Abstract
Background
Personality correlates highly with both cocaine and nicotine dependencies (CD, ND), and their co-morbid psychopathologies. However, little is known about the nature of these relationships. This study examined if environment (marriage) or genetics (a single SNP, CHRNA5*rs16969968) would moderate the correlation of personality with CD, ND and cocaine-induced paranoia (CIP) in African and European Americans (AAs, EAs).
Methods
1432 EAs and 1513 AAs were examined using logistic regression. Personality was assessed by NEO-PI-R, while CD, ND and CIP were diagnosed according to DSM-IV. ND and CD were examined as binary traits and for the analysis of CIP, subjects were divided into 3 groups: (A) Controls with no CIP; (B) CD cases without CIP; and (C) CD cases with CIP. Multiple testing was Bonferroni-corrected.
Results
For CD and ND in the EA population, marital status proved to be a significant moderator in their relationship with openness only (OR = 1.90, 95%CI = 1.36–2.64, p = 1.54e-04 and OR = 2.12, 95%CI = 1.52–2.90, p = 4.65e-06 respectively). For CIP, marriage was observed to moderate its correlation with openness and neuroticism (OR = 1.39, 95%CI = 1.18–1.63, p = 7.64e-04 and OR = 1.26, 95%CI = 1.12–1.42, p = 1.27e-03 respectively). The correlations moderated by rs16969968 were those of conscientiousness and CD (OR = 1.62, 95%CI: 1.23–2.12, p = 8.94e-04) as well as CIP (OR = 1.21, 95%CI: 1.11–1.32, p = 4.93e-04 when comparing group A versus group C). No significant interactions were observed in AA population. The Bonferroni-corrected significance threshold was set to be 1.67e-03.
Conclusion
The role of personality in CD and CIP may be interceded by both environment and genetics, while in ND by environment only.
Introduction
Substance use disorders pose a significant problem for society, with serious health-related, personal and economic consequences [1]–[3]. According to the National Epidemiologic Survey on Alcohol and Related Conditions, approximately 10% of adults in the US experienced a drug-use disorder during their lifetimes [4] and about 20% are smoking cigarettes [5].
One of the most commonly exploited substances is nicotine [6] and it is frequently used in combination with other substances including cocaine [6]–[9]. Both nicotine and cocaine have been shown to act as excitants for each other: the use of cocaine reportedly elevates nicotine intake [10]–[12], while tobacco smoking increases both the frequency and the dosage of cocaine administration [7], [10]. Cocaine and nicotine dependences (ND and CD) are both known to be highly correlated with personality measures. In addition, they also share similarities in their etiological factors, such as marital status and CHRNA5.
Personality characteristics have been consistently associated with substance use disorders. Although the hypothesis of “addictive personality” is not well supported [13], [14], it is still unclear whether different personality traits predict distinct forms of drug dependence and whether the effect of personality would still hold if co-morbid psychopathology were accounted for. In general, substance users can be characterized by higher impulsivity, aggression, disinhibition and novelty seeking [15], [16] as well as by high neuroticism, low agreeableness and low conscientiousness [17], [18]. Similarly, nicotine and cocaine dependences have both been found to be associated with neuroticism, agreeableness and conscientiousness [19]–[21]. In addition, cigarette smoking has been linked to extraversion [21]–[23] and CD to openness [17].
Marriage has been cited as a reproducible protective factor against drug use, with rates of abuse and dependence being higher among individuals who are divorced or separated or have never been married [4], [24]. Cessation of cocaine use as well as cigarette smoking has also been linked to one's marital status [21], [25]–[30], showing that being married maintains its preservative effect on these two dependencies too.
Biologically, one of the most replicable genetic risk factors for ND is rs16969968, a non-synonymous SNP in CHRNA5 [31]–[45]. This polymorphism leads to the substitution of aspartic acid with asparagine (Asp398Asn), which has proven to be functionally relevant in the activity of nicotinic acetylcholine receptor's (nAChR) alpha 5 subunit [41]. In addition, human mRNA clones and expressed sequence tags display evidence of alternative splicing of CHRNA5 in exon 5, in which rs16969968 resides. Possibly, a decrease in inclusion of rs16969968 in the mature mRNA could decrease the risk for ND [37]. Furthermore, rs16969968 may also act together with the variability in CHRNA5's mRNA expression in human brain: occurrence of non-risk allele of rs16969968 on the background of the low mRNA expression allele of CHRNA5 leads to a significantly lower risk for cigarette smoking compared to its co-occurrence with the high expression allele [46].
Apart from being functionally relevant and consistently associated with ND, rs16969968 has also been associated with dependence on cannabis [36], alcohol [36], [47], opiates [48], [49] and cocaine [32], [49]. This shared genetic vulnerability of nicotine and cocaine addictions is particularly interesting as the risk for these two dependencies is conferred by the opposite alleles of rs16969968 [32], [49].
Substance dependence traits cannot be explained solely by either personality or by genetic and environmental factors. The purpose of this study was to investigate the complex interplay among personality measures and the elements of environment (marriage) and genetics (CHRNA5). The main emphasis was placed on the relationship between personality and CD, ND as well as co-morbid psychopathology in the form of CIP; we hypothesized that marital status and CHRNA5 could act as moderators in these correlations. Firstly, the possible moderating effect of marriage was examined in the relationship of personality and CD, ND. The alternate explanation for the association between these dependences and personality domains is that it could be altered by the protective environment a marriage may provide: people who get divorced may have similar traits of personality to those who develop CD and ND, making marital status a moderating factor in the correlation between personality and substance dependence. Thus, we postulated that the association of CD and ND with personality measures may not be independent from marital status.
Secondly, the intermediate role of CHRNA5 SNP rs16969968 was also evaluated in the relationship of the personality measures with CD and ND. Rs16969968 has consistently been associated with both cocaine and nicotine dependences, while personality traits have a strong relationship with the same traits. Since both rs16969968 and personality are associated with ND and CD, there is a possibility of interaction between these two explanatory factors.
Finally, on the account of co-morbid psychopathology predisposing cocaine-dependent patients to developing such specific symptoms as paranoia [50], [51], possible interplay between personality and CHRNA5 as well as marital status was additionally examined in participants displaying symptoms of cocaine-induced paranoia (CIP).
Materials and Methods
Subject recruitment and assessment
Recruitment was conducted at four sites: 1437 participants recruited at the University ofConnecticut Health Center (UCONN, Farmington, CT), 885 participants at Yale University School of Medicine (New Haven, CT), 242 participants at the University of Pennsylvania School of Medicine (UPENN, Philadelphia, PA) and 382 participants at the Medical University of South Carolina (MUSC, Charleston, SC). The protocol and consent forms were approved by the institutional review boards at each site: the UCONN institutional review board, Yale University human research protection program, UPENN office of regulatory affairs- institutional review board and MUSC institutional review board of human research. Written informed consent was obtained from all participants.
All subjects were assessed with the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA) [52], [53], which yields a DSM-IV based lifetime diagnoses for a variety of psychiatric and substance use disorders. Individuals with a primary diagnosis of bipolar affective disorder or schizophrenia were excluded.
Personality traits were characterized in terms of five factors based on the NEO (the Big Five Model-BFM), which comprise behavioral, emotional and cognitive patterns: Neuroticism, Extraversion, Openness to new experiences, Agreeableness and Conscientiousness [54], [55]. The BFM has a hierarchical construction, where each of the five domains includes six facets. The NEO-PI-R (NEO-PI-R) [56] was applied to evaluate these facets of participants' personality.
NEO-PI-R scores were normalized with respect to age and sex of participants as women generally score higher than men on Neuroticism and Agreeableness, while college-age men and women tend to score higher on Openness, Neuroticism, and Extraversion, and lower on Conscientiousness, than do older individuals [57].
Population assignment as either African American (AA) or European American (EA) was done empirically by ancestry proportions estimated in STRUCTURE on the basis of ancestry-informative marker genotypes [58].
Genotyping
SNP rs16969968 was genotyped with a standard TaqMan technique (assay-on-demand), using the ABI PRISM1 7900 Sequence Detection System (ABI, Foster City, CA). All genotyping was performed in duplicate and compared to ensure validity of the data. Mismatched genotypes were discarded.
Statistical analyses
All relationships were examined using logistic regression or multinomial logistic regression models adjusted for age and sex. Subjects classified as abusers (as opposed to dependent) on a particular substance were excluded from all analyses for that substance. To avoid confounding due to population stratification, AA and EA subjects were analyzed separately.
ND and CD were coded as dichotomous variables, dividing participants into cases and comparison subjects (controls) and allowing the use of binomial logistic regression for their analyses. The CD affected group consisted of CD patients regardless of their ND status; likewise, the ND. Since CD and ND were the foci of the analyses, these two dependencies only were considered in the definition of “control” and only those individuals with neither of these diagnoses were classified into this group.
