Abstract
Measuring response to chemotherapy is a backbone of the clinical management of patients with acute leukemia. This task has historically relied on the ability to identify leukemic cells among normal bone marrow cells by their morphology. However, more accurate ways to identify leukemic cells have been developed, which allow their detection even when they are present in small numbers that would be impossible to be recognized by microscopic inspection. The levels of such minimal residual disease (MRD) are now widely used as parameters for risk assignment in acute lymphoblastic leukemia (ALL) and increasingly so in acute myeloid leukemia (AML). However, different MRD monitoring methods may produce discrepant results. Moreover, results of morphologic examination may be in stark contradiction to MRD measurements, thus creating confusion and complicating treatment decisions. This review focusses on the relation between results of different approaches to measure response to treatment and define relapse in childhood acute leukemia.
Keywords: Acute lymphoblastic leukemia, Acute myeloid leukemia, Flow cytometry, Polymerase chain reaction, Minimal residual disease, Remission
INTRODUCTION
In patients with acute leukemia, treatment decisions are based on the status of peripheral blood and bone marrow cellularity. This provides a measure of the efficacy of therapy and can reveal leukemia relapse. The reliability of morphologic examination of peripheral blood and bone marrow largely depends on the hematologist's expertise, and its sensitivity is fundamentally limited by the similarities in appearance between leukemic cells and normal lympho-hematopoietic progenitors. Therefore, patients in complete morphologic remission may still have a large number of residual leukemic cells (potentially up to 1010) [1].
The introduction of methods for minimal residual disease (MRD) detection has revolutionized monitoring of treatment response in acute leukemia. These methods can not only recognize leukemic cells by objective criteria, thus potentially improving the reliability of blood and marrow examination, but they also allow the detection of leukemic cells well past the resolution of microscopic examination. The concept that patients with leukemia in morphologic remission could have measurable levels of MRD was first demonstrated in the early 80s [2]. Since then, much data has been collected supporting this notion in both acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) [3-6].
During its initial phases of development, concerns were raised as to whether MRD monitoring could be clinically useful. The evidence accumulated in subsequent studies, however, overwhelmingly dispelled these concerns. The prognostic significance of MRD in childhood ALL was demonstrated in many studies involving newly diagnosed patients, patients with first-relapse ALL, and those undergoing hematopoietic stem cell transplant [7-32]. There is also strong evidence pointing to the clinical significance of MRD in adult ALL [33-38]. Evidence has also accumulated in AML, with several studies reporting significant associations between MRD and relapse [31, 39-53].
With the increasing availability of MRD assays, clinicians may find themselves in the awkward situation in which MRD results contradict morphologic findings, or two different MRD assays produce discordant results. This creates uncertainty regarding the best treatment approach to offer, and can be a source of considerable anxiety for patients and parents.
1. Measurements of treatment response in ALL
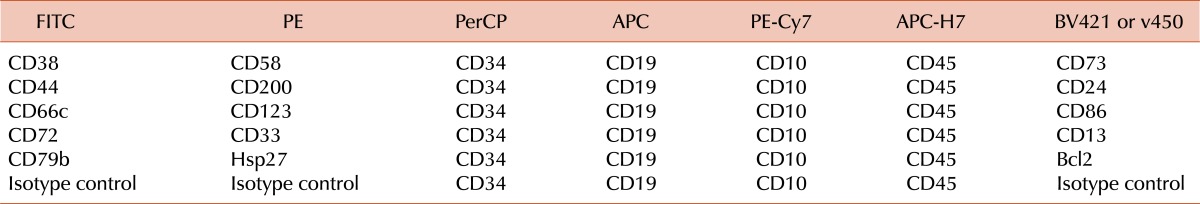
The proven theory behind all MRD assays is that leukemic cells express molecular features that are not expressed by normal lympho-hematopoietic cells. One of the distinctive features of ALL cells is the clonal rearrangement of the genes encoding immunoglobulin (IG) and T-cell receptor (TCR) proteins [4]. The standard process for using these rearrangements for MRD monitoring is to identify them in each patient at diagnosis, determine their unique sequence, synthesize specific primers for a polymerase chain reaction (PCR), optimize the PCR assay, and then apply the assay to follow-up samples [4]. Approximately 90% of childhood ALL cases will have suitable rearrangements for MRD monitoring [54]. We found that 475 of 539 (88.1%) cases of newly diagnosed B-lineage ALL had rearrangements sufficiently diverse for monitoring of MRD with a sensitivity of at least 0.001% [21]. Although the test is accurate and sensitive (it allows the routine detection of one leukemic cell in 10,000 to 100,000 normal cells), the complexity of its set-up typically prevents its application during the very early phases of therapy (e.g., day 8, day 15). Leukemic lymphoblasts can also be recognized by the presence of chromosomal abnormalities and their resulting gene fusions and transcripts, such as BCR-ABL1, MLL-AFF1, TCF3-PBX1, and ETV6-RUNX1 [4]. The most recurrent abnormalities are found in about one-third or less of patients and allow the detection of one leukemic cell in 1,000 to 100,000 normal bone marrow cells by PCR [4]. Finally, ALL cells can be recognized by virtue of unique cell markers combinations visualized with monoclonal antibodies and flow cytometry [55]. Current instruments allow the detection of 6 or more markers providing a comprehensive description of the leukemic cell phenotype which facilitates their identification (Table 1). Virtually every case of ALL expresses several abnormal cell marker profiles, affording a sensitivity of detection of 1 leukemic cell in 10,000 normal cells [55]. In the St Jude Total XV study, MRD could be monitored by flow cytometry with a 0.01% sensitivity in 482 of 492 patients (98%) [56].
Table 1.
Antibody and fluorochrome combinations currently used in our laboratory for MRD monitoring in B-lineage ALL by flow cytometry.a)
a)Using the markers listed in this table, a leukemia-associated signature can be identified in virtually all cases of B-lineage ALL at diagnosis. For the few remaining cases, additional markers that can be tested include CD133, CD15, anti-NG2, CD164, CD304, CD97, CD102, CD99, and CD300a [80].
Abbreviations: FITC, Fluorescein Isothiocyanate; PE, R-Phycoerythrin; PerCP, Peridinin Chlorophyll Protein; APC, Allophycocyanin; PE-Cy7, Phycoerythrin-Cyanine 7; APC-H7, Allophycocyanin-Cyanine 7 analog; BV421, Brilliant Violet 421; v450, BD Horizon v450.
