Abstract
Experience often does not produce veridical memory. Understanding false attribution of events constitutes an important problem in memory research. “Peak shift” is a well-characterized, controllable phenomenon in which human and animal subjects that receive reinforcement associated with one sensory stimulus later respond maximally to another stimulus in post-training stimulus generalization tests. Peak shift ordinarily develops in discrimination learning (reinforced CS+, unreinforced CS−) and has long been attributed to the interaction of an excitatory gradient centered on the CS+ and an inhibitory gradient centered on the CS−; the shift is away from the CS−. In contrast, we have obtained peak shifts during single tone frequency training, using stimulation of the cholinergic nucleus basalis (NB) to implant behavioral memory into the rat. As we also recorded cortical activity, we took the opportunity to investigate the possible existence of a neural frequency gradient that could account for behavioral peak shift. Behavioral frequency generalization gradients (FGGs, interruption of ongoing respiration) were determined twice before training while evoked potentials were recorded from the primary auditory cortex (A1), to obtain a baseline gradient of “habituatory” neural decrement. A post-training behavioral FGG obtained 24 h after three daily sessions of a single tone paired with NB stimulation (200 trials/day) revealed a peak shift. The peak of the FGG was at a frequency lower than the CS while the cortical inhibitory gradient was at a frequency higher than the CS frequency. Further analysis indicated that the frequency location and magnitude of the gradient could account for the behavioral peak shift. These results provide a neural basis for a systematic case of memory misattribution and may provide an animal model for the study of the neural bases of a type of “false memory”.
Keywords: Acetylcholine, Associative learning, Auditory cortex, Conditioning, Frequency generalization, Implanted memory, Nucleus basalis
1. Introduction
Learning and memory concern the acquisition and storage, respectively, of experience. Experience consists of the multiplicity of sensory events in the several sensory systems. How experiences are represented and retained in the brain constitute central problems in neuroscience. An accurate record of experience requires precision by perceptual, acquisition and storage processes. Yet even when they are all functioning in an optimal manner, the content of the resultant memory can be different from the actual experience. “Peak shift” constitutes such a case. It consists of the systematic displacement of behavioral performance from a training stimulus (e.g., tone) to another stimulus (e.g., another tonal frequency) despite the fact that only the former had been reinforced. Peak shift is found in both classical and instrumental discrimination training, across sensory modalities and dimensions, in both humans and animals (Purtle, 1973). For example, if a 1.0 kHz tone is rewarded (CS+) while a 1.2 kHz tone is not rewarded (CS−), then the peak of the post-training frequency generalization gradient will probably not be found at the CS+ frequency but rather at a lower frequency, e.g., 0.7 kHz. Note that the peak is shifted away from the CS− to a lower frequency, in this case, because the CS− is higher than the CS+. (The opposite occurs when the CS− is lower than the CS+.) In short, although discrimination training might be thought to promote accuracy of memory of the CS+, actually peak shift reveals an impairment of veridicality of the memory of an experience.
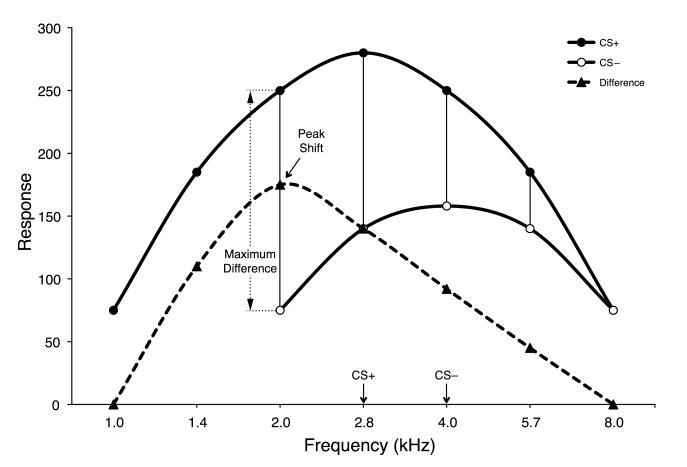
Spence (1937, 1942) proposed that within a stimulus dimension (e.g., acoustic frequency) rewarded stimuli (S+) produce a surrounding gradient of excitation and non-rewarded stimuli (S−) produce a gradient of inhibition. He further held that the two gradients combine algebraically with the sum dictating the gradient of resultant behavior. Insofar as the sum of the gradients would cause the peak of excitation to be displaced from the CS+, away from the CS−, Spence’s theory could in principle explain peak shift (Fig. 1). Although Spence’s ideas were published about 75 years ago, his formulation remains the dominant explanation (Bouton, 2007).