For the multinomial logistic regression analysis of CIP, CD cases (including those co-morbid with ND) and controls were divided into 3 groups: (A) controls; (B) CDs with no CIP symptoms; (C) CDs exhibiting CIP symptoms. Cases with nicotine dependence only were excluded from these analyses. Marital status was coded as (1) never married, (2) separated or divorced and (3) married. Subjects who were widowed at the time of recruitment were excluded (because this would not reasonably be expected to correlate with the subject's personality). Rs16969968 genotype was coded as a dichotomous variable depending on whether an individual carried at least one copy of the minor “A” allele of this SNP.
Before examining the possibility of a moderating effect of marital status and rs16969968 on the role of personality in CD, ND and CIP, exploratory logistic regression was performed to evaluate if there was a significant correlation between personality domains and CD, ND and CIP in our sample at all. In addition, Spearman correlations between all explanatory variables (personality measures, marital status and rs16969968 genotype) were also examined in order to avoid potential co-linearity.
All of the five personality measures revealed significant correlation with CD, ND and CIP; and because these dimensions are known not to be independent from each other, each personality domain was evaluated separately, culminating in five regressions examined for each of the outcomes of CD, ND and CIP. Since none of the explanatory variables showed high correlation with each other (all Spearman's rho values <0.25), regression models were constructed to include the main effects of personality domain (one at a time), marital status and rs16969968, and all of the pair-wise two-way interactions. After evaluating the two-way interactions, the interplay between personality, marital status and rs16969968 in CD, ND and CIP was examined with saturated models by adding a three-way interaction term (personality domain x marital status x rs16969968).
Adjustment for multiple testing was done by Bonferroni correction. Since personality scores were not independent, Bonferroni correction was performed based on the number of autonomous tests only: three models for outcomes of CD, ND, and CIP with two-way interactions, and another three saturated models including the three-way interaction. In addition, the overall significance threshold was reduced to a more stringent level of 0.01, to avoid false positive results. Thus, the Bonferroni-corrected significance threshold p-value was 1.67e-03 (0.01/6) in this study.
Results
Subject recruitment and assessment
In total, 2946 (1432 EA and 1514 AA) unrelated subjects were included. Among EAs, the average age was 38.3 (SD = 11.7) years and 46.9% of individuals were females. Among AAs, the average age was 41.3 (SD = 9.08) years and 44.4% of participants were females. Information on lifetime substance dependence diagnoses is summarized in Table 1.
Table 1. Summary of DSM-IV based, life-time diagnoses of substance addictions in study participants.
| Number of Individuals | ||
| European Americans | African Americans | |
| Cocaine Dependence | ||
| Cases | 1014 | 1178 |
| controls | 418 | 335 |
| Co-morbid ND in cases | 786 (77.51%) | 762 (64.68%) |
| Co-morbid ND in controls | None | None |
| Co-morbid AD in cases | 639 (63.02%) | 657 (55.77%) |
| Co-morbid AD in controls | 59 (14.11%) | 55 (16.42%) |
| Co-morbid OD in cases | 697 (68.74%) | 312 (26.48%) |
| Co-morbid OD in controls | 34 (8.13%) | 19 (5.67%) |
| Co-morbid MDD in cases | 208 (20.51%) | 162 (13.75%) |
| Co-morbid MDD in controls | 36 (8.61%) | 29 (8.66%) |
| Nicotine Dependence | ||
| Cases | 1029 | 845 |
| controls | 418 | 335 |
| Co-morbid CD in cases | 786 (76.38%) | 762 (90.28%) |
| Co-morbid CD in controls | None | None |
| Co-morbid AD in cases | 630 (61.22%) | 510 (60.35%) |
| Co-morbid AD in controls | 59 (14.11%) | 247 (73.73%) |
| Co-morbid OD in cases | 718 (69.78%) | 260 (30.77%) |
| Co-morbid OD in controls | 34 (8.13%) | 103 (30.75%) |
| Co-morbid MDD in cases | 213 (20.70%) | 130 (15.38%) |
| Co-morbid MDD in controls | 36 (8.61%) | 72 (21.49%) |
| Cocaine-Induced Paranoia | ||
| CD cases without CIP | 328 | 364 |
| CD cases with CIP | 686 | 814 |
| controls | 418 | 335 |
| Co-morbid ND in cases without CIP | 242 (73.78%) | 226 (62.09%) |
| Co-morbid ND in cases with CIP | 544 (79.30%) | 536 (65.85%) |
| Co-morbid ND in controls | None | None |
| Co-morbid AD in cases without CIP | 186 (56.71%) | 173 (47.53%) |
| Co-morbid AD in cases with CIP | 453 (66.03%) | 484 (59.46%) |
| Co-morbid AD in controls | 59 (14.11%) | 55 (16.42%) |
| Co-morbid OD in cases without CIP | 228 (69.51%) | 98 (26.92%) |
| Co-morbid OD in cases with CIP | 469 (68.37%) | 214 (26.29%) |
| Co-morbid OD in controls | 34 (8.13%) | 19 (5.67%) |
| Co-morbid MDD in cases without CIP | 61 (18.60%) | 49 (13.46%) |
| Co-morbid MDD in cases with CIP | 147 (21.43%) | 113 (13.88%) |
| Co-morbid MDD in controls | 36 (8.61%) | 29 (8.66%) |
CD = cocaine dependence, ND = nicotine dependence, AD = alcohol dependence, OD = opiate dependence, MDD = major depression disorder, CIP = cocaine induced paranoia.
The scores of main five domains in NEO-PI-R followed normal distribution. Their mean and standard deviations are presented in Table 2.
Table 2. Mean and standard deviation (SD) of NEO-PI-R normalized scores in the participants of the study.
| European American Population | |||||
| Mean (Standard Deviation) score of | |||||
| Neuroticism | Extraversion | Openness to experience | Agreeableness | Conscientiousness | |
| CD Cases | 60.15 (9.63) | 48.60 (9.61) | 47.63 (9.12) | 41.20 (10.38) | 38.77 (10.81) |
| CD Controls | 51.51 (11. 12) | 50.92 (10.45) | 50.69 (10.07) | 47.89 (12.29) | 46.96 (12.30) |
| ND Cases | 59.99 (9.51) | 48.22 (9.49) | 47.71 (9.21) | 51.29 (10.63) | 38.69 (10.75) |
| ND Controls | 48.61 (10.59) | 52.21 (10.66) | 51.39 (10.15) | 50.49 (11.79) | 50.28 (11.72) |
| CD cases without CIP | 58.18 (9.84) | 48.64 (9.78) | 47.36 (8.99) | 42.13 (9.98) | 39.29 (11.08) |
| CD cases with CIP | 61.08 (9.39) | 48.58 (9.54) | 47.75 (9.19) | 40.76 (10.55) | 38.53 (10.68) |
| CIP Controls (no CD and no CIP) | 48.61 (10.59) | 52.21 (10.66) | 51.39 (10.14) | 50.49 (11.79) | 50.28 (11.72) |
CD = cocaine dependence, ND = nicotine dependence, CIP = cocaine induced paranoia.
Genotyping of rs16969968
All included subjects were successfully genotyped for rs16969968. Consistent with previous reports (minor allele frequency ranges from 33% to 43% in EA; and ∼5% in AA [31], [32], [41]), the observed minor allele frequency for this SNP was 35.3% in EA subjects and 6.6% in AA participants. Genotype distributions were consistent with Hardy-Weinberg equilibrium expectations in both populations overall, as well as in case and control groups of AAs and EAs.
Statistical analyses
For examining the interaction between personality measures and marital status, significant moderating effects of both marriage and divorce were observed in the relationship between the “openness to experience” domain and CD, ND as well as CIP in the EA population only (Tables 3, 4 and 5). The strongest impact appeared to be generated by “married” status compared to the “never married” state on the correlations between “openness to experience” and ND (OR: 2.12, 95%CI: 1.36–2.64, p-value: 4.65e-06), followed by CD (OR: 1.90, 95%CI:1.55–2.90, p-value:1.54e-04) and CIP (OR: 1.39, 95%CI: 1.18–1.63, p-value: 7.64e-04).
