Abstract
Background
Gelsolin and matrix metalloproteinase 12 (MMP12) expression has been reported in Langerhans cell histiocytosis (LCH), but the clinical significance of this expression is unknown. We investigated the associations of these proteins with clinical manifestations in patients diagnosed with LCH.
Methods
We performed a retrospective analysis of clinical data from patients diagnosed with LCH and followed up between 1998 and 2008. Available formalin-fixed, paraffin-embedded specimens were used for gelsolin and MMP12 immunohistochemical staining. We analyzed the expression levels of these proteins and their associations with LCH clinical features.
Results
Specimens from 36 patients (20 males, 16 females) with a diagnosis of LCH based on CD1a positivity with clinical manifestations were available for immunohistochemical staining. Median patient age was 62 months (range, 5 to 207). The expression of gelsolin varied; it was high in 17 patients (47.2%), low in 11 patients (30.6%), and absent in 8 patients (22.2%). The high gelsolin expression group had a higher tendency for multi-organ and risk organ involvement, although the trend was not statistically significant. MMP12 was detected only in 7 patients (19.4%) who showed multi-system involvement (P=0.018) and lower event-free survival (P=0.002) in comparison to patients with negative MMP12 staining.
Conclusion
Gelsolin and MMP12 expression may be associated with the clinical course of LCH, and MMP12 expression may be particularly associated with severe LCH. Further studies of larger populations are needed to define the precise role and significance of gelsolin and MMP12 in the pathogenesis of LCH.
Keywords: Histiocytosis, Langerhans cells, Immunohistochemistry, Gelsolin, Matrix Metalloproteinase 12
INTRODUCTION
The pathogenic mechanism of Langerhans cell histiocytosis (LCH) remains unclear; it is also unclear why some patients develop single system disease while others experience fatal multi-system lesions [1, 2]. A genetic alteration may have a significant effect on the cellular mechanisms controlling proliferation and apoptosis of Langerhans cells (LCs), and may be one of the causes of LCH [3, 4].
Gelsolin is the primary regulator of the actin cytoskeleton, which is widely expressed in mammalian tissues [5, 6]. Gelsolin regulates the actin cytoskeleton integrity and dynamics and maintains proper cellular morphology and motility [5-7]. Gelsolin expression seems to act downstream of Ras and phosphoinositides to promote cancer cell invasion [5-7]. High expression of gelsolin mRNA and protein in LCH has been reported but the clinical significance of gelsolin has not been clarified [1].
Matrix metalloproteinase 12 (MMP12) hydrolyzes substrates such as elastin, type IV collagen, fibronectin, laminin, gelatin, and chondroitin sulfates [8, 9]. High MMP12 expression has been associated with tumor progression and poor prognosis in skin, vulvar, and pancreatic cancer [10-12]. In LCH, expression of MMP12 mRNA was observed most abundantly in multi-system disease associated with poor prognosis, suggesting expression of MMP12 might play a role in the progression of LCH [1]. However, the association between MMP12 and multi-system disease requires additional study.
We investigated the association between gelsolin and MMP12 expression and clinical outcomes in pediatric patients diagnosed with LCH. Our objective was to study the expression of gelsolin and MMP12 by immunohistochemistry to determine whether their level of expression could predict disease outcomes.
MATERIALS AND METHODS
1. Patients and materials
Children with LCH diagnosed between March 1998 and March 2008 were retrospectively recruited. The medical records of 50 patients (28 males, 22 females) with LCH were reviewed for organ involvement at diagnosis, disease course, and relapse. According to disease extent at diagnosis, all patients were classified as having single system LCH (involvement of one organ or multifocal involvement of a single organ system) or multi-system LCH (involvement of multiple organ systems, with or without organ dysfunction). Risk organ involvement was defined as involvement of at least one of the following organ systems: liver, spleen, hematological system, and lungs [13]. We analyzed the association between immunohistochemical protein expression and clinical features, including outcomes.