Fig. 1.
Hypothetical schema to explain peak shift in frequency discrimination learning (after Spence, 1937). Spence postulated that in discrimination training within a sensory dimension, two gradients are formed: a gradient of excitation centered on the CS+ (●) and inhibition centered on the CS− (◯). The algebraic sum was thought to determine the behavioral gradient of generalization. The vertical black lines show the difference between the two gradients. Note that the maximum difference (▴) is not at the CS+ (2.8 kHz) but is shifted to the adjacent stimulus frequency value on the low frequency side (2.0 kHz), away from the CS− (4.0 kHz). The dashed line shows the hypothesized resultant behavioral frequency generalization gradient, exhibiting a peak shift. For illustrative purposes, the inhibitory gradient is depicted above the response baseline rather than as negative values below the baseline.
As research on peak shift has been confined almost exclusively to behavioral studies, the existence of putative explanatory neural gradients has remained unexplored. In approaches to the neural bases of this phenomenon, the hypothesized “inhibitory” gradient is of prime consideration because it is said to be the mechanism of peak shift. However, the term “inhibition” should be considered in an appropriate context. Spence’s theory refers to behavioral gradients related to excitatory and inhibitory conditioning discovered by Pavlov (1927), not to neurophysiological excitation or inhibition. Thus, neural gradients that could account for peak shift need not be gradients of cellular inhibition per se, e.g., strengthening of inhibitory post-synaptic potentials (IPSPs). Other neural processes can oppose excitation, such as decrement in pre-synaptic transmitter release (Castellucci & Kandel, 1974), depotentiation (Fujii, Saito, Miyakawa, Ito, & Kato, 1991) and synaptic depression (Simons-Weidenmaier, Weber, Plappert, Pilz, & Schmid, 2006). Our use of the term “inhibition” is thus intended only in a general functional sense, i.e., to refer to neural processes that could reduce the effectiveness of neural excitation regardless of their particular neuronal bases.
We have reliably observed peak shift along the dimension of acoustic frequency in our previous studies of “implanted memory” (e.g., Miasnikov, Chen, & Weinberger, 2006, 2011). Memory is implanted by pairing a tone with electrical stimulation of the nucleus basalis (NBstm) (McLin, Miasnikov, & Weinberger, 2002; Miasnikov et al., 2006, 2011; Weinberger, Miasnikov, & Chen, 2006, 2009), which projects to (Mesulam, Mufson, Wainer, & Levey, 1983) and releases acetylcholine (ACh) into the cerebral cortex (Lehmann, Nagy, Atmadja, & Fibiger, 1980). Post-training behavioral measures reveal that animal subjects behave as though they had undergone standard training with a normal environmental reinforcement, i.e., they exhibit conditioned responses. Implanted memory has the major characteristics of natural associative memory. It is associative, can be acquired rapidly, is specific (conditioned responses to the CS and adjacent frequencies), and shows consolidation over days and long-term retention over weeks (reviewed in Weinberger, 2007; see also Miasnikov et al., 2011). Moreover, implanted memory is not an artifact of unintended reward or punishment because NBstm as used in our studies is motivationally neutral (Miasnikov, Chen, Gross, Poytress, & Weinberger, 2008a). Rather, the nucleus basalis appears to be “downstream” of motivational systems and may constitute a “final common path” to the cortex. Implanted auditory memory requires that NBstm release ACh that engages muscarinic receptors (Miasnikov, Chen, & Weinberger, 2008b). Accordingly, elucidation of the neural bases of peak shifts of implanted memory may be applicable to natural memory given that the former has the major attributes of the latter.
