Abstract
In this study, we examined the expression of Sonic Hedgehog, Patched, Gli1, Gli2, Gli3 and Myocardin in the developing bladders of male and female normal and megabladder (mgb−/−) mutant mice at embryonic days 12 through 16 by in situ hybridization. This analysis indicated that each member of the Sonic Hedgehog signaling pathway as well as Myocardin displayed distinct temporal and spatial patterns of expression during normal bladder development. In contrast, mgb−/− bladders showed both temporal and spatial changes in the expression of Patched, Gli1 and Gli3 as well as a complete lack of Myocardin expression. These changes occurred primarily in the outer mesenchyme of developing mgb−/− bladders consistent with the development of an amuscular bladder phenotype in these animals. These results provide the first comprehensive analysis of the Sonic Hedgehog signaling pathway during normal bladder development and provide strong evidence that this key signaling cascade is critical in establishing radial patterning in the developing bladder. In addition, the lack of detrusor smooth muscle development observed in mgb−/− mice is associated with bladder-specific temporospatial changes in Sonic Hedgehog signaling coupled with a lack of Myocardin expression that appears to result in altered patterning of the outer mesenchyme and poor initiation and differentiation of smooth muscle cells within this region of the developing bladder.
Introduction
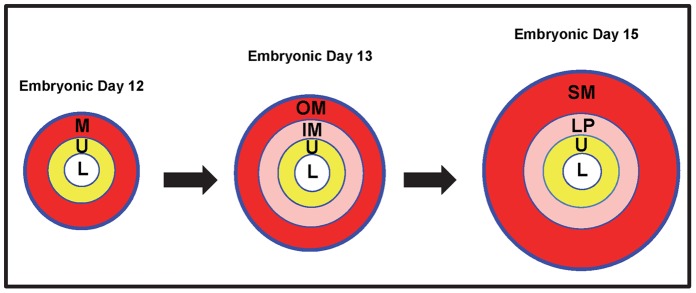
The mammalian urinary bladder develops from a partitioning of the cloaca by the urorectal septum with the superior region of the developing urogenital sinus, giving rise to the definitive urinary bladder [1], [2]. The developing bladder is initially composed of three distinct layers that include the 1) innermost urothelium, 2) intermediate-placed mesenchyme and 3) outer serosal layer (Fig. 1) [2], [3]. The innermost urothelium is a derivative of the embryonic endoderm and is composed of an expandable transitional epithelium that provides a watertight barrier for urine storage [4], [5]. The mesodermal-derived bladder mesenchyme initially differentiates into two distinct compartments that include the inner and outer mesenchyme. The inner mesenchyme, adjacent to the urothelium, differentiates into the lamina propria composed of loose connective tissue [1], [4], [5]. This layer enables the bladder to expand as it fills, while still maintaining a low pressure to protect the kidneys. The outer mesenchyme differentiates into detrusor smooth muscle that generates the tensile strength and functional contractility necessary to store and expel urine [1], [4], [5].
Figure 1. Model of murine bladder development.
Timeline showing the approximate embryonic stage at which the lumen (L), urothelium (U), inner mesenchyme (IM), outer mesenchyme (OM), lamina propria (LP) and detrusor smooth muscle (SM) first become evident.
Bladder organogenesis is highly dependent upon the reciprocal interactions of differentiating urothelium and bladder mesenchyme [6]–[8]. Tissue recombination experiments indicate that bladder urothelium is required to induce bladder mesenchyme to differentiate into smooth muscle via a diffusible signaling molecule [3], [7], [9]. Of particular interest, Sonic Hedgehog (Shh) and it's downstream signaling molecules have been shown to be involved in many epithelial-mesenchymal interactions during development, including proper bladder development and differentiation [10]–[16]. Shh is a secreted signaling molecule that interacts with the transmembrane receptor Patched (Ptch) resulting in repression of Ptch, and activation of Gli transcription factors in the target cell. Work by numerous labs indicates that this canonical Shh signaling pathway is involved in multiple processes, including cell fate determination, morphogenesis, differentiation, and apoptosis [13], [17].