MRD assays can identify leukemic cells in many samples where these cannot be detected by morphology. For example, in a study performed with 248 bone marrow samples collected after 2 weeks of remission induction therapy from children with newly diagnosed ALL, we found that only 32 (12.9%) had leukemic lymphoblasts identifiable by morphologic analysis and all of these had at least 0.01% cells expressing leukemia-specific immunophenotypes [12]. However, among the 216 samples without leukemic lymphoblasts recognizable by their morphologic features, 102 (47.2%) had leukemic lymphoblasts detectable by flow cytometry, ranging from 0.01% to 16% (median, 0.1%) [12]. It should be noted that in 2 samples with 9% and 16% leukemic cells on flow cytometry, the morphologic analysis revealed only apparently mature normal lymphocytes (9% and 45%, respectively) [12]. In the St Jude Total XV study, 100 of 492 (20.3%) samples studied at the end of remission induction therapy (day 43), had leukemic lymphoblasts detectable by flow cytometry [56]. In sum, it is clear that a considerable fraction of "remission" samples collected during treatment for childhood ALL are MRD-positive, with a prevalence of MRD being higher during the early phases of therapy and progressively decreasing thereafter.
Bone marrow samples collected after a temporary stop in chemotherapy, after the end of treatment, or after hematopoietic stem cell transplantation may contain a high proportion of recovering immature lymphoid cells whose morphology resembles that of ALL lymphoblasts ("hematogones") [57-60]. Therefore, morphologic assessment of these samples is difficult and may result in erroneous conclusions; the application of MRD assays can clarify the identity of the morphologically ambiguous cells. Among MRD methods, flow cytometry is the one that is most affected by the state of bone marrow recovery [61]. In this regard, it is critical that flow cytometric analysis of MRD relies on markers that truly distinguish ALL cells from normal cells, including lymphoid progenitors; otherwise, the risk of false-positive MRD results is high. In fact, the samples studied at the end of remission induction therapy in the St Jude Total Studies were particularly rich in hematogones, as they were collected on day 43-46 of therapy, approximately two weeks after completion of remission induction therapy; despite their high concentration of hematogones, MRD measurements could be performed reliably and were strongly correlated with clinical outcome [9, 11, 56].
To determine the relation between results by flow cytometry and by PCR amplification of IG and TCR genes, we measured MRD using the assays in tandem in 1375 samples obtained from 227 patients with B-lineage ALL. By both assays, MRD was <0.01% in 1200, and ≥0.01% in 129 with an excellent correlation between the results of the two methods [62]. Of the remaining 46 samples, 28 had MRD ≥0.01% by flow cytometry but <0.01% by PCR. However, PCR was positive in 26 of these 28 samples at levels lower than 0.01%. Conversely, in 18 additional samples, MRD was ≥0.01% by PCR and <0.01% by flow cytometry but flow cytometry detected ALL cells in 8 of the 9 samples where a sensitivity of 0.001% could be achieved [62]. Thus, the results of the two methods were highly concordant overall. Kerst et al. analysed 105 samples from 30 patients with ALL and also found highly concordant results [63]. Malec et al. reported a study of 71 samples from 22 patients with ALL in which concordant results between flow cytometry and PCR were found in 89% of samples if the cutoff level of 0.01% to define MRD-positivity was applied [64]. However, there were significant differences in MRD level estimates in some samples, most likely due to technical shortcomings. Irving et al. studied MRD by flow cytometry and PCR in samples collected from 134 patients enrolled in the UKALL 2003 trial on day 28 (end of remission induction) and week 11 (completion of consolidation) [65]. Overall, 90 samples were MRD <0.01% and 25 were MRD ≥0.01% by both methods. Most of the 19 discordant samples were around the threshold level and MRD was detectable by both techniques in 8 [65]. With the improvement in methodologies, the concordance between MRD assays should improve [66].
Conclusive studies on the relation between PCR detection of fusion transcripts and other MRD assays in ALL are lacking. To this end, Metzler et al. compared MRD results of PCR amplification of ETV6-RUNX1 fusion transcripts and of antigen-receptor gene rearrangements in 12 patients with t(12;21) ALL and found concordance of results in 10, while in 2 patients ETV6-RUNX1 persisted while MRD was negative by IG/TCR; these patients were in complete remission at the time of the report [67]. Zaliova et al. performed a similar study but targeting BCR-ABL1 transcripts in 218 samples from 17 children with Philadelphia chromosome-positive ALL and found a poor correlation with IG/TCR studies: 20% of the samples studied were positive for the fusion transcript but negative by IG/TCR gene rearrangements [68].
It should be noted that the proportion of MRD-positive samples at any given time point during the course of treatment for children with ALL is highly dependent on the preceding therapy and hence varies widely among different studies. As shown in Table 2, patients with newly diagnosed ALL studied at the end of remission induction therapy had a prevalence of MRD positivity (≥0.01%) ranging from 19.4% to 83.5% in studies from different groups. Consequently, the interpretation of the clinical significance of MRD results needs to be considered in the context of each treatment regimen.
Table 2.
Prevalence of MRD positivity at the end of remission induction therapy in patients with newly diagnosed childhood ALL enrolled in different treatment protocols.
a)Treatment intensification for patients with MRD ≥1% on day 19. On the preceding Total XIII study, 42 of 165 patients studied were MRD-positive on day 46 [12].
Abbreviations: COG, Children's Oncology Group; St. Jude, St. Jude Children's Research Hospital; DFCI, Dana-Farber Cancer Institute; BFM, Berlin-Frankfurt-Münster; EORTC, European Organization for Research and Treatment of Cancer; UKALL, United Kingdom Medical Research Council acute lymphoblastic leukaemia; Ma-Spore, Malaysia-Singapore Acute Lymphoblastic Leukemia Study; AIEOP-BFM, Associazione Italiana di Ematologia Oncologia Pediatrica and the Berlin-Frankfurt-Münster; NOPHO, the Nordic Society of Pediatric Haematology and Oncology.