However, in contradistinction to natural associative memory, we obtained peak shifts in the absence of overt discrimination training. Rather, peak shift appears in post-training frequency generalization gradients, after a single tone has been merely paired with brief stimulation of the nucleus basalis (Miasnikov et al., 2006, 2011). Thus, while behavioral responses to the CS frequency were enhanced compared to non-associative controls, the peak of the post-training generalization gradient was at a neighboring frequency.
Previous studies of implanted memory have used relatively weak (~65 μA) levels of NBstm that produce only a few seconds of cortical activation. The present study was initiated to determine if a higher, but still moderate, level of stimulation (~100 μA) that activates the cortex would maximize the specificity of learning, that is produce the largest behavioral responses precisely at the CS frequency. However, it soon became apparent that peak shift still developed. Therefore, we took the opportunity to explore its possible neural mechanisms.
2. Materials and methods
The materials and methods were same as those previously reported (Weinberger et al., 2009), and will be described only briefly. All procedures were performed in accordance with the University of California, Irvine, Animal Research Committee and the NIH Animal Welfare guidelines. During training and testing, subjects were continuously monitored by video cameras.
2.1. Subjects and surgery
The subjects were 16 adult male Sprague–Dawley rats (412 ± 28 g, mean ± sd), housed individually with ad libitum food and water, on a 12/12 h light/dark cycle (lights on at 6:15 am). Following several days of adaptation to the vivarium, they were handled and learned to sit calmly during attachment of a thermistor assembly and a cable to their skull pedestal. Under general anesthesia (sodium pentobarbital, 40 mg/kg, i.p.), a stainless steel recording epidural screw electrode was threaded into the bone over the right primary auditory cortex at the locus showing the largest amplitude evoked potential to a contralateral noise burst. This placement maximized the opportunity to record activity across the frequency spectrum of the 70 dB tones used later (see 2.2. Stimuli) by a single epidural electrode, as well as to record the EEG. Two stainless steel screws over the frontal sinus served as reference electrodes. The EEG from the auditory cortex and respiration recordings were used to assess arousal state during training and testing. The EEG was also used to ensure the NBstm-elicited cortical activation, which is an index of the cortical release of acetylcholine during natural behavior and by NBstm (e.g., Celesia & Jasper, 1966; Détári, Rasmusson, & Semba, 1997, 1999; Duque, Balatoni, Détári, & Zaborszky, 2000). A concentric bipolar stainless steel stimulating electrode was implanted vertically into the right (ipsilateral) caudal nucleus basalis (ventrolateral internal capsule, ventromedial lateral globus pallidus and nucleus basalis of Meynert), which are sites of cholinergic projections to the auditory cortex (Bigl, Woolf, & Butcher, 1982; Moriizumi & Hattori, 1992). The effective locus was confirmed by repeatedly obtaining several seconds of auditory cortical EEG activation to NBstm (0.2 s train). After recovery from surgery, NBstm effectiveness in activating the EEG was confirmed while subjects were in a state of slow wave sleep (SWS). This functional confirmation was deemed acceptable in lieu of histology insofar as six prior studies using identical NB implantation targets and procedures had always yielded placements within the NB (McLin et al., 2002; Miasnikov et al., 2006, 2008a, 2008b, 2011; Weinberger et al., 2006). A dental acrylic pedestal was built with two aluminum hex threaded standoffs embedded therein (for mounting the subject-adjusted thermistor assembly during experiments) and all leads connected to a miniature socket that could be led to a commutator via a multi-conductor cable. Subjects were allowed 1–2 weeks to recover from surgery.