Our lab has identified a unique transgenic mouse model for studying bladder development, function, and pathogenesis [18]. Homozygous megabladder (mgb−/−) mice develop greatly enlarged bladders in utero as a result of a tissue specific defect in detrusor smooth muscle development. In this current study, we characterized the expression patterns of the Shh ligand, Ptch receptor, downstream transcription factors Gli1, Gli2, & Gli3 and a potential target gene of the pathway, Myocardin (Myocd), in the bladders of male and female wild type (normal) and mgb−/− (mutant) mice at key developmental time-points for smooth muscle differentiation-embryonic days (E) 12, 13, 14, 15, and 16- by in situ hybridization. This analysis indicated that members of the Shh signaling pathway and Myocd were expressed in distinct temporal and spatial patterns during normal bladder development. The observed patterns of expression were consistent with the Shh signaling pathway playing a key role in radial patterning of the developing bladder. Defects in the expression of Ptch, Gli1 and Gli3 in developing mgb−/− bladders suggest that changes in radial patterning coupled with a lack of Myocd expression are responsible for the lack of detrusor smooth muscle development in these animals.
Results
No gender-specific differences in expression of Shh, Ptch, Gli1, Gli2, Gli3, or Myocd were observed at any of the developmental time points examined in this study. The expression of Shh, Ptch, Gli1, Gli2, Gli3, and Myocd were examined by antisense and sense RNA probes at each developmental time point assessed. Representative images for each of these are provided to evaluate signal vs. background (Fig. 2–16) and these results are summarized in Table 1. A positive control probe, β-tubulin (β-tub), was examined by antisense and sense RNA probes. β-tub expressed in multiple tissues, including the central nervous system, in all the developmental time points examined in this study (Fig. 17).
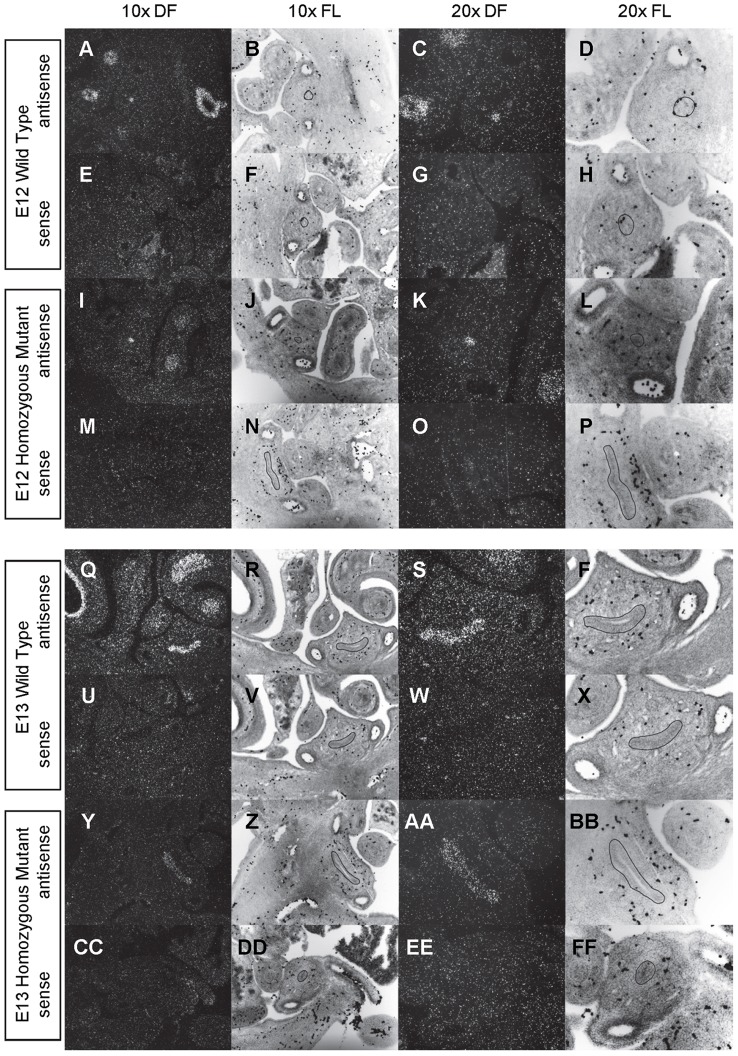
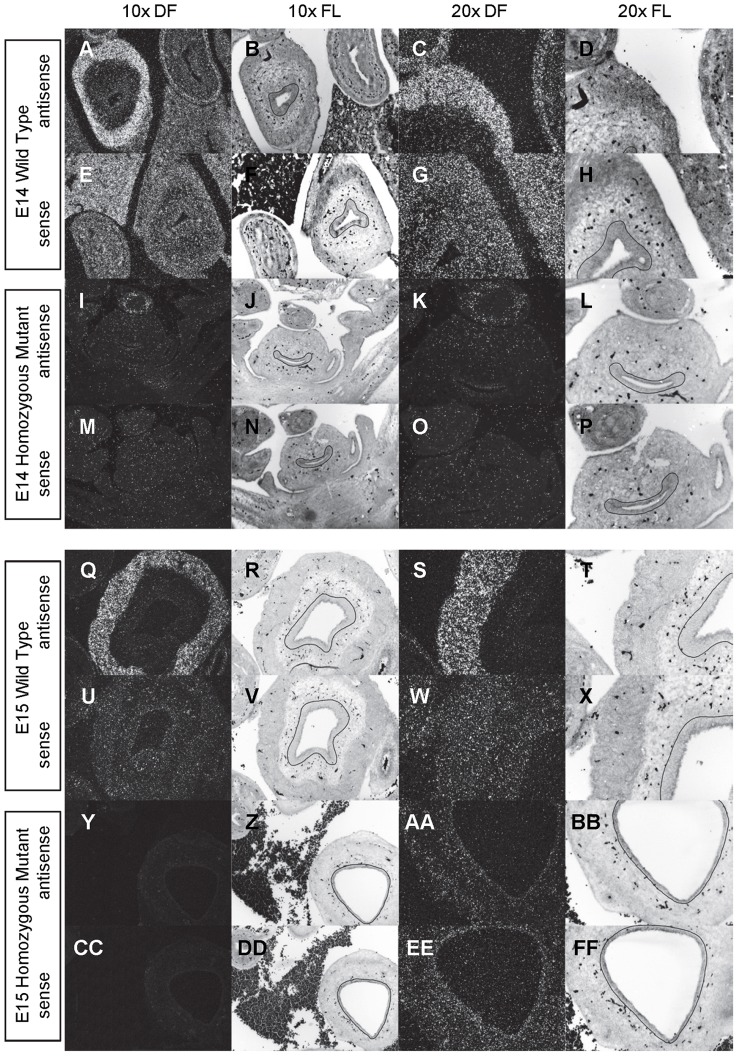
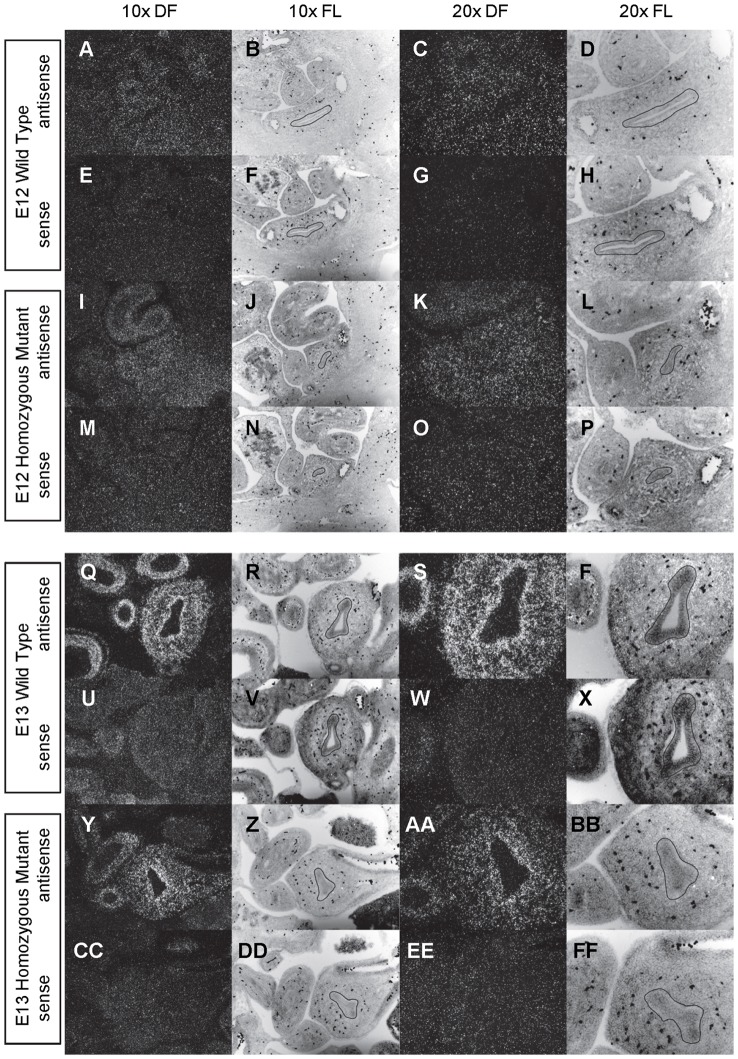
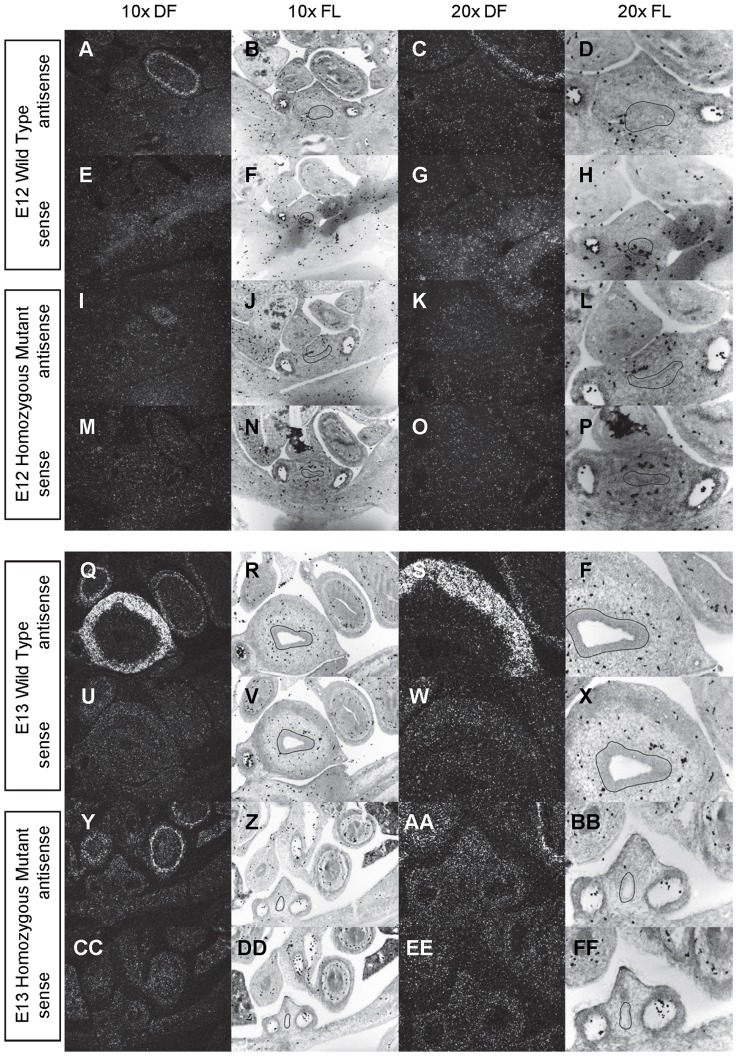
Figure 2. Expression of Shh in the bladder of E12 and E13 mice.
In situ hybridization analysis of Shh expression in E12 (A-P) and E13 (Q-FF) bladders using antisense (A-D, I-L, Q-T, Y-BB) and sense (E-H, M-P, U-X, CC-FF) riboprobes on transverse sections of wild type (A-H, Q-X) and mgb−/− (I-P, Y-FF) mice. Sections are shown at 10X dark field (DF; A, E, I, M, Q, U, Y, CC), 10X fluorescence (FL; B, F, J, N, R, V, Z, DD), 20X dark field (DF; C, G, K, O, S, W, AA, EE) and 20X fluorescence (FL; D, H, L, P, T, X, BB, FF). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
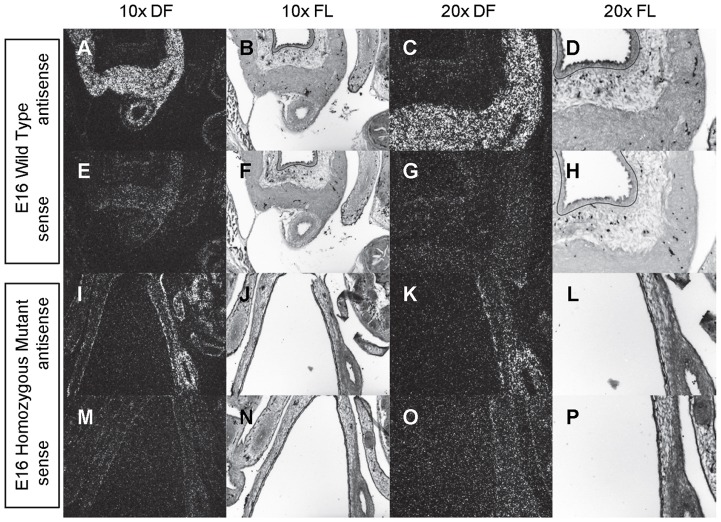
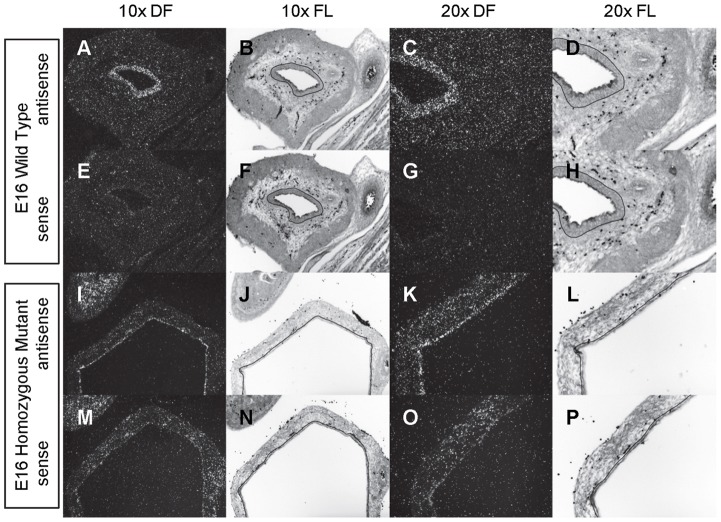
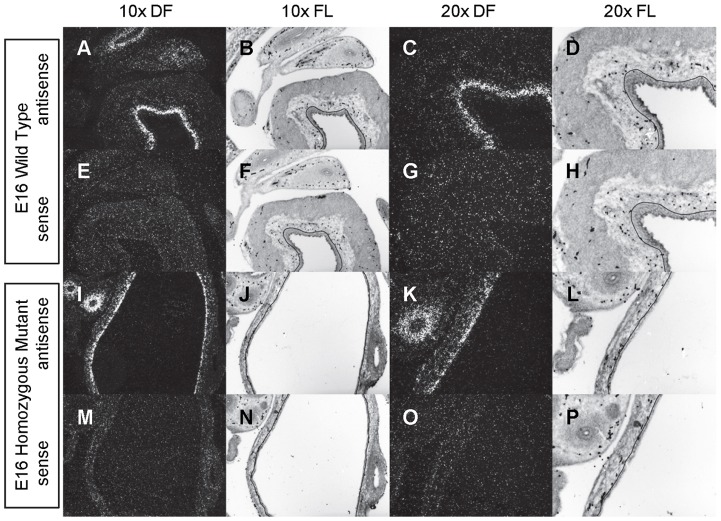
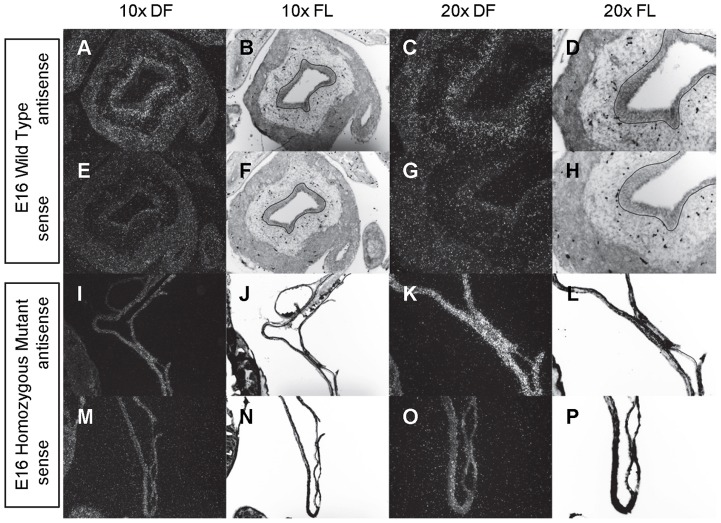
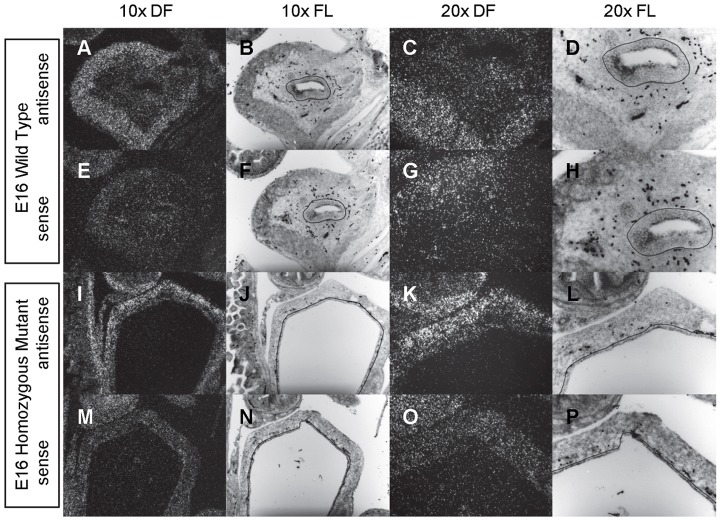
Figure 16. Expression of Myocd in the bladder of E16 mice.