2. Measurements of treatment response in AML
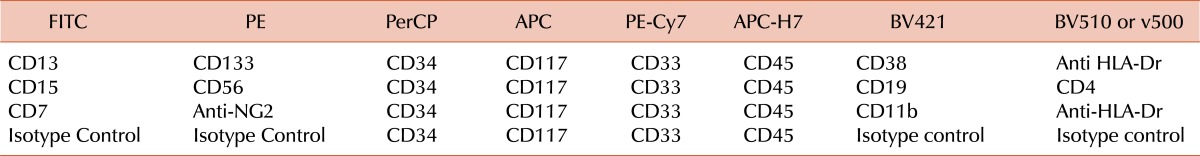
The targets most frequently used to monitor MRD in AML are transcripts originating from gene fusions, mutations, or overexpression, and leukemia-associated immunophenotypes [5, 6]. Rearrangements of IG and TCR genes are infrequent in AML [69]. Gene transcripts amenable to routine monitoring by PCR in childhood AML include RUNX1-RUNX1T1, CBFB-MYH11 and MLL-containing transcripts (in addition to PML-RARA in acute promyelocytic leukemia); these allow monitoring of MRD in approximately one-third of patients, with a sensitivity of one in 10,000 or higher [5]. NPM1 mutations and FLT3-internal tandem duplications have been described in approximately 8% and 15% of children with AML and can be a target for MRD studies by PCR [70, 71]. Leukemia-associated immunophenotypes can also be identified in most patients (200 of 210 patients enrolled in the AML02 study), although in approximately 40% of patients the routine sensitivity that can be achieved is not higher than one in 1,000 due to a partial overlap between the phenotype of leukemic cells and those of normal myeloid progenitor cells [49, 72]. Some of the marker combinations currently used in our laboratory are shown in Table 3.
Table 3.
Antibody and fluorochrome combinations currently used in our laboratory for MRD monitoring in AML by flow cytometry.a)
a)The FITC, PE, BV421 and V500/BV510 antibody conjugates can be changed between tubes for the final MRD combinations according to the abnormal patterns defined at diagnosis. In addition, CD41 may be useful in some cases of AML M7.
Abbreviations: FITC, Fluorescein Isothiocyanate; PE, R-Phycoerythrin; PerCP, Peridinin Chlorophyll Protein; APC, Allophycocyanin; PE-Cy7, Phycoerythrin-Cyanine 7; APC-H7, Allophycocyanin-Cyanine 7 analog; BV421, Brilliant Violet 421; BV510, Brilliant Violet 510; v500, BD Horizon v500.
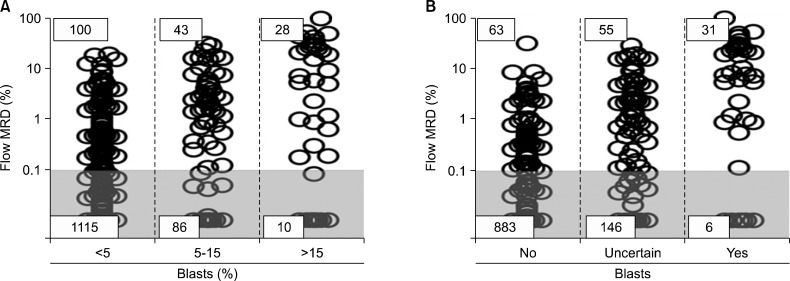
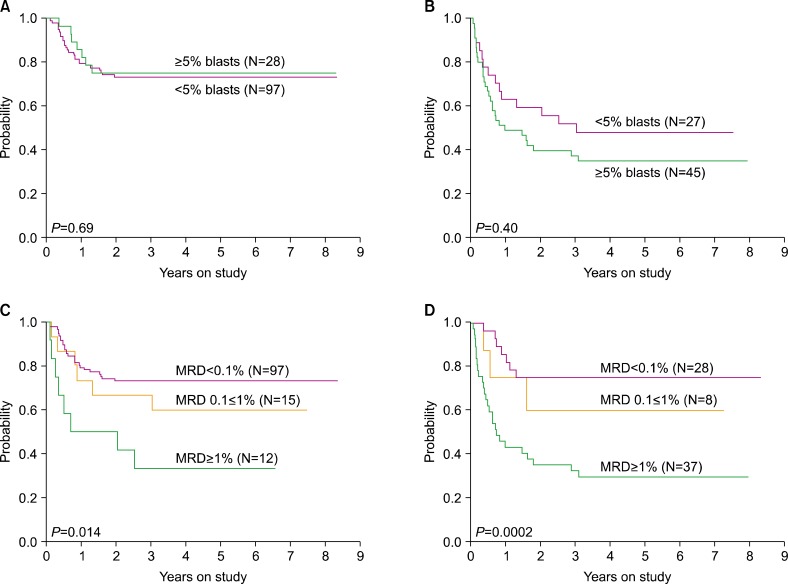
In AML, response to initial treatment dictates the intensity of subsequent therapy and identifies candidates for allogeneic stem cell transplantation. Because of the increasing availability of MRD monitoring, the clinical usefulness of morphologic assessment of treatment response is now questionable. To address this issue, we recently analyzed the results of flow cytometric monitoring of MRD in 1,514 bone marrow samples obtained from 203 children and adolescents with newly diagnosed AML enrolled in the St Jude AML02 study during and after completion of therapy [73]. Of the 1,514 bone marrow samples studied, 202 (13.3%) had MRD ≥0.1% by flow cytometry. Data on cell morphology was available in 1,382 (91.3%) of the 1,514 samples. MRD was positive in 28 of the 38 (73.7%) samples with >15% myeloblasts, 43 of the 129 (33.3%) with 5-15% myeloblast, and in 100 of the 1,215 (8.2%) samples with <5% myeloblasts (Fig. 1). Therefore, a considerable number of samples with no morphologic evidence of disease contained leukemic cells, while some samples appearing to contain myeloblasts lacked detectable leukemic cells by flow cytometry. Flow cytometric measurements of MRD after Induction I or II were strongly associated with event-free survival [73]. Importantly, the percentage of myeloblasts by morphology did not affect the relation between MRD by flow cytometry and treatment outcome; by contrast, MRD measured by flow cytometry was a significant predictor of relapse regardless of the morphologic results (Fig. 2) [73].
Fig. 1.
Relation between morphologic and flow cytometric detection of residual disease during and after treatment in childhood AML. (A) Percentage of bone marrow mononucleated cells expressing leukemia-associated immunophenotypes as measured by flow cytometry within groups defined by the percentage of myeloblasts counted by morphology. Gray area corresponds to measurements below the 0.1% threshold used to define MRD positivity. (B) Flow cytometric MRD data within groups defined by the hemopathologists' judgement regarding the presence of leukemic myeloblasts. From Inaba et al. [73] with permission.
Fig. 2.
