Abstract
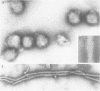
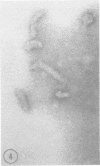
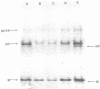
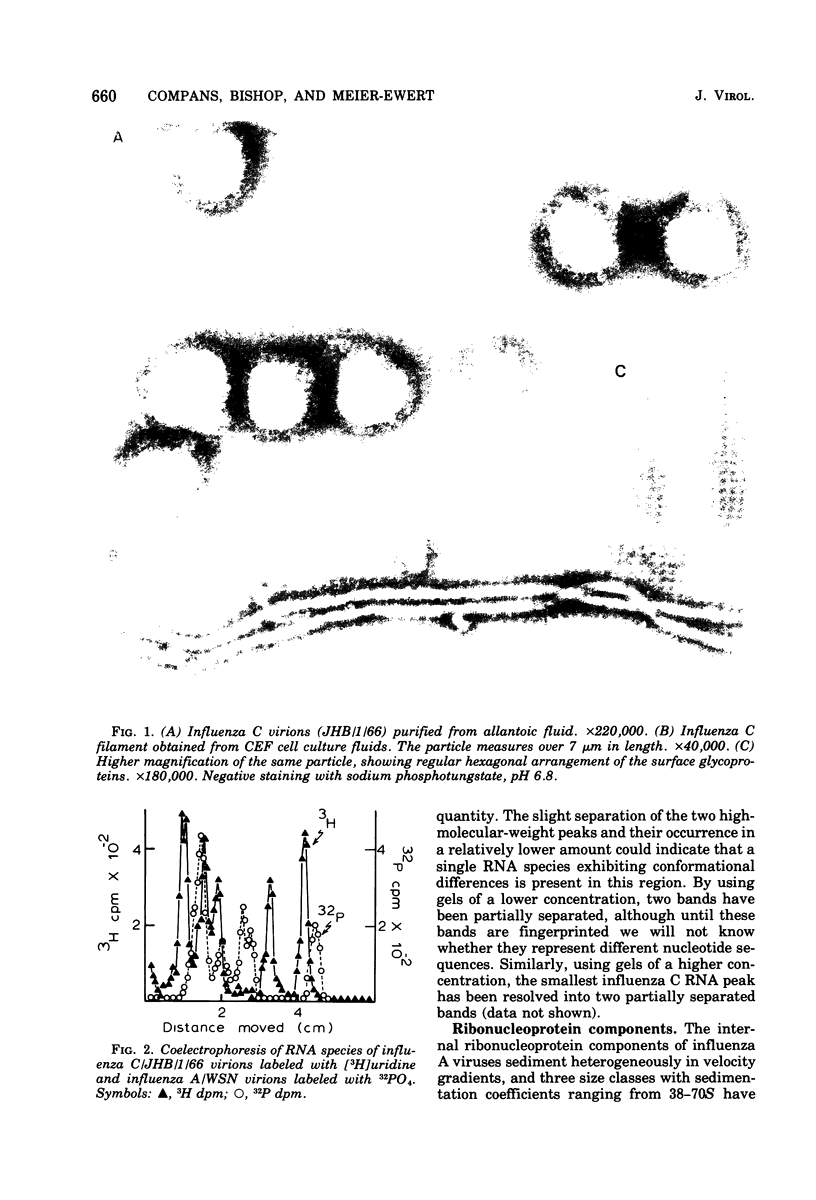
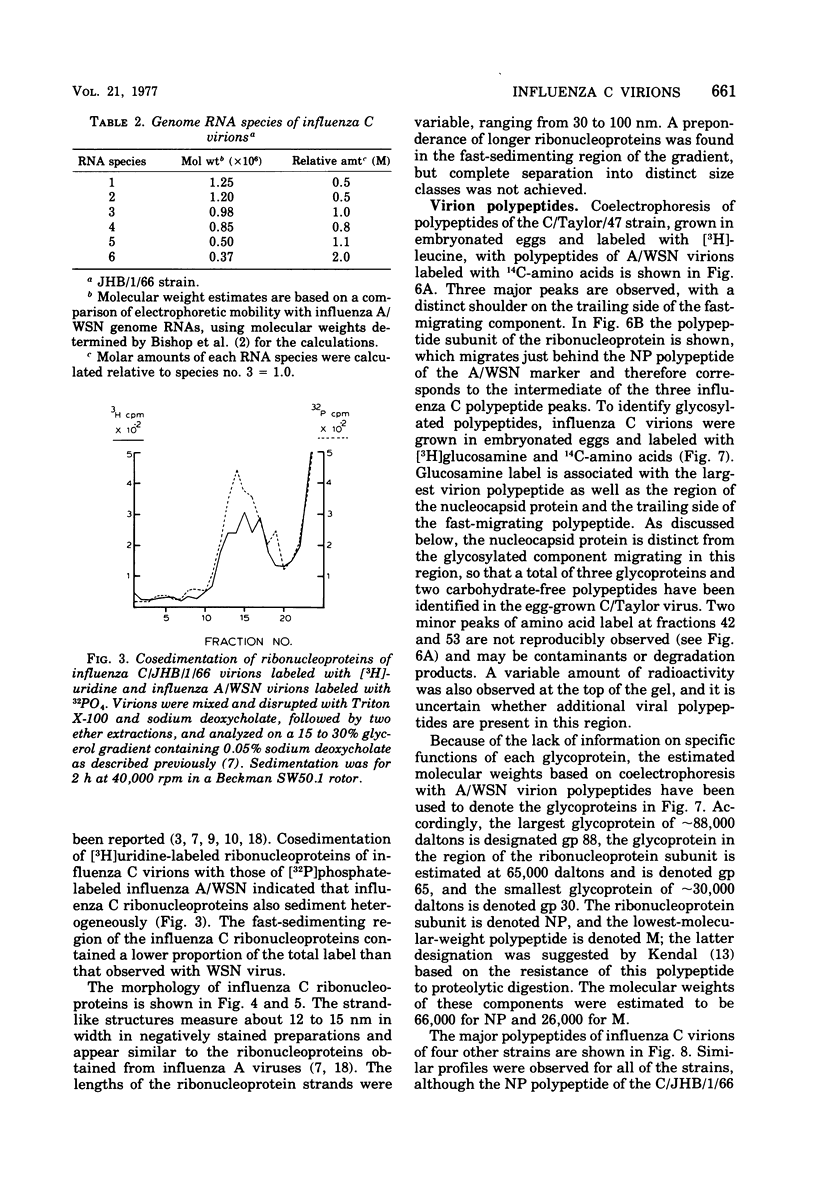
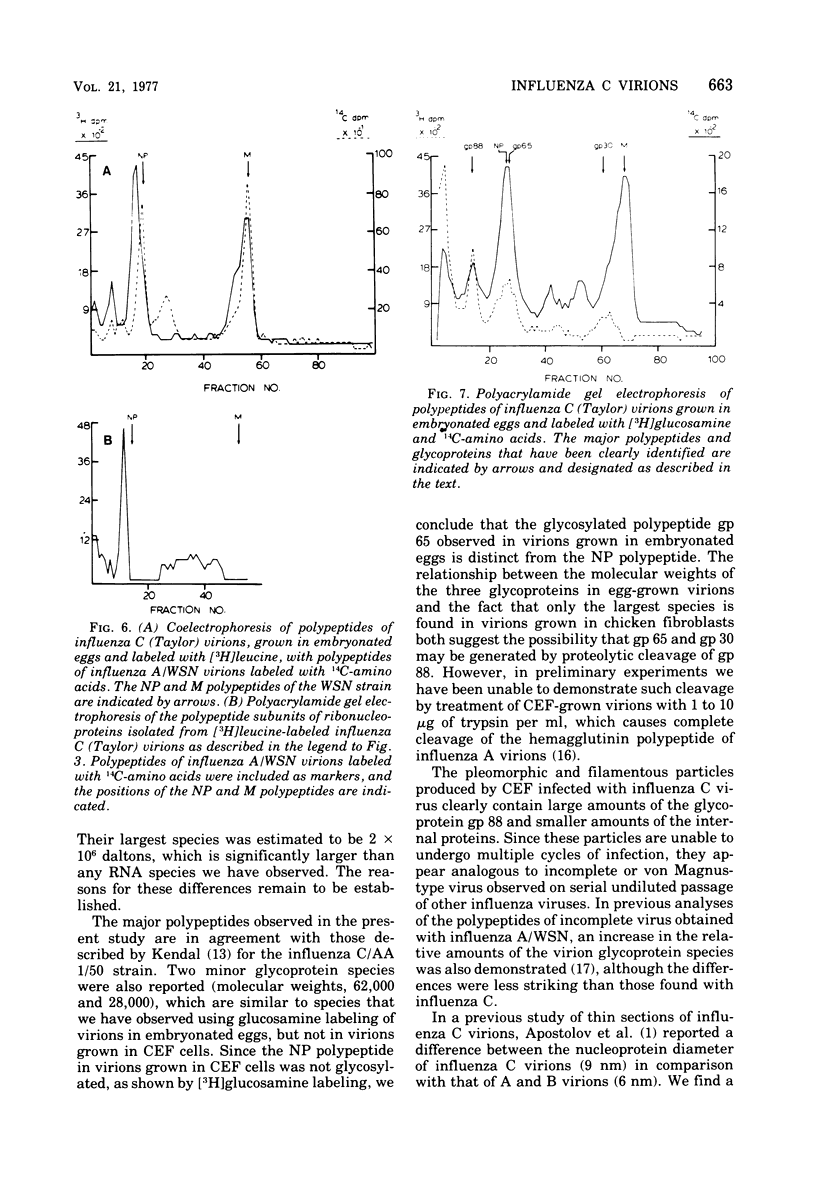
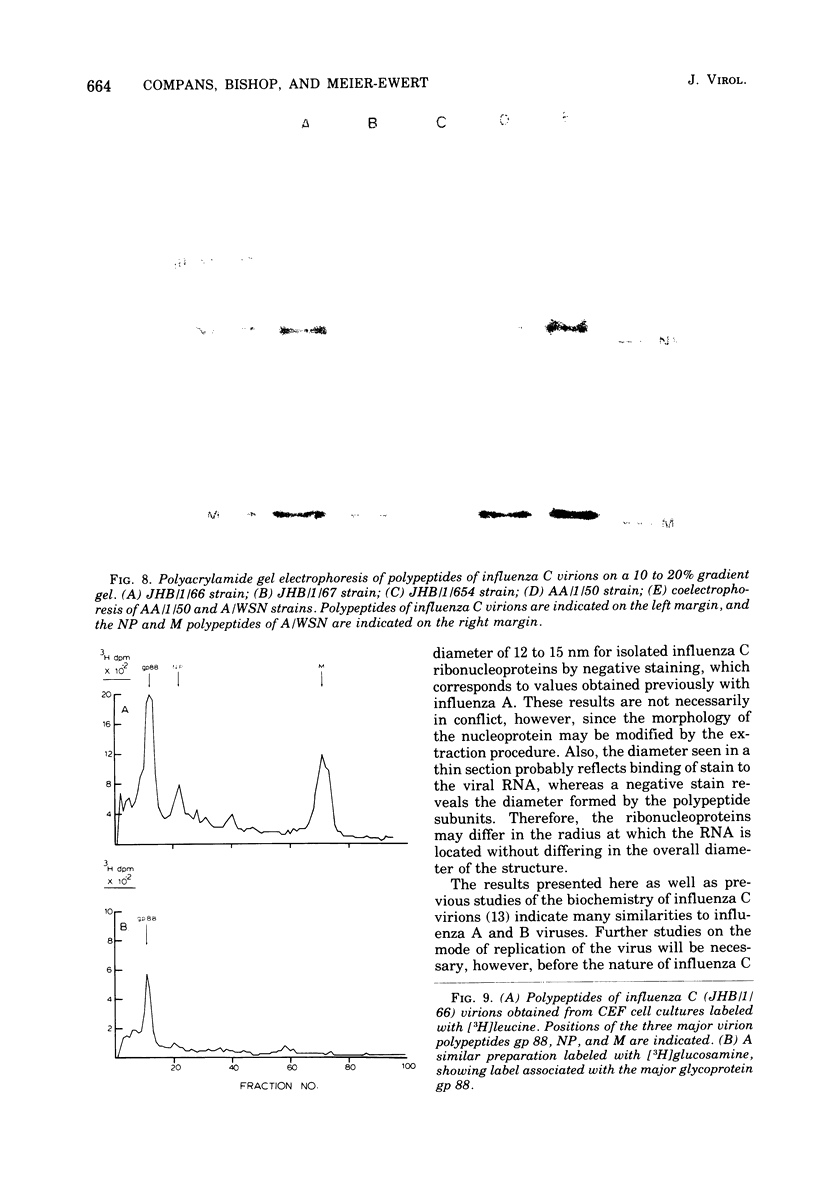
The genome RNA species of influenza type C virions were analyzed by polyacrylamide gel electrophoresis. The pattern obtained was found to resemble those of other influenza viruses. Six RNA species were resolved, with estimated sizes ranging from 0.37 X 10(6) to 1.25 X 10(6) daltons. The internal ribonucleoproteins of influenza C virions were found to sediment heterogeneously in glycerol velocity gradients as demonstrated previously with influenza A/WSN virus. The ribonucleoproteins possessed diameters of 12 to 15 nm, with lengths ranging from 30 to 100 nm. Of the three major virion polypeptides (molecular weights, 88,000, 66,000, and 26,000), only the largest is glycosylated. Similar polypeptide species were present in influenza C virions of five different strains. All three major proteins of influenza C virions possess electrophoretic mobilities distinguishable from those of the major