Abstract
We report a case of diffuse large B-cell lymphoma (DLBCL) treated successfully with clarithromycin (CAM) and prednisolone (PSL). A 71-year-old woman presented with fever and cervical pain. DLBCL was diagnosed based on histological results from lymph node biopsy. Cervical pain was thought to be caused by the invasion of lymphoma cells into the cervical vertebrae. She initially received radiotherapy for the cervical lesion. She did not receive conventional chemotherapy because of the risk of recurrent non-tuberculous mycobacteria infection; therefore, she was treated with 20 mg/day PSL and 800 mg/day CAM to induce apoptosis in lymphoma cells. Complete remission was achieved after 6 months. The present findings suggest that CAM and PSL may be effective in some cases of DLBCL.
Keywords: Diffuse large B-cell lymphoma, Clarithromycin, Prednisolone, Apoptosis
INTRODUCTION
Macrolides have been proposed as potential antineoplastic and immunomodulatory agents [1]. This class of antibiotic agents has an anti-lymphoproliferative effect via down-regulation of the anti-apoptotic proteins bcl-2 and bcl-xL [2, 3]; therefore, macrolides may be clinically applicable in the treatment of malignancy, particularly lymphoid malignancy. There have been several previous reports indicating that malignant lymphoma and plasma cell disorders can be successfully treated with the macrolide clarithromycin (CAM) alone or in combination with a corticosteroid [1-4]. Corticosteroids are key agents for the treatment of lymphoid malignancy due to their ability to induce apoptosis of malignant lymphoid cells. We encountered a case, in which a positive outcome was achieved with the use of CAM and prednisolone (PSL) for the treatment of advanced diffuse large B-cell lymphoma (DLBCL).
CASE REPORT
A 71-year-old woman was admitted to hospital with fever and cervical pain. For the previous year, she had been treated with antituberculars for non-tuberculous mycobacteria (NTM) infection of the lung after detection of Mycobacterium avium in her sputum. Her symptoms reappeared approximately 10 months after completion of antitubercular therapy, and just prior to admission, she had received levofloxacin then amoxicillin for fever based on suspicion of recurrent NTM or another pulmonary infection. However, while chest radiography revealed no new lesions, her symptoms did not improve.
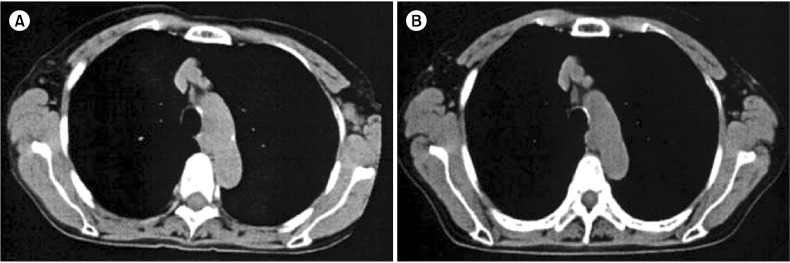
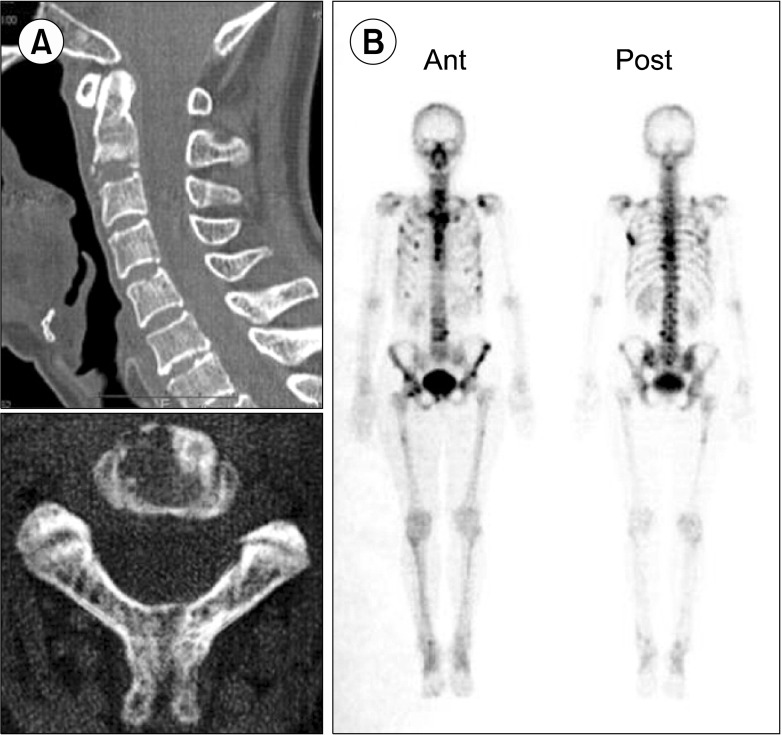
