Abstract
Mesorhizobium loti is the microsymbiont of Lotus species, including the model legume L. japonicus. M. loti differs from other rhizobia in that it contains two copies of the key nitrogen fixation regulatory gene nifA, nifA1 and nifA2, both of which are located on the symbiosis island ICEMlSymR7A. M. loti R7A also contains two rpoN genes, rpoN1 located on the chromosome outside of ICEMlSymR7A and rpoN2 that is located on ICEMlSymR7A. The aims of the current work were to establish how nifA expression was activated in M. loti and to characterise the NifA-RpoN regulon. The nifA2 and rpoN2 genes were essential for nitrogen fixation whereas nifA1 and rpoN1 were dispensable. Expression of nifA2 was activated, possibly in response to an inositol derivative, by a novel regulator of the LacI/GalR family encoded by the fixV gene located upstream of nifA2. Other than the well-characterized nif/fix genes, most NifA2-regulated genes were not required for nitrogen fixation although they were strongly expressed in nodules. The NifA-regulated nifZ and fixU genes, along with nifQ which was not NifA-regulated, were required in M. loti for a fully effective symbiosis although they are not present in some other rhizobia. The NifA-regulated gene msi158 that encodes a porin was also required for a fully effective symbiosis. Several metabolic genes that lacked NifA-regulated promoters were strongly expressed in nodules in a NifA2-dependent manner but again mutants did not have an overt symbiotic phenotype. In summary, many genes encoded on ICEMlSymR7A were strongly expressed in nodules but not free-living rhizobia, but were not essential for symbiotic nitrogen fixation. It seems likely that some of these genes have functional homologues elsewhere in the genome and that bacteroid metabolism may be sufficiently plastic to adapt to loss of certain enzymatic functions.
Introduction
Mesorhizobium loti is the natural microsymbiont of Lotus species, including the model legume L. japonicus. The genes required for nodule formation and nitrogen fixation in M. loti strain R7A are located on a 502-kb chromosomally located symbiosis island [1], [2], which was subsequently named ICEMlSymR7A [3] as it belongs to the family of mobile genetic elements collectively termed integrative and conjugative elements (ICEs) [4]. Sequence analysis of ICEMlSymR7A revealed that it shares 248 kb of DNA with the 611-kb symbiosis island of the sequenced M. loti strain MAFF303099 [5], including all the genes likely to be required for Nod factor synthesis and the formation of a functional nitrogenase enzyme. In addition, it contains mobility genes, a type IV secretion system similar to that of the vir system from Agrobacterium tumefaciens and a diverse range of regulators, metabolic genes, and transporters that may contribute to nodule function [6], [7].
Compared to several other rhizobial species, very little is known about how M. loti genes required for symbiotic nitrogen fixation are regulated. In most nitrogen-fixing bacteria, the NifA protein binds to an upstream activating sequence (UAS) and acts in association with the RNA polymerase sigma factor RpoN (σ54) to activate nif gene expression and, in rhizobia, the expression of several other symbiotic genes (reviewed in [8]). M. loti differs from other rhizobia examined to date in that it contains two copies of the nifA gene, nifA1 and nifA2, both of which are located on ICEMlSymR7A. The nifA1 gene is most similar to and in the same genomic context (between fixX and nifB) as nifA from Rhizobium etli, R. leguminosarum, Rhizobium sp. strain NGR234 and Sinorhizobium meliloti [9], [10], [11], [12], [13]. In contrast, nifA2 is most similar to nifA from Bradyrhizobium japonicum and is not located adjacent to known nitrogen fixation genes. The two genes are not functionally redundant as M. loti nifA2 mutants form Fix− nodules [14], [15] whereas nifA1 mutants are not symbiotically impaired [14].
NifA activity in rhizobia is oxygen-sensitive and it is thought that conserved cysteine residues present within NifA are involved in sensing and reacting to the cellular oxygen status (reviewed in [8]). In addition, in most rhizobia the nifA gene is subject to transcriptional regulation although the mechanisms vary depending on the rhizobial strain. In S. meliloti nifA expression is activated by the FixLJ two-component regulatory system in response to low oxygen tension. In addition nifA is located downstream of fixABCX and nifA expression is enhanced by NifA-mediated expression via the fixA promoter [8]. In B. japonicum the fixR-nifA operon is controlled by the redox-responsive two-component system RegSR acting on the fixRp1 promoter [16]. In R. leguminosarum bv. viciae strain UPM791, nifA is expressed only under symbiotic conditions, through autoregulation via the promoter which precedes the orf71-orf79-fixW-orf5-fixABCX-nifA operon. Basal symbiotic expression of nifA occurs from an unidentified promoter upstream of the 3′-end of fixX [17]. For R. etli, expression of nifA occurs independently of cellular oxygen status and no genetic regulatory elements have been identified. However nifA expression is upregulated under symbiotic conditions, suggesting that it may be under some form of symbiosis-specific regulation [18].
RpoN recognizes and binds a −24/−12 promoter sequence with the consensus 5′-TGGCACG-N4-TTGCW-3′. The G situated at position −24 and C situated at −12 relative to the transcription start site (shown in bold in consensus) are almost invariant although the nifH promoters of M. loti and R. etli have an A instead of C at the −12 position. Sixteen candidate NifA-regulated promoters were defined on ICEMlSymR7A on the basis of their containing a potential NifA upstream activator sequence (TGT-N10-AGA) and a −24/−12 promoter sequence [6]. Of these, 15 are located upstream of annotated genes, including eight that precede known nif/fix gene clusters (Table 1). One potential promoter region was found upstream of msi281 but in reverse orientation, facing a 2.3-kb region that contains a fragment of the nodulation gene noeL but no annotated complete genes. The msi320-msi321 cluster is the only potential NifA-regulated cluster present on ICEMlSymR7A that is not present in MAFF303099 [6]. Whether the M. loti genes in the putative NifA-regulated clusters other than the well-characterised nif/fix genes are required for symbiotic nitrogen fixation remains unknown. However many of them have predicted functions that may be of symbiotic relevance (Table 1).
Table 1. Potential NifA-regulated operons on ICEMlSymR7A.
| Gene or operon | −24/−12 promoter seq. | Putative gene/operon function |
| omp2b (msi036) | TTGGCACGTCATTTGCG | Outer-membrane porin (Omp2 family) |
| msi071-msi064 | TTGGCACGAGTTTTGAA | Diterpenoid synthesis |
| msi158 | TTGGCACGACACATGCG | Outer-membrane porin (OmpW family) |
| msi262-msi263 | CTGGCACGTTCTGTGCA | Msi262 iron-sulfur cluster assembly, HesB family, IscN; Msi263 FeS cluster assembly, NifU N-terminal homology |
| acdS (msi273) | TTGGCACGGTACATGCT | 1-aminocyclopropane-1-carboxylate deaminase |
| fixV frag, hypC frag, msi276-274 | CTGGCATGACGTTTGCT | Msi276 DUF683 (found in nif clusters); Msi275 FdxB Ferredoxin III [4Fe-4S], nif-specific; Msi274 partial similarity (SyrA superfamily) |
| msi280 | CTGGCACGTTCGATGCA | L-lysine 6-monooxygenase |
| Nr msi281 | CTGGCACGGCCTTTGCT | no annotated genes |
| nifHDKENX-msi288 | TTGGCACGAGTTTTGAA | Nitrogenase enzyme synthesis; msi288 unknown function DUF269, NifX-associated protein |
| nifH frag, msi321-320 | TTGGCACGAGTTTTGAA | Msi321 methyltransferase; Msi320 unknown |
| nifQ frag, msi332-331 | TTGGCACGACTTTTGAA | Msi332 cytochrome P450 monooxygenase; Msi331 DUF1271 superfamily, possible ferredoxin |
| prxS-rpoN2 (msi334-msi335) | ATGGCACGGCGCTTGCG | peroxiredoxin; Sigma 54 subunit of RNA polymerase |
| nifS-nifW (msi340-341) | TTGGCACGGTCCATGCG | NifS cysteine desulfurase, iron-sulfur cluster synthesis; NifW nitrogenase-stabilizing/protective protein |
| fixABCX-nifA1 (msi342-346) | TTGGCACGAATGATGCT | Electron transport to nitrogenase; Nif-regulatory protein |
| nifB-fdxN-nifN-fixU-msi351 (msi347-351) | TTGGCATATCTCTTGCG | Nitrogenase synthesis; Msi351 conserved hypothetical, prokaryotic sirtuin-like family |
| ccpR (msi380) | TTGGCACGACTTTTGAT | Cytochrome c peroxidase |
M. loti also contains two rpoN genes, rpoN1 (mll3196 in strain MAFF303099) located on the chromosome outside of the symbiosis island and rpoN2 that is located on the island (msi335 in strain R7A). R. etli also contains rpoN1 and rpoN2 genes and RpoN1 is required for the metabolism of C4-dicarboxylic acids and several nitrogen sources during free-living growth [19] while RpoN2 is involved in symbiotic nitrogen fixation. The rpoN2 gene is part of a prxS-rpoN2 operon that is activated by NifA. An additional symbiosis-specific weak promoter is located between prxS and rpoN2 [20], [21]. In M. loti the rpoN2 gene is also downstream of prxS as part of a potential NifA-regulated operon (Table 1). B. japonicum also has two rpoN genes but both RpoN1 and RpoN2 are functional in free-living and symbiotic conditions [22]. In contrast, S. meliloti and R. leguminosarum bv. viciae strain VF39SM have a single copy of rpoN that in R. leguminosarum is negatively autoregulated [23], [24].
A transcriptome macroarray analysis based on the M. loti MAFF303099 genome revealed clusters of genes within the symbiosis island that were up-regulated during symbiosis compared to free-living growth, whereas genes outside the island were in general down-regulated. The up-regulated genes included island genes involved in metabolism as well as nif-fix genes and the duplicate fixNOQP genes outside the symbiosis island [25].
The aims of the current work were to characterise the NifA-RpoN regulon in M. loti and to establish how nifA expression is activated. In addition we wished to determine the symbiotic phenotypes of selected metabolic genes found to be up-regulated in nodules and to determine whether their expression depended on NifA. We show that symbiotic gene expression in M. loti is under novel regulation and identify several new symbiotic genes. The availability of the M. loti mutants described in this paper should assist future studies of the physiological functioning of nodules formed on the model legume Lotus japonicus.
