Abstract
The antibacterial triclocarban (TCC) concentrates in the cellular fraction of blood. Consequently, plasma levels are at least two-fold lower than the TCC amount present in blood. Utilizing whole blood sampling, a low but significant absorption of TCC from soap during showering is demonstrated for a small group of human subjects.
Keywords: Antibacterial, Triclocarban, Biomonitoring, Whole blood
1. Introduction
Triclocarban (TCC) is commonly used as an antimicrobial in bar soaps. Recently we showed, that TCC is a potent inhibitor (IC50 24 ± 5 nM) of the human soluble epoxide hydrolase, an enzyme of the arachidonic cascade (Liu et al., 2011; Schebb et al., 2011b,c). Similarly potent inhibitors have pronounced pharmacological effects on regulation of inflammation and pain (Inceoglu et al., 2011). Moreover, TCC may act as an endocrine disruptor at high dose by enhancing the action of steroids (Chen et al., 2008). In the aquatic environment, TCC accumulates in sludge and shows a pronounced bio-concentration in algae, snail and fish (Schebb et al., 2011a). Particularly the combination of off-target activities on mammals and accumulation of TCC in the biota, moved the question of human exposure to TCC into the focus of public interest.
Studies on human subjects, unveiled that a low but significant amount of TCC is absorbed during showering with TCC-containing antibacterial soap (Scharpf et al., 1975; Schebb et al., 2011c). Based on the urinary excretion of TCC metabolites it was calculated that 0.6% of the applied TCC, corresponding to ~70 μg, were absorbed during a single shower (Schebb et al., 2011c). However, previous studies from the 1980's failed to detect TCC in human blood and plasma after exposure by showering or bathing (limit of detection ranging from 30 to 80 nM) (Scharpf et al., 1975; Howes and Black, 1976; Taulli et al., 1977). Recent studies utilizing liquid chromatography tandem mass spectrometry (LC–MS/MS) with a limit of detection of 0.3 nM indicated very low serum levels of adults in the US with a mean concentration of 1.42 nM compared to more than 10 fold higher urine TCC-metabolite levels (Ye et al., 2011). This significant urinary excretion of TCC indicates that absorbed TCC must be systemically available and thus be present in blood. This compelled us to hypothesize that the lack of detection in plasma and serum does not represent the blood concentration of TCC. In order to test this hypothesis, we investigated the distribution of TCC among whole blood, plasma and the cellular fraction in vitro and in vivo. Finally whole blood sampling was successfully utilized to assess the systemic TCC levels in exposed humans.
1.1. In vitro experiments
Blood from male Swiss Webster mice (Charles River, Boston, MI) was collected by cardiac puncture in EDTA-containing tubes. The fresh blood from four animals was pooled, and three aliquots were spiked with 100 nM TCC (mixing 5 μL of a 10 μM TCC solution in 50/50 ACN/water with 495 μL of blood) and incubated at 37 °C. After 10 and 30 min two samples were taken from the incubation mixture. One 200 μL aliquot was immediately separated into plasma and a cellular fraction by centrifugation at 800g at 4 °C for 10 min. Whole blood was sampled by mixing the blood 1:5 (20 μL blood to 100 μL water) with deionized water, to obtain a clear solution as described previously (Schebb et al., 2010). The whole blood/water mixture and cellular fraction samples were subjected to liquid/liquid extraction with ethyl acetate followed by LC–MS (Liu et al., 2011). Plasma samples were analyzed for their TCC level directly by online-SPE–LC–MS (Schebb et al., 2011a,c). Each incubation was carried out in three independent replicates.
1.2. In vivo experiments
TCC blood distribution was investigated in vivo in two male mice (34.3 ± 0.7 g) and three male Sprague Dawley rats (475 ± 40 g). TCC was administered by oral gavage of a 1 mg/mL TCC solution in 80/20 oleic oil/PEG400 at dose of 1–5mg kg−1 bodyweight. Blood was sampled and plasma and whole blood levels were measured as described above after 4 h by cardiac puncture for the mice and by tail vein puncture after 4 h, 24 h, and 48 h for rats.
1.3. Bio-monitoring of TCC
Whole blood samples were obtained from human subjects by finger-prick as described earlier (Schebb et al., 2010). Samples were collected during the same human exposure study previously published (Schebb et al., 2011c). In brief, the subjects were exposed by showering for 15 min with 0.6% TCC containing soap while covering hands with plastic gloves. Blood was collected before (t = 0) and 0.5–48 h after exposure. This study was reviewed and approved by the UC Davis Institutional Review Board and informed consent was obtained from the subjects prior to the study. All studies involving laboratory animals were approved by the UC Davis Institutional Animal Care and Use Committee.