Table 3. Effect of marital status and CHRNA5 (rs16969968) on the relationship between NEO-PI-R personality domains and CD in AA and EA subjects.
| NEO-PI-R domain and Population | |||||||||||
| Neuroticism | Extraversion | Openness to experience | Agreeableness | Conscientiousness | |||||||
| AAs | EAs | AAs | EAs | AAs | EAs | AAs | EAs | AAs | EAs | ||
| Main effect of NEO-PI-R domain | OR (95% CI) | 3.00 (2.33–3.88) | 2.91 (2.22–3.83) | 0.66 (0.53–0.82) | 0.54 (0.42–0.68) | 0.59 (0.48–0.74) | 0.44 (0.34–0.57) | 0.51 (0.50–0.52) | 0.43 (0.34–0.55) | 0.43 (0.35–0.52) | 0.30 (0.23–0.39) |
| p-value | 2.00e-16 | 1.40e-14 | 1.70e-04 | 4.60e-07 | 3.28e-06 | 1.65e-10 | 5.90e-12 | 4.69e-12 | 2.00e-16 | 2.00e-16 | |
| Main effect of “Divorced” marital status | OR (95% CI) | 1.03 (0.81–1.32) | 1.09 (0.89–1.33) | 0.96 (0.77–1.21) | 0.81 (0.68–0.95) | 0.75 (0.60–0.94) | 0.76 (0.64–0.91) | 1.17 (0.97–1.42) | 1.08 (0.91–1.27) | 0.94 (0.78–1.13) | 0.94 (0.80–1.11) |
| p-value | 0.780 | 0.389 | 0.767 | 0.011 | 0.013 | 2.27e–03 | 0.100 | 0.368 | 0.496 | 0.475 | |
| Main effect of “Married” marital status | OR (95% CI) | 0.84 (0.65–1.08) | 0.64 (0.51–0.82) | 0.86 (0.70–1.05) | 0.72 (0.61–0.85) | 0.77 (0.63–0.95) | 0.62 (0.53–0.74) | 1.06 (0.84–1.20) | 0.98 (0.83–1.16) | 1.03 (0.84–1.28) | 0.97 (0.825–1.14) |
| p-value | 0.180 | 2.72e-04 | 0.147 | 1.21e-04 | 0.013 | 4.43e-08 | 0.947 | 0.859 | 0.750 | 0.736 | |
| Main effect of rs16969968 | OR (95% CI) | 0.77 (0.54–1.09) | 1.01 (0.85–1.34) | 0.81 (0.64–1.03) | 0.95 (0.83–1.10) | 0.94 (0.73–1.21) | 0.93 (0.80–1.07) | 1.01 (0.99–1.03) | 0.92 (0.82–1.05) | 1.03 (0.81–1.31) | 0.80 (0.70–0.91) |
| p-value | 0.136 | 0.886 | 0.083 | 0.507 | 0.656 | 0.317 | 0.953 | 0.225 | 0.795 | 1.05-03 | |
| Interaction between NEO-PI-R domain and “Divorced” marital status | OR (95% CI) | 0.92 (0.57–1.51) | 0.88 (0.62–1.25) | 1.05 (0.67–1.64) | 1.65 (1.20–2.26) | 1.77 (1.13–2.77) | 1.80 (1.29–2.52) | 0.71 (0.49–1.03) | 0.95 (0.68–1.31) | 1.14 (0.79–1.65) | 1.16 (0.83–1.62) |
| p-value | 0.756 | 0.466 | 0.845 | 2.02e-03 | 0.015 | 5.24e-04 | 0.085 | 0.734 | 0.499 | 0.351 | |
| Interaction between NEO-PI-R domain and “Married” marital status | OR (95% CI) | 1.18 (0.69–1.97) | 1.91 (1.24–2.95) | 1.09 (0.72–1.65) | 1.45 (1.06–1.98) | 1.35 (0.88–2.08) | 1.90 (1.36–2.64) | 0.79 (0.55–1.15) | 0.80 (0.57–1.12) | 0.75 (0.49–1.15) | 0.81 (0.58–1.13) |
| p-value | 0.505 | 3.58e-03 | 0.676 | 0.020 | 0.169 | 1.54e-04 | 0.239 | 0.206 | 0.185 | 0.218 | |
| Interaction between NEO-PI-R domain and rs16969968 | OR (95% CI) | 1.73 (0.87–3.44) | 0.96 (0.72–1.29) | 1.58 (0.99–2.53) | 1.08 (0.84–1.40) | 1.17 (0.70–1.95) | 1.90 (1.44–2.49) | 1.03 (0.67–1.58) | 1.18 (0.92–1.53) | 0.95 (0.60–1.53) | 1.62 (1.23–2.12) |
| p-value | 0.111 | 0.817 | 0.056 | 0.534 | 0.538 | 0.383 | 0.887 | 0.206 | 0.850 | 8.94e-04 | |
| Interaction between rs16969968 and “Divorced” marital status | OR (95% CI) | 1.05 (0.91–1.21) | 1.03 (0.96–1.10) | 1.00 (0.90–1.12) | 1.02 (0.96–1.09) | 1.01 (0.90–1.13) | 1.03 (0.97–1.10) | 1.00 (0.89–1.12) | 1.01 (0.94–1.08) | 0.97 (0.87–1.09) | 1.04 (0.97–1.11) |
| p-value | 0.464 | 0.419 | 0.975 | 0.500 | 0.841 | 0.326 | 0.981 | 0.830 | 0.668 | 0.299 | |
| Interaction between rs16969968 and “Married” marital status | OR (95% CI) | 0.98 (0.92–1.04) | 1.01 (0.94–1.08) | 1.00 (0.89–1.11) | 1.03 (0.97–1.09) | 1.00 (0.90–1.11) | 1.04 (0.98–1.11) | 0.96 (0.86–1.08) | 1.01 (0.95–1.08) | 0.95 (0.85–1.07) | 1.03 (0.96–1.10) |
| p-value | 0.755 | 0.853 | 0.951 | 0.389 | 0.968 | 0.193 | 0.551 | 0.681 | 0.407 | 0.456 | |
Reference group for marital status were subjects who have never been married. Reference group for the genotypes of rs16969968 were subjects who did not have any copies of minor allele. All odds ratios (OR) refer to 10 units change in personality scores, meaning that the presented odds of an individual developing CD reflect the NEO-PI-R domain score change in the magnitude of 10. Presented p-values are not adjusted for multiple testing. Bonferroni-corrected significance threshold was set to 1.67e-03. Significant p-values are highlighted in bold.
Table 4. Effect of marital status and CHRNA5 (rs16969968) on the relationship between NEO-PI-R personality domains and ND in AA and EA subjects.
| NEO-PI-R domain and Population | |||||||||||
| Neuroticism | Extraversion | Openness to experience | Agreeableness | Conscientiousness | |||||||
| AAs | EAs | AAs | EAs | AAs | EAs | AAs | EAs | AAs | EAs | ||
| Main effect of NEO-PI-R domain | OR (95% CI) | 3.22 (2.45–4.24) | 2.89 (2.19–3.80) | 0.63 (0.51–0.78) | 0.58 (0.46–0.74) | 0.57 (0.45–0.72) | 0.45 (0.35–0.59) | 0.50 (0.41–0.60) | 0.47 (0.37–0.59) | 0.41 (0.33–0.51) | 0.32 (0.25–0.41) |
| p-value | 2.00e-16 | 2.26e-14 | 6.13e-05 | 7.57e-06 | 3.10e-06 | 3.87e-10 | 1.39e-11 | 7.47e-11 | 5.16e-16 | 2.00e-16 | |
| Main effect of “Divorced” marital status | OR (95% CI) | 1.02 (0.78–1.32) | 1.09 (0.89–1.34) | 1.00 (0.79–1.27) | 0.85 (0.72–1.00) | 0.75 (0.59–0.94) | 0.76 (0.64–0.91) | 1.20 (0.98–1.47) | 1.09 (0.92–1.28) | 0.92 (0.76–1.11) | 0.96 (0.81–1.12) |
| p-value | 0.895 | 0.381 | 0.898 | 0.057 | 0.014 | 2.82e-03 | 0.074 | 0.305 | 0.400 | 0.593 | |
| Main effect of “Married” marital status | OR (95% CI) | 0.85 (0.65–1.12) | 0.64 (0.50–0.81) | 0.86 (0.68–1.08) | 0.77 (0.65–0.91) | 0.76 (0.61–0.94) | 0.59 (0.50–0.70) | 1.02 (0.84–1.23) | 0.96 (0.81–1.13) | 1.12 (0.88–1.43) | 1.01 (0.85–1.20) |
| p-value | 0.258 | 2.77e-04 | 0.188 | 2.29e-03 | 0.012 | 6.52e-10 | 0.873 | 0.612 | 0.357 | 0.886 | |
| Main effect of rs16969968 | OR (95% CI) | 0.82 (0.58–1.15) | 0.98 (0.82–1.16) | 0.77 (0.60–0.99) | 1.01 (0.88–1.16) | 0.91 (0.70–1.17) | 0.93 (0.81–1.08) | 0.97 (0.79–1.19) | 0.96 (0.85–1.09) | 1.04 (0.80–1.34) | 0.84 (0.73–0.96) |
| p-value | 0.250 | 0.777 | 0.041 | 0.875 | 0.450 | 0.357 | 0.771 | 0.542 | 0.763 | 9.10e-03 | |
| Interaction between NEO-PI-R domain and “Divorced” marital status | OR (95% CI) | 0.96 (0.58–1.60) | 0.88 (0.62–1.25) | 1.00 (0.62–1.60) | 1.46 (1.07–2.00) | 1.79 (1.12–2.86) | 1.77 (1.27–2.47) | 0.67 (0.44–1.01) | 0.91 (0.66–1.24) | 1.17 (0.81–1.70) | 1.12 (0.80–1.56) |
| p-value | 0.876 | 0.462 | 0.997 | 0.020 | 0.017 | 8.48e-04 | 0.057 | 0.559 | 0.394 | 0.517 | |