2. Immunohistochemical staining
Available formalin-fixed, paraffin-embedded specimens were used for gelsolin and MMP12 immunohistochemistry according to standard laboratory methods. In summary, 5-mm sections from representative tissue blocks were cut and mounted on saline-coated slides, deparaffinized in xylene, and then rehydrated with gelsolin antibody (monoclonal mouse anti-human gelsolin, clone GS-2C4; Sigma Chemical Co., St. Louis, MO) and MMP12 antibody (anti-human MMP-12 monoclonal hemopexin-like domain antibody clone 4D2; R&D systems, Wiesbaden, Germany). Immunohistochemical staining of all the slides was evaluated by an experienced pathologist who was blinded to the clinical outcome.
Grading was performed in fields where LCs were identified morphologically and immunohistochemically by comparison of sections stained with the relevant antibody to adjacent H&E and CD1a-stained sections. Staining was considered positive when brown coloration was observed in the cytoplasm of the LCs (Figs. 1, 2). At least 500 LCs were counted on each slide using a grid eyepiece at 400× magnification. The numbers of LCs expressing the relevant antigen were then counted in the same fields and the result was expressed as the degree of staining in LCs vs. lymphocytes surrounding the LCs. Negative staining was defined as the absence of gelsolin and MMP12 staining, whereas positivity was defined as the appearance of brown stain in the cytoplasm of pathologic LCs. A semi-quantitative evaluation of gelsolin was based on the following grading system: lower expression included negative staining and staining similar to lymphocytes surrounding the LCs; higher expression included stronger staining than lymphocytes.
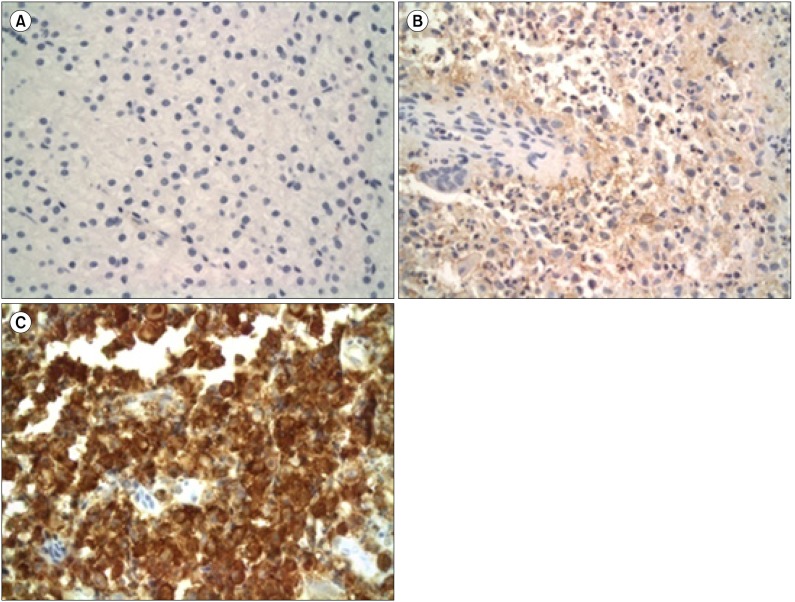
Fig. 1.
Light micrographs of LCH lesions after gelsolin immunohistochemical staining (×400). Scattered LCs with reddish brown reactions in the cytoplasm were considered positive. A semi-quantitative evaluation was made using the following grading system: negative (A), lower expression group (LEG) (B) and higher expression group (HEG) (C) according to the extent of cytoplasmic staining.
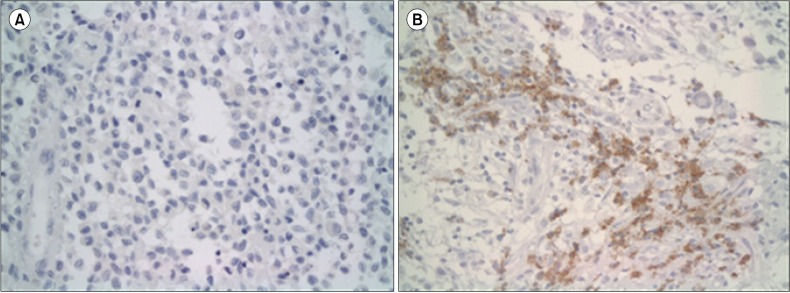
Fig. 2.
