Abstract
Background
Emerging evidence showed that resistin induces vascular smooth muscle cell (VSMC) migration, a critical step to initiating vascular restenosis. Mechanistically, adhesion molecule expression and cytoskeletal rearrangement have been observed in this progress. Given that matrix metalloproteinases (MMPs) also regulates cell migration, we hypothesized that MMPs may mediate resistin-induced VSMC migration.
Materials and Methods
Human VSMCs were treated with recombinant human resistin at physiological (10 ng/mL) and pathological (40 ng/mL) concentrations for 24 hours. Cell migration was determinate by Boyden chamber assay. MMP and TIMP mRNA and protein levels were measured with real-time PCR and ELISA. MMP enzymatic activity was measured by zymography on precast gels. In another experiment, neutralizing antibodies against MMP-2 and MMP-9 were co-incubated with resistin in cultured VSMCs. The regulation of MMP by protein kinase C (PKC) was determined by εV1–2, a selective PKCε inhibitor.
Results
Resistin-induced SMC migration was confirmed by Boyden chamber assay. 40ng/mL Resistin increased SMC migration by 3.7 fold. Molecularly, resistin stimulated MMP-2 and - MMP9 mRNA and protein expressions. In contrast, the TIMP-1 and TIMP-2 mRNA levels were inhibited by resistin. Neutralizing antibodies against MMP-2 and MMP-9 effectively reversed VSMC migration. Furthermore, resistin activated PKCε and selective PKCε inhibitor suppressed resistin-induced MMP expression, activity and cell migration.
Conclusions
Our study confirmed that resistin increases vascular smooth muscle cell migration in vitro. Mechanistically, resistin-stimulated cell migration was associated with increased MMP expression and activity, which was dependent on PKCε activation.
Keywords: diabetes mellitus, obesity, resistin, smooth muscle cell migration, restenosis
Introduction
Obesity continues to pose significant public health problems in the developed nations and is steadily increasing in the developing world. Patients with obesity are at an increased risk of developing cardiovascular complications [1, 2] including atherosclerosis [3, 4] and restenosis [5–8]. Ngo et al [6] demonstrated the relationship between obesity and aortic stenosis. Nikolsky and colleagues [7] confirmed that obesity was a significant risk factor for restenosis and major adverse cardiac events following bare-metal stent placement in the TAXUS-IV trial. Other studies showed increased resistin levels in obese patients [4]. Recently, Owens et al [8] reported that decreased adiponectin and elevated resistin levels are associated with poor outcomes following lower extremity revascularization with autogenous vein. Several adipokines, such as adiponectin and leptin, were implicated in connecting obesity to vascular complications. Resistin is a newly-identified adipokine that was found increased in obese/diabetics patients. The pathological plasma concentration of resistin is around 40ng/mL while its normal level is around 10 ng/mL [8–14]. Accumulating evidence suggested that resistin may play a role in the development of vascular complications [8, 15–17]. However, little is known about the mechanisms.
Vascular smooth muscle cell (VSMC) migration is involved in initiating neointimal hyperplasia [18–20] and ultimately restenosis. Two pieces of evidence from scrape-wound assay showed that resistin promoted the VSMC migration [17, 21]. Mechanistically, matrix metalloproteinases (MMPs), particularly MMP-2 and MMP-9, are known to be involved in VSMC migration [22, 23] and protein kinase C (PKC) can regulate MMP expression [24]. We, therefore, hypothesize that MMPs may be involved in resistin-mediated VSMC migration and that PKCε, one detectable PKC isozyme in VSMCs, might modulate MMP expression. In this study, we used Boyden chamber assay, an alternative migration model, to confirm that resistin at high (pathological) concentration can stimulate VSMC migration. We also examined the effects of resistin on the expression of MMP-2 and -9 and activation of PKCε. The involvements of MMPs and PKCε in resistin-induced VSMC migration were further confirmed using specific blocking antibodies.
Materials and Methods
Materials and reagents
Human coronary artery smooth muscle cells (HCASMC) and SMC growth medium were from Genlantis (San Diego, CA). Cells came at passage 2 and were used at passages 5–8. Recombinant human resistin was obtained from EMD Biosciences Inc (San Diego, CA). Polycarbonate membrane transwell inserts (pore size 8.0 µm) and plates for Boyden chamber assay were from Corning Inc (Corning, NY). The Protein and RNA Isolation System (PARIS) kits were obtained from Ambion (Austin, TX). 10% zymogram precast gels for MMP activity detection were purchased from Bio-Rad (Hercules, CA). Antibodies against human MMP-2 and MMP-9 were from Abcam (Cambridge, MA). The enzyme-linked immunosorbent assay (ELISA) kits for human MMP-2 and MMP-9 were from Calbiochem (San Diego, CA). The high capacity RNA-to-DNA kit and power SYBR Green PCR master mix were from Applied Biosystems (Foster City, CA). DAPI (4, 6-diamidino-2-phenylindole), trypsin, and non-specific immunoglobulin (IgG) were from Sigma (St. Louis, MO). PKCε antibody was from Millipore (Billerica, MA). Selective PKCε inhibitor εV1–2 was from Dr. Daria Mochly-Rosen’s laboratory.
SMC migration
Boyden chamber assay was employed in this study as an alternative migration model to scrape-wound assay. Serum-starved HCASMCs (1×105 cells/well) were placed into the membrane-embedded insert with 1 mL of DMEM. The bottom chamber contained 2 mL of DMEM. Resistin (10 or 40 ng/mL) was added to medium in the bottom chamber. After 12 hours of incubation, non-migrated cells were scraped off from the top side of the insert membrane. Migrated cells on the bottom side were fixed in 4% formalin in PBS, stained with DAPI and checked under a fluorescent microscope. Migrated cells in each well were counted in four high-power (400×) fields. In addition, resistin at 40ng/ml was added to both top and bottom chamber to examine its chemkinetic effects on VSMCs.
Pharmacological interventions
Drug intervention on resistin-induced cell migration was tested using a Boyden chamber with the addition of anti-MMP-2 and MMP-9 antibodies, or PKCε-selective inhibitor, εV1–2 [25]. Anti-MMP-2, MMP-9, or non-specific IgG (10µg/mL) was added in the bottom chamber and pre-incubated for 2 hours with SMCs before introducing resistin (40ng/mL). PKCε inhibitor (1µmol/L) was also pre-incubated 2 hours before the addition of resistin. Migrated cells in different treatments were counted in the same way as mentioned above.