| Interaction between NEO-PI-R domain and “Married” marital status | OR (95% CI) | 1.14 (0.67–1.93) | 1.97 (1.26–3.10) | 1.08 (0.69–1.70) | 1.27 (0.93–1.74) | 1.40 (0.90–2.21) | 2.12 (1.55–2.90) | 0.77 (0.51–1.16) | 0.85 (0.62–1.17) | 0.63 (0.38–1.02) | 0.76 (0.54–1.09) |
| p-value | 0.640 | 3.23e-03 | 0.737 | 0.129 | 0.143 | 4.65e-06 | 0.212 | 0.325 | 0.068 | 0.131 | |
| Interaction between NEO-PI-R domain and rs16969968 | OR (95% CI) | 1.60 (0.81–3.18) | 1.05 (0.77–1.44) | 1.80 (1.15–2.83) | 0.97 (0.75–1.25) | 1.31 (0.79–2.18) | 1.12 (0.85–1.47) | 1.16 (0.75–1.79) | 1.09 (0.85–1.41) | 0.97 (0.58–1.62) | 1.48 (1.12–1.94) |
| p-value | 0.173 | 0.757 | 0.021 | 0.828 | 0.305 | 0.391 | 0.502 | 0.491 | 0.918 | 6.15e-03 | |
| Interaction between rs16969968 and “Divorced” marital status | OR (95% CI) | 1.03 (0.91–1.17) | 1.02 (0.95–1.09) | 1.02 (0.91–1.13) | 1.02 (0.96–1.09) | 1.03 (0.91–1.15) | 1.03 (0.97–1.10) | 1.00 (0.89–1.13) | 1.01 (0.94–1.08) | 0.98 (0.87–1.10) | 1.03 (0.97–1.11) |
| p-value | 0.626 | 0.574 | 0.771 | 0.543 | 0.661 | 0.368 | 0.970 | 0.789 | 0.717 | 0.347 | |
| Interaction between rs16969968 and “Married” marital status | OR (95% CI) | 1.02 (0.90–1.15) | 1.01 (0.94–1.08) | 1.01 (0.90–1.13) | 1.01 (0.95–1.08) | 1.01 (0.90–1.12) | 1.03 (0.97–1.09) | 0.99 (0.88–1.11) | 1.01 (0.94–1.07) | 0.96 (0.85–1.08) | 1.00 (0.93–1.07) |
| p-value | 0.811 | 0.846 | 0.879 | 0.662 | 0.903 | 0.387 | 0.845 | 0.839 | 0.478 | 0.998 | |
Reference group for marital status were subjects who have never been married. Reference group for the genotypes of rs16969968 were subjects who did not have any copies of minor allele. All odds ratios (OR) refer to 10 units change in personality scores, meaning that the presented odds of an individual developing ND reflect the NEO-PI-R domain score change in the magnitude of 10. Presented p-values are not adjusted for multiple testing. Bonferroni-corrected significance threshold was set to 1.67e-03. Significant p-values are highlighted in bold.
Table 5. Effect of marital status and CHRNA5 (rs16969968) on the relationship between NEO-PI-R personality domains and CIP in AA and EA subjects.
| NEO-PI-R domain and Population | |||||||||||
| Neuroticism | Extraversion | Openness to experience | Agreeableness | Conscientiousness | |||||||
| AAs | EAs | AAs | EAs | AAs | EAs | AAs | EAs | AAs | EAs | ||
| Main effect of NEO-PI-R domain | OR (95% CI) | 1.57 (1.49–1.65) | 1.60 (1.47–1.74) | 0.84 (0.80–0.89) | 0.76 (0.69–0.84) | 0.79 (0.76–0.83) | 0.70 (0.63–0.78) | 0.78 (0.74–0.82) | 0.70 (0.65–0.77) | 0.72 (0.69–0.75) | 0.64 (0.59–0.69) |
| p-value | 2.00e-16 | 2.00e-16 | 5.28e-04 | 1.65e-05 | 1.57e-05 | 4.75e-08 | 9.49e-10 | 4.19e-11 | 2.00e-16 | 2.00e-16 | |
| Main effect of “Divorced” marital status | OR (95% CI) | 1.10 (1.05–1.16) | 1.03 (0.96–1.10) | 0.92 (0.88–0.97) | 0.92 (0.86–0.98) | 0.88 (0.84–0.92) | 0.87 (0.81–0.94) | 0.99 (0.95–1.04) | 0.99 (0.94–1.05) | 0.92 (0.87–0.96) | 0.95 (0.90–0.99) |
| p-value | 0.031 | 0.502 | 0.101 | 0.035 | 8.99e-03 | 1.55e-03 | 0.848 | 0.821 | 5.97e-03 | 0.085 | |
| Main effect of “Married” marital status | OR (95% CI) | 0.93 (0.88–0.97) | 0.85 (0.79–0.90) | 0.92 (0.88–0.97) | 0.90 (0.83–0.97) | 0.89 (0.85–0.93) | 0.78 (0.72–0.85) | 1.08 (1.03–1.13) | 1.00 (0.94–1.07) | 1.04 (0.99–1.09) | 1.02 (0.96–1.07) |
| p-value | 0.157 | 3.90e-05 | 0.154 | 0.30 | 0.028 | 6.97e-07 | 0.051 | 0.953 | 0.371 | 0.634 | |
| Main effect of rs16969968 | OR (95% CI) | 0.98 (0.93–1.03) | 1.00 (0.95–1.06) | 0.93 (0.88–0.97) | 0.95 (0.89–1.00) | 0.86 (0.91–1.01) | 0.96 (0.90–1.02) | 1.00 (0.95–1.05) | 0.96 (0.92–1.01) | 1.03 (0.98–1.08) | 0.91 (0.88–0.95) |
| p-value | 0.705 | 0.930 | 0.173 | 0.112 | 0.028 | 0.223 | 0.985 | 0.165 | 0.475 | 1.38e-04 | |
| Interaction between NEO-PI-R domain and “Divorced” marital status | OR (95% CI) | 0.84 (0.80–0.88) | 0.95 (0.85–1.07) | 1.17 (1.12–1.23) | 1.22 (1.07–1.39) | 1.30 (1.24–1.36) | 1.35 (1.18–1.55) | 1.01 (0.96–1.06) | 1.05 (0.94–1.17) | 1.22 (1.16–1.28) | 1.13 (1.02–1.26) |
| p-value | 0.027 | 0.477 | 0.108 | 0.0127 | 8.98e-03 | 4.56e-04 | 0.871 | 0.470 | 4.69e-03 | 0.057 | |
| Interaction between NEO-PI-R domain and “Married” marital status | OR (95% CI) | 1.10 (1.05–1.16) | 1.26 (1.12–1.42) | 1.08 (1.03–1.13) | 1.06 (0.91–1.23) | 1.18 (1.12–1.24) | 1.39 (1.18–1.63) | 0.79 (0.75–0.83) | 0.88 (0.77–1.00) | 0.86 (0.82–0.90) | 0.86 (0.77–0.96) |
| p-value | 0.347 | 1.27e-03 | 0.485 | 0.546 | 0.156 | 7.64e-04 | 3.91e-03 | 0.091 | 0.079 | 0.024 | |
| Interaction between NEO-PI-R domain and rs16969968 | OR (95% CI) | 1.07 (1.02–1.12) | 0.97 (0.89–1.07) | 1.21 (1.15–1.27) | 1.09 (0.98–1.22) | 1.14 (1.08–1.19) | 1.06 (0.94–1.19) | 1.05 (1.00–1.10) | 1.06 (0.97–1.17) | 0.96 (092–1.01) | 1.21 (1.11–1.32) |
| p-value | 0.521 | 0.634 | 0.082 | 0.209 | 0.156 | 0.424 | 0.605 | 0.299 | 0.705 | 4.93e-04 | |
| Interaction between rs16969968 and “Divorced” marital status | OR (95% CI) | 1.00 (0.95–1.05) | 1.02 (0.99–1.04) | 1.00 (0.95–1.05) | 1.02 (0.99–1.04) | 0.99 (0.94–1.04) | 1.02 (0.99–1.04) | 0.99 (0.94–1.04) | 1.01 (0.98–1.03) | 0.99 (0.94–1.04) | 1.02 (0.99–1.05) |
| p-value | 0.999 | 0.249 | 0.902 | 0.353 | 0.996 | 0.296 | 0.697 | 0.630 | 0.690 | 0.191 | |
| Interaction between rs16969968 and “Married” marital status | OR (95% CI) | 1.00 (0.95–1.05) | 1.00 (0.97–1.03) | 0.98 (0.93–1.03) | 1.02 (0.99–1.05) | 0.98 (0.93–1.03) | 1.02 (0.99–1.05) | 0.99 (0.94–1.04) | 1.01 (0.98–1.04) | 0.98 (0.94–1.03) | 1.00 (0.98–1.03) |
| p-value | 0.950 | 0.906 | 0.945 | 0.376 | 0.994 | 0.215 | 0.751 | 0.716 | 0.541 | 0.834 | |
Reference group for marital status were subjects who have never been married. Reference group for the genotypes of rs16969968 were subjects who did not have any copies of minor allele. All odds ratios (OR) refer to 10 units change in personality scores, meaning that the presented odds of an individual developing CIP reflect the NEO-PI-R domain score change in the magnitude of 10. Results shown in the table are those of comparison between groups A (controls with no CIP) and C (CDs with CIP). Presented p-values are not adjusted for multiple testing. Bonferroni-corrected significance threshold was set to 1.67e-03. Significant p-values are highlighted in bold.