2.2. Stimuli
Training and testing took place while each subject was in an acoustically damped box (23 × 23 × 31 cm) with an open top, supplied with fresh bedding, contained in a double-walled acoustic chamber (Industrial Acoustics Co., Bronx, NY). Acoustic stimuli were 9 pure tones, 1.00–27.64 kHz, separated by ~0.58 octaves, each 2.0 s duration, cosine 10 ms rise/fall time [10–90%], 70 dB SPL, produced by Tucker–Davis Technologies (TDT, Alachua, FL) System 3 components. They were delivered to two calibrated speakers: electrodynamic (#40-1421, RadioShack, Fort Worth, TX) for delivering frequencies below 2.0 kHz and electrostatic (#ES-1, TDT) for higher frequencies, to avoid tonal distortions. The speakers were positioned ~35 cm above the floor of the box. Stimulation of the nucleus basalis consisted of a 0.2 s train of 100 Hz (pairs of 0.2 ms opposite polarity) pulses, 100 μA (S88 stimulator and linked pair of the PSIU6 isolation units, Grass Instrument Co., Quincy, MA). NBstm was subthreshold to affect ongoing, or initiate new, behavior.
2.3. Respiration behavior: state control, recording and analysis
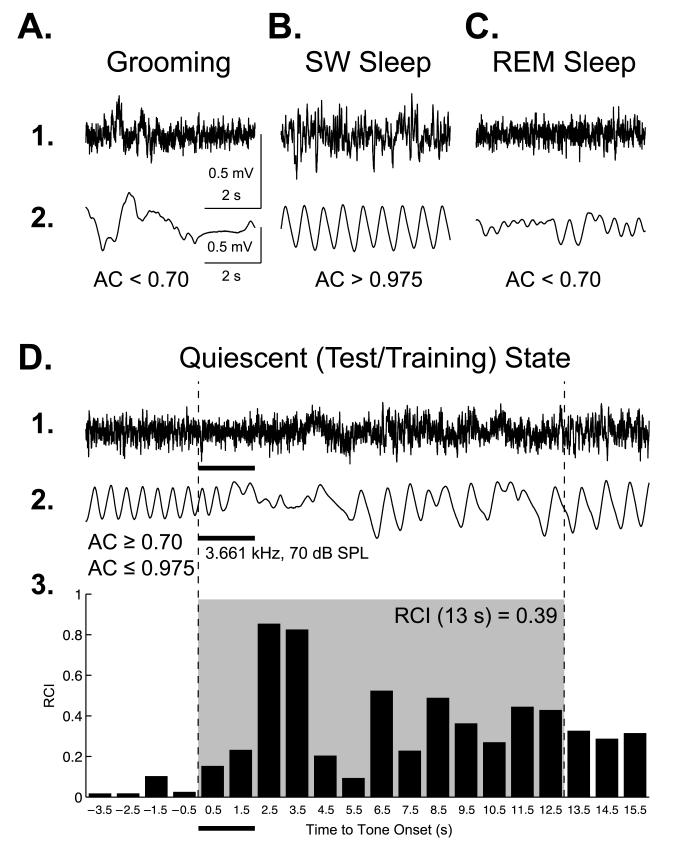
To assess the implantation of memory, we measured disruption of the ongoing pattern of regular respiration to all of the tones, before and after training, as previously described (e.g., Weinberger et al., 2006). Respiration is a sensitive measure of behavioral state and associative learning, the latter first described by Sherrington (1900). Respiration was detected as breathing-related thermal fluctuations by a glass-encapsulated thermistor attached to a lightweight pedestal-mounted assembly pre-adjusted in such way that a sensor is positioned in front of a naris, as described previously (McLin et al., 2002). The amplified output signal was fed to an ADC module, and the autocorrelation function (AC) was calculated on-line. The AC was used to present tones only when the subject was in a quiescent behavioral state, defined as four (4) consecutive seconds of 0.700 ≤ AC ≤ 0.975. Prior studies had found that animals were exploring, grooming, or in paradoxical sleep, etc. when the AC was < 0.700 and in slow wave sleep when AC was > 0.975 (e.g., Weinberger et al., 2006) (Fig. 2A–C). This state control was employed to avoid giving stimuli when very high levels of ACh were being released in the cortex, as during exploration or paradoxical sleep, or very low, as during SWS (Giovannini, Rakovska, Benton, Pazzagli, Bianchi, & Pepeu, 2001; Jasper & Tessier, 1971) to prevent ceiling or floor effects.