Results
NifA2 but not NifA1 is required for symbiotic nitrogen fixation although nifA1 encodes a functional protein
Previous work showed that M. loti nifA2 mutants form Fix− nodules [14], [15] whereas nifA1 mutants are not symbiotically impaired [14]. For the current work, marker exchange deletion mutants JS01 (ΔnifA1:: Ωkan) and JS02 (ΔnifA2:: Ωkan) were constructed. As expected, JS01 formed Fix+ nodules on Lotus corniculatus whereas JS02 was Fix− (Table 2). Plasmid pJS100 that contained nifA2 and the preceding 626 bp cloned into vector pFAJ1700 (Table 3) complemented strain JS02 to Fix+, confirming that the Fix− phenotype of the nifA2 mutation was not due to a polar effect on downstream genes.
Table 2. M. loti mutants constructed in this study and their symbiotic phenotypes.
| Strain Background | Descriptiona | R7A mutant Fix phenotypeb | ||
| R7A | JS01 | JS02 | ||
| JS01 | ΔnifA1:: Ωkan, gene replacement deletion | + | ||
| JS02 | ΔnifA2:: Ωkan, gene replacement deletion | − | ||
| JS03 | JS229 | nifA1::lacZ, pFUS2 IDM | + | |
| JS04 | JS121 | JS221 | rpoN1::lacZ, pFUS2 IDM | + |
| JS05A | JS114 | JS214 | ΔrpoN2:: Ωkan, gene replacement deletion | − |
| JS05B | rpoN2::lacZ, pFUS2 IDM | − | ||
| JS06A | JS112 | JS212 | prxS::lacZ, pFUS2 IDM | − |
| JS06B | JS113 | JS213 | ΔprxS, markerless in-frame deletion | + |
| JS07 | prxS::lacZ, pFUS2 CMD | + | ||
| JS08 | fixK::lacZ, pFUS2 IDM | + | ||
| JS09 | fixJ::lacZ, pFUS2 IDM | + | ||
| JS10 | regR::lacZ, pFUS2 IDM | + | ||
| JS11 | regS::lacZ, pFUS2 IDM | + | ||
| JS12 | ΔregR, markerless deletion mutant | + | ||
| JS13 | JS12 fixK::lacZ, pFUS2 IDM | + | ||
| JS14 | JS12 fixJ::lacZ, pFUS2 IDM | + | ||
| JS15 | JS119 | nifA2::lacZ, pFUS2 CMD, 536 bp of nifA2 promoter preceding lacZ | + | |
| JS16 | nifA2::lacZ, pFUS2 CMD, 426 bp of nifA2 promoter preceding lacZ | − | ||
| JS17 | nifA2::lacZ, pFUS2 CMD, 293 bp of nifA2 promoter preceding lacZ | − | ||
| SB01 | ΔfixV:: Ωkan, gene replacement deletion of msi360, renamed fixV in this work | − | ||
| JS18 | fixV::lacZ, pFUS2 IDM | − | ||
| JS19 | SB01 nifA2::lacZ, pFUS2 CMD | − | ||
| JS20 | SB01 fixA::lacZ, pFUS2 CMD | − | ||
| JS21 | JS111 | JS211 | nifH::lacZ, pFUS2 IDM | − |
| JS22 | JS116 | JS216 | nifS::lacZ, pFUS2 IDM | − |
| JS23 | JS118 | JS218 | nifB::lacZ, pFUS2 IDM | − |
| JS24 | JS103 | JS203 | msi158::lacZ, pFUS2 IDM | P |
| JS25 | JS101 | JS201 | msi036::lacZ, pFUS2 IDM | + |
| JS26 | Δmsi036:: Ωkan msi158::lacZ, gene replacement deletion of msi036, pFUS2 IDM of msi158 | P | ||
| JS27 | Δ [msi262-msi263]:: Ωkan, gene replacement deletion of msi262-msi263 | P | ||
| JS28 | Δ [fdxN-fixU]:: Ωkan, gene replacement deletion of fdxN-nifZ-fixU | − | ||
| JS29 | Δ [nifZ-fixU]: Ωkan, gene replacement | − | ||
| JS30 | ΔfixU:: Ωkan, gene replacement deletion | + | ||
| JS31 | msi351::lacZ, pFUS2 IDM | + | ||
| JS32 | JS120 | JS220 | ccpR::lacZ, pFUS2 IDM | + |
| JS33 | JS07 ccpR::lacZ, pFUS2 IDM | + | ||
| JS34 | JS102 | JS202 | msi071::lacZ, pFUS2 IDM | + |
| JS35 | JS124 | JS224 | msi083::lacZ, pFUS2 IDM | + |
| JS36 | JS123 | JS223 | metE::lacZ, pFUS2 IDM | + |
| JS37 | JS122 | JS222 | metK::lacZ, pFUS2 IDM | + |
| JS38 | JS126 | JS226 | pepM::lacZ, pFUS2 IDM | + |
| JS39 | JS104 | JS204 | msi260::lacZ, pFUS2 IDM | + |
| JS40 | JS105 | JS205 | msi262::lacZ, pFUS2 CMD | P |
| JS41 | JS106 | JS206 | acdS::lacZ, pFUS2 IDM | + |
| JS42 | JS128 | JS228 | aatA::lacZ, pFUS2 IDM | + |
| JS43 | JS127 | JS227 | asnB::lacZ, pFUS2 IDM | + |
| JS44 | JS125 | JS225 | exsA::lacZ, pFUS2 IDM | + |
| JS45 | JS115 | JS215 | nifQ::lacZ, pFUS2 IDM | P |
| JS46 | msi338::lacZ, pFUS2 CMD | + | ||
| JS47 | Δmsi337:: Ωkan, gene replacement deletion | P | ||
| JS48 | Δmsi338:: Ωkan, gene replacement deletion | P | ||
| JS49 | JS108 | JS208 | msi280::lacZ, pFUS2 IDM | + |
| JS50 | Δ [msi274-276]:: Ωkan, gene replacement deletion of msi274-msi275-msi276 | + | ||
| JS51 | JS107 | JS207 | msi276::lacZ, pFUS2 CMD | + |
| JS52 | JS109 | JS209 | msi321::lacZ, pFUS2 IDM | + |
| JS53 | JS110 | JS210 | msi332::lacZ, pFUS2 CMD | + |
| JS54 | JS117 | JS217 | fixA::lacZ, pFUS2 CMD | + |
IDM = insertion duplication mutant in which coding sequence disrupted; CMD = cis-merodiploid insertion mutant in which gene is not inactivated as mutant retains wild-type copy of gene including entire promoter region downstream of lacZ fusion (except for JS16 and JS17 in which promoter is truncated).
Symbiotic effectiveness of mutants determined by measuring the wet weights of 15 L. corniculatus seedlings at 6 weeks post-inoculation. Data were compared with those obtained for seedlings inoculated with the wild-type and uninoculated controls. + = Fix+ (fully effective); − = Fix− (ineffective); P = partially effective (see Table 5).
Table 3. Plasmids used in this study.
| Plasmid | Description | Reference |
| pFAJ1700 | Broad-host-range IncP plasmid, TcR | [69] |
| pFAJ1708 | pFAJ1700 containing nptII promoter | [69] |
| pFUS2 | oriC ColE1 oriT RK2 lacZ transcriptional reporter; suicide vector, GmR | [62] |
| pIJ3200 | Broad-host-range IncP plasmid, TcR | [70] |
| pPH1JI | IncP plasmid, GmR | [71] |
| pJQ200SK | Suicide vector containing sacB gene, GmR | [65] |
| pJS100 | pFAJ1700 containing nifA2 and preceding 626 bp | This study |
| pJS101 | pFAJ1700 containing the 626 bp that precedes nifA2 fused at the start codon to the complete nifA1 gene | This study |
| pJS102 | pFAJ1700 containing rpoN1 and preceding 118 bp | This study |
| pJS103 | pFAJ1700 containing the 570 bp that precedes prxS fused at the start codon to the complete rpoN2 gene | This study |
| pJS104 | pFAJ1700 containing fixV and preceding 295 bp | This study |
| pJS105 | pFAJ1700 containing msi158 and preceding 392 bp | This study |
| pJS106 | pFAJ1700 containing msi262 and preceding 739 bp | This study |
| pJS107 | pFAJ1700 containing 279 bp upstream of nifB, with in-frame deletion of nifB and complete fdxN, nifZ and fixU genes | This study |
| pJS108 | pFAJ1700 containing the nifB promoter region, with the 5′ end of nifB fused in-frame to the 3′ end of fdxN, and complete nifZ and fixU genes. | This study |
| pJS109 | pFAJ1700 containing the nifB promoter region, with the 5′ end of nifB fused in-frame to the 3′ end of nifZ, and complete fixU gene. | This study |
| pJS110 | pFAJ1700 containing nifQ cloned behind nptII promoter | This study |
To determine whether nifA1 was expressed in nodules, an insertion-duplication (IDM) mutant with a transcriptional fusion between the 5′-end of the mutated gene and lacZ was constructed by integration through homologous recombination of the suicide vector pFUS2 containing a cloned internal fragment of the gene. Examination of expression of the lacZ fusion in JS03 (nifA1::lacZ) revealed that nifA1 was expressed. However the same fusion was not expressed in a nifA2 mutant strain (Table 4), suggesting that nifA1 transcription initiated from the fixA promoter and not the region immediately upstream of nifA1. To determine if nifA1 encoded a functional protein, the region upstream of nifA2 spanning from the 3′ end of msi360, the gene that precedes nifA2, to the nifA2 start codon was joined to the nifA1 gene at the start codon by extension overlap PCR. The product was cloned into pFAJ1700 and the resulting plasmid pJS101 complemented the nifA2 mutant JS02 to a fully Fix+ phenotype. Taken together these results indicate that nifA1 is functional in nodules formed by R7A, but its expression is dependent on NifA2.