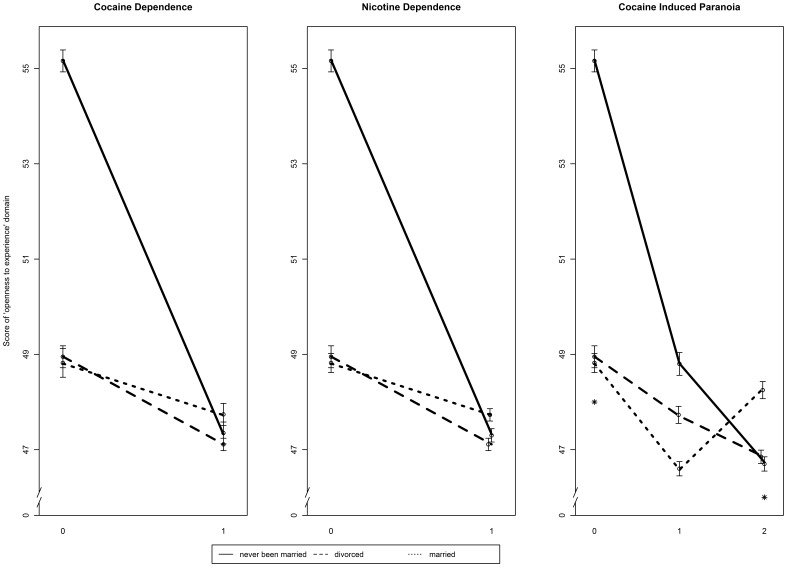
Never-marrieds consistently scored higher in the “openness to experience” domain compared to either divorced or married participants in control groups for CD, ND and CIP (Figure 1). This pattern was not present among affected individuals; in the substance dependent subjects, divorced and never married subjects appeared to present somewhat lower scores than those who were married, especially in subjects suffering from both CD and CIP (Figure 1).
Figure 1. Interaction plot reflecting significant moderation of marital status on correlation between “openness to experience” NEO-PI-R domain and the risk of CD, ND and CIP in EAs.
The Y axis represents the score of “openness to experience” domain, while the X axis shows the phenotype examined (for cocaine and nicotine dependences zero stands for controls and one stands for cases; for cocaine induced paranoia zero stands for controls, one stands for cocaine dependent cases without CIP and two stands for cocaine dependent cases with CIP). The error bars represent standard error of calculated “openness to experience” scores in each group of marital status in cases and controls.
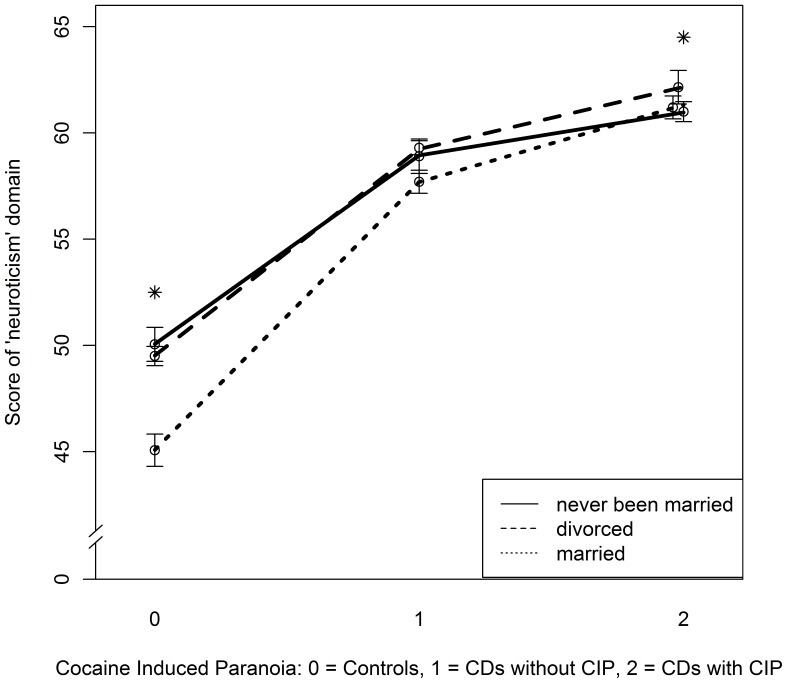
In addition to the observed moderating effect of marital status on the association between “openness to experience” and all three examined outcomes (i.e. CD, ND and CIP), a significant interaction also occurred between marriage and the domain of “neuroticism” in the development of CIP in the EA population only (OR:1.26, 95%CI:1.12–1.42, p-value:1.27e-03, Table 5). Similarly to the moderating trend marriage displayed on the “openness to experience” domain, never-married controls (with neither CD nor CIP) revealed the highest score in “neuroticism” domain as well (Figure 2). Cases (with CD and CIP) demonstrated a contrasting pattern, with divorced individuals scoring the highest (Figure 2).
Figure 2. Interaction plot reflecting significant moderation of marital status on correlation between “neuroticism” NEO-PI-R domain and the risk of CIP in EAs.
The Y axis represents the score of “neuroticism” domain, while the X axis shows the categories of CIP: zero stands for controls, one stands for cocaine dependent cases without CIP and two stands for cocaine dependent cases with CIP. The error bars represent standard error of calculated “neuroticism” scores in each group of marital status in cases and controls. The asterisks indicate the two groups (controls and CD cases with symptoms of paranoia) that revealed significant interaction (there was no significant result observed between controls and CD cases without symptoms of paranoia or CD cases with and without symptoms of paranoia).
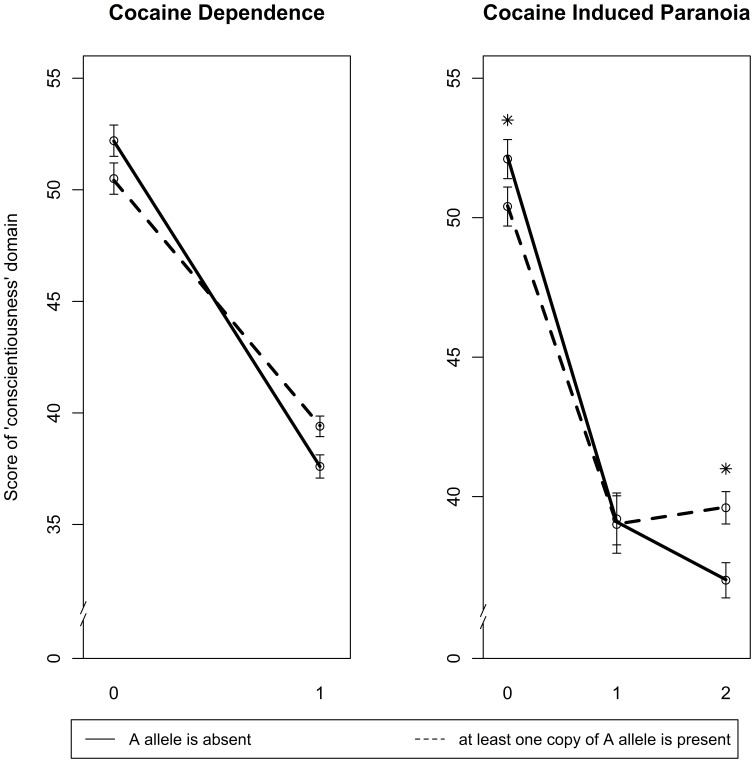
For evaluating the possibility of interaction between rs16969968 and NEO-PI-R domains, a significant result was noted for the risk of CD and CIP in the EA population only (Table 5). Among subjects with the diagnosis of CD, those with at least one copy of protective allele “A” of rs16969968 were more conscientious (revealed higher score in “conscientiousness” domain) than those without it, while reverse scoring was observed in subjects who did not suffer from CD (Figure 3). Similar interaction pattern was revealed in the risk of CIP: individuals who displayed symptoms of CIP in addition to CD scored higher on conscientiousness if they carried at least one rs16969968 “A” allele compared to those without it, with the contrary scores in the control group (Figure 3).