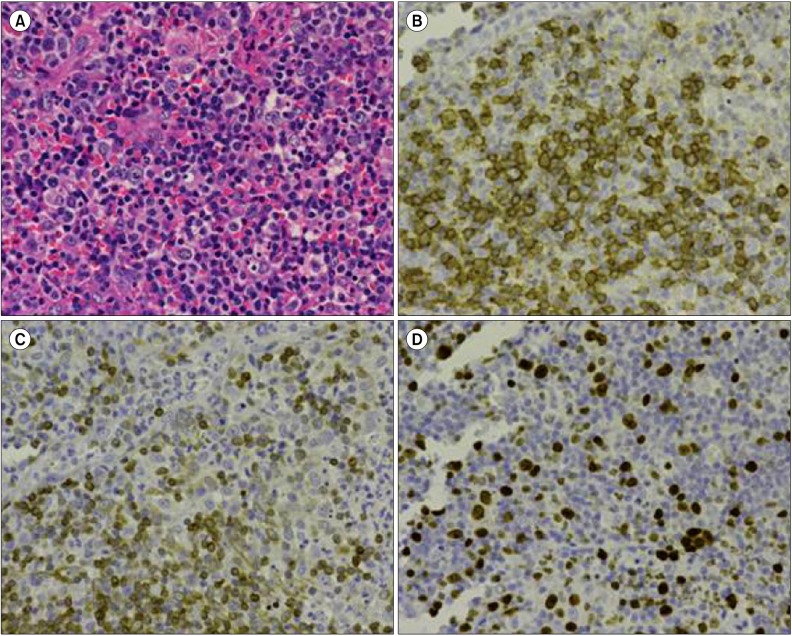
On admission, physical examination showed several swollen lymph nodes in the left axillary region. Lactate dehydrogenase levels were 148 IU/L and serum soluble interleukin-2 receptor (sIL-2R) levels were increased to 928 U/mL. Chest computed tomography (CT) revealed axillary lymphadenopathy (Fig. 1A) accompanied by slight infiltration shadows of NTM in the right lung field. Abdominal CT revealed neither para-aortic nor inguinal lymphadenopathy. Bone CT revealed bone destruction of the axis (Fig. 2A). Bone scintigram revealed uptake into the vertebrae, sternum, ribs, left scapula, pelvis, and right femur (Fig. 2B). Magnetic resonance imaging revealed high-intensity areas in the cervical and thoracic vertebrae on T2-weighted imaging. Fluorodeoxyglucose (FDG)-positron emission tomography (PET) demonstrated increased spotty uptake of FDG into the vertebrae, sternum, ribs, pelvis, right femur, and bilateral axillary lymph nodes (Fig. 3A). Along with spotty uptake, moderate serial uptake into the cervical to lumbar-region vertebrae was also evident (Fig. 3A). Esophagogastroduodenoscopy and colonoscopy showed no abnormal lesions associated with malignancy. No malignant gynecological lesions were found. Biopsy specimens from the left axillary lymph nodes revealed an absence of follicular structures and diffusely proliferative large lymphoid cells with pleomorphic, irregular nuclei and prominent nucleoli, accompanied by small lymphoid cells (Fig. 4A). Immunohistochemical staining revealed that the large lymphoid cells were positive for CD20, bcl-2, and MIB-1 (Fig. 4B-D), but negative for CD3, CD30, and EBER. MIB-1 proliferation index was 40%. The small lymphoid cells were positive for CD3 but negative for CD20. Ziehl-Neelsen staining for acid-fast bacilli yielded negative results. Biopsy specimens were inadequate for conducting chromosomal analysis or determining genetic rearrangements in the axillary lymph nodes. Although neither bone marrow aspiration specimens nor bone marrow biopsy specimens from the ileum revealed large lymphoid cells, the results of bone CT, bone scintigraphy, and FDG-PET indicated multiple lesions of the bone and bone marrow. Based on these findings, she was diagnosed with clinical stage IV DLBCL, with low-to-intermediate risk according to the international prognostic index [5]. Her cervical pain was thought to be due to invasion of the lymphoma cells into the cervical vertebrae.