Real-time polymerase chain reaction (Real time RT-PCR)
Total RNA from HCASMCs was isolated with the PARIS kit as instructed by the manufacturer. Complementary DNA (cDNA) was generated by reverse transcription from messenger RNA (mRNA) with the high capacity RNA-to-DNA kit. The power SYBR green PCR master mix was used for Real time RT-PCR reaction. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene was used as an internal control to account for variations in sample loading. Human MMP-2, MMP-9, TIMP-1, TIMP-2 and GAPDH primers were listed in Table 1. Real time RT-PCR was performed in a Mastercycler RT-PCR detection system (Eppendorf, Westbury, NY). The thermal cycle conditions used for reverse transcription were as follows: 5 minutes at 25°C, 60 minutes at 37°C, and 5 minutes at 95°C. The conditions used for RT-PCR were as follows: 3 minutes at 95°C, 40 cycles of 20 seconds at 95°C, and 1 minute at 60°C. Controls were performed with no reverse transcription (mRNA sample only) or no mRNA (water only) to demonstrate the specificity of the primers and the lack of DNA contamination in samples. The relative level of target (MMP and TIMP) mRNA in each group was normalized against internal housekeeping gene GAPDH using the calculation formula of 2 [Ct GAPDH-CtMMP]. The target mRNA levels in drug treated groups were further normalized against the control group.
Table.
The primer sequences of human MMPs and TIMPs for real-time polymerase chain reaction
| Gene name | GenBank number | Forward | Reverse |
|---|---|---|---|
| MMP-2 | NM_004530 | CAACTACAACTTCTTCCCTCGCA | GGTCACATCGCTCCAGACTTG |
| MMP-9 | NM_004994 | GCATAAGGACGACGTGAATGGC | CGGTGTGGTGGTGGTTGGAG |
| TIMP-1 | NM_003254 | AAGGCTCTGAAAAGGGCTTC | GAAAGATGGGAGTGGGAACA |
| TIMP-2 | NM_003255 | CCAAGCAGGAGTTTCTCGAC | GACCCATGGGATGAGTGTTT |
| GAPDH | NM_002046 | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
GAPDH, Glyceraldehydes-3-phosphate dehydrogenase; MMP, matrix metalloproteinase; TIMP, tissue inhibitor metalloproteinase.
Enzyme-linked immunosorbent assay (ELISA)
Total cell lysates were collected and the quantification of MMP-2 and MMP-9 proteins was carried out with the ELISA kit according to the manufacturer's instructions. The assay was quantified with a spectrophotometric microplate reader (iMark, Bio-Rad) at a dual wavelength of 450/595 nm. Data were presented as a percentage of controls.
Gelatin zymography
Equal amounts of cell lysates (20 µg protein per lane) were analyzed by gel zymography on 10% zymogram precast gels. Protein-embedded gels were incubated in 2.5% Triton X-100 for 30 minutes. Gels were then incubated in the developing buffer (50 mM Tris-HCl, pH 7.8, 200mM NaCl, 5 mM CaCl2 and 0.02% Brij 35) for 16 hours at 37°C to enable MMPs cleaving activity. After rinsing in water, each gel was stained with Coomassie blue for 2 hours and destained in 10% ethanol, 5% acetic acid for 24–36 hours. Proteolytic activities of MMPs were detected by clear bands which indicated the lysis of the substrate. MMP activity quantification was performed with NIH ImageJ software program.
Membrane-bound PKCε determination
Activation of PKCε, as measured by its translocation to the membrane fraction was determined by Western blot of the membrane extraction of HCASMCs. Briefly, 80–90% confluent HCASMCs were starved in serum-free DMEM for 24 hours. Starved cells were then treated with vehicle (0.9% normal saline), resistin (40ng/mL), or resistin plus PKCε inhibitor (1µmol/L, 2 hours prior to resistin administration) for another 24 hours. Cellular membrane extraction and western blot were conducted as described by Christopher et al [26]. The dilution ratios of PKCε primary antibody and corresponding secondary antibody were 1:1000 and 1:5000 respectively. Immunoreactive bands were visualized with the use of a chemiluminescence reagent (Pierce ECL Western Blotting Substrate, Pierce; Rockford, IL) according to the manufacturer’s protocol. Densitomitric analysis was performed using the NIH Image J program.
Statistical analysis
Concentration-dependent data from RT-PCR, ELISA and cell migration were compared by one-factor analysis of variance (ANOVA); drug interference data from antibody blocking and PKCε inhibitor experiments were analyzed using two-factor ANOVA. Statistical significance was considered if the P-value was < 0.05.
Results
Effect of resistin on HCASMC migration
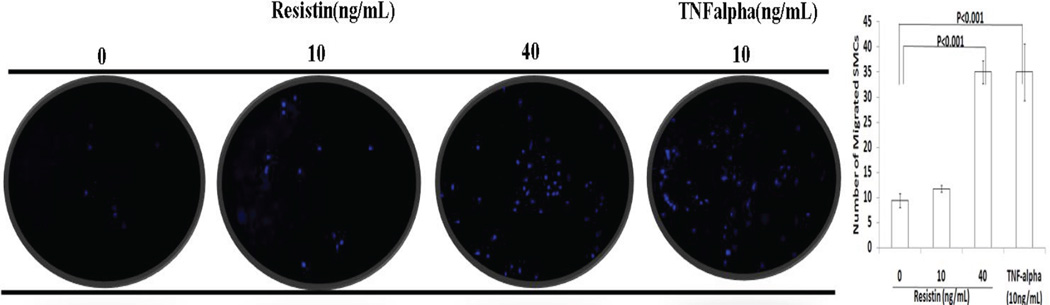
The effect of resistin on SMC migration was studied in the Boyden chamber assay. Cells were counted and averaged from four randomly observed fields. As shown in Figure 1, the average numbers of migrated SMCs in the control, 10 ng/mL of resistin, and 40 ng/mL of resistin groups were 9.4 (±1.5), 11.7 (±0.7), and 35.0 (±2.3) respectively. This reflected a 1.2 fold increase in cell migration after treatment with 10ng/mL resistin (P>0.05) and a 3.7 fold increase after treatment with 40ng/mL resistin (P<0.001). We used TNF-alpha as a positive control, which significantly induced cell migration at 10ng/mL (P<0.001).
Figure 1.
In vitro effect of resistin on human coronary artery smooth muscle cell migration in Boyden chamber assay. Two concentrations of resistin at physiological (10ng/mL) and pathological (40 ng/mL) concentrations were tested. TNF-alpha was used as a positive control. Values are expressed as the number of migrated cells, and each bar represents the mean±SEM of quadruplicate determinations.