Fig. 2.
Behavioral state control and quantification of changes in ongoing respiration. Examples of measures of respiration/EEG corresponding to major behavioral states: exploring/grooming, slow-wave sleep, REM sleep and quiet waking. Shown are the EEG from primary auditory cortex (line 1) and respiration records (line 2). (A) During periods of ongoing activity such as exploration or grooming while the EEG is low-voltage fast (A1), the respiration pattern lacks regularity and is represented by the wide range of waveform shapes and amplitudes due to the variable depth of breathing supporting whole body movements and more nimble movements of naris affecting the flow of warm air during exhalation captured by the thermistor (A2). (B) During deep slow-wave sleep, the EEG is higher voltage, lower frequencies (B1); animals are not moving and respiration is very regular (B2). (C) During REM sleep, the EEG is low-voltage fast (C1) and breathing is shallower (hence, lower amplitude) and less regular (C2). (D) During quiet waking (quiescent state), which was the state when stimuli were presented during testing and training, the EEG is less desynchronized (D1); the animals are not moving and respiration is regular and can be easily disrupted by tones (thick horizontal bars). (D2) Throughout the experiment, the respiration autocorrelation function (AC) was continuously calculated on-line over 4-s long epochs. When a randomly selected inter-trial interval had passed, the software compared the current value of the AC with pre-selected thresholds (0.70 ≤ AC ≤ 0.975) and triggered a stimulus when that criterion was satisfied. (D3) Quantification of a regular sinusoidal baseline (first 4 s) respiration record disrupted by CS tone presentation 24 h post-training. The “Respiration Change Index” (RCI, see Methods) is sensitive to both increases and decreases in signal amplitude and frequency. The shaded area indicates the first 13-s portion of the peristimulus respiratory record containing the majority of the tone-evoked change in respiration. The RCI values found within this epoch were used in the behavioral data analysis.
The methods of analysis of respiratory behavior were identical to prior studies (e.g., Miasnikov et al., 2011). Briefly, major evoked changes in respiration occurred within the first 13 s after tone onset. The collected data were used to calculate a “Respiration Change Index” (RCI), on a second-by-second basis. The index was sensitive to increases and decreases of both frequency and amplitude of respiration response. RCIs were calculated as: RCIi = (∣Posti – Pre∣) / (Posti + Pre) where Post and Pre were the values of the power of the respiration signal (McLin et al., 2002). An RCI value of zero would indicate no change and a value of 1.0 would indicate complete cessation of respiration. An example of tone-elicited disruption of respiration and its RCI quantification is provided in Fig. 2D. Statistical analyses used SPSS v.17 software (SPSS, Chicago, IL).
2.4. Recording and analysis of auditory evoked potentials
Evoked potentials and the ongoing EEG were recorded by a DAM-50 pre-amplifier (1–1,000 Hz, 1,000×, WPI, Sarasota, FL) linked to an A/D converter of Power 1401 System (CED, Cambridge, England, UK) and stored on a computer. Evoked potentials typically had three components whose polarity was opposite to those typically recorded at depth because the recording electrode was epidural: peak latencies, P1= 15 ± 3 ms; N1 = 27 ± 2 ms; P2 = 41 ± 5 ms, (mean ± sd), based on evoked responses to each of the 9 test frequencies during Days 1 and 2. Average evoked potentials were computed for each test frequency on Days 1 and 2 (below).