Relation between event-free survival (EFS) for patients with childhood AML according to flow cytometry and morphology after Induction I. (A) EFS of patients who were MRD-negative (<0.1%) by flow cytometry according to percentage of blasts by morphology. (B) EFS of patients who were MRD-positive (≥0.1%) by flow cytometry according to percentage of blasts by morphology. (C) EFS of patients with <5% blasts by morphology according to MRD levels by flow cytometry. (D) EFS of patients with ≥5% blasts by morphology according to MRD levels by flow cytometry. From Inaba et al. [73] with permission.
The relation between MRD results obtained by flow cytometry and those obtained by PCR amplification of fusion transcripts is unclear. Of the 203 patients enrolled in the study, 80 had RUNX1-RUNX1T1, CBFB-MYH11, RBM15-MKL1, or MLL-containing fusion transcripts [73]. Of the 311 follow-up samples classified as MRD-negative by PCR, 308 (99.0%) were also MRD-negative by flow cytometry. However, only 19 of the 197 (9.6%) MRD-positive samples by PCR were also MRD-positive by flow cytometry, with RUNX1-RUNX1T1 and CBFB-MYH11 accounting for most discrepancies [73]. MRD measurements by PCR were not significantly related to outcome either by using the 0.1% cut-off level used for flow cytometry or by using a lower 0.01% cut-off level [73]. Moreover, PCR testing did not identify patients with a worse outcome among those who were MRD-negative by flow cytometry. The reason for the lack of relation between MRD by flow cytometry and PCR is unclear. It is possible that low levels of MRD by PCR (undetectable by flow cytometry) may not be associated with relapse as low levels of disease might be suppressed by subsequent treatment. We also speculate that fusion transcripts might signal the persistence of pre-leukemic cells, or partially differentiated leukemic cells with low or no leukemogenic potential (Fig. 3).
Fig. 3.
Possible scenarios that may explain concordant or discordant MRD results by flow cytometry and PCR after chemotherapy. This can either be ineffective (top), result in leukemia cell death (middle), or induce leukemic cell differentiation (bottom). In the latter case, MRD by flow cytometry might be negative, owing to the loss of aberrant immunophenotypes but MRD by PCR would be positive as the cells retain leukemia fusion transcripts. However, these cells may also lack clonogenic potential.
Overall, the results of this study indicate the value of morphologic monitoring is limited if MRD monitoring by flow cytometry is available, and that PCR results, particularly those targeting RUNX1-RUNX1T1 and CBFB-MYH11 are difficult to interpret, suggesting that these tests should be used with caution or not done at all in childhood AML.
3. Useful methods and time points for routine MRD testing in childhood leukemia
In patients with ALL, flow cytometry and PCR amplification of antigen-receptor genes provide similar results if MRD is present at levels of 0.01% or above, and hence the choice between these two methods is primarily dictated by the facilities and expertise available. In general, flow cytometry is more widely available because of its use in many diagnostic laboratories for cell marker profiling. The main limitation of flow cytometry is the requirement for data interpretation, which in turns relies on the expertise of the operator. In this respect, PCR methods are somewhat easier to standardize and, typically, the results are easier to interpret.
In patients with ALL, MRD is usually measured at the end of remission induction therapy and at various intervals during the post-remission period. The value of extensive post-remission monitoring for patients with MRD negative results at the end of remission induction is questionable, as most MRD-negative patients at this time point will remain in long-term remission [11]. Nevertheless, some groups base their MRD risk-classification on two time points including end of remission induction and post-consolidation [20, 74]. It is important to stress that measurements of MRD during remission induction therapy can also provide valuable prognostic information, allowing the simultaneous identification of patients with poor or excellent response to initial therapy [12, 18, 19, 75, 76]. In patients with T-lineage ALL, MRD is equally distributed in blood and in bone marrow [13, 77]. In these patients, MRD can be monitored in peripheral blood. In patients with B-lineage ALL, early MRD measurements in peripheral blood (e.g., on day 8) may also be predictive of outcome [17].
Flow cytometry is the only method that can be applied to monitor MRD in the majority of patients with AML. As discussed above, studies on MRD by PCR amplification of fusion transcripts can be used in only a fraction of children with AML and results are difficult to interpret. The most informative time points are those after the initial blocks of remission induction therapy, which allow the identification of poor responders and candidates for transplant.
For patients with either ALL or AML who achieve MRD-negativity, conversion to MRD positivity strongly suggests the possibility of relapse and should trigger careful monitoring. A further increase in MRD levels is usually followed by overt relapse. Levels of MRD before transplant are strongly related to relapse post-transplant. A study analysing the significance of MRD pre-transplant in 190 children with very high-risk ALL or AML found that survival probability was mostly dependent on MRD levels before transplant in addition to whether patients were transplanted in first remission or with more advanced disease [31]. In a subsequent analysis focusing on 122 children with very-high-risk ALL or AML, higher MRD levels at the time of transplant independently predicted a poorer survival [78]. Interestingly, the increase in risk of death associated with any given level of MRD was greater in ALL than in AML, suggesting that a pre-transplantation reduction of leukemia burden would have a higher impact in the former [78]. MRD measurements can also be used to trigger retrieval efforts post-transplant, e.g., tapering immunosuppression, administration of donor lymphocyte infusions, and a second hematopoietic cell transplant.
Because of its prognostic significance, MRD before transplant is being increasingly applied to optimize the timing of transplant and guide post-transplant management. To this end, in a study by Lankester at al. children with ALL who were MRD ≥0.01% before transplant were eligible for early tapering of cyclosporine post-transplant as well as to receive donor lymphocyte infusions, resulting in an apparent delay in the occurrence of relapse, suggesting some leukemia-controlling effect, although overall event-free survival was not superior to that of previous trials [79].
4. Considerations for the future
The introduction of MRD monitoring has transformed the way in which patients with acute leukemia are managed. MRD results can be applied to most children with ALL and AML, and can be delivered in a timely fashion to satisfy the requirements for rapid changes in treatment timing and intensity [49, 56]. Despite this progress there are areas for continuing development.
Methods to study MRD by flow cytometry are constantly being refined by the introduction of new markers [80], which take advantage of the capacity of newer instruments to detect an increasingly higher number of fluorochromes. Technologies relying on mass spectrometry-based detection of elements conjugated to antibodies can further increase this capability [81]; their utility for MRD detection is yet untested. The traditional ways to analyze flow cytometry data are clearly inadequate when applied to the amount of information acquired with contemporary instruments and hence a parallel development in analytical software must take place. To this end, efforts to generate programs that can take full advantage of newer technologies and markers are being reported [82, 83]. An alternative approach to immunophenotypic analysis of MRD, based on high-speed cell imaging scanning technology, was also recently proposed [84]. The initial data indicate that the method has the potential to identify MRD with a very high sensitivity and ensure that the signals detected originate from viable cells.