In situ hybridization analysis of Myocd expression in E16 (A-P) bladders using antisense (A-D, I-L) and sense (E-H, M-P) riboprobes on transverse sections of wild type (A-H) and mgb−/− (I-P) mice. Sections are shown at 10X dark field (DF; A, E, I, M), 10X fluorescence (FL; B, F, J, N), 20X dark field (DF; C, G, K, O) and 20X fluorescence (FL; D, H, L, P). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
Table 1. Summary of Shh, Ptch, Gli1, Gli3, and Myocd mRNA Expression in the Developing Bladder of WT and mgb−/− Mice.
| Probe | Stage | WT Urothelium | WT Inner Mesenchyme | WT Outer Mesenchyme | mgb−/− Urothelium | mgb−/− Inner Mesenchyme | mgb−/− Outer Mesenchyme |
| Shh | E12 | ++ | − | − | ++ | − | − |
| E13 | ++ | − | − | ++ | − | − | |
| E14 | ++ | − | − | ++ | − | − | |
| E15 | ++ | − | − | ++ | − | − | |
| E16 | ++ | − | − | ++ | − | − | |
| Ptch | E12 | − | + | + | − | + | + |
| E13 | − | + | −/+ | − | −/+ | − | |
| E14 | − | +++ | − | − | ++ | − | |
| E15 | − | +++ | − | − | ++ | − | |
| E16 | − | +++ | − | − | ++ | − | |
| Gli1 | E12 | − | −/+ | −/+ | − | −/+ | −/+ |
| E13 | − | ++ | ++ | − | + | −/+ | |
| E14 | − | ++ | + | − | ++ | ++ | |
| E15 | − | ++ | ++ | − | ++ | + | |
| E16 | − | ++ | − | − | ++ | ++ | |
| Gli3 | E12 | − | − | − | − | − | − |
| E13 | − | − | ++ | − | − | − | |
| E14 | − | − | ++ | − | − | + | |
| E15 | − | − | ++ | − | − | ++ | |
| E16 | − | − | ++ | − | − | ++ | |
| Myocd | E12 | − | − | − | − | − | − |
| E13 | − | − | +++ | − | − | − | |
| E14 | − | − | +++ | − | − | −/+ | |
| E15 | − | − | +++ | − | − | − | |
| E16 | − | − | +++ | − | − | −/+ |
Shh, Ptch, Gli1, Gli3, and Myocd mRNA expression in the developing bladder of wild type (WT) and megabladder (mgb−/−) mice at embryonic day (E) 12, 13, 14, 15, and 16. Relative expression is reported for mRNA as: (−) no expression, (−/+) faint, (+) low, (++) moderate, and (+++) and high levels of expression. The relative levels of mRNA expression reported for each time-point represent an average of multiple sections processed in a minimum of three separate runs.
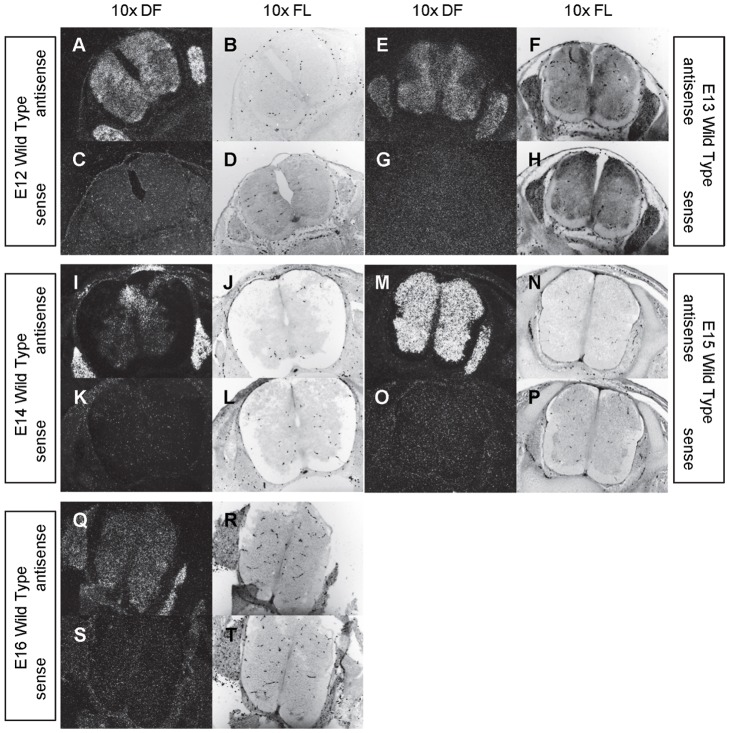
Figure 17. Expression of β-tubulin in the central nervous system of E12 through E16 mice.
In situ hybridization analysis of β-tubulin expression in E12 (A-D), E13 (E-H), E14 (I-L), E15 (M-P), and E16 (Q-T) central nervous system using antisense (A-B, E-F, I-J, M-N, Q-R) and sense (E-D, G-H, K-L, O-P, S–T) riboprobes on transverse sections of wild type (A-T) mice. Sections are shown at 10X dark field (DF; A, C, E, G, I, K, M, O, Q, S) and 10X fluorescence (FL; B, D, F, H, J, L, N, P, R, T).
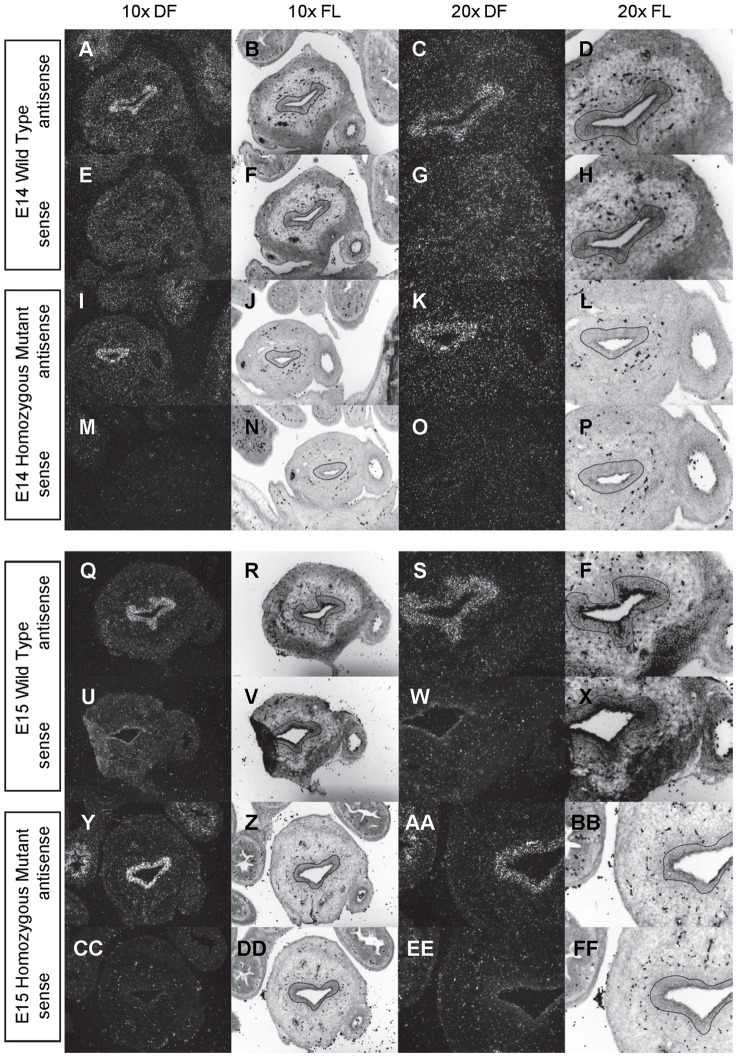
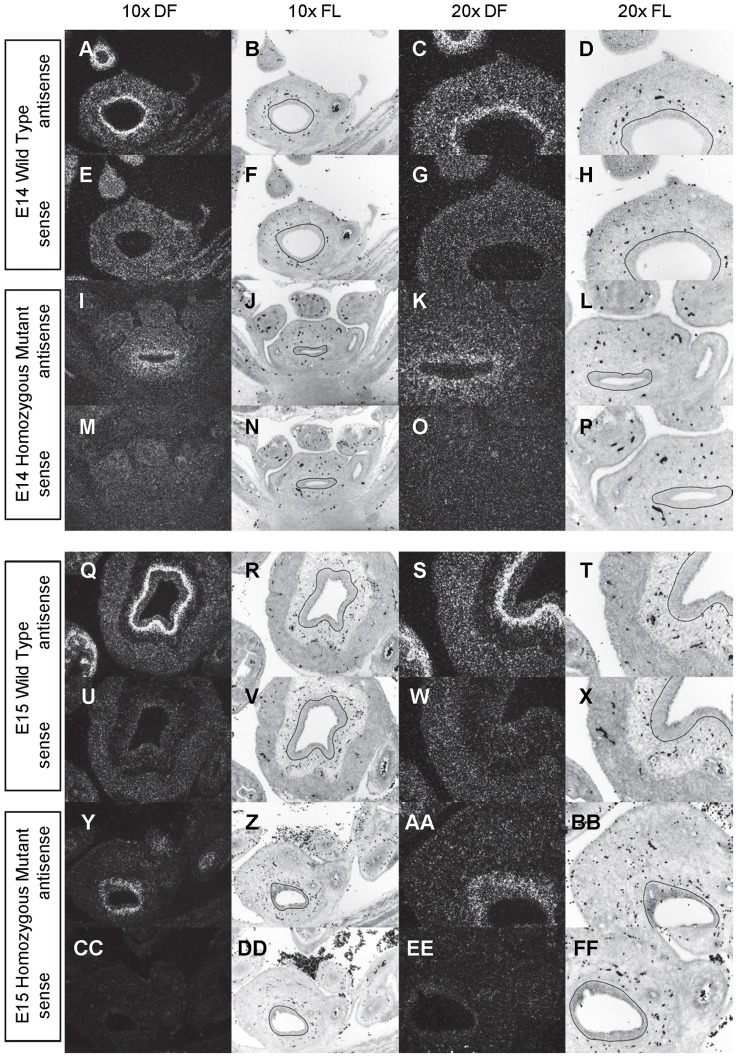
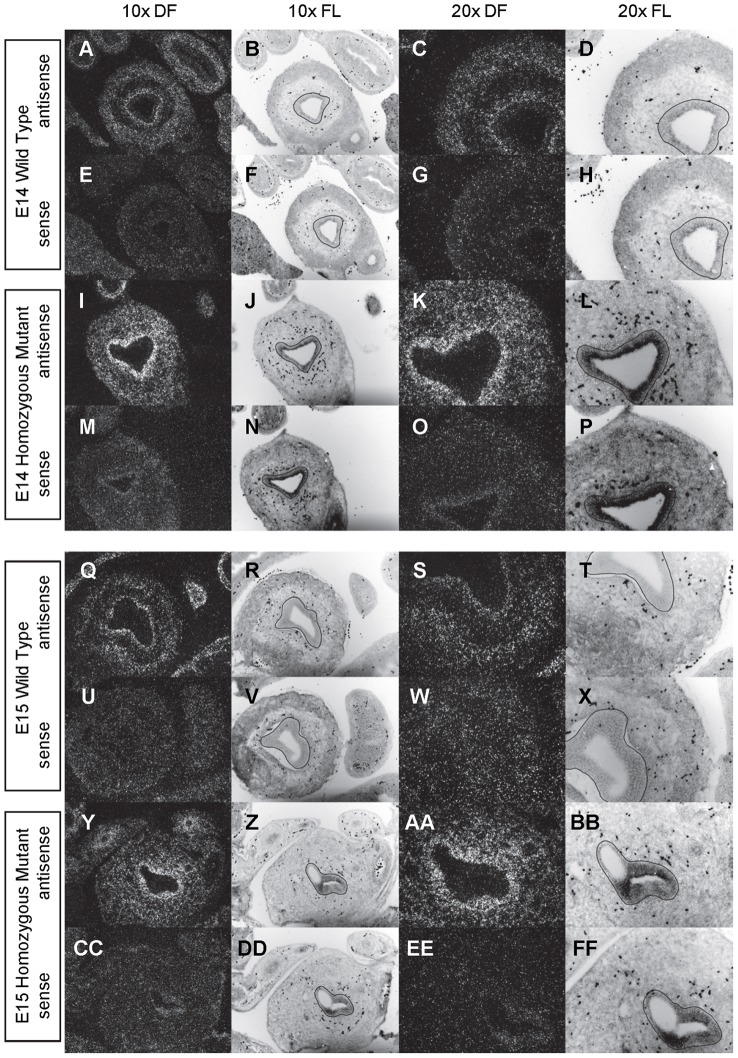
Figure 3. Expression of Shh in the bladder of E14 and E15 mice.
