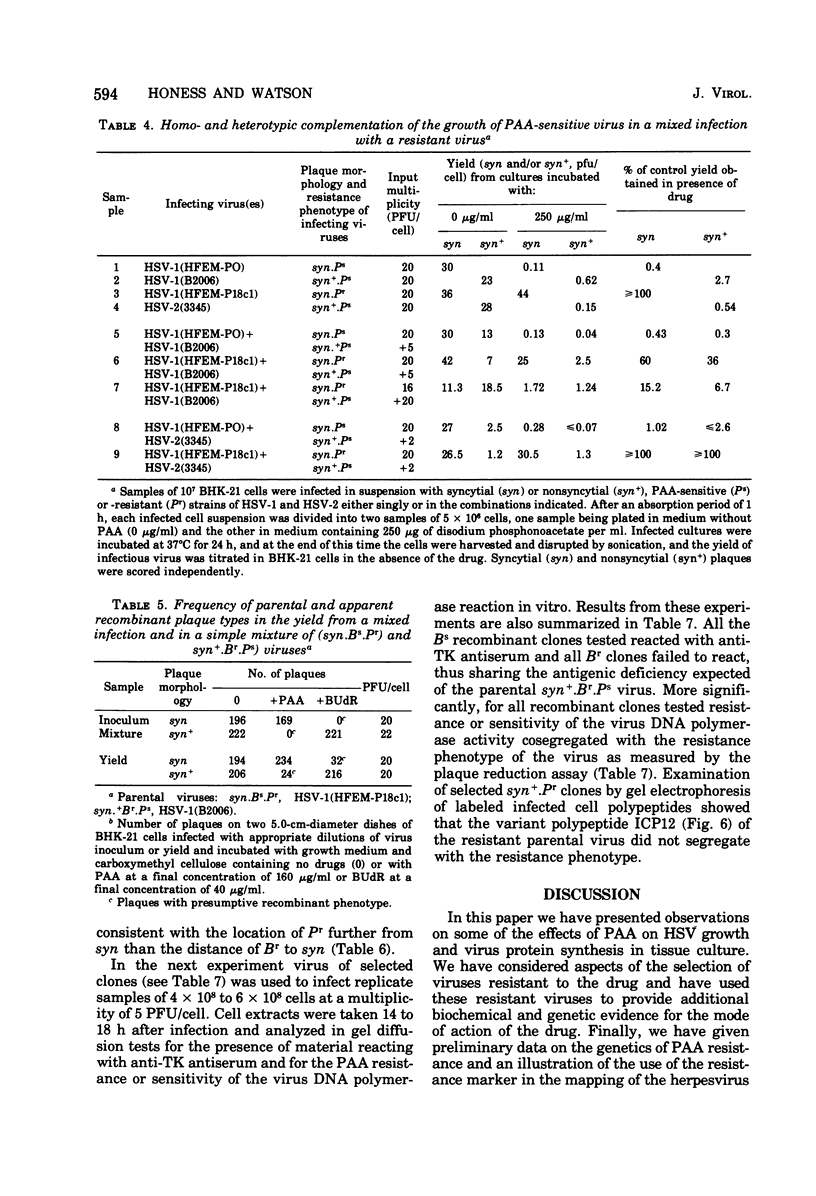
Phosphonoacetic acid (PAA) inhibited the synthesis of herpes simplex virus DNA in infected cells and the activity of the virus-specific DNA polymerase in vitro. In the presence of concentrations of PAA sufficient to prevent virus growth and virus DNA synthesis, normal amounts of early virus proteins (alpha- and beta-groups) were made, but late virus proteins (gamma-group) were reduced to less than 15% of amounts made in untreated infected cells. This residual PAA-insensitive synthesis of gamma-polypeptides occurred early in the virus growth cycle when rates were identical in PAA-treated and untreated infected cells. Passage of virus in the presence of PAA resulted in selection of mutants resistant to the drug. Stable clones of mutant viruses with a range of drug sensitivities were isolated and the emergence of variants resistant to high concentrations of PAA involved the sequential selection of mutants progressively better adapted to growth in the presence of the drug. Increased drug resistance of virus yield or plaque formation was correlated with increased resistance of virus DNA synthesis, gamma-protein synthesis, and resistance of the virus DNA polymerase reaction in vitro to the inhibitory effects of the drug. PAA-resistant strains of herpes simplex virus type 1 (HSV-1) complemented the growth of sensitive strains of homologous and heterologous types in mixed infections in the presence of the drug. Complementation was markedly dependent upon the proportions of the resistant and sensitive partners participating in the mixed infection. Intratypic (HSV-1A X HSV-1B) recombination of the PAA resistance marker(s), Pr, occurred at high frequency relative to plaque morphology (syn) and bromodeoxyuridine resistance (Br, thymidine kinase-negative phenotype) markers, with the most likely order being syn-Br-Pr. Recombinant viruses were as resistant or sensitive to PAA as the parental viruses, and viruses recombinant for their PAA resistance phenotype were also recombinant for the PAA resistance character of the virus DNA polymerase. The results provide additional evidence that the herpesvirus DNA polymerase is the site of action of PAA and illustrate the potential usefulness of PAA-resistant mutants in genetic studies of herpesviruses.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aron G. M., Purifoy D. J., Schaffer P. A. DNA synthesis and DNA polymerase activity of herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1975 Sep;16(3):498–507. doi: 10.1128/jvi.16.3.498-507.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden A., Aucker J., Weissbach A. Synthesis of herpes simplex virus, vaccinia virus, and adenovirus DNA in isolated HeLa cell nuclei. I. Effect of viral-specific antisera and phosphonoacetic acid. J Virol. 1975 Dec;16(6):1584–1592. doi: 10.1128/jvi.16.6.1584-1592.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone D. R., Courtney R. J. A temperature-sensitive mutant of herpes simplex virus type 1 defective in the synthesis of the major capsid polypeptide. J Gen Virol. 1974 Jul;24(1):17–27. doi: 10.1099/0022-1317-24-1-17. [DOI] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A. Genetic studies with herpes simplex virus type 1. Analysis of mixed plaque-forming virus and its bearing on genetic recombination. Virology. 1975 Mar;64(1):32–42. doi: 10.1016/0042-6822(75)90076-8. [DOI] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Buchan A., Watson D. H., Dubbs D. R., Kit S. Serological study of a mutant of herpesvirus unable to stimulate thymidine kinase. J Virol. 1970 Jun;5(6):817–818. doi: 10.1128/jvi.5.6.817-818.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. MUTANT STRAINS OF HERPES SIMPLEX DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Apr;22:493–502. doi: 10.1016/0042-6822(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Esparza J., Benyesh-Melnick B., Schaffer P. A. Intertypic complementation and recombination between temperature-sensitive mutants of herpes simplex virus types 1 and 2. Virology. 1976 Apr;70(2):372–384. doi: 10.1016/0042-6822(76)90279-8. [DOI] [PubMed] [Google Scholar]