The BIOMED-2 Concerted Action BMH4-CT98-3936 project optimized methods for PCR amplification of IG and TCR which are now used by most laboratories that employ these methods for MRD studies in childhood ALL [4, 85]. A recent advance in this area is the application of high-throughput sequencing technology to sequence all antigen-receptor gene rearrangements amplified using a panel of universal consensus primers [86-88]. This method should facilitate molecular MRD studies in ALL and could replace current molecular methods.
The implementation of MRD studies in treatment protocols requires a strong interaction between MRD specialists and pediatric oncologists. MRD studies can be relatively expensive compared to routine clinical laboratory tests but, in general, their cost is similar to that of other high complexity tests and, when executed and applied properly, have the potential for reducing clinical care costs by improving treatment effectiveness. The establishment of regional or national expert reference centers which can proficiently perform MRD for multicenter studies can reduce costs by avoiding erroneous results from less experienced laboratories as well as the time-consuming and costly standardization process that is required to ensure homogeneous results when multiple laboratories perform the same test. One should also make sure that unnecessary procedures and reagents are avoided. For example, testing MRD at later time points during therapy or off therapy in patients with ALL appears to have little informative value unless supported by a clinical suspicion of relapse, and hence it might be given low priority. In addition, flow cytometric testing during remission induction therapy in patients with B-lineage ALL can be performed with a reduced number of antibodies, further saving costs [89, 90].
It seems inevitable that MRD will increasingly be adopted as an eligibility criteria for clinical trials of novel anti-leukemic therapies and a surrogate marker of response. In this context, no changes in MRD levels after exposure to a new agent could allow quick shift to different agents or schedules and avoid lengthy trials with ineffective agents, ultimately rendering Phase II studies more nimble and efficient.
Footnotes
This study was supported by National Medical Research Council of Singapore.
References
- 1.Campana D, Pui CH. Detection of minimal residual disease in acute leukemia: methodologic advances and clinical significance. Blood. 1995;85:1416–1434. [PubMed] [Google Scholar]
- 2.Bradstock KF, Janossy G, Tidman N, et al. Immunological monitoring of residual disease in treated thymic acute lymphoblastic leukaemia. Leuk Res. 1981;5:301–309. doi: 10.1016/0145-2126(81)90002-3. [DOI] [PubMed] [Google Scholar]
- 3.Campana D. Minimal residual disease in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2010;2010:7–12. doi: 10.1182/asheducation-2010.1.7. [DOI] [PubMed] [Google Scholar]
- 4.Brüggemann M, Schrauder A, Raff T, et al. Standardized MRD quantification in European ALL trials: proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18-20 September 2008. Leukemia. 2010;24:521–535. doi: 10.1038/leu.2009.268. [DOI] [PubMed] [Google Scholar]
- 5.Shook D, Coustan-Smith E, Ribeiro RC, Rubnitz JE, Campana D. Minimal residual disease quantitation in acute myeloid leukemia. Clin Lymphoma Myeloma. 2009;9(Suppl 3):S281–S285. doi: 10.3816/CLM.2009.s.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buccisano F, Maurillo L, Del Principe MI, et al. Prognostic and therapeutic implications of minimal residual disease detection in acute myeloid leukemia. Blood. 2012;119:332–341. doi: 10.1182/blood-2011-08-363291. [DOI] [PubMed] [Google Scholar]
- 7.Brisco MJ, Condon J, Hughes E, et al. Outcome prediction in childhood acute lymphoblastic leukaemia by molecular quantification of residual disease at the end of induction. Lancet. 1994;343:196–200. doi: 10.1016/s0140-6736(94)90988-1. [DOI] [PubMed] [Google Scholar]
- 8.Cavé H, van der Werff ten Bosch J, Suciu S, et al. European Organization for Research and Treatment of Cancer-Childhood Leukemia Cooperative Group. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. N Engl J Med. 1998;339:591–598. doi: 10.1056/NEJM199808273390904. [DOI] [PubMed] [Google Scholar]
- 9.Coustan-Smith E, Behm FG, Sanchez J, et al. Immunological detection of minimal residual disease in children with acute lymphoblastic leukaemia. Lancet. 1998;351:550–554. doi: 10.1016/S0140-6736(97)10295-1. [DOI] [PubMed] [Google Scholar]
- 10.van Dongen JJ, Seriu T, Panzer-Grümayer ER, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352:1731–1738. doi: 10.1016/S0140-6736(98)04058-6. [DOI] [PubMed] [Google Scholar]
- 11.Coustan-Smith E, Sancho J, Hancock ML, et al. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood. 2000;96:2691–2696. [PubMed] [Google Scholar]
- 12.Coustan-Smith E, Sancho J, Behm FG, et al. Prognostic importance of measuring early clearance of leukemic cells by flow cytometry in childhood acute lymphoblastic leukemia. Blood. 2002;100:52–58. doi: 10.1182/blood-2002-01-0006. [DOI] [PubMed] [Google Scholar]
- 13.Coustan-Smith E, Sancho J, Hancock ML, et al. Use of peripheral blood instead of bone marrow to monitor residual disease in children with acute lymphoblastic leukemia. Blood. 2002;100:2399–2402. doi: 10.1182/blood-2002-04-1130. [DOI] [PubMed] [Google Scholar]
- 14.Nyvold C, Madsen HO, Ryder LP, et al. Precise quantification of minimal residual disease at day 29 allows identification of children with acute lymphoblastic leukemia and an excellent outcome. Blood. 2002;99:1253–1258. doi: 10.1182/blood.v99.4.1253. [DOI] [PubMed] [Google Scholar]
- 15.Dworzak MN, Fröschl G, Printz D, et al. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood. 2002;99:1952–1958. doi: 10.1182/blood.v99.6.1952. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Goldwasser MA, Li A, et al. Quantitative analysis of minimal residual disease predicts relapse in children with B-lineage acute lymphoblastic leukemia in DFCI ALL Consortium Protocol 95-01. Blood. 2007;110:1607–1611. doi: 10.1182/blood-2006-09-045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood. 2008;111:5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basso G, Veltroni M, Valsecchi MG, et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol. 2009;27:5168–5174. doi: 10.1200/JCO.2008.20.8934. [DOI] [PubMed] [Google Scholar]