Light micrographs of LCH lesions after MMP12 immunohistochemical staining (400×). Slides with no staining were regarded as negative (A), whereas LCs with light brown reactions in the cytoplasm were considered positive (B).
3. Statistical analysis
Statistical analysis was performed using SPSS 20.0 (SPSS Standard version 20.0, IBM). Chi-square test was used to compare differences between categorical variables. P<0.05 was considered significant.
RESULTS
1. Patient characteristics
Among the 50 enrolled patients (28 males, 22 females), slides from 36 patients (20 males, 16 females) were available for immunohistochemical staining. The median age of the 36 patients was 62 (range, 5-207) months. The diagnosis of LCH was made by demonstrating pathological LCs identified by a positive immunohistochemical reaction to CD1a antibodies with clinical manifestations. Biopsy was performed at the primary site including bone, skin, bone marrow, and lymph node. Bone involvement with or without involvement of another site was the most common manifestation and was observed in 72.2% of cases (26/36). Eight patients (22.2%) were diagnosed with multi-system disease and 4 patients (11.1%) with risk organ involvement. Four patients (11.1%) relapsed during follow-up. The median follow-up duration was 69 months (range, 6-132).
2. Gelsolin immunohistochemical staining
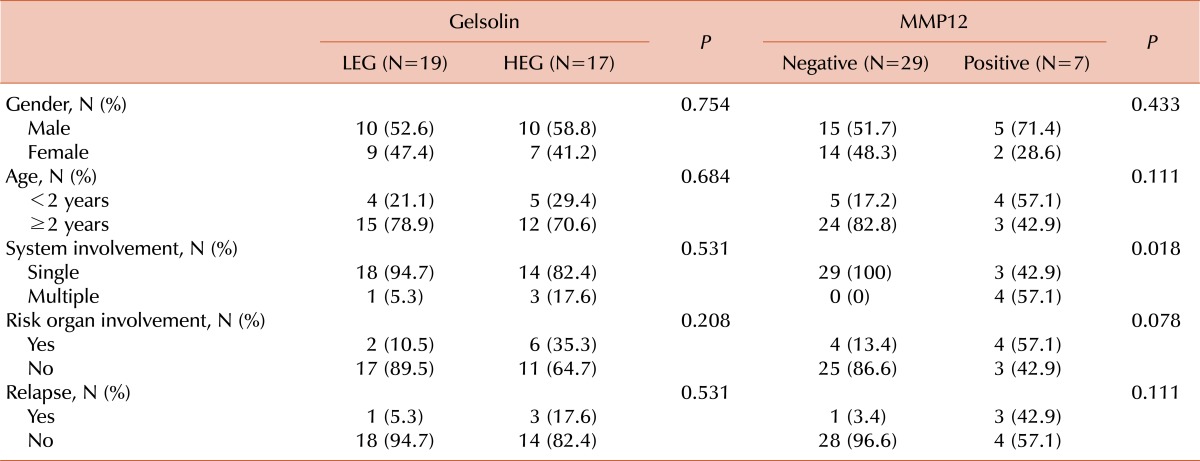
Most patients (28/36, 77.8%) showed diffuse cytoplasmic staining of pathologic LCs (Fig. 1). To determine the predictive power of gelsolin expression for LCH outcomes, immunohistochemical expression of gelsolin was divided into a lower expression group (LEG) including negative and low gelsolin expression and a higher expression group (HEG) (Fig. 1). Nineteen patients (52.8%) were assigned to the LEG including 8 (22.2%) with negative expression; 17 patients (47.2%) were assigned to the HEG. There was no difference between groups in terms of sex and age less than 2 years old (Table 1). Only one patient (5.3%) of the LEG had multi-system involvement, vs. 3 patients (17.6%) of the HEG. In addition, risk organ involvement was more frequent in HEG (6/17, 35.3%) than LEG (2/19, 10.5%) as was the relapse rate (HEG: 3/17, 17.6% vs. LEG 1/19, 5.3%) (Table 1). However, these differences were not statistically significant (P>0.05).
