Abstract
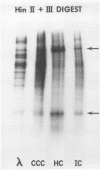
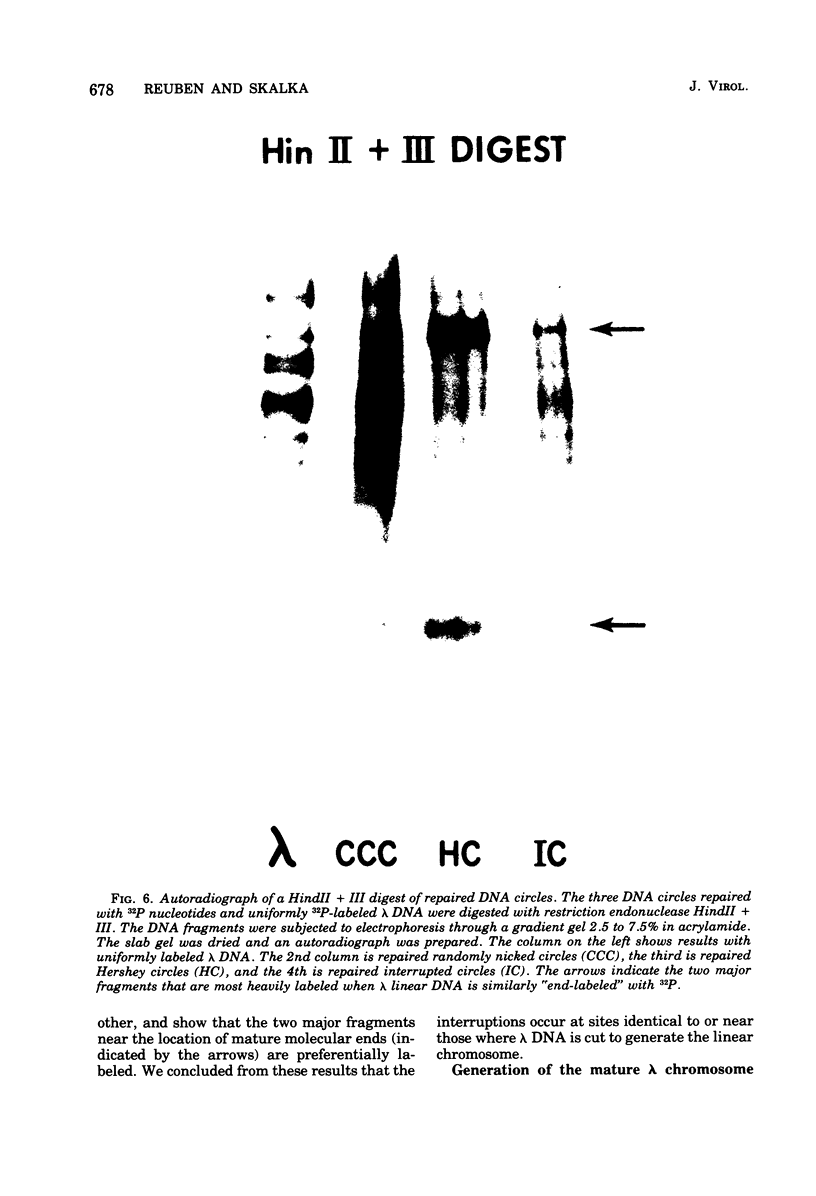
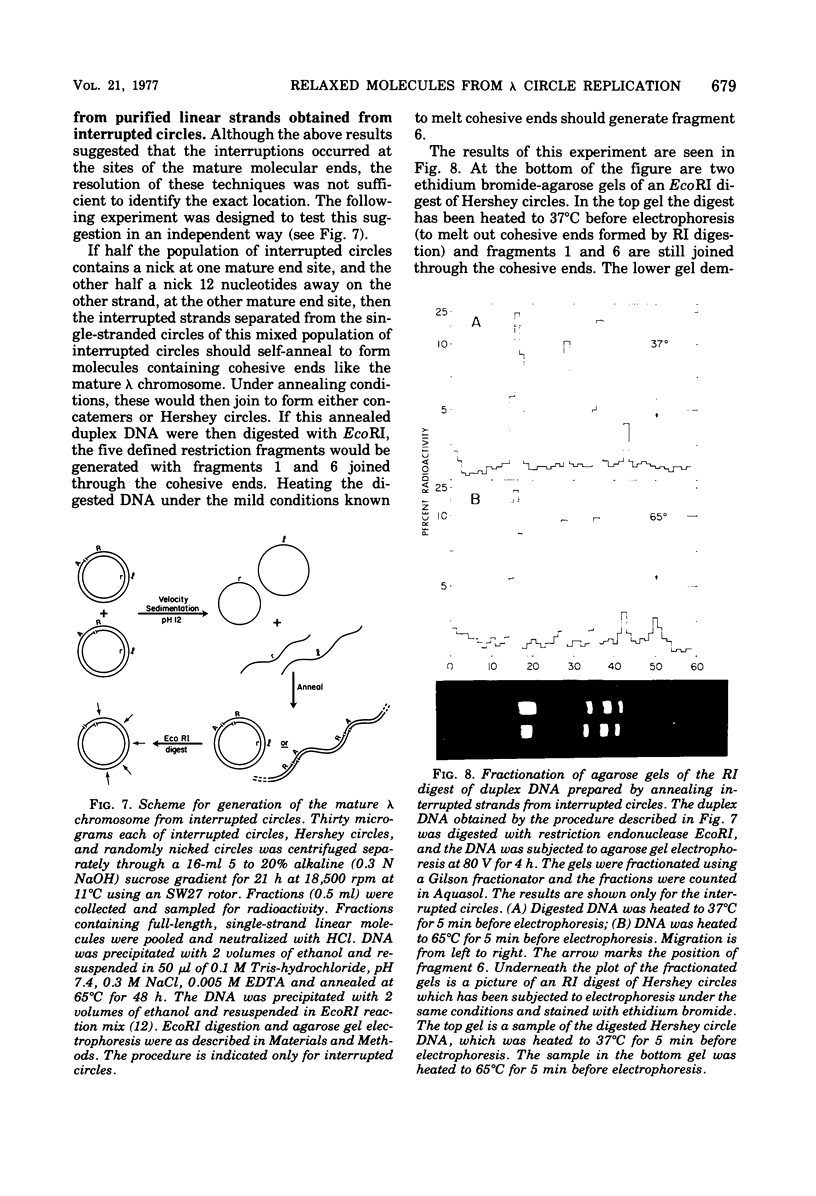
The DNA that accumulates in the lambda infection restricted to the early (circular) stage of replication consists of approximately two-thirds covalently closed circles and one-third relaxed circles bearing a single interruption in either strand of the duplex. The latter molecules are presumed to be a unique class in that the interruption is not repairable by DNA polymerase and ligase. Preferential radioisotopic labeling of the region immediately adjacent to the interruption, followed by hybridization to sheared fragments of the lambda chromosome with varying guanine plus cytosine content, suggested that the nick resides at the position of the mature molecular ends of the lambda chromosome. Digestion of the labeled molecules with restriction enzymes and reconstruction experiments in which Hershey circles were generated by annealing of interrupted strands isolated from the relaxed circles support this interpretation. The results indicate that the relaxed circles consist of a population containing one interruption in either of the two strands of the duplex jointly representing the two "nicks" contained in Hershey circles (in which the cohesive ends are annealed). These molecules could result from the inability of the maturation function to make the required staggered endonucleolytic cuts when the DNA substrate is a monomeric circle rather than a multimeric linear molecule. Alternatively, this interruption could be the result of an endonucleolytic cutting event critical to DNA replication.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Solem R. Separation and analysis of promoter sites in bacteriophage lambda DNA by specific endonucleases. J Mol Biol. 1974 Jan 5;85(4):475–484. doi: 10.1016/0022-2836(74)90310-6. [DOI] [PubMed] [Google Scholar]