- 19.Sutton R, Venn NC, Tolisano J, et al. Clinical significance of minimal residual disease at day 15 and at the end of therapy in childhood acute lymphoblastic leukaemia. Br J Haematol. 2009;146:292–299. doi: 10.1111/j.1365-2141.2009.07744.x. [DOI] [PubMed] [Google Scholar]
- 20.Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115:3206–3214. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- 21.Stow P, Key L, Chen X, et al. Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood. 2010;115:4657–4663. doi: 10.1182/blood-2009-11-253435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaji K, Okamoto T, Yokota S, et al. Minimal residual disease-based augmented therapy in childhood acute lymphoblastic leukemia: a report from the Japanese Childhood Cancer and Leukemia Study Group. Pediatr Blood Cancer. 2010;55:1287–1295. doi: 10.1002/pbc.22620. [DOI] [PubMed] [Google Scholar]
- 23.Katsibardi K, Moschovi MA, Braoudaki M, Papadhimitriou SI, Papathanasiou C, Tzortzatou-Stathopoulou F. Sequential monitoring of minimal residual disease in acute lymphoblastic leukemia: 7-year experience in a pediatric hematology/oncology unit. Leuk Lymphoma. 2010;51:846–852. doi: 10.3109/10428191003682734. [DOI] [PubMed] [Google Scholar]
- 24.Meleshko AN, Savva NN, Fedasenka UU, et al. Prognostic value of MRD-dynamics in childhood acute lymphoblastic leukemia treated according to the MB-2002/2008 protocols. Leuk Res. 2011;35:1312–1320. doi: 10.1016/j.leukres.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Eckert C, Biondi A, Seeger K, et al. Prognostic value of minimal residual disease in relapsed childhood acute lymphoblastic leukaemia. Lancet. 2001;358:1239–1241. doi: 10.1016/S0140-6736(01)06355-3. [DOI] [PubMed] [Google Scholar]
- 26.Coustan-Smith E, Gajjar A, Hijiya N, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia after first relapse. Leukemia. 2004;18:499–504. doi: 10.1038/sj.leu.2403283. [DOI] [PubMed] [Google Scholar]
- 27.Paganin M, Zecca M, Fabbri G, et al. Minimal residual disease is an important predictive factor of outcome in children with relapsed 'high-risk' acute lymphoblastic leukemia. Leukemia. 2008;22:2193–2200. doi: 10.1038/leu.2008.227. [DOI] [PubMed] [Google Scholar]
- 28.Raetz EA, Borowitz MJ, Devidas M, et al. Reinduction platform for children with first marrow relapse of acute lymphoblastic leukemia: A Children's Oncology Group Study. J Clin Oncol. 2008;26:3971–3978. doi: 10.1200/JCO.2008.16.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krejci O, van der Velden VH, Bader P, et al. Level of minimal residual disease prior to haematopoietic stem cell transplantation predicts prognosis in paediatric patients with acute lymphoblastic leukaemia: a report of the Pre-BMT MRD Study Group. Bone Marrow Transplant. 2003;32:849–851. doi: 10.1038/sj.bmt.1704241. [DOI] [PubMed] [Google Scholar]
- 30.Bader P, Kreyenberg H, Henze GH, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27:377–384. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 31.Leung W, Campana D, Yang J, et al. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood. 2011;118:223–230. doi: 10.1182/blood-2011-01-333070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao XS, Liu YR, Zhu HH, et al. Monitoring MRD with flow cytometry: an effective method to predict relapse for ALL patients after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2012;91:183–192. doi: 10.1007/s00277-011-1285-1. [DOI] [PubMed] [Google Scholar]
- 33.Brüggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107:1116–1123. doi: 10.1182/blood-2005-07-2708. [DOI] [PubMed] [Google Scholar]
- 34.Raff T, Gökbuget N, Lüschen S, et al. Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood. 2007;109:910–915. doi: 10.1182/blood-2006-07-037093. [DOI] [PubMed] [Google Scholar]
- 35.Holowiecki J, Krawczyk-Kulis M, Giebel S, et al. Status of minimal residual disease after induction predicts outcome in both standard and high-risk Ph-negative adult acute lymphoblastic leukaemia. The Polish Adult Leukemia Group ALL 4-2002 MRD Study. Br J Haematol. 2008;142:227–237. doi: 10.1111/j.1365-2141.2008.07185.x. [DOI] [PubMed] [Google Scholar]
- 36.Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL) Blood. 2009;113:4153–4162. doi: 10.1182/blood-2008-11-185132. [DOI] [PubMed] [Google Scholar]
- 37.Patel B, Rai L, Buck G, et al. Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: final results of the international trial UKALL XII/ECOG2993. Br J Haematol. 2010;148:80–89. doi: 10.1111/j.1365-2141.2009.07941.x. [DOI] [PubMed] [Google Scholar]
- 38.Gökbuget N, Kneba M, Raff T, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120:1868–1876. doi: 10.1182/blood-2011-09-377713. [DOI] [PubMed] [Google Scholar]
- 39.Guerrasio A, Rosso C, Martinelli G, et al. Polyclonal haemopoieses associated with long-term persistence of the AML1-ETO transcript in patients with FAB M2 acute myeloid leukaemia in continous clinical remission. Br J Haematol. 1995;90:364–368. doi: 10.1111/j.1365-2141.1995.tb05160.x. [DOI] [PubMed] [Google Scholar]
- 40.Marcucci G, Livak KJ, Bi W, Strout MP, Bloomfield CD, Caligiuri MA. Detection of minimal residual disease in patients with AML1/ETO-associated acute myeloid leukemia using a novel quantitative reverse transcription polymerase chain reaction assay. Leukemia. 1998;12:1482–1489. doi: 10.1038/sj.leu.2401128. [DOI] [PubMed] [Google Scholar]
- 41.Tobal K, Newton J, Macheta M, et al. Molecular quantitation of minimal residual disease in acute myeloid leukemia with t(8;21) can identify patients in durable remission and predict clinical relapse. Blood. 2000;95:815–819. [PubMed] [Google Scholar]