Table 1.
Clinical manifestations according to the immunohistochemistry.
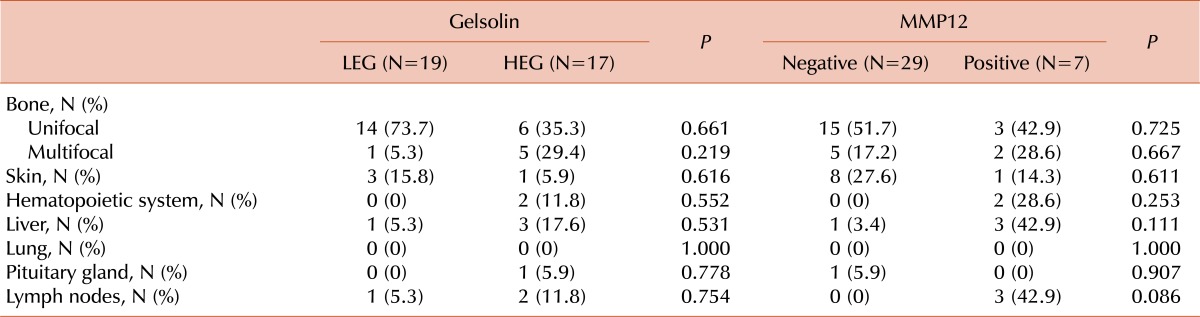
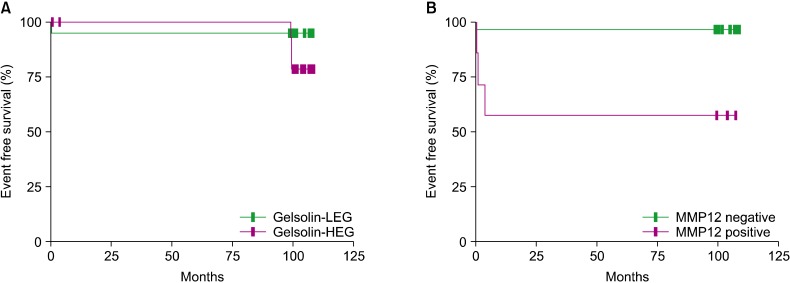
Organ involvement differed between groups. In fact, bone is the most common organ involved in LCH, and this study showed that the HEG had a higher frequency of multifocal bone involvement (HEG: 5/17, 29.4% vs. LEG: 1/19, 5.3%). Among the risk organs, hematopoietic system (HEG: 2/17, 11.8% vs. LEG: 0/19, 0%) and liver (HEG: 3/17, 17.6% vs. LEG: 1/19, 5.3%) involvement were higher in the HEG, while lung and spleen involvement was absent in both groups (Table 2); however, these differences were statistically insignificant (Table 2). The probability of event-free survival (EFS) was 94.7% for LEG patients and 78.6% for HEG patients, although the log-rank tested indicated the difference was not significant (P=0.222) (Fig. 3).
Table 2.
Organ involvement according to immunohistochemistry.
Fig. 3.
Event-free survival (EFS) rates according to immunohistochemical grade. (A) The probability of EFS according to the gelsolin expression. The EFS was 94.7% for LEG patients and 78.6% for HEG patients. The difference was not statistically significant according to the log-rank test (P=0.222). (B) The probability of EFS in terms of MMP positivity. EFS for the negative group was 96.6%, while that of the positive group was 57.1%, a statistically significant difference according to the log-rank test (P=0.002).