Effect of resistin on MMP-2 and MMP-9 expression in VSMCs
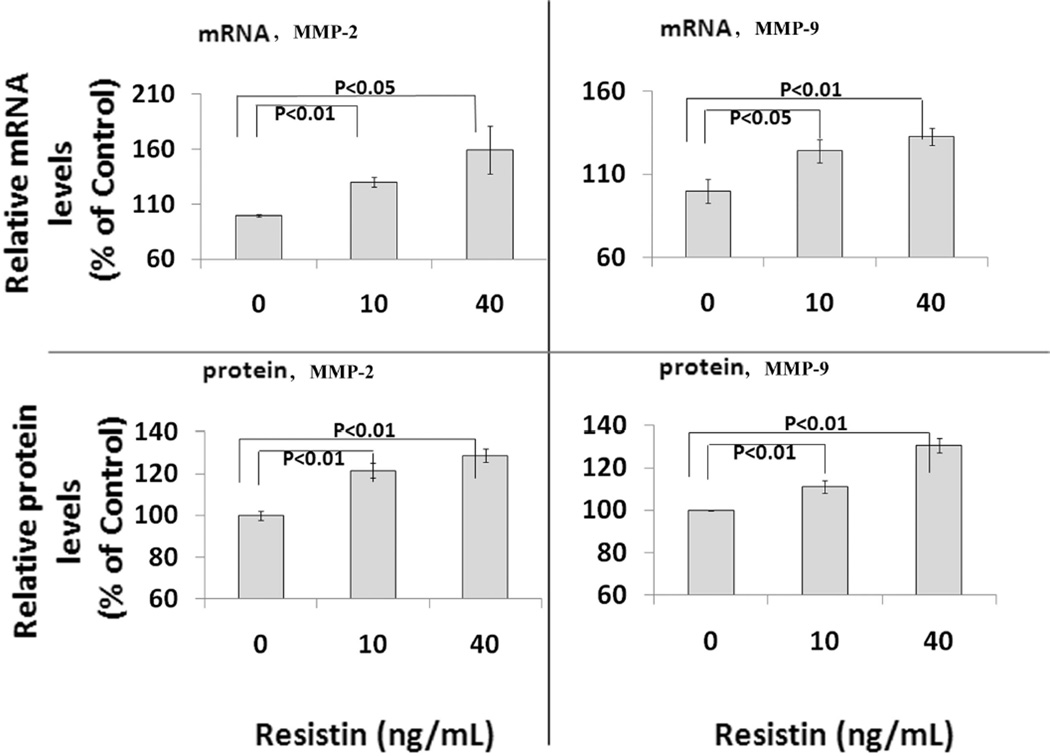
SMCs were treated with 10 or 40 ng/mL of resistin for 24 hours. Cellular MMP-2 and -9 were measured on both mRNA and protein levels. As shown in Figures 2A and 2B, resistin induced an increase in MMP mRNA expression in a concentration-dependent manner. The MMP-2 mRNA levels after 10 and 40 ng/mL of resistin treatments were 130.3% (±4.5%) (p<0.01) and 159.7% (±21.7%) compared to the control (P<0.001); the post-treatment MMP-9 mRNA levels were 124.2% (±7.1%) (p<0.01) and 132.8% (±5.4%) compared to the control (P<0.01). The effects of resistin on protein expression are shown in Figure 2C and 2D. MMP-2 protein levels after treatments were 121.6% (±3.6%) and 128.7% (±3.1%) compared to the control (P<0.01); the MMP-9 protein levels were 111.1% (±3.0%) and 130.6% (±3.3%) compared to the control (P<0.01). Regression analysis showed positive and significant correlations between resistin concentration and MMP expression. The correlation coefficients between resistin concentrations and MMP-2 and MMP-9 protein levels were 0.73 (p<0.01) and 0.96 (p<0.001), respectively.
Figure 2.
Effect of resistin on MMP-2 and MMP-9 expression in human coronary artery smooth muscle cells. Resistin was tested at physiological (10ng/mL) and pathological (40 ng/mL) concentrations. Left panels, MMP-2 mRNA and protein data; right panels, MMP-9 mRNA and protein data. Values are expressed as percentage of the control, and each bar represents the mean±SEM of triplicate determinations in 2–3 independent experiments.
Effect of resistin on TIMP-1 and TIMP-2 expression in VSMCs
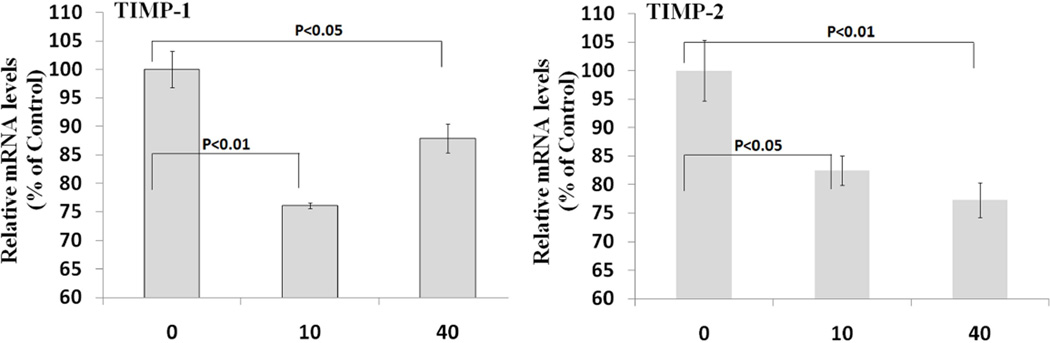
As shown in Figure 3, after 24 hours incubation with VSMCs, resistin induced a decrease in both TIMP-1 and TIMP-2 mRNA expression. The TIMP-1 mRNA levels in 10 and 40 ng/mL resistin treatment groups were 76.1% (±0.5%) (P<0.001) and 87.9% (±2.6%) (P<0.05) compared to the saline control; and the TIMP-2 mRNA levels were 82.5% (±2.5%) (P<0.05) and 77.3.0% (±3.0%) (P<0.01), respectively. Regression analysis showed a negative and significant correlation between resistin concentration and TIMP-2 (R=0.63; P<0.01).
Figure 3.
Effect of resistin on TIMP mRNA expression in human coronary artery smooth muscle cells. Resistin was tested at physiological (10ng/mL) and pathological (40 ng/mL) concentrations. Left panel, TIMP-1 mRNA data; right panel, TIMP-2 mRNA data. Values are expressed as percentage of the control, and each bar represents the mean±SEM of triplicate determinations in 2–3 independent experiments.
