Abstract
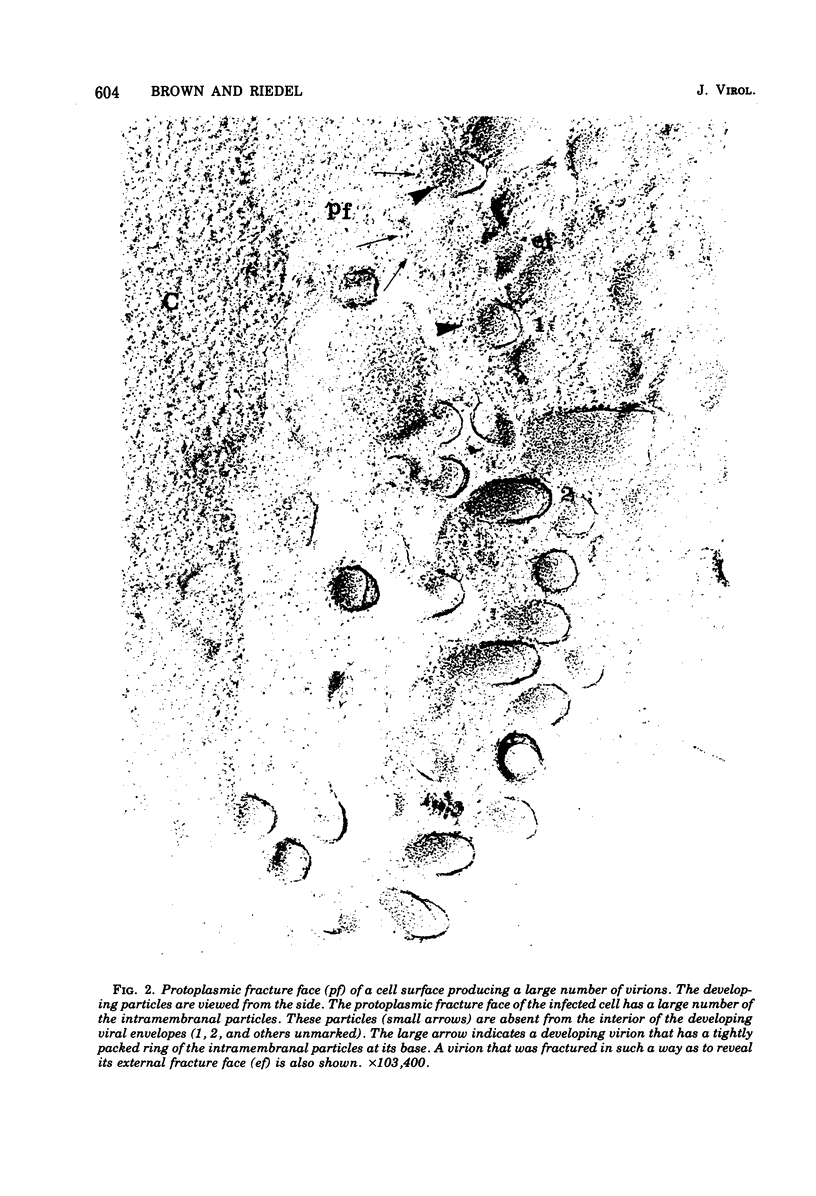
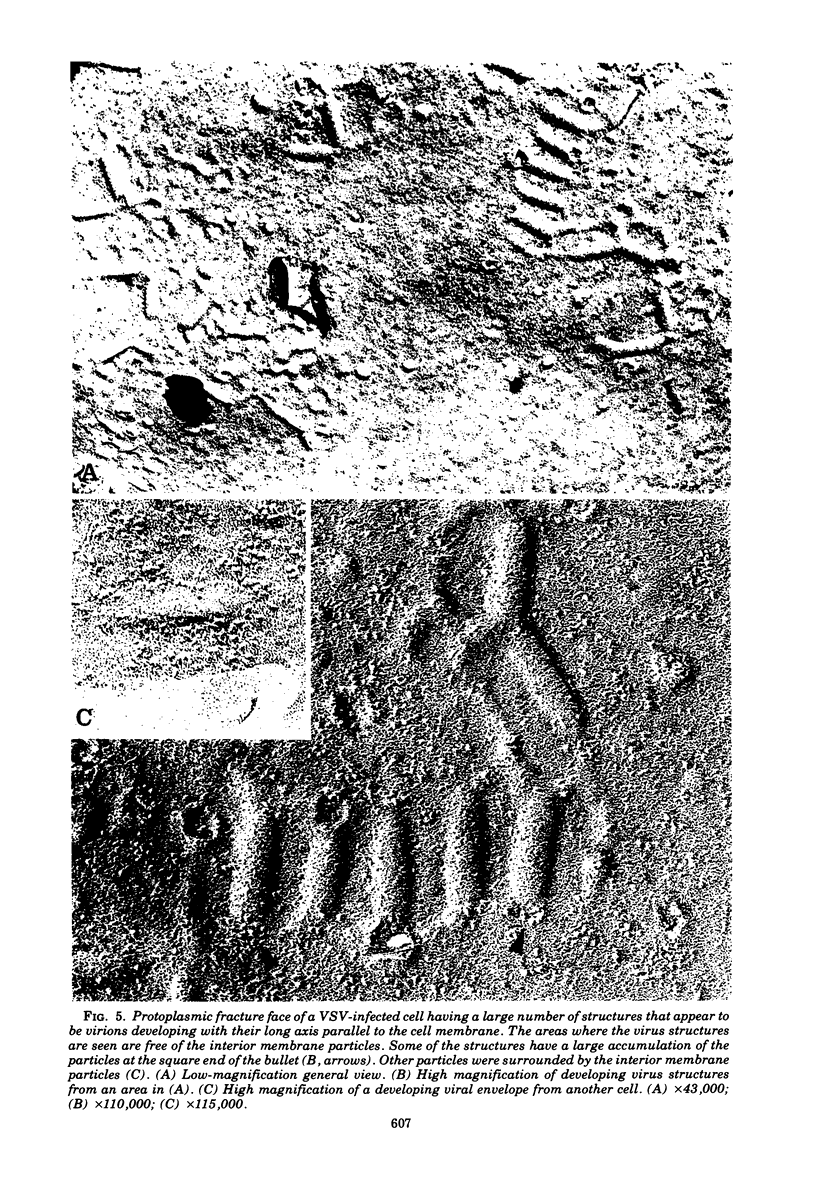
The morphogenesis of vesicular stomatitis virus was examined using freeze-fracture techniques, and the results obtained were compared with those from previously published experiments carried out with influenza viruses and togaviruses. The process of conversion of the host cell plasma membrane into the vesicular stomatitis virus envelope was accompanied by a loss of the intramembranal particles abundant in cell membranes. Frequently a dense accumulation of intramembranal particles could be seen at the base of the developing virion, suggesting that these structures might play some role in the generation of viral envelope. In addition to the viral structures that were seen to develop in the classical fashion, with their long axis perpendicular to the cell surface, structures were also found that suggested the initiation of a process similar to budding, with the long axis of the viral capsid parallel to the plasma membrane. In this situation, as in the "perpendicular" process, intramembranal particles were excluded from the viral structure, and an accumulation of these particles could be seen adjacent to the developing viral membrane.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Waite M. R., Pfefferkorn E. R. Morphology and morphogenesis of Sindbis virus as seen with freeze-etching techniques. J Virol. 1972 Sep;10(3):524–536. doi: 10.1128/jvi.10.3.524-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächi T., Gerhard W., Lindenmann J., Mühlethaler K. Morphogenesis of influenza A virus in Ehrlich ascites tumor cells as revealed by thin-sectioning and freeze-etching. J Virol. 1969 Nov;4(5):769–776. doi: 10.1128/jvi.4.5.769-776.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F., Hull R. Model for vesicular stomatitis virus. J Virol. 1972 Aug;10(2):256–260. doi: 10.1128/jvi.10.2.256-260.