Fig. 1.
Computed tomography (CT). (A) Chest CT revealing left axillary lymphadenopathy at admission. (B) Chest CT revealing no left axillary lymphadenopathy 6 months after initiation of clarithromycin and prednisolone treatment.
Fig. 2.
Bone CT and bone scintigram. (A) Bone CT revealing bone destruction of the axis at admission. (B) Bone scintigram revealing abnormal uptake into vertebrae, sternum, ribs, left scapula, pelvis, and right femur at admission.
Fig. 3.
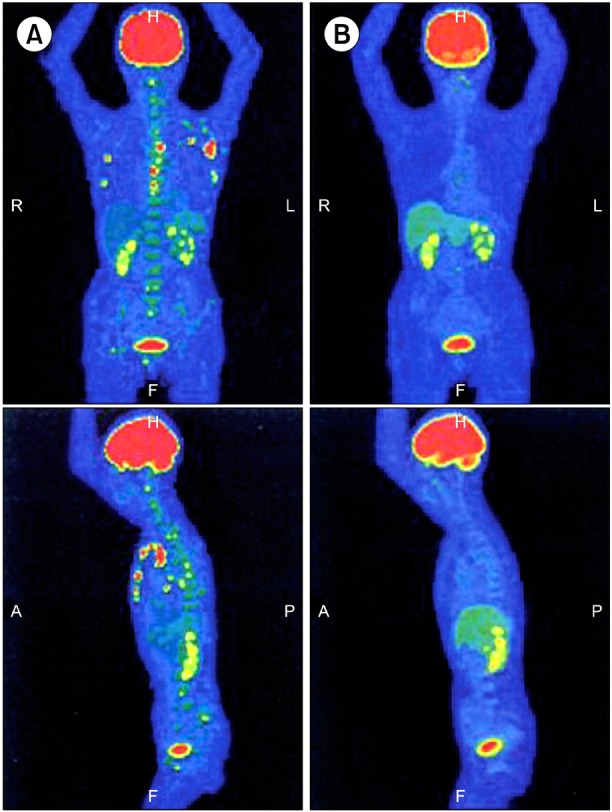
Fluorodeoxyglucose (FDG)-positron emission tomography (PET). (A) FDG-PET revealing increased spotty uptake into vertebrae, sternum, rib bones, pelvis, right femur, and bilateral axillary lymph nodes, and moderate serial uptake into the cervical to lumbar-region vertebrae at admission. (B) FDG-PET showing no abnormal uptake 6 months after initiation of clarithromycin and prednisolone treatment.
Fig. 4.
Histological and immunohistochemical examination of left axillary lymph node biopsy specimens. (A) Histological examination revealing proliferation of atypical large lymphocytes with pleomorphic, irregular nuclei and prominent nucleoli, accompanied by small lymphocytes (hematoxylin and eosin stain ×400). (B) Immunohistochemical examination for CD20 exhibiting positive staining in large lymphocytes (immunohistochemical stain ×400). (C) Immunohistochemical examination for bcl-2 exhibiting positive staining in large lymphocytes (immunohistochemical stain ×400). (D) Immunohistochemical examination for MIB-1 exhibiting positive staining in large lymphocytes (immunohistochemical stain ×400).
She initially received radiotherapy for the cervical lesions to avoid neurological symptoms. She subsequently declined conventional chemotherapy due to the risk of recurrent NTM infection. We therefore began treatment with CAM (800 mg/day) and PSL (20 mg/day) on the condition that conventional chemotherapy would be started immediately if DLBCL exacerbation was observed. Fever improved within a few days. The PSL dosage was initially 20 mg/day for 2 weeks and subsequently 15 mg/day for 2 weeks. The maintenance dosage was 10 mg/day. Her sIL-2R levels decreased from 928 to 619 U/mL approximately 2 months after beginning CAM and PSL treatment. At that time, contraction of the axillary lymph nodes was found on CT. Complete remission (CR) was achieved after 6 months, with improvements in lymphadenopathy and lesions of the bone and bone marrow apparent on both CT (Fig. 1B) and FDG-PET (Fig. 3B). The sIL-2R levels decreased to 215 U/mL. Slightly reduced taste sensation was reported, and was considered a side effect of long-term CAM use. She remains in CR 7 months after initiation of treatment.