Effect of MMP-2 and MMP-9 antibodies on HCASMC migration
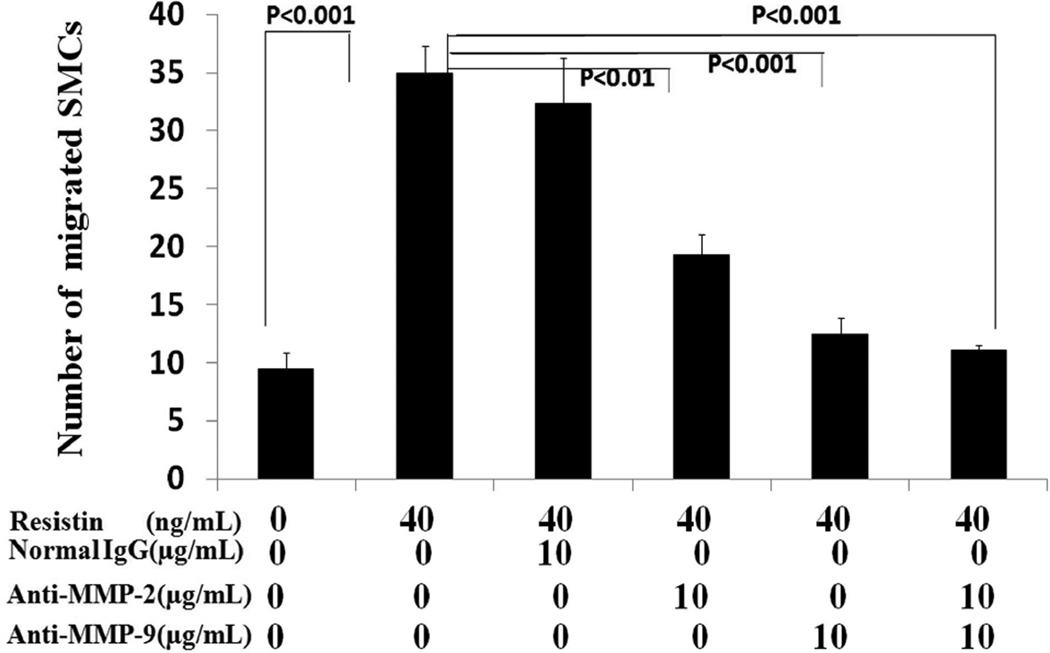
Neutralizing antibodies against human MMP-2 and MMP-9 were used to pre-treat the cells for 2 hours before adding resistin. Nonspecific IgG was used as a sham control. As shown in Figure 4, the average number of migrated cells in the vehicle control, resistin alone, resistin plus non-specific IgG, anti-MMP-2, anti-MMP-9 and anti-MMP-2 plus MMP-9 groups were 9.4 (±1.5), 35.0 (±2.3), 32.3 (±4.0), 19.3 (±1.8), 12.4 (±1.4), and 11.1(±0.4) respectively. The data again showed that resistin at 40ng/mL significantly increased cell migration and, MMP-2 and MMP-9 antibodies at a concentration of 10 µg/mL effectively blocked resistin-induced SMC migration (P<0.001). Nonspecific IgG showed no similar blocking effect.
Figure 4.
Effect of MMP-2 and MMP-9 antibodies on resistin-induced cell migration of human coronary artery smooth muscle cells (Boyden chamber assay). The migratory effect of resistin was exerted at the concentration of 40 ng/mL. Anti MMP-2 and MMP-9 as well as nonspecific IgG was tested at the same concentration of 10µg/mL. Values are expressed as the number of migrated cells, and each bar represents the mean±SEM of quadruplicate determinations.