- 42.Sievers EL, Lange BJ, Alonzo TA, et al. Immunophenotypic evidence of leukemia after induction therapy predicts relapse: results from a prospective Children's Cancer Group study of 252 patients with acute myeloid leukemia. Blood. 2003;101:3398–3406. doi: 10.1182/blood-2002-10-3064. [DOI] [PubMed] [Google Scholar]
- 43.Coustan-Smith E, Ribeiro RC, Rubnitz JE, et al. Clinical significance of residual disease during treatment in childhood acute myeloid leukaemia. Br J Haematol. 2003;123:243–252. doi: 10.1046/j.1365-2141.2003.04610.x. [DOI] [PubMed] [Google Scholar]
- 44.San-Miguel JF, Vidriales MB, Orfão A. Immunological evaluation of minimal residual disease (MRD) in acute myeloid leukaemia (AML) Best Pract Res Clin Haematol. 2002;15:105–118. doi: 10.1053/beha.2001.0193. [DOI] [PubMed] [Google Scholar]
- 45.Langebrake C, Creutzig U, Dworzak M, et al. Residual disease monitoring in childhood acute myeloid leukemia by multiparameter flow cytometry: the MRD-AML-BFM Study Group. J Clin Oncol. 2006;24:3686–3692. doi: 10.1200/JCO.2005.05.4312. [DOI] [PubMed] [Google Scholar]
- 46.Maurillo L, Buccisano F, Del Principe MI, et al. Toward optimization of postremission therapy for residual disease-positive patients with acute myeloid leukemia. J Clin Oncol. 2008;26:4944–4951. doi: 10.1200/JCO.2007.15.9814. [DOI] [PubMed] [Google Scholar]
- 47.Lane S, Saal R, Mollee P, et al. A >or=1 log rise in RQ-PCR transcript levels defines molecular relapse in core binding factor acute myeloid leukemia and predicts subsequent morphologic relapse. Leuk Lymphoma. 2008;49:517–523. doi: 10.1080/10428190701817266. [DOI] [PubMed] [Google Scholar]
- 48.Corbacioglu A, Scholl C, Schlenk RF, et al. Prognostic impact of minimal residual disease in CBFB-MYH11-positive acute myeloid leukemia. J Clin Oncol. 2010;28:3724–3729. doi: 10.1200/JCO.2010.28.6468. [DOI] [PubMed] [Google Scholar]
- 49.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–552. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Velden VH, van der Sluijs-Geling A, Gibson BE, et al. Clinical significance of flowcytometric minimal residual disease detection in pediatric acute myeloid leukemia patients treated according to the DCOG ANLL97/MRC AML12 protocol. Leukemia. 2010;24:1599–1606. doi: 10.1038/leu.2010.153. [DOI] [PubMed] [Google Scholar]
- 51.Krönke J, Schlenk RF, Jensen KO, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29:2709–2716. doi: 10.1200/JCO.2011.35.0371. [DOI] [PubMed] [Google Scholar]
- 52.Yin JA, O'Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012;120:2826–2835. doi: 10.1182/blood-2012-06-435669. [DOI] [PubMed] [Google Scholar]
- 53.Loken MR, Alonzo TA, Pardo L, et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children's Oncology Group. Blood. 2012;120:1581–1588. doi: 10.1182/blood-2012-02-408336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flohr T, Schrauder A, Cazzaniga G, et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. 2008;22:771–782. doi: 10.1038/leu.2008.5. [DOI] [PubMed] [Google Scholar]
- 55.Coustan-Smith E, Campana D. Immunologic minimal residual disease detection in acute lymphoblastic leukemia: a comparative approach to molecular testing. Best Pract Res Clin Haematol. 2010;23:347–358. doi: 10.1016/j.beha.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Longacre TA, Foucar K, Crago S, et al. Hematogones: a multiparameter analysis of bone marrow precursor cells. Blood. 1989;73:543–552. [PubMed] [Google Scholar]
- 58.Rimsza LM, Larson RS, Winter SS, et al. Benign hematogone-rich lymphoid proliferations can be distinguished from B-lineage acute lymphoblastic leukemia by integration of morphology, immunophenotype, adhesion molecule expression, and architectural features. Am J Clin Pathol. 2000;114:66–75. doi: 10.1309/LXU4-Q7Q9-3YAB-4QE0. [DOI] [PubMed] [Google Scholar]
- 59.van Wering ER, van der Linden-Schrever BE, Szczepanski T, et al. Regenerating normal B-cell precursors during and after treatment of acute lymphoblastic leukaemia: implications for monitoring of minimal residual disease. Br J Haematol. 2000;110:139–146. doi: 10.1046/j.1365-2141.2000.02143.x. [DOI] [PubMed] [Google Scholar]
- 60.McKenna RW, Washington LT, Aquino DB, Picker LJ, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4-color flow cytometry. Blood. 2001;98:2498–2507. doi: 10.1182/blood.v98.8.2498. [DOI] [PubMed] [Google Scholar]
- 61.Luria D, Rosenthal E, Steinberg D, et al. Prospective comparison of two flow cytometry methodologies for monitoring minimal residual disease in a multicenter treatment protocol of childhood acute lymphoblastic leukemia. Cytometry B Clin Cytom. 2010;78:365–371. doi: 10.1002/cyto.b.20532. [DOI] [PubMed] [Google Scholar]
- 62.Neale GA, Coustan-Smith E, Stow P, et al. Comparative analysis of flow cytometry and polymerase chain reaction for the detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia. 2004;18:934–938. doi: 10.1038/sj.leu.2403348. [DOI] [PubMed] [Google Scholar]
- 63.Kerst G, Kreyenberg H, Roth C, et al. Concurrent detection of minimal residual disease (MRD) in childhood acute lymphoblastic leukaemia by flow cytometry and real-time PCR. Br J Haematol. 2005;128:774–782. doi: 10.1111/j.1365-2141.2005.05401.x. [DOI] [PubMed] [Google Scholar]
- 64.Malec M, Björklund E, Söderhäll S, et al. Flow cytometry and allele-specific oligonucleotide PCR are equally effective in detection of minimal residual disease in ALL. Leukemia. 2001;15:716–727. doi: 10.1038/sj.leu.2402091. [DOI] [PubMed] [Google Scholar]
- 65.Irving J, Jesson J, Virgo P, et al. Establishment and validation of a standard protocol for the detection of minimal residual disease in B lineage childhood acute lymphoblastic leukemia by flow cytometry in a multi-center setting. Haematologica. 2009;94:870–874. doi: 10.3324/haematol.2008.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Denys B, van der Sluijs-Gelling AJ, Homburg C, et al. Improved flow cytometric detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia. 2012 doi: 10.1038/leu.2012.231. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 67.Metzler M, Mann G, Monschein U, et al. Minimal residual disease analysis in children with t(12;21)-positive acute lymphoblastic leukemia: comparison of Ig/TCR rearrangements and the genomic fusion gene. Haematologica. 2006;91:683–686. [PubMed] [Google Scholar]