2. Results and Discussion
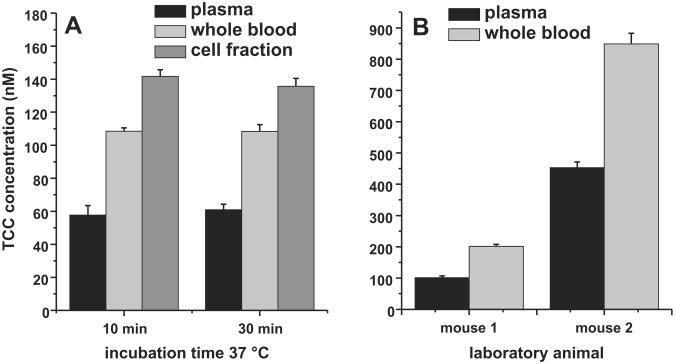
The analysis of spiked mice blood confirmed the hypothesis that TCC concentrates in the cellular fraction of blood. After 10 min of incubation, the TCC plasma concentration was only 57.6 ± 5.8 nM of the 100 nM TCC spiked to the blood (Fig. 1). Accordingly the cellular fraction contained a higher TCC concentration of 141.8 nM ± 3.9 nM. Taking a hematocrit of murine blood of 0.4– 0.6 into account (Hedrich, 2004), TCC mass balance was very good with a calculated whole blood concentration of 99 ± 8 nM. Comparing the measured whole blood concentrations, a factor of 1.9 ± 0.2 between whole blood concentration and plasma resulted, which remained stable over the incubation time (Fig 1). The same distribution was found in vivo in mice after oral gavage of TCC, with a blood/plasma concentration ratio of 1.9 ± 0.2, though the observed TCC whole blood levels between mice varied 202 ± 7 nM and 792 ± 34 nM (Fig. 1B). By contrast, the investigation of rat blood led to a varying blood/plasma ratio between 2 and 15, trending toward a higher factor with lower TCC concentrations. This indicated that binding of TCC to the cellular fraction in blood is concentration and species dependent, which warrants further investigation. This preliminary study clearly demonstrated that the whole blood concentration of TCC better reflect the total amount of TCC present in blood than the plasma concentration, which is at least 2-fold lower.
Fig. 1.
Distribution of TCC in mice blood. In vitro A: Fresh blood from Swiss Webster mice was spiked with 100 nM TCC and incubated at 37 °C. In vivo B: The TCC blood and plasma levels 4 h after oral gavage. Concentrations are presented as mean and SD of analyses in triplicate.
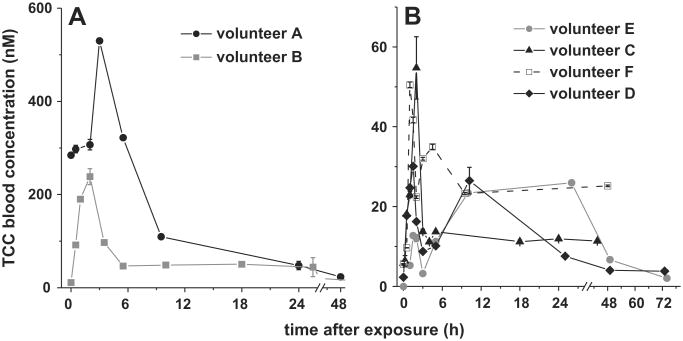
Whole blood analysis was therefore utilized for the bio-monitoring of TCC after exposing human subjects by a single shower with antibacterial soap. As shown in Fig. 2, this analysis demonstrated the detection of TCC in whole blood after human exposure with TCC-containing personal care products. In accordance to the previously reported urine concentration of TCC-N-glucuronides(Schebb et al., 2011c), Volunteer A and B showed the highest TCC concentration with a Cmax of 530 nM and 240 nM TCC, respectively detected 2–3 h after exposure (Fig. 2A). A surprisingly high level 285 ± 5 nM TCC was observed in the blood of volunteer A at baseline. This volunteer regularly used TCC-containing soap. This indicates that frequent users of these personal care products may have a significant body burden of TCC.
Fig. 2.
TCC whole blood concentrations of human subjects after showering with TCC containing soap: (A) TCC levels in the blood of volunteer A (a regular TCC-soap user) and volunteer B. (B) The TCC blood concentration of volunteers C–F.
Similarly as described for the urinary excretion (Schebb et al., 2011c), strong inter-individual differences were observed in blood levels of the subjects (Fig. 2). Despite a standardized exposure protocol, the blood levels of volunteer C–F were about 10-fold lower than those of volunteer A–B. Moreover, the observed pharmacokinetics varied widely among the six volunteers. Because of these inter-individual variations, human exposure to TCC by the use of antibacterial soaps cannot be broadly extrapolated to the general population from such a small group of volunteers. Nevertheless, a single shower with an antibacterial soap led to a detectable TCC whole blood concentration in all exposed volunteers (Cmax between 23 and 530 nM, Fig. 2). This clearly demonstrates that measuring the systemic TCC levels is feasible by analyzing whole blood as sample matrix. Several convenient techniques are available for sample preparation of whole blood, such as dried-blood-spot analysis (Li and Tse, 2010), or the mixing with an excess of water followed by liquid/liquid or solid phase extraction (Schebb et al., 2010). These techniques shall be used in combination with sensitive analysis by LC–MS (Sapkota et al., 2007; Liu et al., 2011; Schebb et al., 2011c) for further studies of human TCC exposure. The possible off-target effects of TCC on mammals merit a comprehensive bio-monitoring of TCC in the population. With preliminary results about human TCC exposure by soaps, we demonstrate that whole blood is the sample matrix of choice for these investigations.