Effects of PKCε on MMP, TIMP expression and HCASMC migration
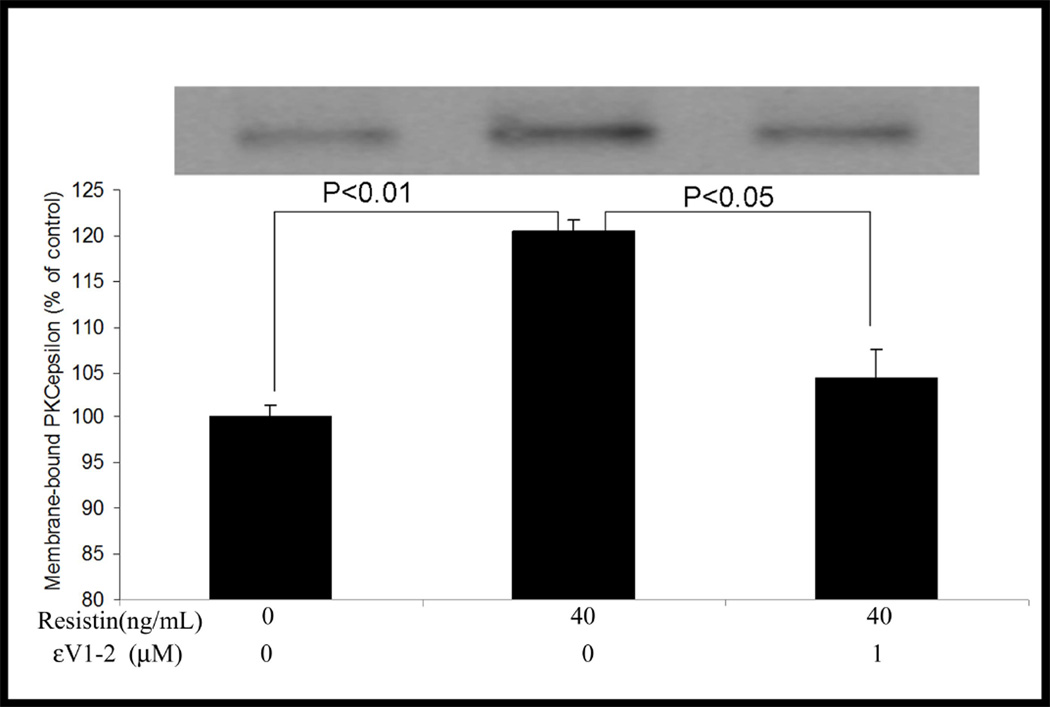
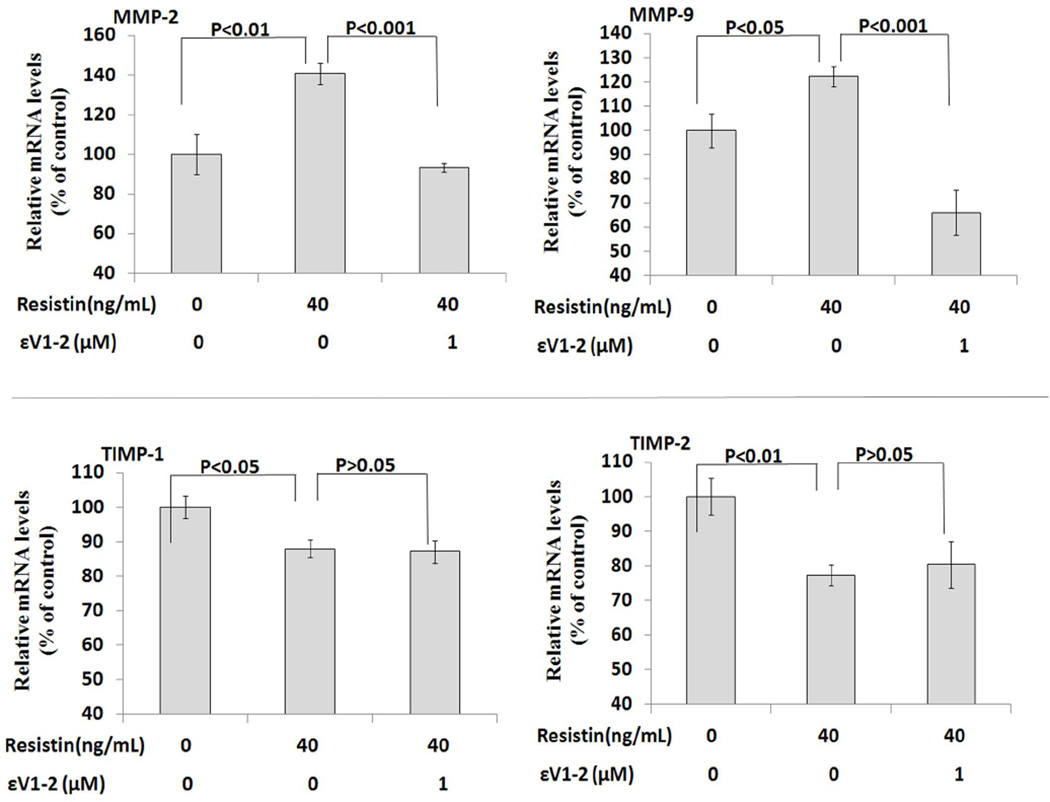
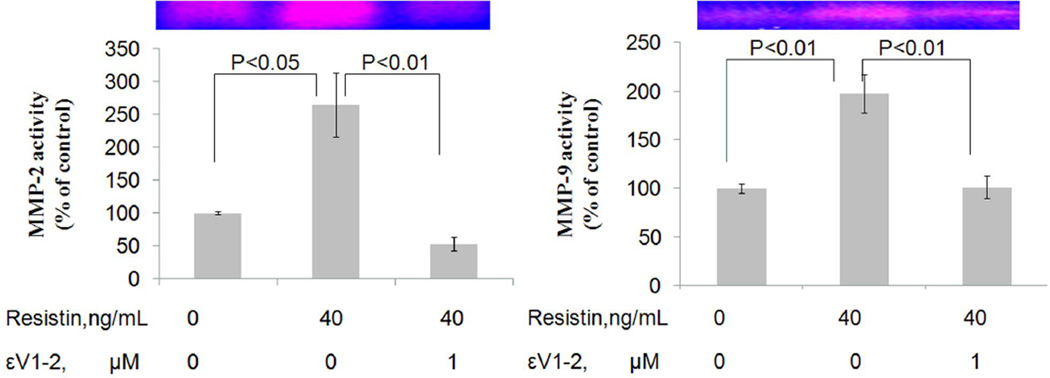
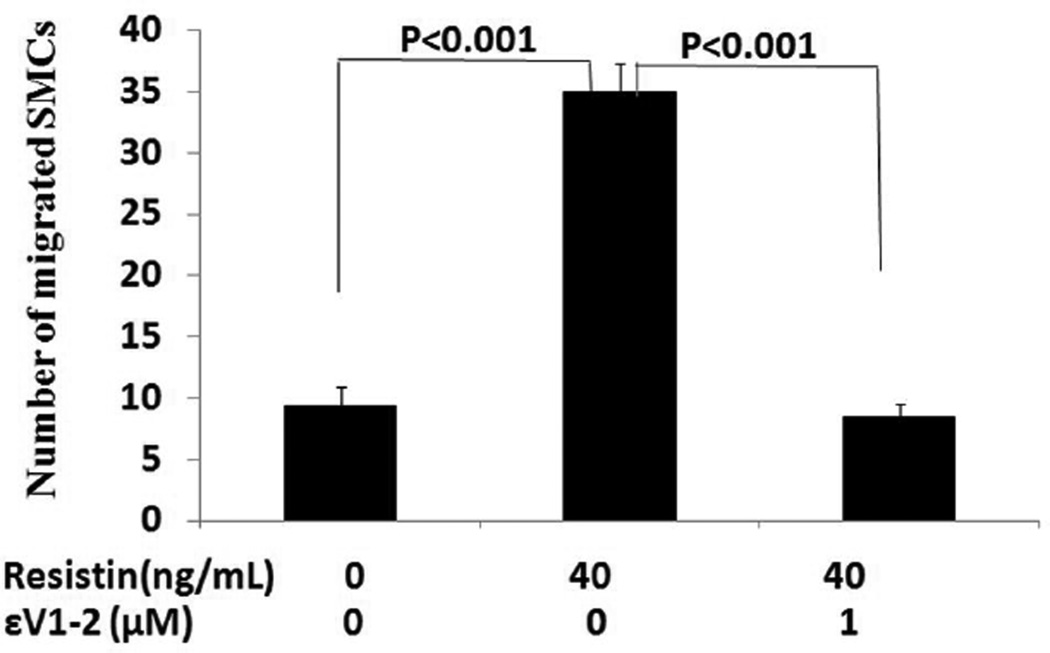
Resistin at 40ng/mL activated PKCε, as characterized by a significant increase in membrane-bound PKCε protein levels (121% of control, P<0.01) (Figure 5). Addition of selective PKCε inhibitor εV1–2 significantly suppressed resistin-induced PKCε activation (104.4% of the control) compared to the resistin alone group (P<0.05). The increases in MMP-2 and MMP-9 mRNA expression triggered by resistin were completely blocked by εV1–2 (For MMP-2, from 141.2% to 93.4% of the control, P<0.001; for MMP-9, from 122.3% to 66.0% of the control, P<0.001). Interestingly, εV1–2 did not affect resistin-induced inhibition on TIMP mRNA expression (Figure 6). To confirm the overall effect of PKCε inhibitor on resistin-associated MMP activity, zymographic analysis was conducted. As shown in Figure 7, resistin-induced increases in MMP-2 and MMP-9 activities were completely blocked by PKCε inhibitor. Morphologically, the migratory effect of resistin on HCASMC was abolished by this inhibitor. The average number of migrated cells decreased from 35.0 to 8.4 (P<0.0001) (Figure 8).