In situ hybridization analysis of Shh expression in E14 (A-P) and E15 (Q-FF) bladders using antisense (A-D, I-L, Q-T, Y-BB) and sense (E-H, M-P, U-X, CC-FF) riboprobes on transverse sections of wild type (A-H, Q-X) and mgb−/− (I-P, Y-FF) mice. Sections are shown at 10X dark field (DF; A, E, I, M, Q, U, Y, CC), 10X fluorescence (FL; B, F, J, N, R, V, Z, DD), 20X dark field (DF; C, G, K, O, S, W, AA, EE) and 20X fluorescence (FL; D, H, L, P, T, X, BB, FF). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
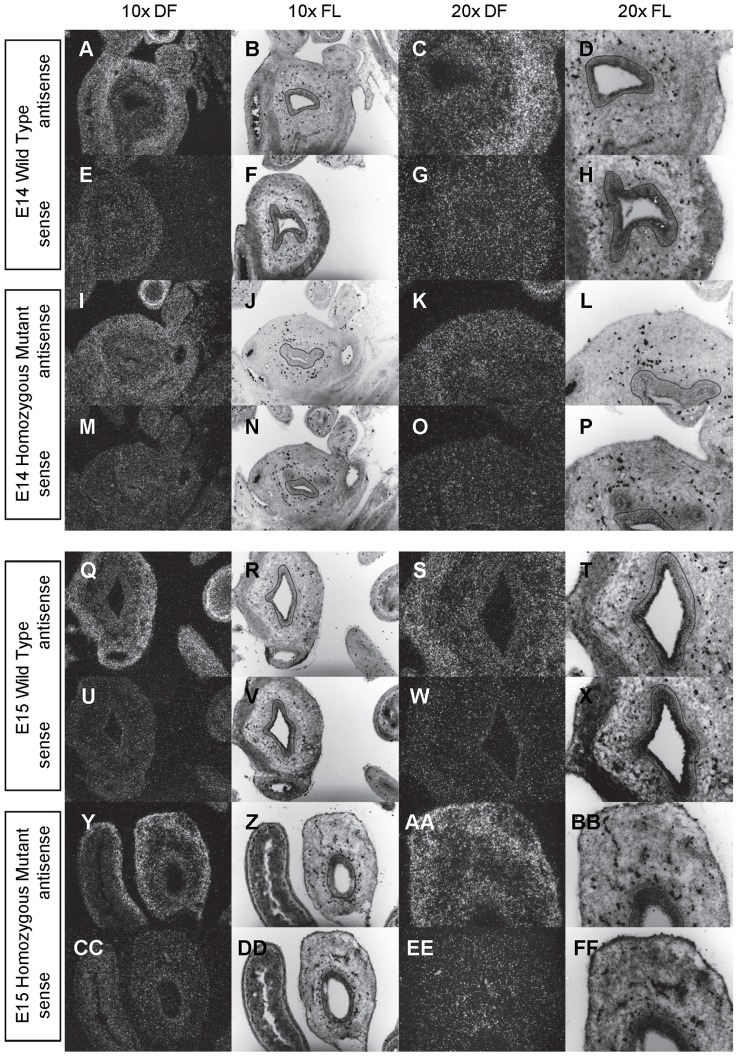
Figure 15. Expression of Myocd in the bladder of E14 and E15 mice.
In situ hybridization analysis of Myocd expression in E14 (A-P) and E15 (Q-FF) bladders using antisense (A-D, I-L, Q-T, Y-BB) and sense (E-H, M-P, U-X, CC-FF) riboprobes on transverse sections of wild type (A-H, Q-X) and mgb−/− (I-P, Y-FF) mice. Sections are shown at 10X dark field (DF; A, E, I, M, Q, U, Y, CC), 10X fluorescence (FL; B, F, J, N, R, V, Z, DD), 20X dark field (DF; C, G, K, O, S, W, AA, EE) and 20X fluorescence (FL; D, H, L, P, T, X, BB, FF). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
Sonic Hedgehog is expressed in bladder urothelium
Shh is uniformly expressed at moderate to high levels in the bladder urothelium of normal mice from E12 to E16 (Fig. 2–4). An identical pattern of urothelium-specific expression of Shh is also observed in the developing bladders of mgb−/− mice (Fig. 2–4). The development of megabladder by E16 results in significant attenuation of the bladder wall and subsequent pattern of Shh expression within the urothelium. A similar epithelial-specific expression of Shh is also noted in other visceral organs including the stomach, intestine, colon and rectum with no differences observed between normal and mgb−/− mice (Fig. 2–4).
Figure 4. Expression of Shh in the bladder of E16 mice.
In situ hybridization analysis of Shh expression in E16 (A-P) bladders using antisense (A-D, I-L) and sense (E-H, M-P) riboprobes on transverse sections of wild type (A-H) and mgb−/− (I-P) mice. Sections are shown at 10X dark field (DF; A, E, I, M), 10X fluorescence (FL; B, F, J, N), 20X dark field (DF; C, G, K, O) and 20X fluorescence (FL; D, H, L, P). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
Patched is expressed in bladder inner mesenchyme
At E12, Ptch is expressed at moderate levels throughout the entire bladder mesenchyme from the base of the urothelium to the outer serosa (Fig. 5). By E13, Ptch expression begins to restrict towards the inner mesenchyme resulting in low levels of Ptch expression throughout the bladder mesenchyme, with an intense band of Ptch expression within the inner mesenchyme underlying the urothelium (Fig. 5). As bladder development progresses from E14 to E16, Ptch expression becomes sequentially more restricted to the base of the bladder urothelium resulting in little or no expression of Ptch in the outer mesenchyme/detrusor smooth muscle and outer half of the inner mesenchyme (Fig. 6–7).
Figure 5. Expression of Ptch in the bladder of E12 and E13 mice.
In situ hybridization analysis of Ptch expression in E12 (A-P) and E13 (Q-FF) bladders using antisense (A-D, I-L, Q-T, Y-BB) and sense (E-H, M-P, U-X, CC-FF) riboprobes on transverse sections of wild type (A-H, Q-X) and mgb−/− (I-P, Y-FF) mice. Sections are shown at 10X dark field (DF; A, E, I, M, Q, U, Y, CC), 10X fluorescence (FL; B, F, J, N, R, V, Z, DD), 20X dark field (DF; C, G, K, O, S, W, AA, EE) and 20X fluorescence (FL; D, H, L, P, T, X, BB, FF). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
Figure 6. Expression of Ptch in the bladder of E14 and E15 mice.
In situ hybridization analysis of Ptch expression in E14 (A-P) and E15 (Q-FF) bladders using antisense (A-D, I-L, Q-T, Y-BB) and sense (E-H, M-P, U-X, CC-FF) riboprobes on transverse sections of wild type (A-H, Q-X) and mgb−/− (I-P, Y-FF) mice. Sections are shown at 10X dark field (DF; A, E, I, M, Q, U, Y, CC), 10X fluorescence (FL; B, F, J, N, R, V, Z, DD), 20X fluorescence (DF; C, G, K, O, S, W, AA, EE) and 20X fluorescence (FL; D, H, L, P, T, X, BB, FF). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
Figure 7. Expression of Ptch in the bladder of E16 mice.
In situ hybridization analysis of Ptch expression in E16 (A-P) bladders using antisense (A-D, I-L) and sense (E-H, M-P) riboprobes on transverse sections of wild type (A-H) and mgb−/− (I-P) mice. Sections are shown at 10X dark field (DF; A, E, I, M), 10X fluorescence (FL; B, F, J, N), 20X fluorescence (DF; C, G, K, O) and 20X (FL; D, H, L, P). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
At E12, Ptch is expressed at moderate levels throughout the entire developing mesenchyme of mgb−/− bladders (Fig. 5). By E13, Ptch expression begins to restrict to the inner mesenchyme of mgb−/− bladders even though the overall intensity of Ptch expression appears reduced versus normal bladders (Fig. 5). At E14, the restriction of Ptch expression to the inner mesenchyme of mgb−/− bladders appears delayed, resembling the pattern observed in E13 normal bladders (Fig. 5 vs. 6). The further restriction and loss of Ptch expression in the distal mesenchyme of E15 mgb−/− bladders remains delayed and appears reduced in intensity versus normal bladders (Fig. 6). The highly attenuated E16 megabladder shows intense Ptch expression in the lamina propria underlying the urothelium similar to normal bladders although, unlike normal bladders, variable regions of diffuse Ptch expression are still observed within the outer mesenchyme (Fig. 7).
Ptch expression is also observed in the developing mesenchyme of the stomach, intestine, colon and rectum in both normal and mgb−/− mice with no differences in genotype observed (Fig. 5–7). The overall intensity and spatial redistribution of Ptch expression during the development of these visceral tissues appears similar to that observed in the bladder with variable maintenance of diffuse Ptch expression throughout the inner and/or outer mesenchyme.
Gli1 is expressed in bladder inner mesenchyme
At E12, Gli1 is diffusely expressed throughout the entire mesenchyme of normal bladders (Fig. 8). By E13, Gli1 is moderately expressed in three concentric rings within the bladder mesenchyme that morphologically correspond to an intense ring of expression immediately underlying the urothelium, a light ring of expression in the remaining inner mesenchyme, and an intense ring of expression in the outer mesenchyme (Fig. 8). As bladder development progresses from E14 to E16, Gli1 expression becomes progressively restricted to the base of the urothelium such that by E16 modest Gli1 expression is principally observed in the inner mesenchyme underlying the bladder urothelium with no more than light and spotty Gli1 expression observed in the outer mesenchyme/detrusor smooth muscle (Fig. 9–10).
Figure 8. Expression of Gli1 in the bladder of E12 and E13 mice.
In situ hybridization analysis of Gli1 expression in E12 (A-P) and E13 (Q-FF) bladders using antisense (A-D, I-L, Q-T, Y-BB) and sense (E-H, M-P, U-X, CC-FF) riboprobes on transverse sections of wild type (A-H, Q-X) and mgb−/− (I-P, Y-FF) mice. Sections are shown at 10X dark field (DF; A, E, I, M, Q, U, Y, CC), 10X fluorescence (FL; B, F, J, N, R, V, Z, DD), 20X dark field (DF; C, G, K, O, S, W, AA, EE) and 20X fluorescence (FL; D, H, L, P, T, X, BB, FF). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
Figure 9. Expression of Gli1 in the bladder of E14 and E15 mice.
In situ hybridization analysis of Gli1 expression in E14 (A-P) and E15 (Q-FF) bladders using antisense (A-D, I-L, Q-T, Y-BB) and sense (E-H, M-P, U-X, CC-FF) riboprobes on transverse sections of wild type (A-H, Q-X) and mgb−/− (I-P, Y-FF) mice. Sections are shown at 10X dark field (DF; A, E, I, M, Q, U, Y, CC), 10X fluorescence (FL; B, F, J, N, R, V, Z, DD), 20X dark field (DF; C, G, K, O, S, W, AA, EE) and 20X fluorescence (FL; D, H, L, P, T, X, BB, FF). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
Figure 10. Expression of Gli1 in the bladder of E16 mice.