- Dawson P., Hohn B., Hohn T., Skalka A. Functional empty capsid precursors produced by lambda mutant defective for late lambda DNA replication. J Virol. 1976 Feb;17(2):576–583. doi: 10.1128/jvi.17.2.576-583.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P., Skalka A. Bacteriophage lambda head morphogenesis: studies on the role of DNA. J Mol Biol. 1975 Apr 5;93(2):167–180. doi: 10.1016/0022-2836(75)90126-6. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Skalka A. Replication of bacteriophage lambda DNA dependent on the function of host and viral genes. I. Interaction of red, gam and rec. J Mol Biol. 1973 Apr 5;75(2):185–212. doi: 10.1016/0022-2836(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Chud L., Levine E. E. Requirement for maturation of Escherichia coli bacteriophage lambda. J Mol Biol. 1974 Mar 15;83(4):503–509. doi: 10.1016/0022-2836(74)90510-5. [DOI] [PubMed] [Google Scholar]
- Gefter M. L. DNA replication. Annu Rev Biochem. 1975;44:45–78. doi: 10.1146/annurev.bi.44.070175.000401. [DOI] [PubMed] [Google Scholar]
- Gellert M. Formation of covalent circles of lambda DNA by E. coli extracts. Proc Natl Acad Sci U S A. 1967 Jan;57(1):148–155. doi: 10.1073/pnas.57.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Goulian M., Lucas Z. J., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXV. Purification and properties of deoxyribonucleic acid polymerase induced by infection with phage T4. J Biol Chem. 1968 Feb 10;243(3):627–638. [PubMed] [Google Scholar]
- Greenstein M., Skalka A. Replication of bacteriophage lambda DNA: in vivo studies of the interaction between the viral gamma protein and the host recBC DNAase. J Mol Biol. 1975 Oct 5;97(4):543–549. doi: 10.1016/s0022-2836(75)80058-1. [DOI] [PubMed] [Google Scholar]
- Hayward G. S., Smith M. G. The chromosome of bacteriophage T5. I. Analysis of the single-stranded DNA fragments by agarose gel electrophoresis. J Mol Biol. 1972 Feb 14;63(3):383–395. doi: 10.1016/0022-2836(72)90435-4. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jeppesen P. G. A method for separating DNA fragments by electrophoresis in polyacrylamide concentration gradient slab gels. Anal Biochem. 1974 Mar;58(1):195–207. doi: 10.1016/0003-2697(74)90458-8. [DOI] [PubMed] [Google Scholar]
- Joyner A., Isaacs L. N., Echols H., Sly W. S. DNA replication and messenger RNA production after induction of wild-type lambda bacteriophage and lambda mutants. J Mol Biol. 1966 Aug;19(1):174–186. doi: 10.1016/s0022-2836(66)80059-1. [DOI] [PubMed] [Google Scholar]