Figure 5.
Effects of resistin on PKCε activation and PKCε inhibitor (εV1–2) on resistin-induced PKCε activation. Values are expressed as percentage of the control, and each bar represents the mean±SEM of duplicate determinations in 2 independent experiments.
Figure 6.
Effect of PKCε inhibitor on MMP and TIMP mRNA expressions in human coronary artery smooth muscle cells. Resistin was tested at the concentration of 40 ng/mL. Top panels, MMP mRNA data; bottom panels, TIMP mRNA data. Values are expressed as percentage of the control, and each bar represents the mean±SEM of triplicate determinations in 2 independent experiments.
Figure 7.
Effect of PKCε inhibitor on MMP activity in human coronary artery smooth muscle cells. Resistin was tested at the concentration of 40 ng/mL. Left panel, MMP-2 activity; right panel, MMP-9 activity. Values are expressed as percentage of the control, and each bar represents the mean±SEM of duplicate determinations in 2 independent experiments.
Figure 8.
Effect of PKCε inhibitor on resistin-induced cell migration of human coronary artery smooth muscle cells (Boyden chamber assay). The migratory effect of resistin was exerted at the concentration of 40 ng/mL. Values are expressed as the number of migrated cells, and each bar represents the mean±SEM of quadruplicate determinations.
Discussion
Long-term success of vascular bypass and angioplasty procedures is limited by neointimal hyperplasia and ultimately restenosis, particularly in obese and diabetic patients [27, 28]. Matrix metalloproteinases are well known to be involved in facilitating VSMC migration, a key initial step in neointimal hyperplasia formation. In this study, we demonstrated that resistin, an elevated adipokine in obese patients with type 2 diabetes, increased vascular SMC migration through mediating MMP-2 and MMP-9 expressions, which themselves may be modulated by PKCε activation. Our study provides additional evidence that resistin can induce vascular SMC migration. Relevant mechanistic data on MMP and PKCε deepens our understanding on resistin-induced migration in human VSMCs and suggests a potential therapeutic strategy for this population of high-risk patients.
Obesity increases the risk of cardiovascular diseases. Adipose tissue releases a large number of bioactive mediators including resistin and leptin, which may be responsible for those complications. For instance, Mu and colleagues showed that resistin induced vascular endothelial cell migration in a dose- and time-dependent manner with the maximal effect at 40 ng/mL and that resistin-induced endothelial cell migration could be effectively blocked by resistin-neutralization antibody [29]. Jung et al used the scrape-wound model to assess the migratory effect of resistin on VSMC and found that resistin at 10 ng/mL had no migratory effect whereas concentration of >=50 ng/mL stimulated cell migration [17]. In this study, we used the Boyden chamber as an alternative migration model confirming the migratory effect of resistin. Consistent with Jung and colleagues’ observations, we found that resistin stimulated vascular smooth muscle cell migration at a higher concentration of 40 ng/mL while the lower, physiological concentration of 10 ng/mL failed to produce that effect. Considering the facts that HCASMC migrated toward the chamber with higher concentration of resistin in the Boyden chamber assay, we postulated that resistin functioned as a chemoattractant. Unlike scrape-wound model, Boyden chamber assay is suitable for chemotactic analysis. However, when resistin was present at equal concentrations in both top and bottom chambers of the Boyden chamber, increased migration was also observed, which indicated chemokinetic effects of resistin on VSMCs. Further studies on the motility mechanisms are warranted.
Generally speaking, there are three factors directly affecting cell migration: intracellular cytoskeletal rearrangement, membrane molecule adhesion and extracellular matrix degradation. The first two factors were recently reported to participate in resistin-induced VSMC migration [21]. MMPs are a family of structurally related, zinc-containing enzymes that degrade the extracellular matrix and connective tissue proteins. Among them, MMP-2 and MMP-9 appear most closely involved in VSMC migration in vitro and intimal hyperplasia in vivo. Selective gene silencing of MMP-2 inhibits migration of rabbit VSMCs [30] and human saphenous vein smooth muscle cells [31]. Interruption of MMP-2 gene expression [32] by chemical inhibitors also suppresses VSMC migration. Similarly, the blockade of MMP-9 expression, either by gene silencing or chemical inhibitors, leads to the suppression of cell migration in human [31, 33, 34] VSMCs. On the other hand, MMP-9 over-expression enhances SMC migration as showed by collagen invasion and Boyden chamber assays [35]. In our study, we found that resistin induced MMP-2 and MMP-9 expressions in HCASMCs at both mRNA and protein levels. Our antibody blocking experiments demonstrated that resistin-induced VSMC migration could be suppressed by anti-MMP-2 and anti-MMP-9 IgG, confirming that these two MMPs were involved in resistin-induced cell migration. It is interesting that activations of MMP-2 and MMP-9 are involved in smooth muscle cell migration in our in vitro model where the extracellular matrix was absent. We postulate that MMP-2 and -9 may facilitate migration of VSMCs through degrading non-matrix substrates. However, the significance of MMP-2 and MMP-9 activations remain to be determined. Our study also showed that resistin at lower concentration (10ng/mL) induced MMP expression but not VSMC migration. It is possible that molecular changes are generally more sensitive and response earlier than the phenotypic change of the cells.
TIMPs are specific endogenous inhibitors that bind MMPs in a 1:1 stoichiometry [36]. The N-terminal domain of TIMP folds as a separate unit and is capable of inhibiting MMPs. Although TIMP-1 and TIMP-2 binds both MMP-2 and MMP-9, TIMP-1 preferentially binds MMP-9 and TIMP-2 preferentially binds MMP-2. Since the biological relevance of MMP expression is substantially affected by TIMP expression, we also measured relative TIMP-1 and TIMP-2 mRNA levels after resistin treatment. We found that while stimulating MMP expression, resistin inhibited TIMP expression. It is possible that resistin-induced increases in MMP-2 and MMP-9 levels in the VSMCs were partially due to decreased TIMP. Relevant mechanism remains to be established.