In situ hybridization analysis of Gli1 expression in E16 (A-P) bladders using antisense (A-D, I-L) and sense (E-H, M-P) riboprobes on transverse sections of wild type (A-H) and mgb−/− (I-P) mice. Sections are shown at 10X dark field (DF; A, E, I, M), 10X fluorescence (FL; B, F, J, N), 20X dark field (DF; C, G, K, O) and 20X fluorescence (FL; D, H, L, P). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
At E12, Gli1 is expressed at low levels throughout the developing mesenchyme of mgb−/− bladders in a manner similar to normal bladders (Fig. 8). At E13, mesenchymal expression of Gli1 in mgb−/− bladders appears reduced and less organized than that observed in normal bladders (Fig. 8). The development of differentially expressed concentric rings of Gli1 within the bladder mesenchyme is absent in E14 mgb−/− bladders, with the overall intensity and pattern of Gli1 expression more reminiscent of E13 normal bladders (Fig. 9C vs. Fig. 9K). This less organized pattern of expression within the mgb−/− bladder remains at E15, with a slight decrease in Gli1 expression observed throughout the mesenchyme except in the region underlying the urothelium (Fig. 9). The highly attenuated E16 mgb−/− megabladder continues to express Gli1 within the mesenchymal compartment but, unlike normal bladders, expression is not restricted to the inner mesenchyme at the base of the urothelium. Rather, Gli1 is expressed throughout the entire E16 bladder mesenchyme (Fig. 10).
A similar mesenchyme-specific pattern of Gli1 expression is also observed in the developing stomach, intestine, colon and rectum in both normal and mgb−/− mice with no differences in genotype observed (Fig. 8–10). The overall intensity and spatial redistribution of Gli1 expression during the development of these visceral tissues appears similar to that observed in the bladder, with moderate intensity expression throughout the mesenchyme at early development, which was restricted to the inner mesenchyme as development progressed.
Gli2 expression undetectable
Specific Gli2 expression was not detected in any of the bladder sections examined in this study even though two different Gli2 probes were tested including one developed in the Joyner lab [19] and one purchased from Open BioSystems (EMM1002-2414207) (data not shown). This observation, coupled with the success of the remaining in situ probes used in this study, suggests that these negative results are not due to technical issues, though it still remains plausible we were unable to detect Gli2 expression in the developing bladder due to probe specificity problems and/or experimental conditions.
Gli3 is expressed in bladder outer mesenchyme
At E12, Gli3 is undetectable in the bladder mesenchyme (Fig. 11). By E13, faint Gli3 expression is visible in the outer mesenchyme of the bladder (Fig. 11). As bladder development progresses, Gli3 expression increases moderately within the outer mesenchyme of E14 bladders and is maintained at this level through E16 (Fig. 12–13).
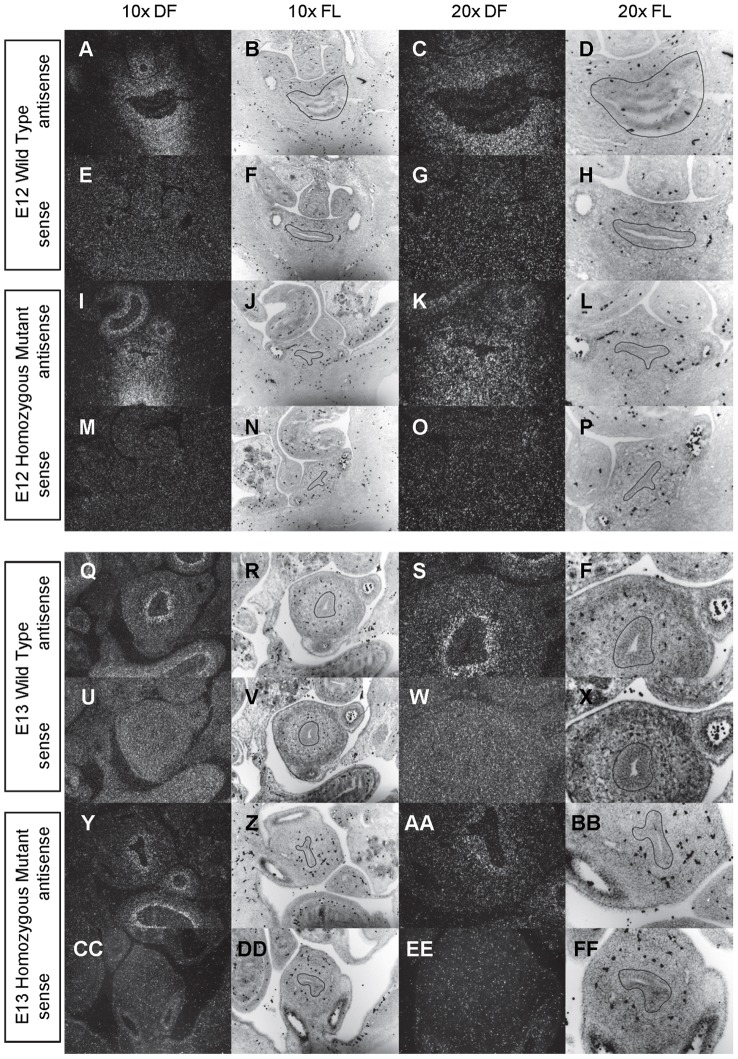
Figure 11. Expression of Gli3 in the bladder of E12 and E13 mice.
In situ hybridization analysis of Gli3 expression in E12 (A-P) and E13 (Q-FF) bladders using antisense (A-D, I-L, Q-T, Y-BB) and sense (E-H, M-P, U-X, CC-FF) riboprobes on transverse sections of wild type (A-H, Q-X) and mgb−/− (I-P, Y-FF) mice. Sections are shown at 10X dark field (DF; A, E, I, M, Q, U, Y, CC), 10X fluorescence (FL; B, F, J, N, R, V, Z, DD), 20X dark field (DF; C, G, K, O, S, W, AA, EE) and 20X fluorescence (FL; D, H, L, P, T, X, BB, FF). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
Figure 12. Expression of Gli3 in the bladder of E14 and E15 mice.
In situ hybridization analysis of Gli3 expression in E14 (A-P) and E15 (Q-FF) bladders using antisense (A-D, I-L, Q-T, Y-BB) and sense (E-H, M-P, U-X, CC-FF) riboprobes on transverse sections of wild type (A-H, Q-X) and mgb−/− (I-P, Y-FF) mice. Sections are shown at 10X dark field (DF; A, E, I, M, Q, U, Y, CC), 10X fluorescence (FL; B, F, J, N, R, V, Z, DD), 20X dark field (DF; C, G, K, O, S, W, AA, EE) and 20X fluorescence (FL; D, H, L, P, T, X, BB, FF). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
Figure 13. Expression of Gli3 in the bladder of E16 mice.
In situ hybridization analysis of Gli3 expression in E16 (A-P) bladders using antisense (A-D, I-L) and sense (E-H, M-P) riboprobes on transverse sections of wild type (A-H) and mgb−/− (I-P) mice. Sections are shown at 10X dark field (DF; A, E, I, M), 10X fluorescence (FL; B, F, J, N), 20X dark field (DF; C, G, K, O) and 20X fluorescence (FL; D, H, L, P). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
Gli3 is expressed in the outer mesenchyme of mgb−/− bladders similar to normal bladders however; its expression appears delayed, not being detected until E14 (Fig. 11–12). By E15, Gli3 is expressed at moderate levels within the outer mesenchyme similar to the pattern observed in E15 normal bladders, although the overall expression appears more irregular than normal bladders (Fig. 12S vs. Fig. 12AA). This moderate level of Gli3 expression within the outer mesenchyme is maintained in the highly attenuated E16 mgb−/− bladder (Fig. 13).
A similar mesenchyme-specific pattern of Gli3 expression is also observed in the developing stomach, intestine, colon and rectum in both normal and mgb−/− mice (Fig. 11–13). The overall intensity and spatial localization of Gli3 expression during the development of these visceral tissues appears similar to that observed in the bladder, with faint expression in the outer mesenchyme at early development and moderate expression in the outer mesenchyme at later developmental time-points.
Myocardin is expressed in bladder outer mesenchyme in normal mice but absent in mgb−/− mice
Myocd expression is not detected in E12 normal bladders (Fig. 14). A high level of Myocd expression is detected at E13 in the outer mesenchyme of the developing bladder and continues through E16 when the outer mesenchyme differentiates into detrusor smooth muscle (Fig. 14–16). In contrast, Myocd shows minimal to no expression in the developing bladders of E12 to E16 mgb−/− mice (Fig. 14–16). A mesenchyme-specific pattern of Myocd expression is also observed in the developing stomach, intestine, colon, and rectum in both normal and mgb−/− mice (Fig. 14–16). The overall intensity and spatial pattern of Myocd expression during the development of these visceral organs appears tissue specific.
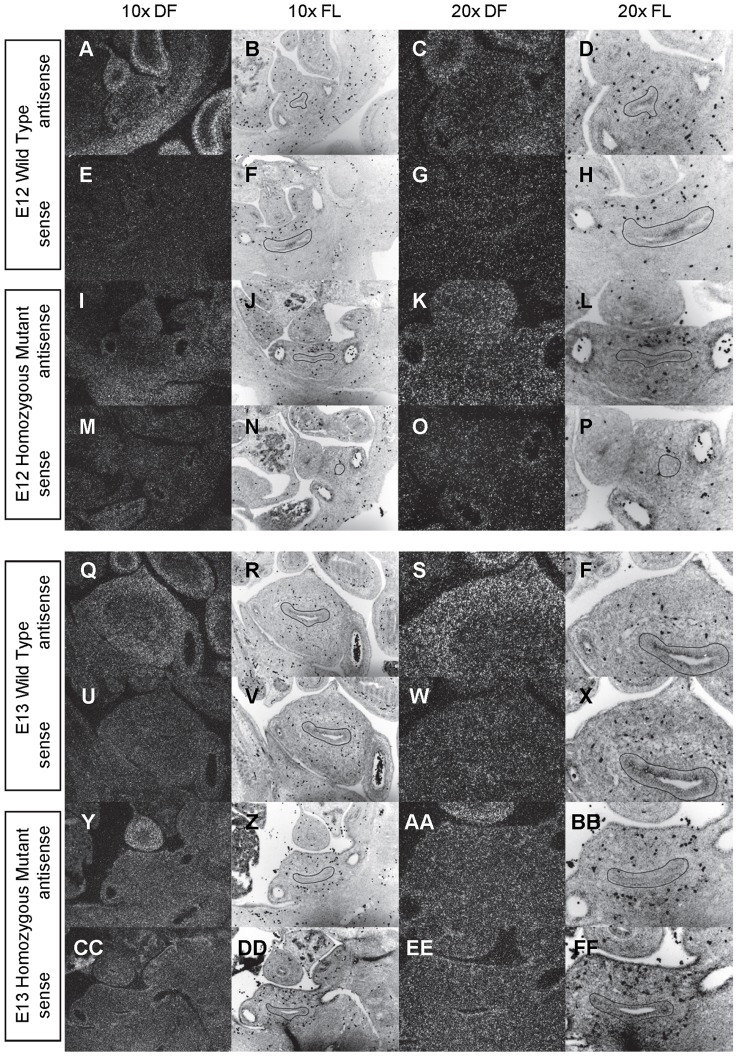
Figure 14. Expression of Myocd in the bladder of E12 and E13 mice.
In situ hybridization analysis of Myocd expression in E12 (A-P) and E13 (Q-FF) bladders using antisense (A-D, I-L, Q-T, Y-BB) and sense (E-H, M-P, U-X, CC-FF) riboprobes on transverse sections of wild type (A-H, Q-X) and mgb−/− (I-P, Y-FF) mice. Sections are shown at 10X dark field (DF; A, E, I, M, Q, U, Y, CC), 10X fluorescence (FL; B, F, J, N, R, V, Z, DD), 20X dark field (DF; C, G, K, O, S, W, AA, EE) and 20X fluorescence (FL; D, H, L, P, T, X, BB, FF). The basement membrane of the developing urothelium has been outlined in black as a point of reference in 10X and 20X FL views.