DISCUSSION
As the patient had been treated for NTM until approximately 10 months prior to admission, fever and lymphadenopathy were initially suspected to have been due to recurrent NTM infection. However, chest CT did not demonstrate any exacerbation of infiltration shadows associated with NTM. Necrotizing granuloma-containing neutrophils, epithelioid cells, and Langhans giant cells, which are suggestive of NTM, were not evident in biopsy specimens from the left axillary lymph nodes. We therefore could not diagnose NTM recurrence. Similarly, we could not diagnose reactive lymphoid hyperplasia because no follicular structure was detectable and large lymphoid cells were positive for bcl-2. The final diagnosis was DLBCL.
Several cases have been reported where NTM developed during chemotherapy for cancer [6], so we initially decided to use CAM to treat the lymphoma, rather than conventional chemotherapy. As CAM is known to be effective in NTM, this treatment was considered advantageous for both the lymphoma and NTM. We prescribed PSL for its anti-inflammatory effects as well as its apoptotic effects on lymphoid cells. CR was achieved following 6-month CAM and PSL treatment.
Serum sIL-2R levels are closely related to disease activity in non-Hodgkin lymphoma patients. Response to chemotherapy is followed by a decrease in sIL-2R levels. Levels of sIL-2R can also be used to predict disease relapse [7]. We therefore continued to monitor sIL-2R levels to determine disease activity, response to therapy, and risk of relapse.
CAM has recently been found to have anti-proliferative activities against some lymphomas [1-3]. Ishimatsu et al. [2] reported 2 cases of pulmonary mucosa-associated lymphoid tissue lymphoma successfully treated with CAM. They speculated that the macrolide induced apoptosis of lymphocytes via the down-regulation of bcl-xL. We have also reported a case of t(14;18)(q32;21)-positive follicular B-cell lymphoma that was successfully treated with CAM [3]. As CAM is known to induce down-regulation of bcl-2 [8], and lymphoma cells from our previous case were positive for bcl-2, we speculated that CAM induced apoptosis in the lymphoma cells.
Chen et al. [9] reported bcl-2 expression in 68.5% and 50.0% of Chinese and Western DLBCL cases, respectively. DLBCL is heterogeneous, and bcl-2 expression is variable within the 2 major DLBCL subgroups, being germinal center B-cell-like (GCB) or activated B-cell-like (ABC) DLBCL [10]. Iqbal et al. [10] reported that t(14;18)(q32;q21) was frequently observed in the GCB subgroup and was strongly associated with bcl-2 expression. Patients with ABC DLBCL did not exhibit t(14;18)(q32;21), but demonstrated a markedly higher frequency of amplification for chromosome 18q21, on which bcl-2 resides.
Chromosomal analysis was not undertaken in this case, so it was unclear to which subgroup the disease belonged. However, because the lymphoma cells were positive for bcl-2, CAM was likely to have induced apoptosis of the lymphoma cells.
Nuclear factor (NF)-κB is a transcription factor that up-regulates anti-apoptotic genes, namely bcl-2, bcl-xL, XIAP, and survivin. NF-κB is closely associated with lymphomas. Heckman et al. reported that NF-κB activates bcl-2 expression in t(14;18) lymphoma cells [11]. Bavi et al. [12] reported that NF-κB was detected in 25.6% of DLBCL tumors, and NF-κB over-expression was found to be a prognostic marker for poor survival in DLBCL. NF-κB inhibition has also been shown to down-regulate expression of the down-stream target gene products bcl-2, bcl-xL, XIAP, and survivin, leading to apoptosis of lymphoma cells.
As CAM has been reported to inhibit NF-κB activation of human blood mononuclear cells (i.e., lymphocytes and monocytes/macrophages) [13], the lymphoma cells in this case may have undergone apoptosis via inhibition of NF-κB activation. Although the above-mentioned case reports did not discuss the relationships between NF-κB and bcl-2/bcl-xL, CAM might also have been effective in those cases through its inhibition of NF-κB activation.
Glucocorticoids (GC) are also known to induce apoptosis of lymphoid cells. In fact, GC-induced apoptosis has been exploited in the treatment of lymphoid malignancies [14]. The lympholytic effect of GCs is mediated by cytoplasmic steroid receptors that are translocated to the nucleus, where they signal apoptosis. GC-induced apoptosis is attenuated by bcl-2 [15]; therefore, down-regulation of bcl-2 by macrolide treatment when combined with GC may result in advantageous apoptotic effects. This synergy between CAM and PSL was effective in the present case of bcl-2-positive DLBCL.