PKC is known to be involved in the regulation of MMP expressions in the vascular system. For example, activation of PKC results in an increase MMP-2 in human [37] endothelial cells. Harja and associates found that activated PKC-beta and increased MMP2 occurred simultaneously in the atheroma in apoE (−/−) mice [38]. Similarly, PKC-alpha activation induced MMP-9 secretion in human vascular endothelial cells [39]. Recently, Inagaki K et al. found that, sustained inhibition of PKCε using εV1–2 reduced perivascular fibrosis and MMP-2 activation in a hypertension-induced heart failure model in rats [40]. εV1–2 is a water-soluble small peptide of eight amino acids that selectively inhibits PKCε activation by preventing the translocation of inactive PKCε from cell cytosol to cell membrane where PKCε transforms into the active form. In this study, we explored the potential involvement of PKCε and εV1–2 in modulating MMP expression stimulated by resistin and demonstrated that both PKCε activation and MMP expression occurred after 24 hours incubation with resistin. Furthermore, selective PKCε inhibitor blocked resistin-induced MMP-2 and MMP-9 mRNA expression. Our study provided the first evidence that resistin-induced VSMC migration was mediated, in part, by increased MMP-2 and MMP-9 expressions which, in turn, were modulated by PKCε activation.
Interestingly, we found that εV1–2 had no effect on resistin-associated inhibition of TIMP mRNA expression, which indicated that PKCε was not involved in resistin-induced inhibition of TIMP production. However, PKCε mediated resistin-induced MMP-2 and MMP-9 expressions and activation. Our data suggested the complex mechanism of resistin-induced VSMC migration and that PKCε was one of the upstream modulators regulating the effects of resistin.
Admittedly, the in vivo effect of resistin was not examined. However, in this mechanistic study, we confirmed that resistin at a pathological concentration stimulated vascular smooth muscle cell migration in vitro. Our data also supported the hypothesis that resistin contributed to the intimal hyperplasia through modulating PKCε signaling pathway and up-regulating MMP expressions. Selective PKCε inhibitor, εV1–2, may potentially be a more effective therapeutic strategy in decreasing resistin-induced vascular restenosis.
Footnotes
Disclosures
DM-R is a founder and shareholder of KAI pharmaceuticals. None of the work was supported by, or performed in collaboration with the company.
References
- 1.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 2.Marinou K, Tousoulis D, Antonopoulos AS, Stefanadi E, Stefanadis C. Obesity and cardiovascular disease: from pathophysiology to risk stratification. Int J Cardiol. 2010;138:3–8. doi: 10.1016/j.ijcard.2009.03.135. [DOI] [PubMed] [Google Scholar]
- 3.Lakka TA, Lakka HM, Salonen R, Kaplan GA, Salonen JT. Abdominal obesity is associated with accelerated progression of carotid atherosclerosis in men. Atherosclerosis. 2001;154:497–504. doi: 10.1016/s0021-9150(00)00514-1. [DOI] [PubMed] [Google Scholar]
- 4.Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, et al. Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab. 2003;88(11):5452–5455. doi: 10.1210/jc.2002-021808. [DOI] [PubMed] [Google Scholar]
- 5.Rana JS, Mittleman MA, Ho KK, Cutlip DE. Obesity and clinical restenosis after coronary stent placement. Am Heart J. 2005;150:821–826. doi: 10.1016/j.ahj.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Ngo MV, Gottdiener JS, Fletcher RD, Fernicola DJ, Gersh BJ. Smoking and Obesity Are Associated with the Progression of Aortic Stenosis. Am J Geriatr Cardiol. 2001;10:86–90. doi: 10.1111/j.1076-7460.2001.00839.x. [DOI] [PubMed] [Google Scholar]
- 7.Nikolsky E, Kosinski E, Mishkel GJ, Kimmelstiel C, McGarry TF, Jr, Mehran R, et al. Impact of obesity on revascularization and restenosis rates after bare-metal and drug-eluting stent implantation (from the TAXUS-IV trial) Am J Cardiol. 2005 Mar 15;95(6):709–715. doi: 10.1016/j.amjcard.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Owens CD, Kim JM, Hevelone ND, Hamdan A, Raffetto JD, Creager MA, et al. Novel adipokines, high molecular weight adiponectin and resistin, are associated with outcomes following lower extremity revascularization with autogenous vein. J Vasc Surg. 2010:1152–1159. doi: 10.1016/j.jvs.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kielstein JT, Becker B, Graf S, Brabant G, Haller H, Fliser D. Increased resistin blood levels are not associated with insulin resistance in patients with renal disease. Am J Kidney Dis. 2003;42:62–66. doi: 10.1016/s0272-6386(03)00409-8. [DOI] [PubMed] [Google Scholar]
- 10.Lu HL, Wang HW, Wen Y, Zhang MX, Lin HH. Roles of adipocyte derived hormone adiponectin and resistin in insulin resistance of type 2 diabetes. World J Gastroenterol. 2006;12:1747–1751. doi: 10.3748/wjg.v12.i11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piestrzeniewicz K, Łuczak K, Komorowski J, Maciejewski M, Jankiewicz Wika J, Goch JH. Resistin increases with obesity and atherosclerotic risk factors in patients with myocardial infarction. Metabolism. 2008;57:488–493. doi: 10.1016/j.metabol.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Stejskal D, Prosková J, Adamovská S, Juráková R, Bartek J. Preliminary experience with resistin assessment in common population. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2002;146:47–49. doi: 10.5507/bp.2002.009. [DOI] [PubMed] [Google Scholar]
- 13.Iacobellis G, Petramala L, Cotesta D, Pergolini M, Zinnamosca L, Cianci R. Adipokines and Cardiometabolic Profile in Primary Hyperaldosteronism. J Clin Endocrinol Metab. 2010;95:2391–2398. doi: 10.1210/jc.2009-2204. [DOI] [PubMed] [Google Scholar]
- 14.Mazaki-Tovi S, Vaisbuch E, Romero R, Kusanovic JP, Chaiworapongsa T, Kim SK, et al. Hyperresistinemia - a Novel Feature in Systemic Infection During Human Pregnancy. Am J Reprod Immunol. 2010;63:358–369. doi: 10.1111/j.1600-0897.2010.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gómez-Ambrosi J, Frühbeck G. Evidence for the involvement of resistin in inflammation and cardiovascular disease. Curr Diabetes Rev. 2005;1:227–234. doi: 10.2174/157339905774574392. [DOI] [PubMed] [Google Scholar]