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A. E. Assembly of the vesicular stomatitis virus envelope: incorporation of viral polypeptides into the host plasma membrane. J Mol Biol. 1973 May 5;76(1):135–148. doi: 10.1016/0022-2836(73)90085-5. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. 3. Intracellular synthesis and extracellular appearance of virus-specific proteins. Virology. 1971 Dec;46(3):678–690. doi: 10.1016/0042-6822(71)90070-5. [DOI] [PubMed] [Google Scholar]
- Kelley J. M., Emerson S. U., Wagner R. R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972 Dec;10(6):1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J., Compans R. W. The membrane structure of lipid-containing viruses. Biochim Biophys Acta. 1974 Apr 8;344(1):51–94. doi: 10.1016/0304-4157(74)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N. F., Kelley J. M., Wagner R. R. Envelope proteins of vesicular stomatitis virions: accessibility to iodination. Virology. 1974 Sep;61(1):292–296. doi: 10.1016/0042-6822(74)90264-5. [DOI] [PubMed] [Google Scholar]
- Mudd J. A. Glycoprotein fragment associated with vesicular stomatitis virus after proteolytic digestion. Virology. 1974 Dec;62(2):573–577. doi: 10.1016/0042-6822(74)90419-x. [DOI] [PubMed] [Google Scholar]
- Nakai T., Howatson A. F. The fine structure of vesicular stomatitis virus. Virology. 1968 Jun;35(2):268–281. doi: 10.1016/0042-6822(68)90267-5. [DOI] [PubMed] [Google Scholar]
- Orenstein J., Johnson L., Shelton E., Lazzarini R. A. The shape of vesicular stomatitis virus. Virology. 1976 May;71(1):291–301. doi: 10.1016/0042-6822(76)90113-6. [DOI] [PubMed] [Google Scholar]
- Orenstein J., Shelton E., Lazzarini R. A. Association of ribosomes with intracellular vesicular stomatitis virus particles. J Virol. 1975 Aug;16(2):447–452. doi: 10.1128/jvi.16.2.447-452.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane splitting in freeze-ethching. Covalently bound ferritin as a membrane marker. J Cell Biol. 1970 Jun;45(3):598–605. doi: 10.1083/jcb.45.