Acknowledgments
This study was supported by NIEHS (R01 ES002710, P42 ES004699), NIOSH (PHS OH07550) and the German Academic Exchange Service. B.D.H is a senior fellow of the American Asthma Society.
References
- Chen J, Ahn KC, Gee NA, Ahmed MI, Duleba AJ, Zhao L, Gee SJ, Hammock BD, Lasley BL. Triclocarban enhances testosterone action: a new type of endocrine disruptor? Endocrinology. 2008;149:1173–1179. doi: 10.1210/en.2007-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich HJ. The Laboratory Mouse. Elsevier Academic Press; Amsterdam: 2004. [Google Scholar]
- Howes D, Black JG. Percutaneous absorption of triclocarban in rat and man. Toxicology. 1976;6:67–76. doi: 10.1016/0300-483x(76)90008-1. [DOI] [PubMed] [Google Scholar]
- Inceoglu B, Wagner K, Schebb NH, Morisseau C, Jinks SL, Ulu A, Hegedus C, Rose T, Brosnan R, Hammock BD. Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proc Natl Acad Sci USA. 2011;108:5093–5097. doi: 10.1073/pnas.1101073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Tse FL. Dried blood spot sampling in combination with LC–MS/MS for quantitative analysis of small molecules. Biomed Chromatogr. 2010;24:49–65. doi: 10.1002/bmc.1367. [DOI] [PubMed] [Google Scholar]
- Liu JY, Qiu H, Morisseau C, Hwang SH, Tsai HJ, Ulu A, Chiamvimonvat N, Hammock BD. Inhibition of soluble epoxide hydrolase contributes to the anti-inflammatory effect of antimicrobial triclocarban in a murine model. Toxicol Appl Pharmacol. 2011;255:200–206. doi: 10.1016/j.taap.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota A, Heidler J, Halden RU. Detection of triclocarban and two co-contaminating chlorocarbanilides in US aquatic environments using isotope dilution liquid chromatography tandem mass spectrometry. Environ Res. 2007;103:21–29. doi: 10.1016/j.envres.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Scharpf LG, Jr, Hill ID, Maibach HI. Percutaneous penetration and disposition of triclocarban in man: body showering. Arch Environ Health. 1975;30:7–14. doi: 10.1080/00039896.1975.10666624. [DOI] [PubMed] [Google Scholar]
- Schebb NH, Flores I, Kurobe T, Franze B, Ranganathan A, Hammock BD, Teh S. Bioconcentration, metabolism and excretion of triclocarban in larval Qurt medaka (Oryzias latipes) Aquat Toxicol. 2011a;105:448–454. doi: 10.1016/j.aquatox.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schebb NH, Huby M, Morisseau C, Hwang SH, Hammock BD. Development of an online SPE–LC–MS-based assay using endogenous substrate for investigation of soluble epoxide hydrolase (sEH) inhibitors. Anal Bioanal Chem. 2011b;400:1359–1366. doi: 10.1007/s00216-011-4861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schebb NH, Inceoglu B, Morisseau C, Ahn KC, Gee SJ, Hammock BD. Investigation of human exposure to triclocarban after showering, and preliminary evaluation of its biological effects. Environ Sci Technol. 2011c;45:3109–3115. doi: 10.1021/es103650m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schebb NH, Inceoglu B, Rose T, Wagner K, Hammock BD. Development of an ultra fast online-solid phase extraction (SPE) liquid chromatography electrospray tandem mass spectrometry (LC–ESI–MS/MS) based approach for the determination of drugs in pharmacokinetic studies. Anal Methods. 2010;3:420–428. doi: 10.1039/C0AY00714E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulli TA, Hill JT, Pounds GW. High-pressure liquid chromatographic studies of TCC and metabolites in experimental animals and man. J Chromatogr Sci. 1977;15:111–118. doi: 10.1093/chromsci/15.3-4.111. [DOI] [PubMed] [Google Scholar]
- Ye X, Zhou X, Furr J, Ahn KC, Hammock BD, Gray EL, Calafat AM. Biomarkers of exposure to triclocarban in urine and serum. Toxicology. 2011;286:69–74. doi: 10.1016/j.tox.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]