3. MMP12 immunohistochemical staining
MMP12 expression was positive in 19.4% (7/37) of patients (Fig. 2). There was no difference between the MMP12-positive and -negative groups with respect to sex and age less than 2 years old (Table 1). Multi-system involvement was present only in patients with MMP12 expression (57.1%) (P=0.018). Involvement of risk organs was also 4.2-fold more frequent in patients with MMP12 expression, although the difference was not statistically significant (P=0.078) (Table 1). Patients with MMP12 expression had a higher probability of multifocal bone disease (2/7, 28.6%) than patients with no MMP12 expression (5/29, 17.2%). Among the risk organs, the involvement of hematopoietic system was found only in patients with MMP12 expression; liver involvement was also higher in this group than in patients with no MMP12 [42.9% (3/7) vs. 3.4% (1/29)]. On the other hand, spleen and lung involvement was absent in both groups (Table 2). We found no statistically significant associations. The probability of EFS in the MMP12-negative group was 96.6%, while that of positive group was 57.1%, a statistically significant difference according to the log-rank test (P=0.002) (Fig. 3).
DISCUSSION
The Working Group of the Histiocyte Society specifies only 2 levels of diagnostic criteria for LCH, namely, a definitive diagnosis and a presumptive diagnosis. A "definitive diagnosis" of LCH requires immunohistochemical identification of LCs by cell surface CD1a or the presence of cells with Birbeck granules observed by electron microscopy [14, 15]. Conventional histology alone can justify only a presumptive diagnosis, but clinical manifestations depend on the site of the lesions, number of involved sites, and the extent to which organ function is compromised. The course of LCH is unpredictable, varying from spontaneous regression and resolution to rapid progression and death or repeated recurrence [16, 17]. Patients with localized disease generally have a good prognosis and require minimal or even no treatment. In contrast, multi-system disease carries the risk of poor outcomes. Thus, the factors influencing LCH outcomes must be understood to prevent disease progression or relapse [18, 19].
Genetic alterations can lead to the development of LCH [1, 4]. These alterations may affect the cellular mechanisms that control proliferation and apoptosis [1, 3, 4]. Some genetic markers associated with LCH have been described in previous reports [1, 18-20]. In patients with LCH, many kinds of immunohistochemical analyses based on functionally relevant genetic markers should be performed [17, 18, 20] to help delineate the different entities of LCH and facilitate an advanced classification system as a basis for selection of appropriate therapeutic approaches.
Gelsolin is well characterized for its functions in cytoskeleton reorganization, cell morphology, and motility [21]. In one study, caspase-3 expression in LCH was significantly more frequent in LCs of patients with single system disease than in those with multi-system disease [22]. Because gelsolin is a substrate for caspase-3, dual roles in promoting apoptosis and protecting cells from apoptosis have been proposed [23, 24]. Gelsolin is highly expressed in LCs and might be associated with disease prognosis [1]. In this study, diffuse expression of gelsolin in both single and multi-system lesions of LCH was observed. Almost all patients assigned to the LEG presented single system involvement (94.7%). A higher tendency of relapse was apparent in the HEG (35.3%) than in LEG (5.3%), and the HEG had a higher tendency for risk organ involvement (17.6%), although these differences were not statistically significant. We expect additional studies of larger patient populations will help clarify the role of gelsolin in the pathogenesis and clinical course of LCH.
MMPs are a family of zinc-dependent endopeptidases that cleave all the major molecules of the extracellular matrix (ECM). In addition to promoting tumor progression and angiogenesis, some MMPs inhibit tumor vascularization [8, 9]. MMP expression is correlated with epithelial dedifferentiation and histological aggressiveness. MMP12 derived from epithelial cells has a different role from that secreted by macrophages [11]. Rust et al. reported MMP12 expression was most abundant in multi-system LCH, which is associated with the worst prognosis [1]. Thus, MMP12 expression may play a role in the progression of LCH. Our study revealed limited expression of MMP12 protein in LCH. The MMP12-negative group presented mainly with single system involvement, whereas the MMP12-positive group showed significant multi-system involvement (P=0.018) and had a higher tendency for risk organ involvement, although this difference was not statistically significant (P=0.078). In addition, MMP12-positive patients has significantly reduced event-free survival (96.6 % vs. 57.1%) (P=0.002). Zyada suggested the possibility of cooperation between macrophages and MMP9 in eosinophilic granuloma, which correlated well with local recurrence. They concluded that MMP9 expression might therefore provide some prognostic indication of the possible aggressive and recurrence behavior of eosinophilic granuloma [25]. Hayashi et al. reported that the reactivity of pathologic LCs in pulmonary LCH was moderate-to-intense for MMP2, weaker for MMP9, and faint for tissue inhibitor of metalloproteinase 1 (TIMP1) and TIMP2. These results indicate an important role for MMPs and TIMPs in LCH [26].