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Association of vesicular stomatitis virus glycoprotein with virion membrane: characterization of the lipophilic tail fragment. J Virol. 1975 Aug;16(2):237–240. doi: 10.1128/jvi.16.2.237-240.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Cellular adsorption function of the sialoglycoprotein of vesicular stomatitis virus and its neuraminic acid. J Virol. 1975 Apr;15(4):882–893. doi: 10.1128/jvi.15.4.882-893.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Sialoglycoprotein of vesicular stomatitis virus: role of the neuraminic acid in infection. J Virol. 1974 Aug;14(2):270–281. doi: 10.1128/jvi.14.2.270-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Gaffney B. J. Effect of the viral proteins on the fluidity of the membrane lipids in Sindbis virus. J Mol Biol. 1974 Dec 5;90(2):343–358. doi: 10.1016/0022-2836(74)90378-7. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Scott R. E., Marchesi V. T. The structure of erythrocyte membranes studied by freeze-etching. II. Localization of receptors for phytohemagglutinin and influenza virus to the intramembranous particles. J Exp Med. 1972 Jun 1;135(6):1209–1227. doi: 10.1084/jem.135.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Heine J. W., Goldstein G., Schnaitman C. A. Use of antiviral-antiferritin hybrid antibody for localization of viral antigen in plasma membrane. J Virol. 1971 Feb;7(2):274–277. doi: 10.1128/jvi.7.2.274-277.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. A., Snyder R. M. Structural proteins of vesicular stomatitis viruses. J Virol. 1969 Apr;3(4):395–403. doi: 10.1128/jvi.3.4.395-403.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Mudd J. A. Iodination of vesicular stomatitis virus with lactoperoxidase. Virology. 1973 Apr;52(2):574–577. doi: 10.1016/0042-6822(73)90353-x. [DOI] [PubMed] [Google Scholar]
- Zee Y. C., Hackett A. J., Talens L. Vesicular stomatitis virus maturation sites in six different host cells. J Gen Virol. 1970;7(2):95–102. doi: 10.1099/0022-1317-7-2-95. [DOI] [PubMed] [Google Scholar]