- 16.Burnett MS, Lee CW, Kinnaird TD, Stabile E, Durrani S, Dullum MK, et al. The potential role of resistin in atherogenesis. Atherosclerosis. 2005;182:241–248. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Jung HS, Park KH, Cho YM, Chung SS, Cho HJ, Cho SY, et al. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc Res. 2006;69:76–85. doi: 10.1016/j.cardiores.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Majesky MW. Neointima formation after acute vascular injury. Role of counteradhesive extracellular matrix proteins. Tex Heart Inst J. 1994;21:78–85. [PMC free article] [PubMed] [Google Scholar]
- 19.Willis AI, Pierre-Paul D, Sumpio BE, Gahtan V. Vascular smooth muscle cell migration: current research and clinical implications. Vasc Endovascular Surg. 2004;38:11–23. doi: 10.1177/153857440403800102. [DOI] [PubMed] [Google Scholar]
- 20.Zargham R. Preventing restenosis after angioplasty: a multistage approach. Clin Sci (Lond) 2008;114:257–264. doi: 10.1042/CS20070228. [DOI] [PubMed] [Google Scholar]
- 21.Jiang C, Zhang H, Zhang W, Kong W, Zhu Y, Zhang H, et al. Homocysteine promotes vascular smooth muscle cell migration by induction of the adipokine resistin. Am J Physiol Cell Physiol. 2009;297:C1466–C1476. doi: 10.1152/ajpcell.00304.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodríguez-Pla A, Bosch-Gil JA, Rosselló-Urgell J, Huguet-Redecilla P, Stone JH, Vilardell-Tarres M, et al. Metalloproteinase-2 and -9 in giant cell arteritis: involvement in vascular remodeling. Circulation. 2005;112:264–269. doi: 10.1161/CIRCULATIONAHA.104.520114. [DOI] [PubMed] [Google Scholar]
- 23.Thomas AC, Newby AC. Effect of matrix metalloproteinase-9 knockout on vein graft remodelling in mice. J Vasc Res. 2010;47:299–308. doi: 10.1159/000265564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 25.Gray MO, Karliner JS, Mochly-Rosen D. A selective epsilon-protein kinase C antagonist inhibits protection of cardiac myocytes from hypoxia-induced cell death. J Biol Chem. 1997;272:30945–30951. doi: 10.1074/jbc.272.49.30945. [DOI] [PubMed] [Google Scholar]
- 26.Christopher J, Velarde V, Zhang D, Mayfield D, Mayfield RK, Jaffa AA. Regulation of B(2)-kinin receptors by glucose in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H1537–H1546. doi: 10.1152/ajpheart.2001.280.4.H1537. [DOI] [PubMed] [Google Scholar]
- 27.Mitra AK, Agrawal DK. In stent restenosis: bane of the stent era. J Clin Pathol. 2006;59:232–239. doi: 10.1136/jcp.2005.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue T, Node K. Molecular basis of restenosis and novel issues of drug-eluting stents. Circ J. 2009;73:615–621. doi: 10.1253/circj.cj-09-0059. [DOI] [PubMed] [Google Scholar]
- 29.Mu H, Ohashi R, Yan S, Chai H, Yang H, Lin P, et al. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res. 2006;70:146–157. doi: 10.1016/j.cardiores.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Hlawaty H, San Juan A, Jacob MP, Jacob MP, Vranckx R, Letourneur D, et al. Inhibition of MMP-2 gene expression with small interfering RNA in rabbit vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;293:H3593–H3601. doi: 10.1152/ajpheart.00517.2007. [DOI] [PubMed] [Google Scholar]
- 31.Turner NA, Hall KT, Ball SG, Porter KE. Selective gene silencing of either MMP-2 or MMP-9 inhibits invasion of human saphenous vein smooth muscle cells. Atherosclerosis. 2007;193:36–43. doi: 10.1016/j.atherosclerosis.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Pinney SP, Chen HJ, Liang D, Wang X, Schwartz A, Rabbani LE. Minocycline inhibits smooth muscle cell proliferation, migration and neointima formation after arterial injury. J Cardiovasc Pharmacol. 2003;42:469–476. doi: 10.1097/00005344-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Suh SJ, Jin UH, Choi HJ, Chang HW, Son JK, Lee SH, et al. Cryptotanshinone from Salvia miltiorrhiza BUNGE has an inhibitory effect on TNF-alpha-induced matrix metalloproteinase-9 production and HASMC migration via down-regulated NF-kappaB and AP-1. Biochem Pharmacol. 2006;72:1680–1689. doi: 10.1016/j.bcp.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Yu YM, Lin HC. Curcumin prevents human aortic smooth muscle cells migration by inhibiting of MMP-9 expression. Nutr Metab Cardiovasc Dis. 2010;20:125–132. doi: 10.1016/j.numecd.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Mason DP, Kenagy RD, Hasenstab D, Bowen-Pope DF, Seifert RA, Coats S, et al. Matrix metalloproteinase-9 overexpression enhances vascular smooth muscle cell migration and alters remodeling in the injured rat carotid artery. Circ Res. 1999;85:1179–1185. doi: 10.1161/01.res.85.12.1179. [DOI] [PubMed] [Google Scholar]
- 36.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foda HD, George S, Conner C, Drews M, Tompkins DC, Zucker S. Activation of human umbilical vein endothelial cell progelatinase A by phorbol myristate acetate: a protein kinase C-dependent mechanism involving a membrane-type matrix metalloproteinase. Lab Invest. 1996;74:538–545. [PubMed] [Google Scholar]
- 38.Harja E, Chang JS, Lu Y, Leitges M, Zou YS, Schmidt AM, et al. Mice deficient in PKCbeta and apolipoprotein E display decreased atherosclerosis. FASEB J. 2009;23:1081–1091. doi: 10.1096/fj.08-120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park MJ, Park IC, Lee HC, Woo SH, Lee JY, Hong YJ, et al. Protein kinase C-alpha activation by phorbol ester induces secretion of gelatinase B/MMP-9 through ERK 1/2 pathway in capillary endothelial cells. Int J Oncol. 2003;22:137–143. [PubMed] [Google Scholar]
- 40.Inagaki K, Koyanagi T, Berry NC, Sun L, Mochly-Rosen D. Pharmacological inhibition of epsilon-protein kinase C attenuates cardiac fibrosis and dysfunction in hypertension-induced heart failure. Hypertension. 2008;51:1565–1569. doi: 10.1161/HYPERTENSIONAHA.107.109637. [DOI] [PMC free article] [PubMed] [Google Scholar]