This study aimed to define the genetic and functional characteristics of LCH that may account for diseases prognosis based on gelsolin and MMP12 expression. Despite the low incidence of this disease, we succeeded in recruiting a number of patients over 10 years, representing various clinical courses of the disease, although statistical significance could not be achieved due to the small sample size. We suggest gelsolin and MMP12 may be involved in the pathogenesis of LCH and may be associated with poorer clinical outcomes and prognosis, but more studies are needed to validate this hypothesis. In future, it will be important to define what induces gelsolin and MMP12 expression and how these proteins affect the pathogenesis of LCH.
Footnotes
This study was supported by a clinical study grant from Chungnam National University Hospital.
References
- 1.Rust R, Kluiver J, Visser L, et al. Gene expression analysis of dendritic/Langerhans cells and Langerhans cell histiocytosis. J Pathol. 2006;209:474–483. doi: 10.1002/path.2003. [DOI] [PubMed] [Google Scholar]
- 2.Sholl LM, Hornick JL, Pinkus JL, Pinkus GS, Padera RF. Immunohistochemical analysis of langerin in langerhans cell histiocytosis and pulmonary inflammatory and infectious diseases. Am J Surg Pathol. 2007;31:947–952. doi: 10.1097/01.pas.0000249443.82971.bb. [DOI] [PubMed] [Google Scholar]
- 3.Amir G, Weintraub M. Association of cell cycle-related gene products and NF-kappaB with clinical parameters in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2008;50:304–307. doi: 10.1002/pbc.21198. [DOI] [PubMed] [Google Scholar]
- 4.Petersen BL, Rengtved P, Bank MI, Carstensen H. High expression of markers of apoptosis in Langerhans cell histiocytosis. Histopathology. 2003;42:186–193. doi: 10.1046/j.1365-2559.2003.01565.x. [DOI] [PubMed] [Google Scholar]
- 5.Kwiatkowski DJ. Functions of gelsolin: motility, signaling, apoptosis, cancer. Curr Opin Cell Biol. 1999;11:103–108. doi: 10.1016/s0955-0674(99)80012-x. [DOI] [PubMed] [Google Scholar]
- 6.Sun HQ, Yamamoto M, Mejillano M, Yin HL. Gelsolin, a multifunctional actin regulatory protein. J Biol Chem. 1999;274:33179–33182. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- 7.Witke W, Li W, Kwiatkowski DJ, Southwick FS. Comparisons of CapG and gelsolin-null macrophages: demonstration of a unique role for CapG in receptor-mediated ruffling, phagocytosis, and vesicle rocketing. J Cell Biol. 2001;154:775–784. doi: 10.1083/jcb.200101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nénan S, Boichot E, Lagente V, Bertrand CP. Macrophage elastase (MMP-12): a pro-inflammatory mediator? Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):167–172. doi: 10.1590/s0074-02762005000900028. [DOI] [PubMed] [Google Scholar]
- 9.Gueders MM, Foidart JM, Noel A, Cataldo DD. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in the respiratory tract: potential implications in asthma and other lung diseases. Eur J Pharmacol. 2006;533:133–144. doi: 10.1016/j.ejphar.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 10.Kerkelä E, Ala-aho R, Klemi P, et al. Metalloelastase (MMP-12) expression by tumour cells in squamous cell carcinoma of the vulva correlates with invasiveness, while that by macrophages predicts better outcome. J Pathol. 2002;198:258–269. doi: 10.1002/path.1198. [DOI] [PubMed] [Google Scholar]
- 11.Kerkelä E, Ala-Aho R, Jeskanen L, et al. Expression of human macrophage metalloelastase (MMP-12) by tumor cells in skin cancer. J Invest Dermatol. 2000;114:1113–1119. doi: 10.1046/j.1523-1747.2000.00993.x. [DOI] [PubMed] [Google Scholar]