polypeptides of influenza A/WSN. The 66,000-dalton protein is associated with the ribonucleoprotein components. Two additional glycosylated polypeptides, with estimated molecular weights of 65,000 and 30,000, were detected in virions grown in embryonated eggs, but not in virus particles obtained from chicken embryo fibroblasts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. II. Nature of the in vitro polymerase product. J Virol. 1971 Jul;8(1):74–80. doi: 10.1128/jvi.8.1.74-80.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Roy P., Bean W. J., Jr, Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. 3. Completeness of the transcription process. J Virol. 1972 Oct;10(4):689–697. doi: 10.1128/jvi.10.4.689-697.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliguiri L. A., Klenk H. D., Choppin P. W. The proteins of the parainfluenza virus SV5. 1. Separation of virion polypeptides by polyacrylamide gel electrophoresis. Virology. 1969 Nov;39(3):460–466. doi: 10.1016/0042-6822(69)90094-4. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Content J., Duesberg P. H. Structure of the ribonucleoprotein of influenza virus. J Virol. 1972 Oct;10(4):795–800. doi: 10.1128/jvi.10.4.795-800.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W. Distinct carbohydrate components of influenza virus glycoproteins in smooth and rough cytoplasmic membranes. Virology. 1973 Oct;55(2):541–545. doi: 10.1016/0042-6822(73)90199-2. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Content J., Duesberg P. H. Base sequence differences among the ribonucleic acids of influenza virus. J Mol Biol. 1971 Dec 14;62(2):273–285. doi: 10.1016/0022-2836(71)90427-x. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Distinct subunits of the ribonucleoprotein of influenza virus. J Mol Biol. 1969 Jun 28;42(3):485–499. doi: 10.1016/0022-2836(69)90237-x. [DOI] [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966 Sep 1;124(3):331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRST G. K. The relationship of the receptors of a new strain of virus to those of the mumps-NDV-influenza group. J Exp Med. 1950 Feb;91(2):177–184. doi: 10.1084/jem.91.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal A. P. A comparison of "influenza C" with prototype myxoviruses: receptor-destroycing activity (neuraminidase) and structural polypeptides. Virology. 1975 May;65(1):87–99. doi: 10.1016/0042-6822(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landsberger F. R., Lenard J., Paxton J., Compans R. W. Spin-labeled electron spin resonance study of the lipid-containing membrane of influenza virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2579–2583. doi: 10.1073/pnas.68.10.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Proteolytic cleavage of the hemagglutinin polypeptide of influenza virus. Function of the uncleaved polypeptide HA. Virology. 1973 Mar;52(1):199–212. doi: 10.1016/0042-6822(73)90409-1. [DOI] [PubMed] [Google Scholar]
- Lenard J., Compans R. W. Polypeptide composition of incomplete influenza virus grown in MDBK cells. Virology. 1975 Jun;65(2):418–426. doi: 10.1016/0042-6822(75)90047-1. [DOI] [PubMed] [Google Scholar]
- Pons M. W., Schulze I. T., Hirst G. K., Hauser R. Isolation and characterization of the ribonucleoprotein of influenza virus. Virology. 1969 Oct;39(2):250–259. doi: 10.1016/0042-6822(69)90045-2. [DOI] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Kilbourne E. D. RNAs of influenza A, B, and C viruses. J Virol. 1976 May;18(2):738–744. doi: 10.1128/jvi.18.2.738-744.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]