Although our patient benefited from CAM and PSL treatment, this combination cannot be considered a recommended therapy at present. Our case demonstrated that this treatment is an option in patients who refuse chemotherapy or in elderly patients with underlying medical conditions.
Clinical trials need to be conducted to better determine the efficacy and tolerability of CAM and PSL in DLBCL before this treatment can be adopted on a wider basis.
References
- 1.Govi S, Dognini GP, Licata G, et al. Six-month oral clarithromycin regimen is safe and active in extranodal marginal zone B-cell lymphomas: final results of a single-centre phase II trial. Br J Haematol. 2010;150:226–229. doi: 10.1111/j.1365-2141.2010.08179.x. [DOI] [PubMed] [Google Scholar]
- 2.Ishimatsu Y, Mukae H, Matsumoto K, et al. Two cases with pulmonary mucosa-associated lymphoid tissue lymphoma successfully treated with clarithromycin. Chest. 2010;138:730–733. doi: 10.1378/chest.09-2358. [DOI] [PubMed] [Google Scholar]
- 3.Ohe M, Hashino S. A case of follicular B-cell lymphoma treated using clarithromycin. Korean J Hematol. 2011;46:203–206. doi: 10.5045/kjh.2011.46.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niesvizky R, Jayabalan DS, Christos PJ, et al. BiRD (Biaxin [clarithromycin]/Revlimid [lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. Blood. 2008;111:1101–1109. doi: 10.1182/blood-2007-05-090258. [DOI] [PubMed] [Google Scholar]
- 5.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto A, Enomoto T, Muroya Y, et al. Pulmonary non-tuberculous mycobacteriosis (Mycobacterium intracellulare) with cavities developing in a non-small cell lung cancer patient during chemotherapy. Nihon Kokyuki Gakkai Zasshi. 2010;48:609–613. [PubMed] [Google Scholar]
- 7.Pavlidis NA, Manoussakis MN, Germanidis GS, et al. Serum soluble interleukin-2 receptors in B-cell lymphoproliferative malignancies. Med Pediatr Oncol. 1992;20:26–31. doi: 10.1002/mpo.2950200106. [DOI] [PubMed] [Google Scholar]
- 8.Ohara T, Morishita T, Suzuki H, Masaoka T, Ishii H, Hibi T. Antibiotics directly induce apoptosis in B cell lymphoma cells derived from BALB/c mice. Anticancer Res. 2004;24:3723–3730. [PubMed] [Google Scholar]
- 9.Chen Y, Han T, Iqbal J, et al. Diffuse large B-cell lymphoma in Chinese patients: immunophenotypic and cytogenetic analyses of 124 cases. Am J Clin Pathol. 2010;133:305–313. doi: 10.1309/AJCP4H6ADGYDZMOA. [DOI] [PubMed] [Google Scholar]
- 10.Iqbal J, Neppalli VT, Wright G, et al. BCL2 expression is a prognostic marker for the activated B-cell-like type of diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:961–968. doi: 10.1200/JCO.2005.03.4264. [DOI] [PubMed] [Google Scholar]
- 11.Heckman CA, Mehew JW, Boxer LM. NF-kappaB activates Bcl-2 expression in t(14;18) lymphoma cells. Oncogene. 2002;21:3898–3908. doi: 10.1038/sj.onc.1205483. [DOI] [PubMed] [Google Scholar]
- 12.Bavi P, Uddin S, Bu R, et al. The biological and clinical impact of inhibition of NF-κB-initiated apoptosis in diffuse large B cell lymphoma (DLBCL) J Pathol. 2011;224:355–366. doi: 10.1002/path.2864. [DOI] [PubMed] [Google Scholar]
- 13.Ichiyama T, Nishikawa M, Yoshitomi T, et al. Clarithromycin inhibits NF-kappaB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells. Antimicrob Agents Chemother. 2001;45:44–47. doi: 10.1128/AAC.45.1.44-47.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ploner C, Schmidt S, Presul E, et al. Glucocorticoid-induced apoptosis and glucocorticoid resistance in acute lymphoblastic leukemia. J Steroid Biochem Mol Biol. 2005;93:153–160. doi: 10.1016/j.jsbmb.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Weller M. Glucocorticoid treatment of primary CNS lymphoma. J Neurooncol. 1999;43:237–239. doi: 10.1023/a:1006254518848. [DOI] [PubMed] [Google Scholar]