PTCH1-LACZ expression provides a functional readout of the Shh pathway and recapitulates the mRNA pattern of expression
We examined a functional readout of PTCH expression within the developing bladder by cross breeding normal and mgb−/− mice with Ptch1-LacZ/+ mice [20]. Intense X-gal staining of PTCH is observed in the inner mesenchyme immediately underlying the urothelium of E16 normal bladders, with little to no staining in the outer half of the inner mesenchyme and no staining in the detrusor smooth muscle/outer mesenchyme (Fig. 18). This pattern is identical to the Ptch mRNA expression pattern observed in E16 normal bladders using in situ hybridization (Fig. 18 vs. Fig. 7).
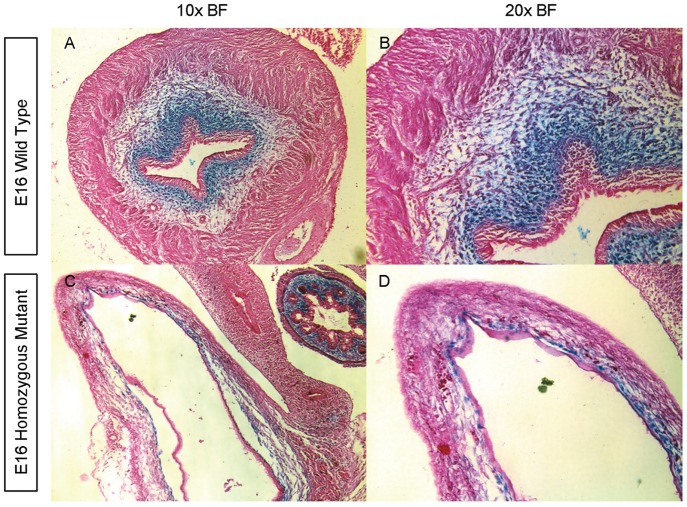
Figure 18. Ptch protein and mRNA expression co-localize in E16 bladders.
X-gal staining of PTCH-LACZ protein (blue) on transverse sections of E16 bladders in wild type (A, B) and mgb−/− (C, D) mice at 10X (A, C) and 20X (B, D) light field.
X-gal staining of PTCH is also present in the inner mesenchyme immediately underlying the urothelium in highly attenuated E16 mgb−/− bladders although the overall pattern appears more intermittent than normal bladders. Little to no X-gal staining of PTCH is observed in the remaining mesenchymal compartment of E16 mgb−/− bladders. This pattern of X-gal staining of PTCH appears identical to the Ptch mRNA expression pattern observed above in E16 mgb−/− bladders (Fig. 18 vs. Fig. 7). X-gal staining of PTCH is also observed in developing mesenchyme of the stomach, intestine, colon, and rectum in both normal and mgb−/− mice with no differences in genotype observed (Fig. 18).
Discussion
Normal bladder development requires the precise temporospatial distribution of key morphogenetic signals to generate radial patterning for the subsequent compartmentalization of distinct cellular phenotypes. Radial patterning in the developing bladder initially involves the subdivision of undifferentiated mesenchyme into an inner and outer compartment, which in turn differentiate into the lamina propria and detrusor smooth muscle, respectively (Fig. 1). Although the Shh signaling pathway has been suggested to play a key role in bladder smooth muscle differentiation [14], [16], its precise role in overall bladder patterning and organogenesis remains to be determined. Shh is a secreted protein whose canonical activity is mediated by binding to the Ptch receptor, de-repression of Smoothened (Smo) and subsequent modulation of the Gli-family of transcription factors. SHH patterns tissue development in PTCH expressing cells based upon both their position within the SHH gradient along with the differential expression and function of distinct Gli-family members.
Previous studies have examined the Shh signaling pathway in the urogenital sinus and early bladder development where it plays a key role in establishing the cellular populations needed for later bladder morphogenesis [11], [14]. Haraguchi et al. characterized the expression of Shh, Ptch and Gli1 in early urogenital development in the mouse (E10.5–13.5). These studies indicated that peri-cloacal mesenchyme is the precursor to multiple urogenital structures, and that Shh mutant mice display hyoplastic external genitalia, pelvic urethra and bladder development. Additional studies by Cheng et al. characterized the expression of Shh, Ptch, Gli1, Gli2 and Gli3 in the mouse urogenital sinus at a single developmental time point (E12.5) and examined the functional role of Shh signaling in cultured fetal mesenchymal cells. Their results indicate that Shh, Gli2 and Bmp4 differentially regulate mesenchymal cell proliferation and differentiation in vitro and in Gli2−/− mice. These studies indicate that SHH signaling is critical in early urogenital development but provide little evidence regarding the precise role that this signaling pathway plays in later bladder organogenesis. Therefore, we examine the expression patterns of Shh, Ptch, Gli1, Gli2, Gli3 and Myocd at sequential developmental time points in the developing bladder ranging from urogenital sinus formation through late bladder organogenesis. These studies were performed using both normal and mgb−/− mice, which are known to contain a previously characterized short-axis bladder mutation that results in detrusor smooth muscle agenesis [18], [21].
Shh is expressed at high levels in the endodermally derived urothelium throughout normal bladder development providing a continuous source of diffusible morphogen in a gradient that is highest immediately adjacent to the urothelium. At the earliest stages of bladder development, Ptch and Gli1 are expressed at low levels throughout the entire undifferentiated mesenchyme suggesting a straightforward readout of the radial SHH diffusion gradient within these cells. As bladder development progresses, the rapid restriction of high levels of Ptch expression by E13 to the region immediately underlying the urothelium provides a potential molecular sink that could blunt the further spread of SHH within the mesenchymal compartment. A similar pattern of expression and functional role for Ptch has been proposed in previous studies [14], [22]. As a consequence, the concentration gradient of SHH within the developing bladder would be significantly altered after E13, creating an inner mesenchymal compartment with high SHH and PTCH expression that differentiates into lamina propria consisting of loose connective tissue and an outer mesenchymal compartment with little or no SHH and PTCH expression that compacts and differentiates into detrusor smooth muscle. These observations are consistent with prior studies indicating that high levels of SHH/PTCH inhibit smooth muscle differentiation, while low levels induce smooth muscle differentiation [11], [14].
The Gli-family of transcription factors has been shown to have distinct and overlapping functions with respect to tissue patterning in development and loss of Gli expression has been shown to result in urogenital defects [11], [19], [23], [24]. A variety of studies indicate that GLI1 is a transcriptional activator, while GLI2 and GLI3 can function as both transcriptional activators and repressors [19], [23]–[26]. Our results indicate that Gli1 is expressed throughout the entire bladder mesenchyme during the early stages of development but becomes restricted to the outer mesenchyme and suburothelial inner mesenchyme by E14 (Fig. 8S vs. Fig. 9C). Interestingly, Gli2 expression in the developing bladder was not detected in our study. This observation is in contrast to Cheng et al., who reported Gli2 expression in the mesenchyme surrounding the ventral urogenital sinus at E12.5 and suggested that it played a key role in modulating mesenchymal proliferation and differentiation [11]. These differences most likely reflect variations in embryonic staging as well as the fact that their functional studies were performed in vitro using cultured fetal bladder mesenchymal cells. We hypothesize that Gli2 expression occurs in the mesenchyme surrounding the urogenital sinus leading up to E12.5, where it may play a key role in initiating Shh pathway signaling by activation of Gli1 in a manner similar to that proposed by Dennler and Bai [26], [27]. Finally, our results indicate that Gli3 expression initiates in and remains restricted to the outer mesenchyme, providing a selective expression pattern that delimits this presumptive smooth muscle region from the rest of the developing bladder as early as E13.
These expression patterns indicate that by E14 the developing bladder is subdivided into four distinct regions that including the 1) ↑ Shh-positive urothelium, 2) ↑ Ptch and ↑ Gli1-positive suburothelial inner mesenchyme, 3) non-Gli expressing inner mesenchyme and 4) ↓ Ptch, ↓ Gli1 and ↑ Gli3-positve outer mesenchyme (Fig. 19). As discussed above, the suburothelial inner mesenchyme that is expressing high levels of Ptch and Gli1 may act as a molecular sink as bladder development progresses, thereby dynamically altering the SHH radial gradient to promote further differentiation of the bladder mesenchyme. The high levels of SHH detected by the Ptch and previously reported Gli2 expressing cells of the inner mesenchyme would be predicted to support cellular proliferation and suppress smooth muscle differentiation within the presumptive lamina propria [11]. In contrast, the Gli3-positive outer mesenchymal cells that also express low levels of Ptch and Gli1 would be exposed to low levels of SHH. Li et al. states that Gli3 functions as an activator in the presence of SHH but acts as a repressor in its absence due to proteolytic cleavage of the C-terminus [23]. Acting as a repressor in the outer mesenchyme of the bladder, Gli3 may block high levels of cell proliferation thereby supporting the subsequent differentiation of detrusor smooth muscle.
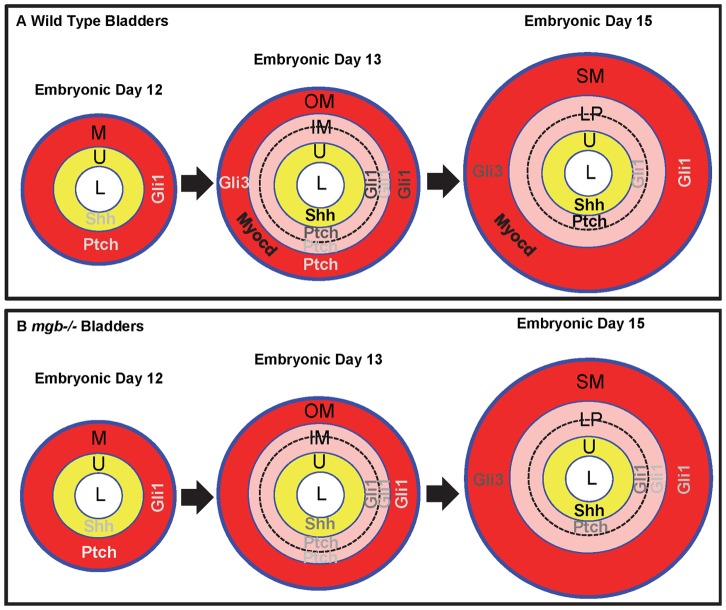
Figure 19. Temporospatial distribution of Shh, Ptch, Gli1, Gli3, and Myocd mRNA expression during murine bladder development.
Differential expression patterns of Shh, Ptch, Gli1, Gli3, and Myocd mRNA at low (light gray), moderate (medium gray) and high (black) levels in the urothelium (U; yellow), mesenchyme (M, red), inner mesenchyme (IM; pink), outer mesenchyme (OM; red), lamina propria (LP; pink) and detrusor smooth muscle (SM; red) are shown in E12, E13 and E15 normal and mgb−/− bladders. Note the temporal and spatial differences in expression in mgb−/− bladders suggestive of a developmental delay in short axis patterning that may contribute to an absence of Myocd expression and subsequent detrusor smooth muscle development.
Myocd is a SAP-family transcriptional co-factor that plays a key role in the development of both cardiac and smooth muscle [28], [29]. At least two distinct Myocd isotypes have been described including a full-length cardiac-Myocd (cMyocd) and truncated smooth muscle-Myocd (sMyocd). sMyocd interacts with serum response factor (SRF) to form a transcriptional complex that activates smooth muscle-specific gene expression [28]–[30]. High levels of sMyocd expression were observed in the outer mesenchyme of the developing bladder from E13 through E16 (Fig. 14–16). The high levels of sMyocd expression occur in concordance with low levels of Gli3 expression within the outer mesenchyme, but appear to precede the more obvious radial compartmentalization demarcated by Ptch and Gli1 expression at later developmental time points. These observations suggest that sMyocd may not be a direct downstream transcriptional target of the Shh signaling pathway but rather is expressed independently during early bladder development, thereby priming the outer mesenchyme for smooth muscle differentiation based upon subsequent radial patterning cues.