Table 4. Symbiotic expression of various genes in wild-type, ΔnifA1 and ΔnifA2 backgrounds.
| Gene fusion | a ß-galactosidase activity (Miller units) in: | ||
| R7A | JS01 (ΔnifA1) | JS02 (ΔnifA2) | |
| No lacZ fusion | 6.4±1.5 | 5.0±0.7 | 10.0±3.2 |
| nifA genes | |||
| nifA1::lacZ | 297.9±191.2 | ND | 10.6±1.6* |
| nifA2CMD::lacZ | 307.5±96.3 | 293.1±134.2 | ND |
| Genes with NifA/RpoN promoters | |||
| acdS::lacZ | 369.4±167.7 | 547.2±111.9 | 7.5±4.4*+ |
| ccpR::lacZ | 347.7±70.6 | 268.8±73.3 | 7.6±1.8**++ |
| fixACMD::lacZ | 527.8±145.4 | 355.5±74.8 | 7.8±2.4**+ |
| msi036::lacZ | 275.8±39.9 | 76.5±16.6•• | 4.3±1.6*+ |
| msi071::lacZ | 618.4±59.6 | 646.0±157.9 | 6.3±1.2*+ |
| msi158::lacZ | 1281.7±251.8 | 1150.0±211.1 | 5.5±0.9**++ |
| msi260::lacZ | 1339.3±72.5 | 685.9±374.5• | 5.1±2.1**+ |
| msi262CMD::lacZ | 613.2±120.7 | 424.5±139.3 | 7.4±1.3*+ |
| msi276CMD::lacZ | 552.9±108.0 | 301.7±106.8 | 14.2±7.8*+ |
| msi280::lacZ | 371.6±125.7 | 268.9±101.4 | 7.3±1.3*+ |
| msi321::lacZ | 319.8±84.7 | 205.4±63.8 | 11.7±2.1*+ |
| msi332::lacZ | 446.6±71.4 | 273.5±89.9 | 14.6±10.2**+ |
| nifB::lacZ | 684.9±79.7 | 535.7±89.8 | 28.6±23.8**+ |
| nifH::lacZ | 854.8±126.1 | 610.6±86.6 | 9.4±7.2**++ |
| nifS::lacZ | 492.5±144.3 | 413.0±125.7 | 5.9±3.7*+ |
| prxS::lacZ | 204.1±31.1 | 188.8±36.1 | 17.5±12.9*+ |
| prxSCMD::lacZ | 779.8±183.1 | 707.3±174.8 | 6.1±3.4*+ |
| rpoN2::lacZ | 138.7±50.8 | 118.0±25.1 | 19.4±2.0*+ |
| Genes without NifA/RpoN promoters | |||
| aatA::lacZ | 2455.8±110.6 | 2289.8±314.0 | 16.2±7.4**++ |
| asnB::lacZ | 861.7±107.8 | 809.2±174.9 | 22.0±6.7**+ |
| exsA::lacZ | 495.5±223.2 | 524.0±166.5 | 8.7±0.7*+ |
| metE::lacZ | 223.6±91.9 | 171.5±43.4 | 28.1±17.3*+ |
| metK::lacZ | 730.2±227.2 | 467.1±157.2 | 11.1±7.3**+ |
| msi083::lacZ | 280.0±85.4 | 259.6±117.0 | 217.8±98.9 |
| nifQ::lacZ | 200.6±112.2 | 149.3±108.3 | 9.7±10.0*+ |
| pepM::lacZ | 827.1±107.4 | 625.1±168.8 | 7.7±2.0**+ |
| rpoN1::lacZ | 29.7±6.0 | 25.1±7.2 | 24.9±8.5 |
ß-galactosidase assays were performed on bacteroid suspensions from nodules harvested 14 days post-inoculation. All activity values are the average of at least two assays ± Standard Deviation. Significant differences in expression observed between R7A and R7AΔnifA1:: Ωkan ** = P<0.005, between R7A and R7AΔnifA2:: Ωkan ++ = P<0.005, between R7AΔnifA2:: Ωkan and R7AΔnifA1:: Ωkan• = P<0.05 (as determined by unpaired t test).
RpoN2 but not RpoN1 is required for symbiotic nitrogen fixation
To determine the roles of the two M. loti genes that each encode the sigma factor RpoN, an IDM mutant of rpoN1 (strain JS04) and marker exchange deletion (R7AΔrpoN2:: Ωkan, strain JS05A) and IDM (rpoN2::lacZ, strain JS05B) mutants of rpoN2 were constructed in the R7A background. When plated on RDM agar containing succinate as carbon source, growth of the rpoN1 mutant was severely reduced whereas the rpoN2 mutants grew at the wild-type rate. The rpoN1 mutant formed microcolonies on the plates after 7 days, presumably as a result of scavenging carbon sources present in the agar. Growth was restored by plasmid pJS102 that contains the rpoN1 gene and the preceding 118 bp cloned into pFAJ1700. When assayed on L. corniculatus, the rpoN1 mutant formed Fix+ nodules whereas the rpoN2 mutants were Fix−. These results suggested that rpoN2 was not expressed in free-living M. loti but was essential for symbiotic nitrogen fixation.
The prxS gene that encodes an atypical 2-Cys peroxiredoxin is located immediately upstream of the rpoN2 gene and is preceded by a potential NifA-regulated promoter (Table 1). A prxS IDM mutant JS06A formed Fix− nodules. To ascertain if the Fix− phenotype of the prxS mutation was due to a polar effect on rpoN2 expression, a prxS markerless in-frame deletion mutant JS06B was constructed. This strain formed Fix+ nodules. The 570-bp region preceding the prxS start codon was then amplified by PCR and fused to the rpoN2 gene to give plasmid pJS103. This plasmid complemented both mutant strains JS06A and JS06B to a Fix+ phenotype, confirming that prxS was not required for an effective symbiosis and that rpoN2 was expressed from the prxS promoter.
FixLJK and RegSR are not required for symbiotic nitrogen fixation
In order to determine if genes known to mediate nifA expression in other rhizobia were involved in regulating nifA2 in M. loti, IDM mutants were constructed for the R7A fixK (mll6606 in MAFF303099), fixJ (mll6578), regR (mlr5308), and regS (mlr5307) genes. The resulting mutants, strains JS08 to JS11, all formed Fix+ nodules. Double mutants JS13 (ΔregR fixK::lacZ) and JS14 (ΔregR fixJ::lacZ) mutants were then constructed and also formed Fix+ nodules. Southern hybridization analysis carried out to confirm the mutants suggested that only a single copy of each of these genes was present in the R7A genome, as is the case for MAFF303099 [5].
Taken together, the above results indicate that the regulation of nifA expression in M. loti differs from that established for other rhizobial species examined to date. The results are most similar for those found with R. etli where no regulators of nifA expression have yet been found.
nifA2 expression is not autoregulatory
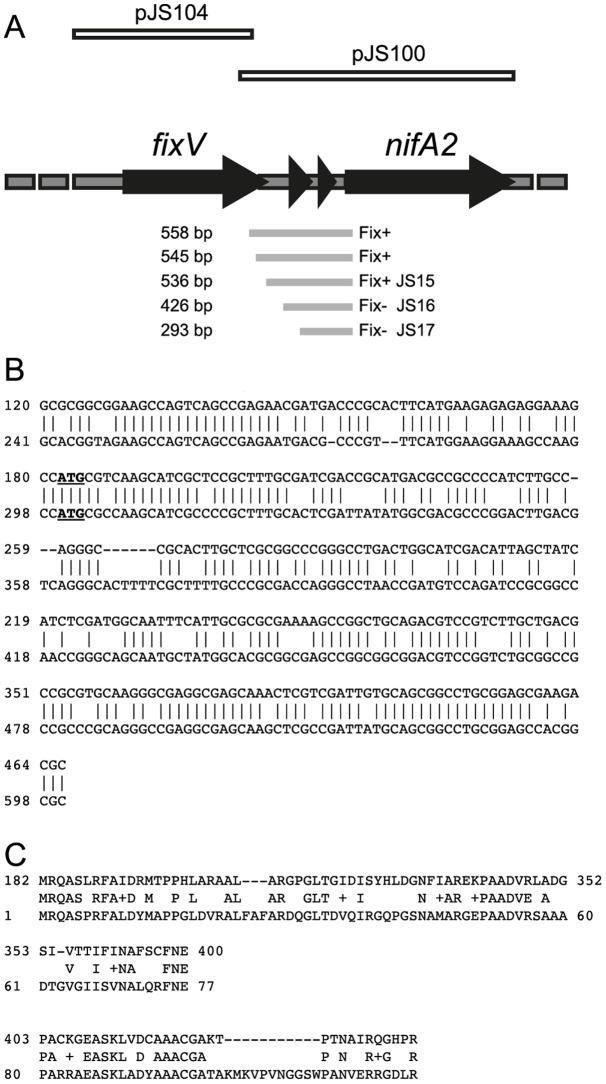
The intergenic region (ICEMlSymR7A coordinates 436876–437433) between msi360, the gene upstream of nifA2, and nifA2 comprises 558 bp (Fig. 1A). BlastN [26] searches carried out using this region as a query showed that it shared 70% nucleotide identity from bp 120–466 with another region conserved between the R7A and MAFF303099 symbiosis islands (Fig. 1B). BlastX analysis showed the presence of two gene fragments spanning bp 182–465 (ICEMlSymR7A coordinates 437058–437341) sharing highest similarity (approximately 45% amino-acid identity) with the N-terminal end of the msi119 gene product that encodes a putative sugar epimerase (COG4130) (Fig. 1A, C).
Figure 1. The fixV-nifA2 region.
A. Map of the fixV-nifA2 region. The location of gene fragments in the intergenic region with homology at the protein level to Msi109 is shown. The inserts in the plasmids pJS104 and pJS100 used for complementation of fixV and nifA2 mutants respectively are indicated above the map. Below the map are the intergenic fragments used to locate the nifA2 promoter. The sizes of the intergenic fragments are shown on the left and the Fix phenotype of the resultant strains on the right. B. BlastN output showing nucleotide identity between the fixV-nifA2 intergenic region and the msi109 region. The ATG corresponding to the start codon of msi109 is bolded and underlined. C. BlastX output showing amino-acid similarity between the two fragments in the fixV-nifA2 intergenic region and Msi109.
To delineate the nifA2 promoter region, a series of nifA2-lacZ nested promoter fusion strains were constructed using the suicide vector pFUS2. The pFUS2 clones were constructed using a series of nested PCR products amplified using a primer nifA2CMDR located within the 5′ end of nifA2 and a series of 5 primers (nifA2CMDL1-5) located at intervals between the 3′ end of msi360 and the 5′ end of nifA2 (Fig. 1A). Insertion of the plasmids into the genome created five cis-merodiploid (CMD) strains. In these strains the full intergenic region along with the 5′-end of the nifA2 gene was fused to lacZ while the amplified promoter region was fused to an intact copy of nifA2 downstream of the inserted plasmid.