- Gerstein D. D., Dawson C. R., O J. O. Phosphonoacetic acid in the treatment of experimental herpes simplex keratitis. Antimicrob Agents Chemother. 1975 Mar;7(3):285–288. doi: 10.1128/aac.7.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliburton I. W., Timbury M. C. Temperature-sensitive mutants of herpes simplex virus type 2: description of three new complementation groups and studies on the inhibition of host cell DNA synthesis. J Gen Virol. 1976 Feb;30(2):207–221. doi: 10.1099/0022-1317-30-2-207. [DOI] [PubMed] [Google Scholar]
- Hay J., Moss H., Jamieson A. T., Timbury M. C. Herpesvirus proteins: DNA polymerase and pyrimidine deoxynucleoside kinase activities in temperature-sensitive mutants of herpes simplex virus type 2. J Gen Virol. 1976 Apr;31(1):65–73. doi: 10.1099/0022-1317-31-1-65. [DOI] [PubMed] [Google Scholar]
- Hay J., Subak-Sharpe J. H. Mutants of herpes simplex virus types 1 and 2 that are resistant to phosphonoacetic acid induce altered DNA polymerase activities in infected cells. J Gen Virol. 1976 Apr;31(1):145–148. doi: 10.1099/0022-1317-31-1-145. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Powell K. L., Robinson D. J., Sim C., Watson D. H. Type specific and type common antigens in cells infected with herpes simplex virus type 1 and on the surfaces of naked and enveloped particles of the virus. J Gen Virol. 1974 Feb;22(2):159–169. doi: 10.1099/0022-1317-22-2-159. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Absence of a requirement for host polypeptides in the herpes virus thymidine kinase. J Gen Virol. 1974 Jul;24(1):215–220. doi: 10.1099/0022-1317-24-1-215. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Herpes simplex virus-specific polypeptides studied by polyacrylamide gel electrophoresis of immune precipitates. J Gen Virol. 1974 Feb;22(2):171–185. doi: 10.1099/0022-1317-22-2-171. [DOI] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. IV. Specific inhibition of virus-induced DNA polymerase activity and viral DNA replication by phosphonoacetic acid. J Virol. 1975 Dec;16(6):1560–1565. doi: 10.1128/jvi.16.6.1560-1565.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir H. M., Hay J., Morrison J. M., Subak-Sharpe H. Altered properties of deoxyribonucleic acid nucleotidyltransferase after infection of mammalian cells with herpes simplex virus. Nature. 1966 Apr 23;210(5034):369–371. doi: 10.1038/210369a0. [DOI] [PubMed] [Google Scholar]
- Klein R. J., Friedman-Kien A. E. Phosphonoacetic acid-resistant herpes simplex virus infection in hairless mice. Antimicrob Agents Chemother. 1975 Mar;7(3):289–293. doi: 10.1128/aac.7.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemperer H. G., Haynes G. R., Shedden W. I., Watson D. H. A virus-specific thymidine kinase in BHK-21 cells infected with herpes simplex virus. Virology. 1967 Jan;31(1):120–128. doi: 10.1016/0042-6822(67)90015-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leinbach S. S., Reno J. M., Lee L. F., Isbell A. F., Boezi J. A. Mechanism of phosphonoacetate inhibition of herpesvirus-induced DNA polymerase. Biochemistry. 1976 Jan 27;15(2):426–430. doi: 10.1021/bi00647a029. [DOI] [PubMed] [Google Scholar]