Since the mgb−/− mouse possesses a tissue-specific defect in detrusor smooth muscle development [18], [21], [31] and prior studies indicate that the Shh signaling cascade plays a key role in radial patterning and visceral smooth muscle development [10], [28], we examined the Shh signaling pathway in mgb−/− bladders. Mgb−/− mice expressed normal levels of Shh within the bladder urothelium suggesting that this key morphogen is in the right place at the right time and at correct levels necessary to direct normal radial patterning and smooth muscle development in these animals. This observation is consistent with prior studies indicating that urothelial development in mgb−/− bladders appears normal [18]. In contrast, the altered levels and pattern of Ptch expression observed within mgb−/− bladder mesenchyme could potentially have a significant effect on radial patterning in these animals. First, the more diffuse and less intense Ptch expression observed within the suburothelial inner mesenchyme of developing mgb−/− bladders would be predicted to generate a less efficient molecular sink for SHH binding. Compounding this potential change in the SHH gradient is the lack of detectable levels of Ptch expression within the outer mesenchyme of mgb−/− bladders versus normal bladders (Fig. 6C vs. Fig. 6K). In addition, mgb−/− bladders also showed a 24-hour developmental delay in the expression of both Gli1 and Gli3. Although delayed, Gli3 expression in mgb−/− bladders initiated and remained restricted to the outer mesenchyme similar to the pattern observed in normal bladders (Fig. 11S vs. Fig. 12K). In contrast, Gli1 expression in mgb−/− bladders is never selectively restricted to the suburothelial inner mesenchyme and outer mesenchyme as observed in normal bladders (Fig. 9). Finally, sMyocd is not expressed within the outer mesenchyme of mgb−/− bladders (Fig. 14–16).
These results indicate that the outer mesenchyme of mgb−/− bladders possess a series of overlapping developmental defects that include 1) potentially higher levels of SHH ligand and lower levels of PTCH receptor, 2) temporal and spatial changes in Gli1 and Gli3 expression, and 3) a complete absence of sMyocd expression (Fig. 19). Although the precise ordering and potential interplay of these defects remains to be determined, it is clear that any one of these changes would be predicted to have profound effects on both the SHH gradient as well as the downstream transcriptional readout of that gradient. The fact that the outer bladder mesenchyme is the precise morphological region that differentiates into detrusor smooth muscle suggests that the combination of these defects within this domain may contribute to the development of an amuscular bladder in mgb−/− mice.
At present, it remains unknown whether Myocd is a direct transcriptional target of the Shh signaling pathway during bladder smooth muscle development. As discussed above, the earliest demarcation of radial patterning observed in the bladder occurs around E13 when low levels of Gli3 and high levels of sMyocd expression are detected within the outer mesenchyme. If sMyocd is transcriptionally activated independent of the Shh signaling pathway at E13, the presence of Gli3 within the outer mesenchyme may provide a SHH-based repression of cellular proliferation within this same region. This decrease in cellular proliferation would be predicted in turn to down-regulate SRF expression to the low levels necessary for SRF-MYOCD complex formation and subsequent transcriptional initiation of the smooth muscle differentiation program [32], [33]. In this manner, Shh signaling pathway could be directly responsible for establishing the overall radial patterning within the bladder, while indirectly supporting the progression of detrusor smooth muscle development independent of direct transcriptional activation of Myocd expression.
It is also plausible that the independent expression of both SHH and PTCH in the E12 urogenital sinus could regulate Myocd expression through non-canonical Shh signaling prior to radial patterning. SHH signaling through PTCH has been shown to drive alterations of cell morphology independent of Gli-mediated transcription [34]. Furthermore, SHH can act independent of PTCH through a second transmembrane receptor known as Hip [35]. Alternatively, in the absence of SHH, PTCH1 has been shown to interact with phosphorylated-cyclinB1 to regulate the G2/M checkpoint, while PTCH can also function as a positive regulator of SMO to promote cell proliferation [36], [37]. Similar non-canonical Shh signaling pathways may be important during early bladder development where they help to initiate Myocd expression within the outer mesenchyme prior to overt morphological signs of radial patterning.
If Myocd is not a direct transcriptional target of either canonical or non-canonical Shh signaling, then the question remains how might it be regulated? Additional key developmental pathways have been identified as important in smooth muscle differentiation including the Wnt/β-catenin and Fgf signaling pathways [38]–[42]. Wnt2−/− null mutants exhibit a loss of Myocd expression that, in turn, results in a loss of smooth muscle cells. Wnt2 appears to activate Myocd, Fgf10 and Wnt7b, promoting smooth muscle development by increasing the expression of smooth muscle-specific genes [39]. Interestingly, Gli3 has been shown to act on Fgf10 to maintain high expression of smooth muscle-specific genes providing a possible regulatory link between the Wnt/β-catenin and Shh signaling pathways [42].
In summary, this study represents the first comprehensive analysis of the Shh signaling pathway and a potential downstream target (Myocd) during key consecutive stages of normal and abnormal bladder development. Shh, Ptch, Gli1, Gli3 and Myocd are all expressed in distinct temporospatial patterns that appear consistent with radial compartmentalization and specialization of the developing bladder. Alterations in both the temporal and spatial distribution of Ptch, Gli1, Gli3 and Myocd were discerned in developing mgb−/− bladders in a pattern consistent with the lack of detrusor smooth muscle development detected in these animals. These observations highlight the importance of the Shh signaling pathway in radial bladder development and indicate that the interplay between this key signaling pathway and Myocd expression is highly critical for normal detrusor smooth muscle development. In conclusion, the results of this study provide important insights into the molecular mechanisms controlling both normal and abnormal bladder development and a basis for future studies designed to evaluate therapeutic strategies for the prevention and treatment of urinary tract diseases and disorders.
Materials and Methods
Ethics Statement
The IACUC board of The Research Institute at Nationwide Children's Hospital approved all of the following animal studies under the Animal Welfare Assurance Number 02105AR.
Animals
Embryonic day (E) 12, 13, 14, 15, and 16 FVB/N wild type mice and mgb−/− mice, previously described, were used for in situ hybridization studies as described below [18], [43], [44]. Mice were maintained according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. E12-E16 wild type and mgb−/− mice were genotyped by real-time quantitative PCR using specific primers sets as previously reported [18]. Male and female embryos of both genotypes (wild type and mgb−/−) at each time-point (E12-E16) were analyzed for every probe evaluated in this study. A minimum of N = 3 (and up to an N = 6) of embryos were assessed for each probe. Timed-pregnancy matings and embryo harvests were performed at the same time of the day to help ensure the embryos assessed for each time-point were at very similar stages of development and accurate representations of the time-point being evaluated.
PCR and Gender-Determination
DNA was isolated using the Spin Doctor Genomic DNA Isolation Kit, according to the manufacturer's protocol (Gerard Biotech). Primers 5′-TGAAGCTTTTGGCTTTGAG-3′ and 5′-CCGCTCGCAAATTCTTTGG-3′ were used to detect the X- and Y-chromosomes by amplification of a X chromosome band and a Y chromosome band as described previously, 95°C for 5 min, (95°C for 30 s, 57°C for 30 s, and 72°C 60 s) ×30 and 72°C for 10 min (Cunningham 2002). The PCR product was confirmed by DNA gel electrophoresis.
In Situ Hybridization
Embryos from timed matings were removed from wild type and homozygous mgb−/− mice and fixed in 4% paraformaldehyde, then transferred to 70% ethanol. Specimens were processed in the Biomorphology Core using standard procedures (TRINCH). Serial paraffin-embedded transverse sections (10 uM) of the bladder were affixed to slides (2–4 sections/slide). In situ hybridization using 35S-UTP radiolabeled riboprobes was performed on the paraffin-embedded sections from E12-E16 normal and mutant embryos as described previously [45]. Briefly, slides carrying the sections were prehybridized with hybridization solution for 90 minutes while riboprobes were labeled with 35S-UTP. The riboprobes were purified with a NucAway kit (Amersham) and the slides were hybridized with 70,000 DPM counts of riboprobe in hybridization solution. Slides were incubated O/N at 50oC in a hybridization oven. Following incubation, the slides were washed in high stringency washes (FSM, STE x2, STE + yeast-tRNA and RNase, STE + BME, FSM x2, 2x SSC, 0.1x SSC) and dehydrated in increasing strength ethanol (30%, 50%, 70%, 85%, 95%, 100%). Slides were air-dried, placed on film, and exposed for 3 days. Following exposure, slides were emulsion coated (Kodak), incubated at 4oC for 10–14 days, and developed (Developer, water, Fixer, water) (Kodak). Lastly, slides were cover slipped with permaslip and observed under dark field and fluorescence. Probes used included:
Shh (courtesy of Herman lab, TRINCH).
Ptch (courtesy of Herman lab, TRINCH).
Gli1 (courtesy of Joyner lab [19]).
Gli2 (courtesy of Joyner lab [19], Openlab BioSystems (EMM1002-2414207)).
Gli3 (courtesy of A. Joyner [19]).
Myocd (McHugh lab) (ttcctgtgcacactgctgtaaagtccaagtctttgggtgacagtaagaaccgccacaaaaagcccaaagaccccaaaccaaaggtgaagaagctcaaataccatcagtacatccccccagaccagaaggcagagaagtctcccccacccatggactctgcctatgcccggctgctccagcaacagcagctattcctgcagctacagatcctcagccagcagcagcaacagcagcagcaacagcagcagcagcaacagcagcagcagcagcagcggttcagctaccctgggatgcaccaaacacacctcaaagaaccaaatgaacagatggccagaaatccgaatccttcttcaacaccactgagcaatacccctctatcccctgtcaaaaatagcatttctggacaaactggtgtttcttctctcaaaccaggccccctcccacccaacctggatgatctcaaggtgtcagagttaagacaacagcttcgaatccggggcttgccagtgtcaggcaccaagacagcgctggtggaccggcttcgtcccttccaggattgtgctggcaaccctgtgcccaactttggggacatcacaactgtcacctttcctgtcacgcccaacaccttgcccagttatcagtcctccccgacaggcttctaccactttggcagcacaagcttcagc).
β-tubulin (McHugh lab) was used as a positive control. (aggagtgtgagcattgcgactgtcttcagggcttccagctcacccactcgctgggcggtggcacgggctcaggcatgggcacactgctcatcagcaagatccgagaggagtacccggaccgcatcatgaacaccttcagcgtcatgccgtcacccaaggtctcagacaccgtggtggagccctacaacgccacattgtcagtgcaccagctggtagagaacaccgacgagacctactgcatcgacaacgaggccctctatgacatctgcttccgcacgctcaagctgaccacacccacttacggggacctcaaccactt).
The primers utilized for both of these novel probe sequences are underlined and derived from published exon sequences.
X-gal Staining
Timed matings were performed with Ptch-lacZ/+; mgb−/+ dames crossed to Ptch-lacZ/+; mgb−/− males (Ptch-lacZ/+ mice courtesy of Herman lab) [20]. At embryonic day (E) 16, embryos were harvested and fixed according to established procedure [46]. Briefly, embryos were fixed in a solution of 0.2% gluteraldehyde, 2% formalin, 5 mM EGTA, 2 mM MgCl2, 100 mM potassium phosphate buffer, pH 7.3, for one hour on a horizontal shaker. Following fixation, embryos were rinsed three times for 30 minutes each on a horizontal shaker in a solution of 0.1% sodium deoxycholate, 0.2% NP40, 2 mM MgCl2, 100 mM potassium phosphate buffer, pH 7.3. Embryos were stained for 60 hours in a solution of 1 mg/mL X-gal, 5 mM potassium ferricyanide, and 5 mM potassium ferrocyanide in the rinse solution on a horizontal shaker. Following staining, embryos were briefly rinsed in 1x PBS and transferred to 70% ethanol. Specimens were processed in the Biomorphology Core using standard procedures (TRINCH). Serial paraffin-embedded transverse sections (4 uM) of the bladder were affixed to slides (4–8 sections/slide). Lastly, slides were deparaffinized, counter stained with eosin, and cover slipped with permaslip. Slides were observed under light field.