The shortest clone that gave a Fix+ phenotype was JS15 that contained a 536-bp region preceding the nifA2 start codon, whereas strains JS16 and JS17 that contained 426-bp and 293-bp regions preceding the start codon respectively were Fix− (Fig. 1A). This indicated that the nifA2 promoter was located upstream of the gene fragments homologous to msi119 located in the msi360-nifA2 intergenic region. ß-galactosidase assays carried out on bacteroids extracted from nodules of plants 14 days post-inoculation with strains JS15, JS16 and JS17 revealed no significant differences in expression measured from the intact nifA2 promoter-lacZ fusion in the strains. The Fix+ strain JS15 that contains the full-length promoter in front of both the nifA2 gene and the nifA2::lacZ fusion gave 307.5±55.6 Miller Units. In comparison, the Fix− strains JS16 and JS17 that contain the same nifA2::lacZ fusion but an inactive nifA2 gene gave 304.8±63.2 and 337.2±103.9 Miller Units, respectively. These data showed that nifA2 was not autoregulated, consistent with the absence of NifA and RpoN binding sites within the putative nifA2 promoter region.
A novel regulatory protein FixV activates nifA2 expression
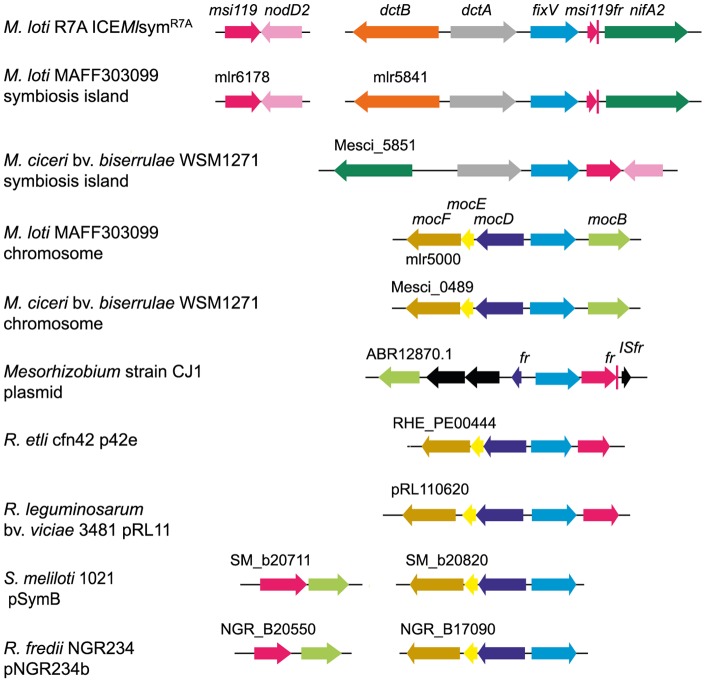
nifA2 is preceded by msi360 which encodes a regulator of the LacI/GalR family. BLAST searches revealed that the most closely related Msi360 orthologs (approx. 60–76% amino-acid identity) are found within other rhizobial species including Mesorhizobium ciceri, R. etli, R. leguminosarum and non-symbiotic Mesorhizobium strain CJ1. In many cases the genes encoding these regulators preceded genes encoding sugar epimerases homologous to Msi119. In several rhizobia, the msi360 homolog was also divergently transcribed from the mocD operon required for catabolism of the rhizopine L-3-O-methyl-scyllo-inosamine (Fig. 2). Mutants in msi360 were constructed by marker replacement (strain SB01; Δmsi360:: Ωkan) and insertion duplication (strain JS18; msi360::lacZ). The mutants were symbiotically defective and wet weights of inoculated plants were not significantly different from uninoculated controls.
Figure 2. Comparison of the genetic organization of gene clusters associated with fixV homologs in a range of rhizobial species.
Genes are shown as arrow symbols and are to scale; colours specific for each gene are used to indicate genes that encode similar proteins in other clusters. Black indicates genes lacking homology to any other genes within the clusters. Fr notes gene fragment, IS denotes insertion sequence.
Several lines of evidence indicated that the msi360 mutations were not polar on nifA2 expression. Both SB01 and JS18 were complemented to a fully Fix+ phenotype by plasmid pJS104. This plasmid contains a PCR product containing msi360 and the preceding 295 bp (Fig. 1A). Furthermore strain JS15 which contains pFUS2 inserted between fixV and nifA2 was Fix+. Finally the nifA2-complementing plasmid pJS100 (Fig. 1A) failed to complement JS18 to Fix+.
To examine nifA2 expression in the msi360 mutant background, strain JS19 (Δmsi360:: Ωkan nifA2::lacZ-CMD) was constructed. Expression of nifA2 in 2-week-old nodules formed by this strain was largely abolished (15.1±6.6 Miller Units compared to 307.5±55.6 Miller Units for strain JS15 (nifA2::lacZ-CMD)), indicating that Msi360 either directly or indirectly activates nifA2 expression. We also introduced a fixA::lacZ-CMD fusion into SB01, creating JS20 (Δmsi360:: Ωkan fixA::lacZ-CMD). Analysis of lacZ expression in bacteroids from nodules formed by this strain showed that fixA expression was abolished in the msi360 mutant background (9.6±7.6 Miller Units compared to 527.8±145.4 Miller Units for strain JS54 (R7A fixA::lacZ-CMD), consistent with a lack of nifA2 expression. These results led us to rename msi360 as fixV in recognition of its contribution to the regulation of nifA2 expression.
Expression analysis of genes preceded by NifA-regulated promoters
Expression studies were carried out to confirm that the 15 putative nifA-regulated promoters present on ICEMlSymR7A that preceded intact genes were subject to NifA-mediated regulation, and to determine whether NifA1 influenced expression from any of these promoters. In most cases, IDM mutants of the first gene downstream of each promoter were constructed using the suicide vector pFUS2 in the wild-type (R7A), ΔnifA1 (JS01) and ΔnifA2 (JS02) strain backgrounds, although in a few cases CMD recombinant strains that did not inactivate the gene were also constructed. The resultant strains contained transcriptional fusions of the mutated gene to lacZ, enabling both the symbiotic phenotype and the expression of the gene to be determined. Mutants in the JS01 background were designated JS101 through to JS128, and those in the JS02 background JS201 through to JS228 (Table 2).
All putative NifA-regulated genes examined were strongly expressed in bacteroids harvested from nodules at two weeks post-inoculation in both the R7A and JS01 backgrounds and expression was abolished in the JS02 background, indicating nifA2 was an absolute requirement for their expression under symbiotic conditions (Table 4). In most cases, expression of the fusion in the JS01 background was less than in the R7A background, but with the exception of msi036::lacZ, the differences were not statistically significant.
Symbiotic phenotypes of NifA-regulated genes
All IDM and CMD mutant strains were assessed for nitrogen-fixing ability on L. corniculatus to determine whether the mutated gene had a symbiotic role, and to confirm that the CMD strains remained Fix+. Visual observations of plant growth and colour were made and wet weights were measured at six weeks post-inoculation. The fixation phenotypes of all recombinant strains are summarised in Table 2. Only strains JS02 JS05A, JS05B, JS06A, JS06B, JS21, JS22, JS23, JS28, and JS29 containing mutations within nifA2, rpoN2, prxS, nifH, nifS, nifB, fdxN and nifZ respectively were completely Fix− (Table 2), producing nodulated seedlings that were otherwise indistinguishable from uninoculated seedlings that displayed severe signs of nitrogen deficiency. However a strain carrying a mutation in msi158 (JS24), a marker exchange mutant in which msi262 and msi263 were deleted (JS27), and JS30, a marker exchange fixU mutant, were partially effective, as plants inoculated with these strains showed growth intermediate between fully Fix+ and Fix− (Tables 2 and 5). The other mutants and all CMD strains tested (with the exception of the nifA2::pFUS2 CMD strains JS16 and JS17, see above) formed fully effective nodules (Table 2).
Table 5. Symbiotic properties of partially effective and ineffective mutants.
| Inoculum strain | Genotype | Mean wet foliage weight in mga | % effectiveness based on wet weight | Acetylene reductionb |
| none | 19.1±3.0** | 22.2 | 0 | |
| R7A | Wild-type | 86.0±32.2 | 100 | 100±35 |
| JS24 | msi158::lacZ | 59.2±9.8* | 68.8 | 51.5±18.2* |
| JS27 | Δ [msi262-263]:: Ωkan | 64.2±22.7 | 74.6 | 72.6±33.5 |
| JS42 | nifQ::lacZ | 61.9±12** | 71.9 | 64.0±15.4* |
| JS28 | Δ [fdxN-fixU]:: Ωkan | 17.46±11.6** | 20.3 | 0 |
| JS29 | Δ [nifZ-fixU]:: Ωkan | 23.6±8.3** | 27.4 | 0 |
| JS30 | ΔfixU:: Ωkan | 67.5±21.7 | 78.4 | 103±22.2 |
Mean wet foliage weight of 30 plants ± Standard Deviation. Data were analysed using the Students T-test. b Percentage of acetylene reduction relative to R7A. Nitrogen fixation activity was measured as the amount of C2H2 reduced (µmol h−1) per plant root for 10 plants ± standard deviation. Data were analysed using the Students T-test. A single asterisk represents P<0.05 and two asterisks P<0.005 when compared to R7A.
msi158 encodes an outer membrane porin with strong similarity to members of the ompW family (COG3047). While only moderate differences in wet weights were observed between seedlings inoculated with the wild-type and strain JS24 (msi158::lacZ) (Table 5), plants infected with the mutant were pale yellow-green in appearance at six weeks post-inoculation in comparison to the wild-type. The plasmid pJS105, containing msi158 and the preceding 392 bp, restored JS24 to a wild-type phenotype. msi036 also encodes a porin (Omp2, COG3452) and is preceded by a NifA-regulated promoter. Msi036 bears no sequence similarity to Msi158. The msi036::lacZ mutant JS25 was fully effective. To determine if msi036 and msi158 were partially functionally redundant, a double mutant JS26 (Δmsi036:: Ωkan msi158::lacZ) was constructed and showed a symbiotic phenotype indistinguishable from that observed for the msi158 mutant.