Figure 3. Interaction plot reflecting significant moderation of rs16969968 on correlation between “conscientiousness” NEO-PI-R domain and the risk of CD and CIP in EA population.
The Y axis represents the score of “conscientiousness” domain, while the X axis shows the phenotype examined (for cocaine and nicotine dependences zero stands for controls and one stands for cases; for cocaine induced paranoia zero stands for controls, one stands for cocaine dependent cases without CIP and two stands for cocaine dependent cases with CIP). The error bars represent standard error of calculated “conscientiousness” scores in each group of marital status in cases and controls. The asterisks indicate the two groups (controls and CD cases with symptoms of paranoia) that revealed significant interaction (there was no significant result observed between controls and CD cases without symptoms of paranoia or CD cases with and without symptoms of paranoia).
No significant interactions were observed in the AA population.
Discussion
This study focused on the role of personality and CHRNA5 variation in CD and ND in both EA and AA populations. The purpose of the analyses described here was to examine potential interaction between NEO-PI-R personality domains with marital status and rs16969968 in CHRNA5 on the risks of CD, ND and CIP.
Consistent with our hypothesis, the measured personality domains did show interaction with both marital status and rs16969968 in their correlations with CD, ND and CIP, although the observed moderating effect did not extend to every domain and each of the examined moderators interceded in different correlations. In addition, there was no significant interaction observed in AA population.
Firstly, the effect of marital status (considered an environmental factor) was evaluated on the relationship of NEO-PI-R personality domains with CD, ND and CIP. Significant interaction was noted between the “openness to experience” domain and marital status on the risks of CD, ND and CIP in the EA population only (Tables 3, 4 and 5). Moreover, a significant moderating effect of marriage was also present in the correlation between “neuroticism” and CIP in EAs (Table 5). These observations suggest that the link between personality and CD, ND and CIP is not trivial and may be affected by the surrounding environment.
Secondly, the role of rs16969968 in the association between NEO-PI-R domains with CD, ND and CIP was assessed. This time, it was the domain of “conscientiousness” that showed significant interaction with rs16969968 in its correlation with CD and CIP in EA subjects only (Tables 3 and 5). No significant result was observed in relation to ND (Table 4), postulating that such genetic factor as rs16969968 in CHRNA5 may be acting differently in etiology of ND compared to CD or CIP.
Since the “big five” are dimensions of perceived personality [59] and represent isomorphic depiction of observed behavior, to interpret its meaning fully, the entire chain of causation from the neuropsychiatric bases of behavior in targets to the inferential processes by which perceivers perceive should be examined [60]. The five factors of the NEO-PI-R could be, however, reformulated to accommodate their social-functional significance for perceivers: Denissen and Penke, for example, portray the five-factor model as individual differences in motivational reactions to situations [61]. Similarly, Tellegen as well as Revelle postulate that the five dimensions could be viewed as stable individual differences in people's reactions to circumscribed environmental cues [62]. Thus, although the big five dimensions are conceptually and empirically related, there is a difference between the content of person's motivations and how he or she will try to achieve them [61]. Below we offer a speculation on how our results could be interpreted in terms of those differences.
“Openness to experience” involves the tendency to be creative, imaginative, attuned to inner feelings and inclined towards new activities [56]. It could be interpreted, in part, as differences in the activation of the inner reward system when engaging in cognitive activity [61]. Considering marriage or divorce as a situational cue (e.g. presence or absence of stress), the score in the “openness to experience” domain may reflect individual differences in how people would react to it. Consistent with this assumption, “openness to experience” has been reported to be a correlate of close relationship [63] and to be associated with several marital outcomes [64] as well as marital satisfaction [65]. Moreover, it may be recognized that an individual's coping skills may be an inevitable part of how he or she would react to an environmental cue. Consequently, individuals scoring high in “openness to experience” tend to engage in more adaptive, flexible coping [66], while those who score low show greater vulnerability to adverse effects of stress [67]. In this study, controls tended to score higher on the “openness to experience” domain, compared to cases (independent of marital status; Figure 1). In addition, married cases appeared to show somewhat higher scores in this domain than never-marrieds, so we hypothesize that not being in a marriage (which could be considered a safe and stable environment) combined with lower scores of “openness to experience” and possibly worse coping structures may increase the risk of developing either CD, ND or CIP.
“Neuroticism” reflects the disposition to be impulsive and to experience negative emotions such as depression, anxiety or anger [56]. It can be characterized, in part, as differences in activation of the punishment system when faced with the cues of social exclusion [61]. In the sense that specific trajectories of social participation may play an important role in the development of paranoia [68] and marriage may be viewed as a criterion for a certain social inclusions or exclusions, it is easy to see that the “neuroticism” domain could interact with marital status in the development of CIP in cocaine dependent subjects. Consistently, subjects who developed symptoms of CIP in addition to CD showed the highest scores of “neuroticism” in the divorced group compared to either married or never married individuals (Figure 2), who could be viewed as socially more acceptable than divorced participants.
Since the interaction between NEO-PI-R domains and marital status on the risk of CIP expanded to more personality domains than that on the risk of CD, marriage might have a different moderating effect on personality and co-morbid psychopathology of CD than it has on CD itself.
“Conscientiousness” appears to reflect self-control, determination and organization. It encompasses several features that may manifest the measure of socialization in a broad manner, involving individual differences in the behavioral predisposition to follow socially prescribed norms for impulse control, to be goal-directed and be able to follow rules [69]. Thus, individuals scoring high on “conscientiousness” tend to use a more active, problem-solving strategies and to avoid emotion-based solutions (e.g. self-blame) [70]. In this study, subjects possessing at least one protective allele “A” of rs16969968 (protective against CD) scored higher on “conscientiousness” than those who had no copies of that allele in the group of CD cases and CD cases with CIP (Figure 3).
CHRNA5 rs16969968 has been previously observed to be involved in phenotype and in gene x environment interaction: it has been reported to interact with age at onset of smoking [71], [72], peer smoking [73] and parent monitoring [74] in the development of ND, as well as relapse likelihood and withdrawal severity [72], cognitive function [75] and BMI [76] in nicotine dependence. These findings refer to nicotine; this study did not observe significant interaction between rs16969968 and NEO-PI-R personality domains on the risk of ND, but on the risk of CD only. Nonetheless, these previously reported moderations along with the one described here, are consistent with the formulation that in the genetics of complex disorders, including substance dependence, individual polymorphisms can generally only account for a small part of phenotypic variance [77]–[79]. Thus, the moderating effect of rs16969968 on the correlation of personality with CD and CIP is small in magnitude.
Since “conscientiousness” has been linked to several different drug dependencies (not only cocaine) and it is still uncertain whether the impact of distinct personality traits would be similar on different substance disorders or co-morbid psychopathology, the interaction between rs16969968 and conscientiousness on the risk of CD would need to be evaluated in the light of possible confounding effects of co-occurring addictions. However, this was not plausible within the logistic regression design of this study because of the observed high correlation between CD and co-morbid alcohol dependence (Spearman's rho = 0.599) as well as opioid dependence (Spearman's rho = 0.573).
None of the moderating effects observed in EAs were present in AA individuals. A possible explanation could be that African Americans may demonstrate unique patterns and pathways of substance use and mental health problems compared to other racial/ethnic groups [80], [81]. Individuals of different ethnic descent have been reported to show disparate nicotine intake and metabolism that may account for variability in nicotine use outcomes: European Americans tend to smoke greater numbers of cigarettes when compared with African Americans [82], [83] and African Americans seem to have greater nicotine intake from tobacco smoke [84]. Similarly, inter-ethnic discrepancies in cocaine use related conditions have also been demonstrated. Although it remains unclear whether substance use and psychological distress influence one another over time, cocaine related suicide in teenagers, for example, appear to be less common in African Americans compared to European Americans [85], suggesting inter-racial variability in psychological consequences of illicit drug use. In addition, African Americans display a higher likelihood of being cocaine users compared to European Americans [86]. Analogously, the social and psychological context of marital dissolution have also been perceived to be contrasting in African and European Americans [87]. These inter-racial differences might account for non-significant interactions in African American population observed in this study.