- 68.Zaliova M, Fronkova E, Krejcikova K, et al. Quantification of fusion transcript reveals a subgroup with distinct biological properties and predicts relapse in BCR/ABL-positive ALL: implications for residual disease monitoring. Leukemia. 2009;23:944–951. doi: 10.1038/leu.2008.386. [DOI] [PubMed] [Google Scholar]
- 69.Boeckx N, Willemse MJ, Szczepanski T, et al. Fusion gene transcripts and Ig/TCR gene rearrangements are complementary but infrequent targets for PCR-based detection of minimal residual disease in acute myeloid leukemia. Leukemia. 2002;16:368–375. doi: 10.1038/sj.leu.2402387. [DOI] [PubMed] [Google Scholar]
- 70.Brown P, McIntyre E, Rau R, et al. The incidence and clinical significance of nucleophosmin mutations in childhood AML. Blood. 2007;110:979–985. doi: 10.1182/blood-2007-02-076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meshinchi S, Woods WG, Stirewalt DL, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97:89–94. doi: 10.1182/blood.v97.1.89. [DOI] [PubMed] [Google Scholar]
- 72.Campana D, Coustan-Smith E. Detection of minimal residual disease in acute leukemia by flow cytometry. Cytometry. 1999;38:139–152. doi: 10.1002/(sici)1097-0320(19990815)38:4<139::aid-cyto1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 73.Inaba H, Coustan-Smith E, Cao X, et al. Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J Clin Oncol. 2012;30:3625–3632. doi: 10.1200/JCO.2011.41.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yeoh AE, Ariffin H, Chai EL, et al. Minimal residual disease-guided treatment deintensification for children with acute lymphoblastic leukemia: results from the Malaysia-Singapore acute lymphoblastic leukemia 2003 study. J Clin Oncol. 2012;30:2384–2392. doi: 10.1200/JCO.2011.40.5936. [DOI] [PubMed] [Google Scholar]
- 75.Panzer-Grümayer ER, Schneider M, Panzer S, Fasching K, Gadner H. Rapid molecular response during early induction chemotherapy predicts a good outcome in childhood acute lymphoblastic leukemia. Blood. 2000;95:790–794. [PubMed] [Google Scholar]
- 76.Koh KN, Park M, Kim BE, et al. Prognostic significance of minimal residual disease detected by a simplified flow cytometric assay during remission induction chemotherapy in children with acute lymphoblastic leukemia. Korean J Pediatr. 2010;53:957–964. doi: 10.3345/kjp.2010.53.11.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van der Velden VH, Jacobs DC, Wijkhuijs AJ, et al. Minimal residual disease levels in bone marrow and peripheral blood are comparable in children with T cell acute lymphoblastic leukemia (ALL), but not in precursor-B-ALL. Leukemia. 2002;16:1432–1436. doi: 10.1038/sj.leu.2402636. [DOI] [PubMed] [Google Scholar]
- 78.Leung W, Pui CH, Coustan-Smith E, et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood. 2012;120:468–472. doi: 10.1182/blood-2012-02-409813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lankester AC, Bierings MB, van Wering ER, et al. Preemptive alloimmune intervention in high-risk pediatric acute lymphoblastic leukemia patients guided by minimal residual disease level before stem cell transplantation. Leukemia. 2010;24:1462–1469. doi: 10.1038/leu.2010.133. [DOI] [PubMed] [Google Scholar]
- 80.Coustan-Smith E, Song G, Clark C, et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2011;117:6267–6276. doi: 10.1182/blood-2010-12-324004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bendall SC, Simonds EF, Qiu P, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pedreira CE, Costa ES, Almeida J, et al. A probabilistic approach for the evaluation of minimal residual disease by multiparameter flow cytometry in leukemic B-cell chronic lymphoproliferative disorders. Cytometry A. 2008;73A:1141–1150. doi: 10.1002/cyto.a.20638. [DOI] [PubMed] [Google Scholar]
- 83.Fišer K, Sieger T, Schumich A, et al. Detection and monitoring of normal and leukemic cell populations with hierarchical clustering of flow cytometry data. Cytometry A. 2012;81:25–34. doi: 10.1002/cyto.a.21148. [DOI] [PubMed] [Google Scholar]
- 84.Liu X, Hsieh HB, Campana D, Bruce RH. A new method for high speed, sensitive detection of minimal residual disease. Cytometry A. 2012;81:169–175. doi: 10.1002/cyto.a.21124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van der Velden VH, Hochhaus A, Cazzaniga G, Szczepanski T, Gabert J, van Dongen JJ. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia. 2003;17:1013–1034. doi: 10.1038/sj.leu.2402922. [DOI] [PubMed] [Google Scholar]
- 86.Boyd SD, Marshall EL, Merker JD, et al. Measurement and clinical monitoring of human lymphocyte clonality by massively parallel VDJ pyrosequencing. Sci Transl Med. 2009;1:12ra23. doi: 10.1126/scitranslmed.3000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu D, Sherwood A, Fromm JR, et al. High-throughput sequencing detects minimal residual disease in acute T lymphoblastic leukemia. Sci Transl Med. 2012;4:134ra63. doi: 10.1126/scitranslmed.3003656. [DOI] [PubMed] [Google Scholar]
- 88.Faham M, Zheng J, Moorhead M, et al. Deep sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012 doi: 10.1182/blood-2012-07-444042. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coustan-Smith E, Ribeiro RC, Stow P, et al. A simplified flow cytometric assay identifies children with acute lymphoblastic leukemia who have a superior clinical outcome. Blood. 2006;108:97–102. doi: 10.1182/blood-2006-01-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koh KN, Park M, Kim BE, et al. Prognostic significance of minimal residual disease detected by a simplified flow cytometric assay during remission induction chemotherapy in children with acute lymphoblastic leukemia. Korean J Pediatr. 2010;53:957–964. doi: 10.3345/kjp.2010.53.11.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schrappe M, Valsecchi MG, Bartram CR, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118:2077–2084. doi: 10.1182/blood-2011-03-338707. [DOI] [PubMed] [Google Scholar]