The msi262 (iscN; COG0316) and msi263 (iscU; COG0822) genes were deleted by marker exchange, producing the double mutant JS27 (Δ [msi262-msi263]:: Ωkan). The mutant showed a partial defect in nitrogen fixation (Table 5). The plasmid pJS106, which contained only msi262 and the preceding 739-bp non-coding region, restored JS27 to the wild-type symbiotic phenotype, indicating that only msi262 was required for a fully effective symbiosis.
fdxN, nifZ, fixU, and msi351 are located within the nifB-fdxN-nifZ-fixU-msi351 cluster. To determine whether these genes have a symbiotic role, three marker exchange mutants, designated JS28 (Δ [fdxN-nifZ-fixU]:: Ωkan), JS29 (Δ [nifZ-fixU]:: Ωkan) and JS30 (ΔfixU:: Ωkan), together with JS31 (msi351::lacZ) were constructed and their symbiotic phenotypes determined. The msi351::lacZ mutant JS31 was fully effective. JS28 and JS29 displayed an ineffective phenotype, whereas JS30 appeared partially effective. Plants inoculated with JS30 were slightly pale in appearance and displayed a slight reduction in average wet shoot weight; however no difference in acetylene reduction was observed (Table 5). Complementation analysis was performed to determine if both fdxN and nifZ were required for a fully effective symbiosis using a series of three plasmids. pJS107 contained the nifB promoter region and the nifB-fdxN-nifZ-fixU cluster with an in-frame deletion within nifB and complemented JS28 and JS29 as expected. Plasmid pJS108 contained an in-frame deletion that removed nifB and fdxN, leaving nifZ and fixU intact, and complemented JS29 but not JS28. Plasmid pJS109 contained an in-frame deletion that fused the 5′ end of nifB to the 3′ end of nifZ leaving only fixU intact. This plasmid did not complement the JS28 or JS29 mutants but complemented JS30. These results indicated that all three genes were required for a fully effective phenotype, although FixU appeared to exert a very slight influence on nitrogen fixation.
As described above, prxS encodes a NifA-regulated peroxiredoxin but is not required for an effective symbiosis. Another NifA-regulated gene present on ICEMlSymR7A, ccpR (msi380), encodes a cytochrome C peroxidase (COG1858). A ccpR::lacZ mutant JS32 was Fix+ on L. corniculatus. In order to determine if functional redundancy existed between prxS and ccpR, a double mutant JS33 (ΔprxS ccpR::lacZ) was constructed. The double mutant also formed Fix+ nodules.
Expression and symbiotic phenotypes of genes not preceded by NifA-regulated promoters
In addition to the NifA-regulated operons, the expression of a selection of ICEMlSymR7A genes encoding metabolic functions and an ABC transporter was examined. The genes were chosen because preliminary screening of lacZ expression from IDM mutants showed the genes were expressed at higher levels in 14-day-old nodules than in G/RDM broth cultures (ß-galactosidase activity of less than 20 Miller Units in broth culture for all mutants). This suggested these genes may be subject to symbiosis-specific regulation. The genes selected included msi083, which encodes the beta subunit of transketolase, metE (msi160; 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase), metK (msi166; S-adenosylmethionine synthetase) pepM (msi327; phosphoenolpyruvate phosphomutase), msi260 (putative diaminobutyrate-2-oxoglutarate transaminase), aatA (msi326; aspartate aminotransferase), asnB (msi323; asparagine synthetase) and exsA (msi339). exsA encodes a MsbA-like saccharide-exporting ABC transporter similar to S. meliloti ExsA (71% amino acid identity) that is involved in the export of the exopolysaccharide succinoglycan [27]. The nifQ gene (msi336) was also selected. nifQ is located with the msi338-msi337-nifQ gene cluster. Homologs of msi337 (fdxB) and msi338 are associated with nif and fix gene clusters preceded by NifA-regulated promoters in some diazotrophs, but a NifA-regulated promoter does not precede the ICEMlSymR7A cluster. The IDM mutants of msi083, metE, metK, pepM, msi260, aatA, asnB, exsA and nifQ were designated JS35, JS36 JS37, JS38, JS39, JS42, JS44 and JS45 respectively. msi337 and msi338 were inactivated by marker exchange mutagenesis producing strains JS47 and JS48.
With the exception of the nifQ (msi339), msi337 and msi338 mutants which formed partially effective nodules compared to R7A (Table 5), all of the mutants formed a fully effective symbiosis. The nifQ gene was amplified by PCR and cloned adjacent to the nptII promoter in pFAJ1708 producing plasmid pJS110. This plasmid complemented JS45, JS47 and JS48 to a fully Fix+ phenotype, indicating that nifQ was the only gene required within the msi338-msi337-nifQ cluster for an effective symbiosis.
ß-galactosidase assays performed on extracts from 14-day-old root nodules formed by IDM mutants revealed that, with the exception of msi083, all of the genes were poorly expressed in the nifA2 mutant background, but were strongly expressed in the wild-type and nifA1 mutant backgrounds. msi083 was strongly expressed in all three backgrounds (Table 4).
Discussion
Our results show that the regulators FixJ, FixK and RegR that initiate symbiotic gene expression in other rhizobia are not required for symbiotic nitrogen fixation in M. loti. Instead M. loti has evolved a different mechanism for the activation of nifA expression. Although nifA1 encodes a functional NifA protein and is in an identical genomic context to the sole nifA gene in several other rhizobial species, it is under the regulation of the product of the second nifA gene, nifA2. The nifA2 gene in turn is under the regulation of a novel regulator FixV that is a member of the LacI/GalR family. A model for the regulatory network governing symbiotic nitrogen fixation in M. loti is shown in Fig. 3.
Figure 3. Model for the regulatory network governing symbiotic nitrogen fixation in M. loti.
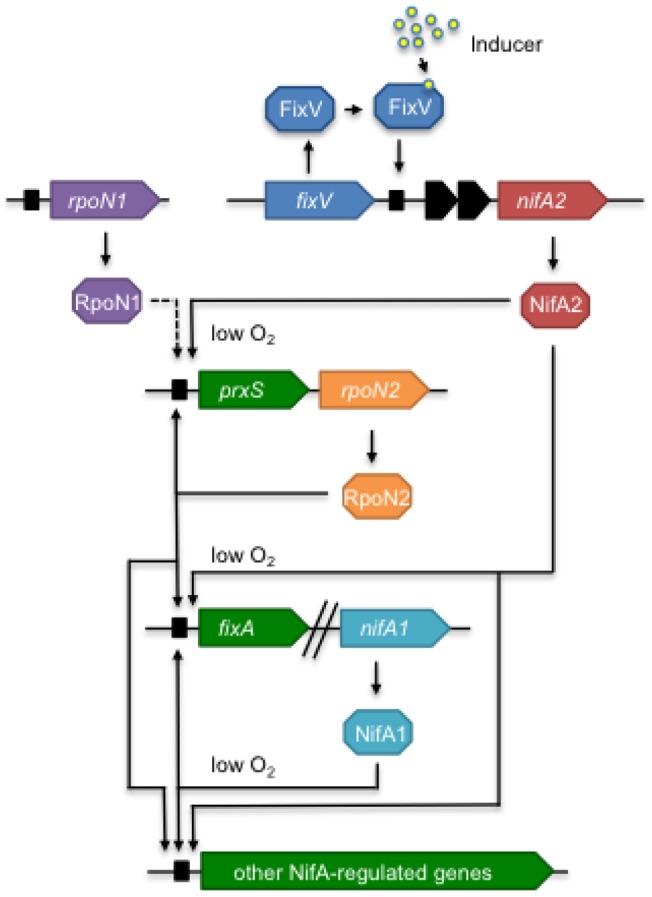
In response to an inducer molecule (possibly an inositol derivative), FixV activates expression of nifA2. NifA2, in conjunction with RpoN1 or basal levels of RpoN2 produced from an unknown promoter, activates expression of the prxS-rpoN2 and fixABCX-nifA1 operons. NifA2 and NifA1, in conjunction with the increased levels of RpoN2, then activate expression of operons required for the production of functional nitrogenase along with other NifA-regulated operons that encode auxiliary metabolic functions.
The fixV gene is located upstream of nifA2 and the 558-bp intergenic region was found to contain gene fragments homologous to an ICEMlSymR7A gene msi119 that encodes a sugar epimerase. Homology was detectable at both the nucleotide and amino acid levels. Analysis of a series of mutants with a nested set of promoter deletions showed that sequences required for nifA2 expression were located downstream of fixV but upstream of the gene fragments. Bioinformatic analysis revealed that homologs of msi119 were located downstream of fixV homologs in M. ciceri, R. etli, R. leguminosarum and Mesorhizobium sp. strain CJ1 (Fig. 2). Taken together, these results suggest that expression of nifA2 was placed under FixV control by a translocation event involving fixV and a downstream promoter that it regulates. Furthermore it seems likely that FixV responds to a carbohydrate signal to initiate nifA2 transcription. The LacI/GalR family of transcriptional regulators consist of an N-terminal helix-turn-helix DNA-binding domain and a C-terminal ligand-binding domain that is structurally homologous to periplasmic sugar-binding proteins [28], [29], [30]. While most family members are repressors, a few members are activators. The most closely related ortholog outside of the analogous copy on the M. loti MAFF303099 symbiosis island was found on the symbiosis island of M. ciceri bv. biserrulae WSM1271 (76% amino-acid identity) where fixV is directly upstream of a msi119 homolog and close to nifA2. The msi119 homolog is also directly downstream of and divergently transcribed from nodD2 as it is in R7A and MAFF303099 (Fig. 2). It appears possible that WSM1271 represents the ancestral genetic organisation and that a series of recombination events may have led to the arrangement observed in R7A and MAFF303099. The next strongest homologs of FixV (approx. 60% identity, 75% similarity over entire protein) are encoded on the M. loti MAFF303099 chromosome, and on plasmids in S. meliloti, Rhizobium sp. NGR234, R. etli and R. leguminosarum. They are located adjacent to a mocDEF cluster involved in rhizopine catabolism. In the case of R. etli and R. leguminosarum, the msi119 homolog is downstream of the fixV homolog. Rhizopine is L-3-O-methyl-scyllo-inosamine, a derivative of inositol [31], and so these observations strongly suggest that Msi119 homologs are involved in catabolism of an inositol derivative. Furthermore, Msi119 is a member of pfam01261, defined by the presence of a TIM alpha/beta barrel structure that is found in xylose isomerase, endonuclease IV and in the N termini of bacterial myo-inositol catabolism proteins. Inositol derivatives play a wide variety of roles in plants and myo-inositol is one of the more abundant non-structural carbohydrates in soybean nodules, where it is largely localized to the peribacteroid space [32]. It thus seems possible that the metabolite FixV senses to activate expression of nifA2 is a derivative of inositol.