- Reuben R., Gefter M., Enquist L., Skalka A. New method for large-scale preparation of covalently closed lambda DNA molecules. J Virol. 1974 Nov;14(5):1104–1107. doi: 10.1128/jvi.14.5.1104-1107.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y., Karu A. E., Linn S., Echols H. Purification and properties of the gamma-protein specified by bacteriophage lambda: an inhibitor of the host RecBC recombination enzyme. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2215–2219. doi: 10.1073/pnas.70.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Skalka A., Burgi E., Hershey A. D. Segmental distribution of nucleotides in the DNA of bacteriophage lambda. J Mol Biol. 1968 May 28;34(1):1–16. doi: 10.1016/0022-2836(68)90230-1. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Greenstein M., Skalka A. The circle mode of replication of bacteriophage lambda: the role of covalently closed templates and the formation of mixed catenated dimers. J Mol Biol. 1976 May 25;103(3):537–562. doi: 10.1016/0022-2836(76)90216-3. [DOI] [PubMed] [Google Scholar]
- Stahl F. W., McMilin K. D., Stahl M. M., Malone R. E., Nozu Y., Russo V. E. A role for recombination in the production of "free-loader" lambda bacteriophage particles. J Mol Biol. 1972 Jul 14;68(1):57–67. doi: 10.1016/0022-2836(72)90262-8. [DOI] [PubMed] [Google Scholar]
- Syvanen M. In vitro genetic recombination of bacteriophage lambda. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2496–2499. doi: 10.1073/pnas.71.6.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpirer J., Brachet P. Relations physiologiques entre les phages tempérés lambda et phi80. Mol Gen Genet. 1970;108(1):78–92. doi: 10.1007/BF00343187. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Valenzuela M. S., Freifelder D. Lack of a unique termination site for the first round of bacteriophage lambda DNA replication. J Mol Biol. 1976 Apr 15;102(3):569–589. doi: 10.1016/0022-2836(76)90335-1. [DOI] [PubMed] [Google Scholar]
- Wright M., Buttin G., Hurwitz J. The isolation and characterization from Escherichia coli of an adenosine triphosphate-dependent deoxyribonuclease directed by rec B, C genes. J Biol Chem. 1971 Nov;246(21):6543–6555. [PubMed] [Google Scholar]