Microscopy
We performed light field, dark field, and fluorescent microscopy using a Zeiss Imaging Microscope equipped with 10x and 20× objectives and a digital camera. We imaged transverse sections of bladder, stomach, intestine, colon, and rectum from normal (wild type) and mutant (mgb−/−) mice. For in situ hybridization analysis, we scored a minimum of three embryos per stage, from E12-E16, for the expression of silver grains. For PTCH-LACZ analysis, we scored E16 embryos for X-gal staining. Identical camera settings were used for dark field and fluorescent images of each stage embryo. We collected the images using OpenLab Software and edited the images with Adobe Photoshop (equal adjustment of Sharpness, Contrast, Shadow, and Brightness; and fluorescent images were inverted).
Acknowledgments
We thank Dr. Gail Herman and Dr. Alexandra L. Joyner for generously sharing resources developed in their labs, the Biomorphology Core at Nationwide Children's Hospital for technical assistance, and Dr. David Cunningham for technical assistance in performing X-gal staining.
Funding Statement
The study was supported by the following: NIH/NIDDK, grant number: R01 DK085242; and NIH/NIDDK, grant number: F32 DK089733; www2.niddk.nih.gov/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cheng W, Jacobs WB, Zhang JJ, Moro A, Park JH, et al. (2006) DeltaNp63 plays an anti-apoptotic role in ventral bladder development. Development (Cambridge, England) 133: 4783–4792. [DOI] [PubMed] [Google Scholar]
- 2. Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P (2003) Mouse urogenital development: A practical approach. Differentiation; Research in Biological Diversity 71: 402–413. [DOI] [PubMed] [Google Scholar]
- 3. Baskin LS, Hayward SW, Young P, Cunha GR (1996) Role of mesenchymal-epithelial interactions in normal bladder development. The Journal of Urology 156: 1820–1827. [PubMed] [Google Scholar]
- 4. Price KL, Woolf AS, Long DA (2009) Unraveling the genetic landscape of bladder development in mice. The Journal of Urology 181: 2366–2374. [DOI] [PubMed] [Google Scholar]
- 5. Baskin LS, Hayward SW, Sutherland RA, DiSandro MJ, Thomson AA, et al. (1996) Mesenchymal-epithelial interactions in the bladder. World Journal of Urology 14: 301–309. [DOI] [PubMed] [Google Scholar]
- 6. Cunha GR (2008) Mesenchymal-epithelial interactions: Past, present, and future. Differentiation; Research in Biological Diversity 76: 578–586. [DOI] [PubMed] [Google Scholar]
- 7.DiSandro MJ, Li Y, Baskin LS, Hayward S, Cunha G (1998) Mesenchymal-epithelial interactions in bladder smooth muscle development: Epithelial specificity. The Journal of Urology 160: 1040–6; discussion 1079. [DOI] [PubMed]
- 8. Liu W, Li Y, Cunha S, Hayward G, Baskin L (2000) Diffusable growth factors induce bladder smooth muscle differentiation. In Vitro Cellular & Developmental Biology. Animal 36: 476–484. [DOI] [PubMed] [Google Scholar]
- 9.Cao M, Liu B, Cunha G, Baskin L (2008) Urothelium patterns bladder smooth muscle location. Pediatric Research. [DOI] [PMC free article] [PubMed]
- 10. Tasian G, Cunha G, Baskin L (2010) Smooth muscle differentiation and patterning in the urinary bladder. Differentiation; Research in Biological Diversity 80: 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng W, Yeung CK, Ng YK, Zhang JR, Hui CC, et al. (2008) Sonic hedgehog mediator Gli2 regulates bladder mesenchymal patterning. The Journal of Urology 180: 1543–1550. [DOI] [PubMed] [Google Scholar]
- 12. Jenkins D, Winyard PJ, Woolf AS (2007) Immunohistochemical analysis of sonic hedgehog signalling in normal human urinary tract development. Journal of Anatomy 211: 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nozaki Y, Kusuhara H, Kondo T, Hasegawa M, Shiroyanagi Y, et al. (2007) Characterization of the uptake of organic anion transporter (OAT) 1 and OAT3 substrates by human kidney slices. The Journal of Pharmacology and Experimental Therapeutics 321: 362–369. [DOI] [PubMed] [Google Scholar]
- 14. Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, et al. (2007) Molecular analysis of coordinated bladder and urogenital organ formation by hedgehog signaling. Development (Cambridge, England) 134: 525 533. [DOI] [PubMed] [Google Scholar]
- 15. Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun TT, et al. (2007) Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development (Cambridge, England) 134: 1967–1975. [DOI] [PubMed] [Google Scholar]
- 16. Cao M, Tasian G, Wang MH, Liu B, Cunha G, et al. (2010) Urothelium-derived sonic hedgehog promotes mesenchymal proliferation and induces bladder smooth muscle differentiation. Differentiation; Research in Biological Diversity 79: 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jenkins D, Winyard PJ, Woolf AS (2007) Immunohistochemical analysis of sonic hedgehog signalling in normal human urinary tract development. Journal of Anatomy 211: 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh S, Robinson M, Nahi F, Coley B, Robinson ML, et al. (2007) Identification of a unique transgenic mouse line that develops megabladder, obstructive uropathy, and renal dysfunction. Journal of the American Society of Nephrology: JASN 18: 461–471. [DOI] [PubMed] [Google Scholar]
- 19. Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL (1994) Expression of three mouse homologs of the drosophila segment polarity gene cubitus interruptus, gli, gli-2, and gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Developmental Biology 162: 402–413. [DOI] [PubMed] [Google Scholar]
- 20. Jiang F, Herman GE (2006) Analysis of nsdhl-deficient embryos reveals a role for hedgehog signaling in early placental development. Human Molecular Genetics 15: 3293–3305. [DOI] [PubMed] [Google Scholar]
- 21. Singh S, Robinson M, Ismail I, Saha M, Auer H, et al. (2008) Transcriptional profiling of the megabladder mouse: A unique model of bladder dysmorphogenesis. Developmental Dynamics: An Official Publication of the American Association of Anatomists 237: 170–186. [DOI] [PubMed] [Google Scholar]
- 22. Chen Y, Stroh G (1996) Dual roles for patched in sequestering and transducing hedgehog. Cell 87: 553–563. [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Zhang H, Choi SC, Litingtung Y, Chiang C (2004) Sonic hedgehog signaling regulates Gli3 processing, mesenchymal proliferation, and differentiation during mouse lung organogenesis. Developmental Biology 270: 214–231. [DOI] [PubMed] [Google Scholar]
- 24. Liu G, Moro A, Zhang JJ, Cheng W, Qiu W, et al. (2007) The role of shh transcription activator Gli2 in chick cloacal development. Developmental Biology 303: 448–460. [DOI] [PubMed] [Google Scholar]
- 25. Rahnama F, Shimokawa T, Lauth M, Finta C, Kogerman P, et al. (2006) Inhibition of GLI1 gene activation by Patched1. The Biochemical Journal 394: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL (2002) Gli2, but not Gli1, is required for initial shh signaling and ectopic activation of the shh pathway. Development (Cambridge, England) 129: 4753–4761. [DOI] [PubMed] [Google Scholar]
- 27. Dennler S, Andre J, Alexaki I, Li A, Magnaldo T, et al. (2007) Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Research 67: 6981–6986. [DOI] [PubMed] [Google Scholar]
- 28. Caubit X, Lye CM, Martin E, Core N, Long DA, et al. (2008) Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development (Cambridge, England) 135: 3301–3310. [DOI] [PubMed] [Google Scholar]
- 29. Phiel CJ, Gabbeta V, Parsons LM, Rothblat D, Harvey RP, et al. (2001) Differential binding of an SRF/NK-2/MEF2 transcription factor complex in normal versus neoplastic smooth muscle tissues. The Journal of Biological Chemistry 276: 34637–34650. [DOI] [PubMed] [Google Scholar]
- 30. Brittingham J, Phiel C, Trzyna WC, Gabbeta V, McHugh KM (1998) Identification of distinct molecular phenotypes in cultured gastrointestinal smooth muscle cells. Gastroenterology 115: 605–617. [DOI] [PubMed] [Google Scholar]
- 31.Saha M, Ingraham SE, Carpenter A, Robinson M, McHugh KE, et al.. (2009) Myocardin splice variants in bladder. In Press. [DOI] [PubMed]
- 32. Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, et al. (2001) Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105: 851–862. [DOI] [PubMed] [Google Scholar]
- 33. Iyer D, Chang D, Marx J, Wei L, Olson EN, et al. (2006) Serum response factor MADS box serine-162 phosphorylation switches proliferation and myogenic gene programs. Proceedings of the National Academy of Sciences of the United States of America 103: 4516–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bijlsma MF, Borensztajn KS, Roelink H, Peppelenbosch MP, Spek CA (2007) Sonic hedgehog induces transcription-independent cytoskeletal rearrangement and migration regulated by arachidonate metabolites. Cellular Signalling 19: 2596–2604. [DOI] [PubMed] [Google Scholar]
- 35. Bourikas D, Pekarik V, Baeriswyl T, Grunditz A, Sadhu R, et al. (2005) Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nature Neuroscience 8: 297–304. [DOI] [PubMed] [Google Scholar]
- 36. Barnes EA, Kong M, Ollendorff V, Donoghue DJ (2001) Patched1 interacts with cyclin B1 to regulate cell cycle progression. The EMBO Journal 20: 2214–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shyamala BV, Bhat KM (2002) A positive role for patched-smoothened signaling in promoting cell proliferation during normal head development in drosophila. Development (Cambridge, England) 129: 1839–1847. [DOI] [PubMed] [Google Scholar]
- 38. Jenkins D (2009) Hedgehog signalling: Emerging evidence for non-canonical pathways. Cellular Signalling 21: 1023–1034. [DOI] [PubMed] [Google Scholar]
- 39. Goss AM, Tian Y, Cheng L, Yang J, Zhou D, et al. (2011) Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf-B and Fgf10 expression. Developmental Biology 356: 541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morrisey EE, Hogan BL (2010) Preparing for the first breath: Genetic and cellular mechanisms in lung development. Developmental Cell 18: 8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Warburton D, Bellusci S, De Langhe S, Del Moral PM, Fleury V, et al. (2005) Molecular mechanisms of early lung specification and branching morphogenesis. Pediatric Research 57: 26R–37R. [DOI] [PubMed] [Google Scholar]
- 42. Veltmaat JM, Relaix F, Le LT, Kratochwil K, Sala FG, et al. (2006) Gli3-mediated somitic Fgf10 expression gradients are required for the induction and patterning of mammary epithelium along the embryonic axes. Development (Cambridge, England) 133: 2325–2335. [DOI] [PubMed] [Google Scholar]
- 43. Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, et al. (1995) Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development (Cambridge, England) 121: 505–514. [DOI] [PubMed] [Google Scholar]
- 44. Zhao H, Yang Y, Rizo CM, Overbeek PA, Robinson ML (2004) Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Investigative Ophthalmology & Visual Science 45: 1930–1939. [DOI] [PubMed] [Google Scholar]
- 45. Poladia DP, Kish K, Kutay B, Hains D, Kegg H, et al. (2006) Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Developmental Biology 291: 325–339. [DOI] [PubMed] [Google Scholar]
- 46. Yamagata T, Urano H, Weeber EJ, Nelson DL, Nishijima I (2008) Impaired hippocampal synaptic function in secretin deficient mice. Neuroscience 154: 1417–1422. [DOI] [PubMed] [Google Scholar]