A combination of mutagenesis and complementation analysis of the prxS-rpoN2 operon showed that rpoN2 but not prxS was essential for an effective symbiosis. In contrast, rpoN1 was required for C4-dicarboxylate usage in free-living M. loti but was not required for symbiosis. These results are similar to those obtained with R. etli, except that nodules formed by the R. etli rpoN2 mutant show a low level of nitrogen fixation activity [19]. The prxS-rpoN2 operon is preceded by an RpoN-dependent promoter and a NifA UAS sequence. It is interesting to note that significant expression of the prxS-rpoN2 operon was observed in bacteroids formed by JS06A (prxS::lacZ) and JS05B (rpoN2::lacZ), given that both these mutants were ineffective as a result of inactivation of rpoN2. This activity was approximately 17% (rpoN2::lacZ) or 25% (prxS::lacZ ) of that obtained with a prxS+::lacZ cis-merodiploid strain (JS07) where the prxS-rpoN2 operon remains intact. For R. etli, expression studies have suggested that a weak symbiosis-specific promoter is located between the end of prxS and start of rpoN2. Symbiosis-specific expression from this promoter initiates rpoN-independent expression of the prxS-rpoN2 operon in an rpoN1 mutant background [21]. However in nodules formed by M. loti R7A, the expression of both prxS and rpoN2 in the nifA2 mutant background appeared wholly dependent on NifA2 (Table 4). Nevertheless, the fact that RpoN1 is not required for symbiotic nitrogen fixation indicates that there must be some NifA-independent expression of rpoN2 in M. loti. Whether this is basal expression from the promoter upstream of prxS or expression from a weak promoter between prxS and rpoN2, as is the case in R. etli, remains to be determined.
It is striking that in R. etli, R. leguminosarum and M. loti, the strongly expressed and symbiotically essential nifH promoter deviates from the −24/−12 consensus at the critical −12 region, with an A instead of the highly conserved C. An A at −12 was found in only 9 out of 186 potential RpoN-regulated promoters identified in silico from 44 species belonging to the Rhizobiales [33]. The RpoN-dependent promoter of the fdxN gene of B. japonicum also has an A at the −12 position and this promoter is active in B. japonicum but not E. coli, unlike other B. japonicum NifA-regulated promoters [34]. The RpoN1 and RpoN2 proteins of R. sphaeroides show specificity to transcribe a particular set of genes that is due in part to the particular nucleotide at the −11 position of the promoter and in part because they only function with their cognate activators [35]. The expression of the prxS-rpoN2 promoter observed in rpoN2 (and rpoN2/nifA1) mutant background(s) rules out the possibility that only RpoN2 and not RpoN1 can interact with NifA2. Hence it seems possible that in M. loti only RpoN2 and not RpoN1 recognizes the atypical nifH promoter.
The construction of IDM mutants allowed us to determine both expression of the mutated gene in its normal genomic context and the symbiotic phenotype of the mutant. The expression patterns of the putative NifA-regulated operons in the nifA2 and nifA1 mutant backgrounds were consistent with their direct activation by NifA-RpoN. The results also confirmed that nifA1 was not required for expression of any of the NifA-regulated genes located on ICEMlSymR7A. Several ICEMlSymR7A-encoded genes not associated with NifA-regulated promoters (msi083, metE, metK, pepM, msi260, aatA, asnB, nifQ, exsA) were also strongly expressed in nodules but, with the exception of msi083, were not expressed in the nifA2 mutant background. It is highly unlikely that their expression is directly activated by NifA. The fact that msi083 and nifA2 expression was readily detected in the nodules formed by nifA2 mutants indicates that expression of these genes would have been detected had it occurred. It seems likely that other factors influenced by NifA expression under symbiotic conditions such as nitrogen and carbon fluxes and oxygen tension induce the expression of these genes in functional nodules. These results contrast with those observed when microarray and proteome analysis was performed on RNA and protein extracted at 11 days post-inoculation from Phaseolus vulgaris nodules formed by wild-type R. etli strain CFN42 and a nifA mutant. This analysis revealed only five genes that were not preceded by RpoN and/or NifA regulatory elements that were down-regulated in the nifA mutant versus the wild-type under symbiotic conditions [36]. None of these genes corresponded to those found to be down-regulated in Lotus nodules in the current study.
In common with studies of other rhizobia, we observed that many genes that were strongly expressed in nodules did not produce an overt symbiotic phenotype when mutated. However, of the genes not previously shown to be required for symbiosis, msi158 and nifZ that are regulated by NifA and nifQ that lacks a NifA-regulated promoter were found to be required for a fully effective symbiosis. The msi158 mutant formed partially effective nodules and the plants were yellowish, indicating nitrogen deficiency. The msi158 gene encodes an outer membrane protein of the OmpW family (COG3047) that shares strong similarity with the gene products of y4MB present on pNGR234a of Rhizobium sp. NGR234 and bll1766 from the symbiotic region of B. japonicum. NifA-regulated promoters are also located upstream of these two orthologs [5], [11], [37]. A bll1766 mutant formed normal nitrogen-fixing nodules on soybean [37]. However a strongly conserved bll1766 ortholog (blr1311) is located elsewhere on the B. japonicum USDA110 genome [38]. No orthologs are present in the S. meliloti 1021, R. leguminosarum bv. viciae strain 3841, R. leguminosarum bv. trifolii WSM1325, or R. etli CFN42 genomes, or in M. loti MAFF303099 outside of the symbiosis island [5], [39], [40], [41], [42], [43], [44]. The E. coli OmpW protein forms an eight-stranded ß-barrel with a hydrophobic channel and may be involved in the transport of small hydrophobic molecules across the bacterial outer membrane [45]. In Salmonella enterica serovar Typhimurium, the ompW gene is part of the SoxRS regulon that protects against oxidative stress and it has been suggested that the porin functions as an efflux channel for toxic compounds generated during oxidative stress [46]. A similar role in M. loti would make msi158 the third member of the NifA-RpoN regulon together with prxS and ccpR likely involved in protection against reactive oxygen species.
The different rhizobial species vary considerably in the complement of nif genes that they share with the paradigm nitrogen-fixing microorganism, the free-living diazotroph Klebsiella pneumoniae, and it is apparent that the nitrogenase assembly machinery is to an extent species-specific in rhizobia (reviewed in [47]). For example, the nifB-fdxN-nifZ-fixU-msi351 cluster found in M. loti is present to varying extents in other rhizobia: it is complete in R. etli CFN42 and M. ciceri bv biserrulae WSM1271, missing msi351 in Rhizobium sp. strain NGR234, missing nifZ and msi351 in S. meliloti 1021 and Bradyrhizobium species ORS278 and BTAi1 (although nifZ is located elsewhere in the latter two species), while in R. leguminosarum only nifB is present [5], [38], [39], [40], [41], [42], [43], [44], [48], [49]. We showed that mutations within the ICEMlSymR7A nifZ and fdxN genes abolished nitrogen fixation. The nifZ gene is found in several diazotrophs and is involved in maturation of the Mo-Fe protein [50]. The FdxN protein is thought to serve in the pathway of electron transfer to nitrogenase. In S. meliloti mutations within fdxN also completely abolish nitrogen fixation [51]. The function of fixU (also called nifT in some diazotrophs) is unknown and inactivation of nifT in K. pneumoniae has no obvious effects on nitrogen fixation [52], [53]. Our results show that active FixU is required for optimal N fixation in M. loti under the growth conditions used.
The msi338-msi337-nifQ gene cluster is not preceded by a NifA-regulated promoter although a NifA-regulated promoter is present upstream of a nifQ fragment that precedes msi332 on ICEMlSymR7A. Homologs of msi337 (fdxB) and msi338 are located within or adjacent to nif and fix clusters preceded by NifA-regulated promoters in S. meliloti, R. etli and B. japonicum. R. leguminosarum possesses fdxB but not msi338 while nifQ is absent from S. meliloti and R. leguminosarum [38], [39], [40], [41], [42], [43], [44]. Homologs of Msi338 are also encoded within nitrogen fixation gene clusters of a wide range of bacteria [54]. NifQ participates as a molybdenum donor for FeMoCo biosynthesis [55]. Our results showed that nifQ was required for a fully effective symbiosis, in contrast to the situation in Rhizobium sp. strain NGR234 where mutation of nifQ had no effect on symbiotic nitrogen fixation [56]. The lack of a symbiotic defect in msi337 and msi338 mutants may reflect functional redundancy as probable orthologs of these genes are present in the msi276-msi275-msi274 gene cluster that is preceded by a NifA-regulated promoter. Consistent with this, a mutant strain JS50 (Δ [msi274-msi276]:: Ωkan) in which all three genes were deleted formed Fix+ nodules.
The msi262 and msi263 genes were renamed iscN and iscU respectively and are likely involved in the production of iron-sulfur clusters for nitrogenase. Msi262 shows 71% identity to the R. etli iscN gene product that is thought to act as a scaffold protein for Fe-S biosynthesis. Mutants of R. etli defective in iscN showed a 90% reduction in nitrogen fixation [57]. Msi263 is a member of the IscU protein family (COG0822). These proteins are similar to the N-terminal region of NifU and are also thought to play a scaffolding role in Fe-S cluster formation. As suggested for R. etli, it seems likely that the IscN and IscU homologs are partially functionally redundant. However the iscN-iscU double mutant was partially effective, suggesting that M. loti may harbor additional genes that can at least partially complement their function.
In summary, a novel regulator FixV together with NifA2 were identified as key regulators of genes required for nodule function in M. loti, with FixV activating nifA2 expression possibly in response to a plant-produced inositol derivative (Fig. 3). Many genes encoded on ICEMlSymR7A were strongly expressed in nodules in a NifA2-dependent manner but not free-living rhizobia. Nevertheless most of these genes were not required for symbiotic nitrogen fixation. It seems likely that some of these genes have functional homologues elsewhere in the genome and that bacteroid metabolism may be sufficiently plastic to adapt to loss of various enzymatic functions.
Materials and Methods
Bacterial strains, plasmids and growth conditions
The wild-type M. loti strain used in this study was R7A, a field reisolate of ICMP 3153 (NZP2238) [2]. Mutant strains constructed in the R7A, JS01 (R7AΔnifA1) and JS02 (R7AΔnifA2) backgrounds are described in Table 2. Plasmids are listed in Table 3. M. loti strains were grown at 28°C in TY [58] or in rhizobium defined medium with 10 mM glucose (G/RDM) or 10 mM succinate (S/RDM) as previously described [59]. Escherichia coli strain S17-1 [60] was used for cloning and as the donor for biparental matings. It was cultured in LB or TY medium. Antibiotics were used at the following concentrations: for E. coli, tetracycline 15 µg mL−1, kanamycin 50 µg mL−1 and gentamicin 25 µg mL−1; and for M. loti tetracycline 2 µg mL−1, neomycin 200 µg mL−1, and gentamicin 50 µg mL−1.