Differences in genetics of AA and EA populations (e.g. large difference in minor allele frequencies of rs16969968 among these two ethnicities rendering this variant less informative in AAs) may account for the lack of observed interaction between CHRNA5 and conscientiousness in AA participants. Such dissimilarity in gene x environment interaction has already been documented [75], [88].
In conclusion, findings of this study suggest that NEO-PI-R personality measures may play an important role in substance use disorders on both environmental (marriage) and genetic (CHRNA5) levels. Although the presented results should be interpreted as exploratory and in need of replication, they do add to a growing body of evidence that personality traits may not necessarily be the cause of drug addiction, but, in combination with other etiological factors, may influence its form or severity, as well as the development of co-occurringpsychopathologies.
Acknowledgments
The presented work benefited from the valuable input of Prof. Henry Kranzler (Department of Psychiatry, University of Pennsylvania, and Philadelphia VAMC, Philadelphia, PA, USA) and Prof. Raymond Anton (Department of Psychiatry and Behavioral Sciences, Center for Drug and Alcohol Programs, Medical University of South Carolina, Charleston, SC, USA), who both contributed to subject recruitment and collection of NEO-PI-R data.
Funding Statement
This study was supported by National Institute on Drug Abuse (NIDA) grants R01 DA12690, R01 DA12849, RC2 DA028909, and K01 DA24758, National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants R01 AA11330 and R01 AA17535, a VA MERIT grant, and the VA Connecticut MIRECC Center, and a Brain and Behavior Research NARSAD Young Investigator award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Muhuri PK, Gfroerer JC (2011) Mortality associated with illegal drug use among adults in the United States. Am J Drug Alcohol Abuse 37: 155–164. [DOI] [PubMed] [Google Scholar]
- 2. Harwood HJ, Fountain D, Fountain G (1999) Economic cost of alcohol and drug abuse in the United States, 1992: a report. Addiction 94: 631–635. [DOI] [PubMed] [Google Scholar]
- 3. McKusick D, Mark TL, King E, Harwood R, Buck JA, et al. (1998) Spending for mental health and substance abuse treatment, 1996. Health Aff (Millwood) 17: 147–157. [DOI] [PubMed] [Google Scholar]
- 4. Compton WM, Thomas YF, Stinson FS, Grant BF (2007) Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry 64: 566–576. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention (2008) Cigarette smoking among adults–United States, 2007. MMWR Morb Mortal Wkly Rep 57: 1221–1226. [PubMed] [Google Scholar]
- 6. Kandel D, Chen K, Warner LA, Kessler RC, Grant B (1997) Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug Alcohol Depend 44: 11–29. [DOI] [PubMed] [Google Scholar]
- 7. Budney AJ, Higgins ST, Hughes JR, Bickel WK (1993) Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse 5: 117–130. [DOI] [PubMed] [Google Scholar]
- 8. Clemmey P, Brooner R, Chutuape MA, Kidorf M, Stitzer M (1997) Smoking habits and attitudes in a methadone maintenance treatment population. Drug Alcohol Depend 44: 123–132. [DOI] [PubMed] [Google Scholar]
- 9. Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL (2010) Tobacco, cocaine, and heroin: Craving and use during daily life. Addict Behav 35: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roll JM, Higgins ST, Budney AJ, Bickel WK, Badger GJ (1996) A comparison of cocaine-dependent cigarette smokers and non-smokers on demographic, drug use and other characteristics. Drug Alcohol Depend 40: 195–201. [DOI] [PubMed] [Google Scholar]
- 11. Roll JM, Higgins ST, Tidey J (1997) Cocaine use can increase cigarette smoking: evidence from laboratory and naturalistic settings. Exp Clin Psychopharmacol 5: 263–268. [DOI] [PubMed] [Google Scholar]
- 12. Mello NK, Newman JL (2011) Discriminative and reinforcing stimulus effects of nicotine, cocaine, and cocaine + nicotine combinations in rhesus monkeys. Exp Clin Psychopharmacol 19: 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nathan PE (1988) The addictive personality is the behavior of the addict. J Consult Clin Psychol 56: 183–188. [DOI] [PubMed] [Google Scholar]
- 14. Kerr JS (1996) Two myths of addiciton: the additive personality and the issue of free choice. Human Psychopharmacology 11: S9–S13. [Google Scholar]
- 15. Allen TJ, Moeller FG, Rhoades HM, Cherek DR (1998) Impulsivity and history of drug dependence. Drug Alcohol Depend 50: 137–145. [DOI] [PubMed] [Google Scholar]
- 16. McGue M, Slutske W, Iacono WG (1999) Personality and substance use disorders: II. Alcoholism versus drug use disorders. J Consult Clin Psychol 67: 394–404. [DOI] [PubMed] [Google Scholar]
- 17. McCormick RA, Dowd ET, Quirk S, Zegarra JH (1998) The relationship of NEO-PI performance to coping styles, patterns of use, and triggers for use among substance abusers. Addict Behav 23: 497–507. [DOI] [PubMed] [Google Scholar]
- 18. Quirk SW, McCormick RA (1998) Personality subtypes, coping styles, symptom correlates, and substances of choice among a cohort of substance abusers. Assessment 5: 157–169. [DOI] [PubMed] [Google Scholar]
- 19. Terracciano A, Costa PT Jr (2004) Smoking and the Five-Factor Model of personality. Addiction 99: 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Terracciano A, Lockenhoff CE, Crum RM, Bienvenu OJ, Costa PT Jr (2008) Five-Factor Model personality profiles of drug users. BMC Psychiatry 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Loon AJ, Tijhuis M, Surtees PG, Ormel J (2005) Determinants of smoking status: cross-sectional data on smoking initiation and cessation. Eur J Public Health 15: 256–261. [DOI] [PubMed] [Google Scholar]
- 22. Lipkus IM, Barefoot JC, Williams RB, Siegler IC (1994) Personality measures as predictors of smoking initiation and cessation in the UNC Alumni Heart Study. Health Psychol 13: 149–155. [DOI] [PubMed] [Google Scholar]
- 23. Cherry N, Kiernan K (1976) Personality scores and smoking behaviour. A longitudinal study. Br J Prev Soc Med 30: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merline AC, O'Malley PM, Schulenberg JE, Bachman JG, Johnston LD (2004) Substance use among adults 35 years of age: prevalence, adulthood predictors, and impact of adolescent substance use. Am J Public Health 94: 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. White HR, Bates ME (1995) Cessation from cocaine use. Addiction 90: 947–957. [DOI] [PubMed] [Google Scholar]
- 26. Moos RH, Nichol AC, Moos BS (2002) Risk factors for symptom exacerbation among treated patients with substance use disorders. Addiction 97: 75–85. [DOI] [PubMed] [Google Scholar]
- 27. Heinz AJ, Wu J, Witkiewitz K, Epstein DH, Preston KL (2009) Marriage and relationship closeness as predictors of cocaine and heroin use. Addict Behav 34: 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Broms U, Silventoinen K, Lahelma E, Koskenvuo M, Kaprio J (2004) Smoking cessation by socioeconomic status and marital status: the contribution of smoking behavior and family background. Nicotine Tob Res 6: 447–455. [DOI] [PubMed] [Google Scholar]
- 29. Lee CW, Kahende J (2007) Factors associated with successful smoking cessation in the United States, 2000. Am J Public Health 97: 1503–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Derby CA, Lasater TM, Vass K, Gonzalez S, Carleton RA (1994) Characteristics of smokers who attempt to quit and of those who recently succeeded. Am J Prev Med 10: 327–334. [PubMed] [Google Scholar]
- 31. Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, et al. (2007) Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet 16: 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grucza RA, Wang JC, Stitzel JA, Hinrichs AL, Saccone SF, et al. (2008) A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol Psychiatry 64: 922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, et al. (2009) Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet 150B: 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, et al. (2009) The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res 69: 6848–6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lips EH, Gaborieau V, McKay JD, Chabrier A, Hung RJ, et al. (2009) Association between a 15q25 gene variant, smoking quantity and tobacco-related cancers among 17 000 individuals. Int J Epidemiol 39: 563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen X, Chen J, Williamson VS, An SS, Hettema JM, et al. (2009) Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet 150B: 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith RM, Alachkar H, Papp AC, Wang D, Mash DC, et al. (2011) Nicotinic alpha5 receptor subunit mRNA expression is associated with distant 5′ upstream polymorphisms. Eur J Hum Genet 19: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, et al. (2010) Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet 6: pii: e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, et al. (2008) Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev 17: 3517–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sherva R, Kranzler HR, Yu Y, Logue MW, Poling J, et al. (1921) Variation in nicotinic acetylcholine receptor genes is associated with multiple substance dependence phenotypes. Neuropsychopharmacology 35: 1921–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, et al. (2008) Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 165: 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Berrettini W, Yuan X, Tozzi F, Song K, Francks C, et al. (2008) Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry 13: 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sherva R, Wilhelmsen K, Pomerleau CS, Chasse SA, Rice JP, et al. (2008) Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction 103: 1544–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, et al. (2008) A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet 4: e1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, et al. (2008) A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452: 638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang JC, Cruchaga C, Saccone NL, Bertelsen S, Liu P, et al. (2009) Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet 18: 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, et al. (2009) Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psychiatry 14: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Erlich PM, Hoffman SN, Rukstalis M, Han JJ, Chu X, et al. (2010) Nicotinic acetylcholine receptor genes on chromosome 15q25.1 are associated with nicotine and opioid dependence severity. Hum Genet 128: 491–499. [DOI] [PubMed] [Google Scholar]
- 49. Sherva R, Kranzler HR, Yu Y, Logue MW, Poling J, et al. (2010) Variation in nicotinic acetylcholine receptor genes is associated with multiple substance dependence phenotypes. Neuropsychopharmacology 35: 1921–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brady KT, Lydiard RB, Malcolm R, Ballenger JC (1991) Cocaine-induced psychosis. J Clin Psychiatry 52: 509–512. [PubMed] [Google Scholar]
- 51. Satel SL, Southwick SM, Gawin FH (1991) Clinical features of cocaine-induced paranoia. Am J Psychiatry 148: 495–498. [DOI] [PubMed] [Google Scholar]
- 52. Pierucci-Lagha A, Gelernter J, Chan G, Arias A, Cubells JF, et al. (2007) Reliability of DSM-IV diagnostic criteria using the semi-structured assessment for drug dependence and alcoholism (SSADDA). Drug Alcohol Depend 91: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, et al. (2005) Diagnostic reliability of the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA). Drug Alcohol Depend 80: 303–312. [DOI] [PubMed] [Google Scholar]
- 54. Goldberg LR (1990) An alternative “description of personality”: the big-five factor structure. J Pers Soc Psychol 59: 1216–1229. [DOI] [PubMed] [Google Scholar]
- 55. Digman JM (1990) Personality Structure: Emergence of the Five-Factor Model. Annual Review of Psychology 41: 417–440. [Google Scholar]
- 56.Costa PT, McCrae RR (1992) NEO-PI-R Professional manual. Odessa, FL: Psychological Assessment Resources, Inc.