- 12.Balaz P, Friess H, Kondo Y, Zhu Z, Zimmermann A, Büchler MW. Human macrophage metalloelastase worsens the prognosis of pancreatic cancer. Ann Surg. 2002;235:519–527. doi: 10.1097/00000658-200204000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladisch S, Gadner H, Aricò M, et al. The Histiocyte Society. LCH-I: a randomized trial of etoposide vs. vinblastine in disseminated Langerhans cell histiocytosis. Med Pediatr Oncol. 1994;23:107–110. doi: 10.1002/mpo.2950230207. [DOI] [PubMed] [Google Scholar]
- 14.Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. The WHO Committee On Histiocytic/Reticulum Cell Proliferations. Reclassification Working Group of the Histiocyte Society. Med Pediatr Oncol. 1997;29:157–166. doi: 10.1002/(sici)1096-911x(199709)29:3<157::aid-mpo1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Satter EK, High WA. Langerhans cell histiocytosis: a review of the current recommendations of the Histiocyte Society. Pediatr Dermatol. 2008;25:291–295. doi: 10.1111/j.1525-1470.2008.00669.x. [DOI] [PubMed] [Google Scholar]
- 16.Garabedian L, Struyf S, Opdenakker G, Sozzani S, Van Damme J, Laureys G. Langerhans cell histiocytosis: a cytokine/chemokine-mediated disorder? Eur Cytokine Netw. 2011;22:148–153. doi: 10.1684/ecn.2011.0290. [DOI] [PubMed] [Google Scholar]
- 17.Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557–4567. doi: 10.4049/jimmunol.0902336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bank MI, Rengtved P, Carstensen H, Petersen BL. p53 expression in biopsies from children with Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 2002;24:733–736. doi: 10.1097/00043426-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Schouten B, Egeler RM, Leenen PJ, Taminiau AH, van den Broek LJ, Hogendoorn PC. Expression of cell cycle-related gene products in Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 2002;24:727–732. doi: 10.1097/00043426-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Kim SY, Kim HJ, Kim HJ, et al. Role of p16 in the pathogenesis of Langerhans cell histiocytosis. Korean J Hematol. 2010;45:247–252. doi: 10.5045/kjh.2010.45.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silacci P, Mazzolai L, Gauci C, Stergiopulos N, Yin HL, Hayoz D. Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci. 2004;61:2614–2623. doi: 10.1007/s00018-004-4225-6. [DOI] [PubMed] [Google Scholar]
- 22.Amir G, Weintraub M. Association of cell cycle-related gene products and NF-kappaB with clinical parameters in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2008;50:304–307. doi: 10.1002/pbc.21198. [DOI] [PubMed] [Google Scholar]
- 23.Geng YJ, Azuma T, Tang JX, et al. Caspase-3-induced gelsolin fragmentation contributes to actin cytoskeletal collapse, nucleolysis, and apoptosis of vascular smooth muscle cells exposed to proinflammatory cytokines. Eur J Cell Biol. 1998;77:294–302. doi: 10.1016/S0171-9335(98)80088-5. [DOI] [PubMed] [Google Scholar]
- 24.Kothakota S, Azuma T, Reinhard C, et al. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- 25.Zyada MM. Expression of matrix metalloproteinase-9 and significance of a macrophage assay in eosinophilic granuloma. Ann Diagn Pathol. 2009;13:367–372. doi: 10.1016/j.anndiagpath.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi T, Rush WL, Travis WD, Liotta LA, Stetler-Stevenson WG, Ferrans VJ. Immunohistochemical study of matrix metalloproteinases and their tissue inhibitors in pulmonary Langerhans' cell granulomatosis. Arch Pathol Lab Med. 1997;121:930–937. [PubMed] [Google Scholar]