DNA manipulations
Plasmid DNA preparations DNA cloning, transformation of DNA into E. coli and Southern hybridisations were carried out using established techniques [61]. Genomic DNA was extracted as described previously [2]. PCR was performed using an Expand HiFi PCR kit (Roche).
Construction of mutants and lacZ promoter fusions
Insertion duplication mutants (IDM) and cis-merodiploid (CMD) lacZ fusions were constructed using the suicide vector pFUS2 [62]. Oligonucleotide primer pairs incorporating restriction sites were used to amplify 350–500 bp regions which comprised either intragenic regions of the target genes to create IDM mutants or the promoter region and 5′ end to create strains containing promoter-lacZ fusions, leaving the associated gene and its promoter region intact. PCR products were then cloned into pFUS2 adjacent to its promoterless lacZ gene and confirmed by sequencing using a lacZ-specific primer. pFUS2 constructs were transferred into M. loti by conjugation from E. coli strain S17-1 donors as described [7] and transconjugants were passaged three times on selective media and then confirmed by Southern hybridization.
Marker exchange mutants were constructed by replacing the gene of interest with the ΩKan interposon [63]. Oligonucleotide primer pairs were designed to amplify 1-kb regions that flanked the target gene and they contained restriction enzyme sites to facilitate cloning. The PCR products were digested with appropriate enzymes and ligated into pIJ3200 along with the ΩKan interposon from pHP45ΩKan [63]. The resulting plasmid was confirmed by DNA sequencing and transferred into R7A by conjugation. Recombination was then forced by plasmid incompatibility using pPH1JI [64] and the mutant confirmed by Southern hybridization. pPH1JI was then removed from the strain by introducing pLAFR1 and an isolate that had lost pLAFR1 was selected as described previously [7].
Markerless deletion mutants of M. loti were constructed using the suicide vector pJQ200SK [65]. One-kilobase regions that flanked the gene were amplified by PCR using primers that included restriction endonuclease sites for cloning. The PCR products were digested and ligated into pJQ200SK. Clones were confirmed by DNA sequencing and then transferred to R7A by conjugation, followed by selection for gentamicin-resistant clones. Integration at the correct site was confirmed by Southern hybridization. Loss of sucrose sensitivity, caused by loss of the sacB gene located on pJQ200SK, was used to select clones that had undergone a second recombination event that removed the vector. Southern hybridization was used to confirm the final deletion derivatives.
Plant assays
Plant studies were performed using L. corniculatus cv. Goldie as previously described [66]. Surface-sterilized seeds were germinated on 0.8% water agar. Seedlings were planted on Jensen's agar slopes in glass test-tubes. For testing mutants for symbiotic effectiveness, 15 plants were inoculated by addition of 100 µl of a cell suspension containing approximately 106 cells. Seedlings were cultivated under environmental conditions of 70% humidity, 25°C, 16 h light, 14°C, 8 h dark. Plants were harvested at six weeks post-inoculation and the effectiveness of the symbiosis determined by visual inspection and by measuring the wet weight of foliage above the first cotyledonary node [66]. Nitrogenase assays were performed on nodulated roots harvested from 15 seedlings as described previously [67]. β-galactosidase assays were performed on bacteroid suspensions as previously described [68] using bacteroid suspensions prepared from nodules harvested 14 days post-inoculation from six plants per inoculum.
Acknowledgments
We thank Rochelle Enright, Natalie Schlegel, Htin Aung, Kien Ly, Regan Murney, Patsarin Rodpothong, Fiona Clow, Damien Fleetwood and Duncan Lash for their contributions to mutant construction and analysis, and nitrogenase assays.
Funding Statement
This work was supported by a grant from the Marsden Fund administered by the Royal Society of New Zealand and grants from the University of Otago. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sullivan JT, Ronson CW (1998) Evolution of rhizobia by acquisition of a 500-kb symbiosis island that integrates into a phe-tRNA gene. Proc Natl Acad Sci USA 95: 5145–5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sullivan JT, Patrick HN, Lowther WL, Scott DB, Ronson CW (1995) Nodulating strains of Rhizobium loti arise through chromosomal symbiotic gene transfer in the environment. Proc Natl Acad Sci USA 92: 8985–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramsay JP, Sullivan JT, Stuart GS, Lamont IL, Ronson CW (2006) Excision and transfer of the Mesorhizobium loti R7A symbiosis island requires an integrase IntS, a novel recombination directionality factor RdfS, and a putative relaxase RlxS. Mol Microbiol 62: 723–734. [DOI] [PubMed] [Google Scholar]
- 4. Wozniak RA, Waldor MK (2010) Integrative and conjugative elements: mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat Rev Microbiol 8: 552–563. [DOI] [PubMed] [Google Scholar]
- 5. Kaneko T, Nakamura Y, Sato S, Asamizu E, Kato T, et al. (2000) Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti . DNA Res 7: 331–338. [DOI] [PubMed] [Google Scholar]
- 6. Sullivan JT, Trzebiatowski JR, Cruickshank RW, Gouzy J, Brown SD, et al. (2002) Comparative sequence analysis of the symbiosis island of Mesorhizobium loti strain R7A. J Bacteriol 184: 3086–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hubber A, Vergunst AC, Sullivan JT, Hooykaas PJJ, Ronson CW (2004) Symbiotic phenotypes and translocated effector proteins of the Mesorhizobium loti strain R7A VirB/D4 type IV secretion system. Mol Microbiol 54: 561–574. [DOI] [PubMed] [Google Scholar]
- 8. Fischer HM (1994) Genetic regulation of nitrogen fixation in rhizobia. Microbiol Rev 58: 352–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Batut J, Terzaghi B, Gherardi M, Huguet M, Terzaghi E, et al. (1985) Localization of a symbiotic fix region on Rhizobium meliloti pSym megaplasmid more than 200 kilobases from the nod-nif region. Mol Gen Genet 199: 232–239. [Google Scholar]
- 10. David M, Domergue O, Pognonec P, Kahn D (1987) Transcription patterns of Rhizobium meliloti symbiotic plasmid pSym: identification of nifA-independent fix genes. J Bacteriol 169: 2239–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freiberg C, Fellay R, Bairoch A, Broughton WJ, Rosenthal A, et al. (1997) Molecular basis of symbiosis between Rhizobium and legumes. Nature 387: 394–401. [DOI] [PubMed] [Google Scholar]
- 12. González V, Bustos P, Ramírez-Romero MA, Medrano-Soto A, Salgado H, et al. (2003) The mosaic structure of the symbiotic plasmid of Rhizobium etli CFN42 and its relation to other symbiotic genome compartments. Genome Biol 4: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scott KF, Rolfe BG, Shine J (1983) Biological nitrogen fixation: primary structure of the Rhizobium trifolii iron protein gene. DNA 2: 149–155. [DOI] [PubMed] [Google Scholar]
- 14. Nukui N, Minamisawa K, Ayabe S, Aoki T (2006) Expression of the 1-aminocyclopropane-1-carboxylic acid deaminase gene requires symbiotic nitrogen-fixing regulator gene nifA2 in Mesorhizobium loti MAFF303099. Appl Environ Microbiol 72: 4964–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sullivan JT, Brown SD, Yocum RR, Ronson CW (2001) The bio operon on the acquired symbiosis island of Mesorhizobium sp. strain R7A includes a novel gene involved in pimeloyl-CoA synthesis. Microbiology 147: 1315–1322. [DOI] [PubMed] [Google Scholar]
- 16. Bauer E, Kaspar T, Fischer HM, Hennecke H (1998) Expression of the fixR-nifA operon in Bradyrhizobium japonicum depends on a new response regulator, RegR. J Bacteriol 180: 3853–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martínez M, Palacios JM, Imperial J, Ruiz-Argüeso T (2004) Symbiotic autoregulation of nifA expression in Rhizobium leguminosarum bv. viciae . J Bacteriol 186: 6586–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benhassine T, Fauvart M, Vanderleyden J, Michiels J (2007) Interaction of an IHF-like protein with the Rhizobium etli nifA promoter. FEMS Microbiol Lett 271: 20–26. [DOI] [PubMed] [Google Scholar]
- 19. Michiels J, Van Soom T, D'Hooghe I, Dombrecht B, Benhassine T, et al. (1998) The Rhizobium etli rpoN locus: DNA sequence analysis and phenotypical characterization of rpoN, ptsN, and ptsA mutants. J Bacteriol 180: 1729–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michiels J, Moris M, Dombrecht B, Verreth C, Vanderleyden J (1998) Differential regulation of Rhizobium etli rpoN2 gene expression during symbiosis and free-living growth. J Bacteriol 180: 3620–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dombrecht B, Heusdens C, Beullens S, Verreth C, Mulkers E, et al. (2005) Defence of Rhizobium etli bacteroids against oxidative stress involves a complexly regulated atypical 2-Cys peroxiredoxin. Mol Microbiol 55: 1207–1221. [DOI] [PubMed] [Google Scholar]
- 22. Kullik I, Fritsche S, Knobel H, Sanjuan J, Hennecke H, et al. (1991) Bradyrhizobium japonicum has two differentially regulated, functional homologs of the sigma-54 gene (RpoN). J Bacteriol 173: 1125–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ronson CW, Nixon BT, Albright LM, Ausubel FM (1987) Rhizobium meliloti ntrA (rpoN) gene is required for diverse metabolic functions. J Bacteriol 169: 2424–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clark SRD, Oresnik IJ, Hynes MF (2001) RpoN of Rhizobium leguminosarum bv. viciae strain VF39SM plays a central role in FnrN-dependent microaerobic regulation of genes involved in nitrogen fixation. Mol Gen Genet 264: 623–633. [DOI] [PubMed] [Google Scholar]
- 25. Uchiumi T, Ohwada T, Itakura M, Mitsui H, Nukui N, et al. (2004) Expression islands clustered on the symbiosis island of the Mesorhizobium loti genome. J Bacteriol 186: 2439–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410. [DOI] [PubMed] [Google Scholar]