- 57.McCrae RR (2002) NEO-PI-R data from 36 cultures: Further intercultural comparisons. In: McCrae RR, Allik J, editors. The Five-Factor Model of personality across cultures. New York: Kluwer Academic/Plenum Publishers.
- 58. Yang BZ, Zhao H, Kranzler HR, Gelernter J (2005) Practical population group assignment with selected informative markers: characteristics and properties of Bayesian clustering via STRUCTURE. Genet Epidemiol 28: 302–312. [DOI] [PubMed] [Google Scholar]
- 59.Saucier G, Goldberg LR (1996) The language of personality: Lexical perscpectives on the five-factor model. In: Wiggins JS, editor. The five-factor model of personality: Theoretical perspectives. New York: NY: Guilford.
- 60. Funder DC (1995) On the accuracy of personality judgment: a realistic approach. Psychol Rev 102: 652–670. [DOI] [PubMed] [Google Scholar]
- 61. Denissen JJ, Penke L (2008) Motivational individual reaction norms underlying the Five-Factor model of personality: First steps towards a theory-based conceptual framework. Journal of Reasearch in Personality 42: 1285–1302. [Google Scholar]
- 62. Revelle W (1995) Personality processes. Annual Review of Psychology 46: 295–328. [Google Scholar]
- 63. Donnellan MB, Conger RD, Bryant CM (2004) The Big Five and enduring marriages. Journal of Research in Personality 38: 481–504. [Google Scholar]
- 64. Botwin MD, Buss DM, Shackelford TK (1997) Personality and mate preferences: five factors in mate selection and marital satisfaction. Journal of Personality 65: 107–136. [DOI] [PubMed] [Google Scholar]
- 65. O'Rourke N, Claxton A, Chou PHB, Smith JZ, Hadjistavropoulos T (2011) Personality trait levels within older couples and between-spouse trait differences as predictors of marital satisfaction. Aging and Mental Health 15: 344–353. [DOI] [PubMed] [Google Scholar]
- 66. Lee-Baggley D, Preece M, DeLongis A (2005) Cooping with interpersonal stress: role of big five traits. Journal of Personality 73: 1141–1180. [DOI] [PubMed] [Google Scholar]
- 67. Williams PG, Rau HK, Cribbet MR, Gunn CH (2009) Openness to experience and stress regulation. Journal of Research in Personality 43: 777–784. [Google Scholar]
- 68. Cromby J, Harper DJ (2009) Paranoia: A social account. Theory and Psychology 19: 335. [Google Scholar]
- 69.John OP, Srivastava S (1999) The Big Five Trait taxonomy: History, measurement, and theoretical perspectives. In: Pervin LA, Oliver JP, editors. Handbook of personality: Theory and research. 2nd ed. New York: Guilford Press. pp. 102–138.
- 70. Watson D, Hubbard B (1996) Adaptational style and dispositional structure: coping in the context of five-factor model. Journal of Personality 64: 737–774. [Google Scholar]
- 71. Grucza RA, Johnson EO, Krueger RF, Breslau N, Saccone NL, et al. (2010) Incorporating age at onset of smoking into genetic models for nicotine dependence: evidence for interaction with multiple genes. Addict Biol 15: 346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Baker TB, Weiss RB, Bolt D, von Niederhausern A, Fiore MC, et al. (2009) Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob Res 11: 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Johnson EO, Chen LS, Breslau N, Hatsukami D, Robbins T, et al. (2009) Peer smoking and the nicotinic receptor genes: an examination of genetic and environmental risks for nicotine dependence. Addiction 105: 2014–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen LS, Johnson EO, Breslau N, Hatsukami D, Saccone NL, et al. (2009) Interplay of Genetic Risk Factors and Parent Monitoring in Risk for Nicotine Dependence. Addiction 104: 1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang H, Kranzler HR, Poling J, Gelernter J (2010) Variation in the nicotinic acetylcholine receptor gene cluster CHRNA5-CHRNA3-CHRNB4 and its interaction with recent tobacco use influence cognitive flexibility. Neuropsychopharmacology 35: 2211–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Freathy RM, Kazeem GR, Morris RW, Johnson PC, Paternoster L, et al. (2011) Genetic variation at CHRNA5-CHRNA3-CHRNB4 interacts with smoking status to influence body mass index. Int J Epidemiol 40: 1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Orr N, Chanock S (2008) Common genetic variation and human disease. Adv Genet 62: 1–32. [DOI] [PubMed] [Google Scholar]
- 78. Goldstein DB (2009) Common genetic variation and human traits. N Engl J Med 360: 1696–1698. [DOI] [PubMed] [Google Scholar]
- 79. Kraft P, Hunter DJ (2009) Genetic risk prediction–are we there yet? N Engl J Med 360: 1701–1703. [DOI] [PubMed] [Google Scholar]
- 80. Brown JS, Meadows SO, Elder GH Jr (2007) Race-ethnic inequality and psychological distress: depressive symptoms from adolescence to young adulthood. Dev Psychol 43: 1295–1311. [DOI] [PubMed] [Google Scholar]
- 81. Grant BF (1997) Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol. Epidemiologic Survey J Stud Alcohol 58: 464–473. [DOI] [PubMed] [Google Scholar]
- 82. Trinidad DR, Perez-Satble EJ, Emery SL, White MM, Grana RA, et al. (2009) Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine Tob Res 11: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Centers for Disease Control and Prevention (1998) Tobacco use among U.S. racial/ethnic minority groups–African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, Hispanics. A Report of the Surgeon General. Executive summary. MMWR Recomm Rep 47: v-xv, 1–16. [PubMed] [Google Scholar]
- 84. Perez-Stable EJ, Herrera B, Jacob P, Benowitz NL (1998) Nicotine metabolism and intake in black and white smokers. JAMA 280: 152–156. [DOI] [PubMed] [Google Scholar]
- 85. Garlow SJ, Purselle DC, Heninger M (2007) Cocaine and alcohol use preceding suicide in African American and white adolescents. J Psychiatr Res 41: 530–536. [DOI] [PubMed] [Google Scholar]
- 86. Bernstein E, Bernstein J, Tassiopoulos K, Valentine A, Heeren T, et al. (2005) Racial and Ethnic Diversity among a Heroin and Cocaine Using Population: Treatment System Utilization. J Addict Dis 24: 43–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Williams DR, Takeuchi DT, Adair RK (1992) Marital Status and Psychiatric Disorders Among Blacks and Whites. Journal of Health and Social Behavior 33 [PubMed] [Google Scholar]
- 88. Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, et al. (2010) Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology 35: 1684–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]