- Levitt J., Becker Y. The effect of cytosine arabinoside on the replication of herpes simplex virus. Virology. 1967 Jan;31(1):129–134. doi: 10.1016/0042-6822(67)90016-5. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Mode of inhibition of herpes simplex virus DNA polymerase by phosphonoacetate. Biochemistry. 1975 Dec 16;14(25):5475–5479. doi: 10.1021/bi00696a015. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E., Overby L. R. Inhibition of DNA polymerase from herpes simplex virus-infected wi-38 cells by phosphonoacetic Acid. J Virol. 1975 May;15(5):1281–1283. doi: 10.1128/jvi.15.5.1281-1283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. F., Varnell E. D., Kaufman H. E. Phosphonoacetic acid in the treatment of experimental ocular herpes simplex infections. Antimicrob Agents Chemother. 1976 Feb;9(2):308–311. doi: 10.1128/aac.9.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii S., Rosenkranz H. S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. 3. Effect of hydroxyurea. J Virol. 1968 Oct;2(10):1163–1171. doi: 10.1128/jvi.2.10.1163-1171.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby L. R., Robishaw E. E., Schleicher J. B., Rueter A., Shipkowitz N. L., Mao J. C. Inhibition of herpes simplex virus replication by phosphonoacetic acid. Antimicrob Agents Chemother. 1974 Sep;6(3):360–365. doi: 10.1128/aac.6.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Cassai E., Honess R. W., Roizman B., Terni M., Nahmias A. Variability in the structural polypeptides of herpes simplex virus 1 strains: potential application in molecular epidemiology. Infect Immun. 1976 Jan;13(1):211–220. doi: 10.1128/iai.13.1.211-220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J., Courtney R. J. The synthesis of herpes simplex virus proteins in the absence of virus DNA synthesis. Biochem Biophys Res Commun. 1975 Sep 2;66(1):262–271. doi: 10.1016/s0006-291x(75)80323-8. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Benyesh-Melnick M. DNA polymerase induction by DNA-negative temperature-sensitive mutants of herpes simplex virus type 2. Virology. 1975 Dec;68(2):374–386. doi: 10.1016/0042-6822(75)90280-9. [DOI] [PubMed] [Google Scholar]
- RUSSELL W. C. A sensitive and precise plaque assay for herpes virus. Nature. 1962 Sep 8;195:1028–1029. doi: 10.1038/1951028a0. [DOI] [PubMed] [Google Scholar]
- Shipkowitz N. L., Bower R. R., Appell R. N., Nordeen C. W., Overby L. R., Roderick W. R., Schleicher J. B., Von Esch A. M. Suppression of herpes simplex virus infection by phosphonoacetic acid. Appl Microbiol. 1973 Sep;26(3):264–267. doi: 10.1128/am.26.3.264-267.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C., Watson D. H. The role of type specific and cross reacting structural antigens in the neutralization of herpes simplex virus types 1 and 2. J Gen Virol. 1973 May;19(2):217–233. doi: 10.1099/0022-1317-19-2-217. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C., Klein G. Inhibition of Epstein-Barr virus DNA synthesis and late gene expression by phosphonoacetic acid. J Virol. 1976 Apr;18(1):151–155. doi: 10.1128/jvi.18.1.151-155.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbury M. C., Calder L. Temperature-sensitive mutants of herpes simplex virus type 2: a provisional linkage map based on recombination analysis. J Gen Virol. 1976 Feb;30(2):179–186. doi: 10.1099/0022-1317-30-2-179. [DOI] [PubMed] [Google Scholar]
- Timbury M. C., Subak-Sharpe J. H. Genetic interactions between temperature-sensitive mutants of types 1 and 2 herpes simplex viruses. J Gen Virol. 1973 Mar;18(3):347–357. doi: 10.1099/0022-1317-18-3-347. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Stevens J. G. Effect of cytosine arabinoside on viral-specific protein synthesis in cells infected with herpes simplex virus. J Virol. 1975 Jan;15(1):71–80. doi: 10.1128/jvi.15.1.71-80.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. H., Shedden W. I., Elliot A., Tetsuka T., Wildy P., Bourgaux-Ramoisy D., Gold E. Virus specific antigens in mammalian cells infected with herpes simplex virus. Immunology. 1966 Oct;11(4):399–408. [PMC free article] [PubMed] [Google Scholar]
- Weissbach A., Hong S. C., Aucker J., Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973 Sep 25;248(18):6270–6277. [PubMed] [Google Scholar]
- Yajima Y., Tanaka A., Nonoyama M. Inhibition of productive replication of Epstein-Barr virus DNA by phosphonoacetic acid. Virology. 1976 May;71(1):352–354. doi: 10.1016/0042-6822(76)90119-7. [DOI] [PubMed] [Google Scholar]