- 27. Becker A, Küster H, Niehaus K, Pühler A (1995) Extension of the Rhizobium meliloti succinoglycan biosynthesis gene cluster: identification of the exsA gene encoding an ABC transporter protein, and the exsB gene which probably codes for a regulator of succinoglycan biosynthesis. Mol Gen Genet 249: 487–497. [DOI] [PubMed] [Google Scholar]
- 28. Fukami-Kobayashi K, Tateno Y, Nishikawa K (2003) Parallel evolution of ligand specificity between LacI/GalR family repressors and periplasmic sugar-binding proteins. Mol Biol Evol 20: 267–277. [DOI] [PubMed] [Google Scholar]
- 29. Nguyen CC, Saier MH (1995) Phylogenetic, structural and functional analyses of the LacI-GalR family of bacterial transcription factors. FEBS Lett 377: 98–102. [DOI] [PubMed] [Google Scholar]
- 30. Weickert MJ, Adhya S (1992) A family of bacterial regulators homologous to Gal and Lac repressors J Biol Chem. 267: 15869–15874. [PubMed] [Google Scholar]
- 31. Murphy PJ, Heycke N, Trenz SP, Ratet P, de Bruijn FJ, et al. (1988) Synthesis of an opine-like compound, a rhizopine, in alfalfa nodules is symbiotically regulated. Proc Natl Acad Sci USA 85: 9133–9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tejima K, Arima Y, Yokoyama T, Sekimoto H (2003) Composition of amino acids, organic acids, and sugars in the peribacteroid space of soybean root nodules. Soil Sci Plant Nutr 49: 293–247. [Google Scholar]
- 33. Dombrecht B, Marchal K, Vanderleyden J, Michiels J (2002) Prediction and overview of the RpoN-regulon in closely related species of the Rhizobiales. Genome Biol 3: 0076.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hauser F, Pessi G, Friberg M, Weber C, Rusca N, et al. (2007) Dissection of the Bradyrhizobium japonicum NifA+sigma54 regulon, and identification of a ferredoxin gene (fdxN) for symbiotic nitrogen fixation. Mol Genet Genomics 278: 255–271. [DOI] [PubMed] [Google Scholar]
- 35. Poggio S, Osorio A, Dreyfus G, Camarena L (2006) Transcriptional specificity of RpoN1 and RpoN2 involves differential recognition of the promoter sequences and specific interaction with the cognate activator proteins. J Biol Chem 281: 27205–27215. [DOI] [PubMed] [Google Scholar]
- 36. Salazar E, Díaz-Mejía JJ, Moreno-Hagelsieb G, Martínez-Batallar G, Mora Y, et al. (2010) Characterization of the NifA-RpoN regulon in Rhizobium etli in free life and in symbiosis with Phaseolus vulgaris . Appl Environ Microbiol 76: 4510–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caldelari Baumberger I, Fraefel N, Göttfert M, Hennecke H (2003) New NodW- or NifA-regulated Bradyrhizobium japonicum genes. Mol Plant-Microbe Interact 16: 342–351. [DOI] [PubMed] [Google Scholar]
- 38. Kaneko T, Nakamura Y, Sato S, Minamisawa K, Uchiumi T, et al. (2002) Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res 9: 189–197. [DOI] [PubMed] [Google Scholar]
- 39. Finan TM, Weidner S, Wong K, Buhrmester J, Chain P, et al. (2001) The complete sequence of the 1,683-kb pSymB megaplasmid of the N2-fixing endosymbiont Sinorhizobium meliloti . Proc Natl Acad Sci USA 98: 9889–9894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barnett MJ, Fisher RF, Jones T, Komp C, Abola AP, et al. (2001) Nucleotide sequence and predicted functions of the entire Sinorhizobium meliloti pSymA megaplasmid. Proc Natl Acad Sci USA 98: 9883–9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Capela D, Barloy-Hubler F, Gouzy J, Bothe G, Ampe F, et al. (2001) Analysis of the chromosome sequence of the legume symbiont Sinorhizobium meliloti strain 1021. Proc Natl Acad Sci USA 98: 9877–9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Young JP, Crossman LC, Johnston AW, Thomson NR, Ghazoui ZF, et al. (2006) The genome of Rhizobium leguminosarum has recognizable core and accessory components. Genome Biol 7: R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. González V, Santamaría RI, Bustos P, Hernández-González I, Medrano-Soto A, et al. (2006) The partitioned Rhizobium etli genome: genetic and metabolic redundancy in seven interacting replicons. Proc Natl Acad Sci USA 103: 3834–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reeve W, O'Hara G, Chain P, Ardley J, Bräu L, et al. (2010) Complete genome sequence of Rhizobium leguminosarum bv. trifolii strain WSM1325, an effective microsymbiont of annual Mediterranean clovers. Stand Genomic Sci 2: 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hong H, Patel DR, Tamm LK, van den Berg B (2006) The outer membrane protein OmpW forms an eight-stranded beta-barrel with a hydrophobic channel. J Biol Chem 281: 7568–7577. [DOI] [PubMed] [Google Scholar]
- 46. Gil F, Hernández-Lucas I, Polanco R, Pacheco N, Collao B, et al. (2009) SoxS regulates the expression of the Salmonella enterica serovar Typhimurium ompW gene. Microbiology 155: 2490–2497. [DOI] [PubMed] [Google Scholar]
- 47. Masson-Boivin C, Giraud E, Perret X, Batut J (2009) Establishing nitrogen-fixing symbiosis with legumes: how many rhizobium recipes? Trends Microbiol 17: 458–466. [DOI] [PubMed] [Google Scholar]
- 48. Giraud E, Moulin L, Vallenet D, Barbe V, Cytryn E, et al. (2007) Legumes symbioses: absence of nod genes in photosynthetic bradyrhizobia. Science 316: 1307–1312. [DOI] [PubMed] [Google Scholar]
- 49.Lucas S, Copeland A, Lapidus A, Cheng JF, Goodwin L, et al. (2011) Complete sequence of chromosome of Mesorhizobium ciceri bv. biserrulae WSM1271. unpublished Genbank accessions CP002447.1, CP002448.1). Available: http://www.ncbi.nlm.nih.gov/bioproject/48991.
- 50. Cotton MS, Rupnik K, Broach RB, Hu Y, Fay AW, et al. (2009) VTVH-MCD study of the ΔnifBΔnifZ MoFe protein from Azotobacter vinelandii . J Am Chem Soc 131: 4558–4559. [DOI] [PubMed] [Google Scholar]
- 51. Klipp W, Reiländer H, Schlüter A, Krey R, Pühler A (1989) The Rhizobium meliloti fdxN gene encoding a ferredoxin-like protein is necessary for nitrogen fixation and is cotranscribed with nifA and nifB . Mol Gen Genet 216: 293–302. [DOI] [PubMed] [Google Scholar]
- 52. Harris GS, White TC, Flory JE, Orme-Johnson WH (1990) Genes required for formation of the apoMoFe protein of Klebsiella pneumoniae nitrogenase in Escherichia coli . J Biol Chem 265: 15909–15919. [PubMed] [Google Scholar]
- 53. Simon HM, Homer MJ, Roberts GP (1996) Perturbation of nifT expression in Klebsiella pneumoniae has limited effect on nitrogen fixation. J Bacteriol 178: 2975–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Buchko GW, Robinson H, Addlagatta A (2009) Structural characterization of the protein cce_0567 from Cyanothece 51142, a metalloprotein associated with nitrogen fixation in the DUF683 family. Biochim Biophys Acta 1794: 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hernandez JA, Curatti L, Aznar CP, Perova Z, Britt RD, et al. (2008) Metal trafficking for nitrogen fixation: NifQ donates molybdenum to NifEN/NifH for the biosynthesis of the nitrogenase FeMo-cofactor. Proc Natl Acad Sci USA 105: 11679–11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fumeaux C, Bakkou N, Kopcinska J, Golinowski W, Westenberg DJ, et al. (2011) Functional analysis of the nifQdctA1y4vGHIJ operon of Sinorhizobium fredii strain NGR234 using a transposon with a NifA-dependent read-out promoter. Microbiology 157: 2745–2758. [DOI] [PubMed] [Google Scholar]
- 57. Dombrecht B, Tesfay MZ, Verreth C, Heusdens C, Napoles MC, et al. (2002) The Rhizobium etli gene iscN is highly expressed in bacteroids and required for nitrogen fixation. Mol Genet Genomics 267: 820–828. [DOI] [PubMed] [Google Scholar]
- 58. Beringer JE (1974) R factor transfer in Rhizobium leguminosarum . J Gen Microbiol 84: 188–198. [DOI] [PubMed] [Google Scholar]
- 59. Ronson CW, Astwood PM, Nixon BT, Ausubel FM (1987) Deduced products of C4-dicarboxylate transport regulatory genes of Rhizobium leguminosarum are homologous to nitrogen regulatory gene products. Nucleic Acids Res 15: 7921–7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Simon R, Priefer U, Pühler A (1983) A broad-host mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Biotechnology (NY) 1: 784–791. [Google Scholar]
- 61.Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A laboratory manual. New York, USA: Cold Spring Harbor Laboratory Press.
- 62. Antoine R, Alonso S, Raze D, Coutte L, Lesjean S, et al. (2000) New virulence-activated and virulence-repressed genes identified by systematic gene inactivation and generation of transcriptional fusions in Bordetella pertussis . J Bacteriol 182: 5902–5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fellay R, Krisch HM, Prentki P, Frey J (1989) Ω-Km – a transposable element designed for in vivo insertional mutagenesis and cloning of genes in gram-negative bacteria. Gene 76: 215–226. [DOI] [PubMed] [Google Scholar]
- 64. Ruvkun GB, Ausubel FM (1981) A general method for site-directed mutagenesis in prokaryotes. Nature 289: 85–88. [DOI] [PubMed] [Google Scholar]
- 65. Quandt J, Hynes MF (1993) Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127: 15–21. [DOI] [PubMed] [Google Scholar]
- 66.Vincent JM (1970) A manual for the practical study of root nodule bacteria. Oxford: Blackwell Scientific Publications.
- 67. Hussain AKMA, Jiang Q, Broughton WJ, Gresshoff PM (1999) Lotus japonicus nodulates and fixes nitrogen with the broad host range Rhizobium sp. NGR234. Plant Cell Physiol 40: 894–899. [Google Scholar]
- 68.Miller JH (1972) Experiments in molecular genetics. New York: Cold Spring Harbor Laboratory.
- 69. Dombrecht B, Vanderleyden J, Michiels J (2001) Stable RK2-derived cloning vectors for the analysis of gene expression and gene function in gram-negative bacteria. Mol Plant-Microbe Interact 14: 426–4300. [DOI] [PubMed] [Google Scholar]
- 70. Liu YN, Tang JL, Clarke BR, Dow JM, Daniels MJ (1990) A multipurpose broad host range cloning vector and its use to characterise an extracellular protease gene of Xanthomonas campestris pathovar campestris. Mol Gen Genet 220: 433–440. [DOI] [PubMed] [Google Scholar]
- 71. Hirsch PR, Beringer JE (1984) A physical map of pPH1JI and pJB4JI. Plasmid 12: 139–141. [DOI] [PubMed] [Google Scholar]