2.5. Experimental design
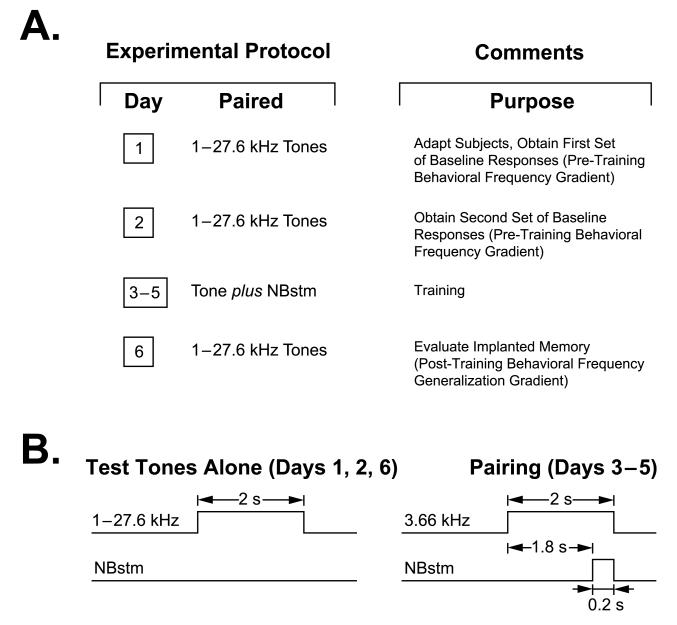
To induce and subsequently evaluate stimulus-specific implanted memory, we used the approach of acquiring behavioral baseline responses to many frequencies, then training with one frequency and later testing the training effects with many frequencies 24 h post-training (Fig. 3). This yielded pre- and post-training behavioral frequency generalization gradients (FGG). Pre-training gradients were obtained twice (Days 1–2), 200 trials/day total for the nine test frequencies (1.00–27.64 kHz), presented randomly at inter-stimulus intervals (ISI) that averaged 53.8 ± 5.8 s. Training was conducted on Days 3–5. Each trial consisted of a 2.0 s CS tone (3.66 kHz) followed after 1.8 s by a 0.2 s train of NBstm, 200 trials/day (inter-trial intervals = 54.5 ± 6.6 s). A post-training gradient was obtained on Day 6 (ISI = 51.3 ± 2.6 s). To determine the effect of pairing the CS with stimulation of the nucleus basalis, the pre-training FGG on Day 2 was subtracted from the post-training FGG, yielding a difference frequency generalization gradient (ΔFGG).
Fig. 3.
Experimental design. (A) The stages of the experiment used to obtain pre-training behavioral frequency gradients and post-training behavioral frequency generalization gradients. (B) Detailed temporal relationships of stimuli for the various phases of the experiment: delivery of test tones (Days 1, 2 and 6) and CS tone paired with NBstm (Days 3–5).
Potential transfer between training and frequency testing sessions was reduced by using different contexts for the two types of sessions. Thus, animals were delivered to the lab via different circuitous routes and they were trained in the dark (red light) but tested (pre- and post-training) in dim incandescent light. The rationale for using different contexts was based on reducing generalization between the training days and the post-training test (Day 6), which is more likely with identical contexts. Estimating the effects of training per se requires that the pre and post-training tests be conducted in the same context. However, if the training and post-training context had been identical, then animals would have been subject to extinction during the Day 6 test as no NB stimulation was given. This would have made it difficult, if not impossible, to assess the effects of training per se because the pre-training (Days 1 and 2) and post-training (Day 6) tests would have occurred under different conditions.
3. Results
3.1. Behavior
Responses to tones on Day 1 index subjects’ initial unconditioned responses while those on Day 2 may reflect decrements in response that are usually attributed to “habituation”, i.e., learning to not respond to stimuli that have no relevant consequences (Thompson, 2009). We observed response decrements, which were significant for three tones: 2.44 kHz, t(15) = 2.157, p < 0.05; 3.66 kHz, t(15) = 2.475, p < 0.03; and 8.22 kHz, t(15) = 2.412, p < 0.03.
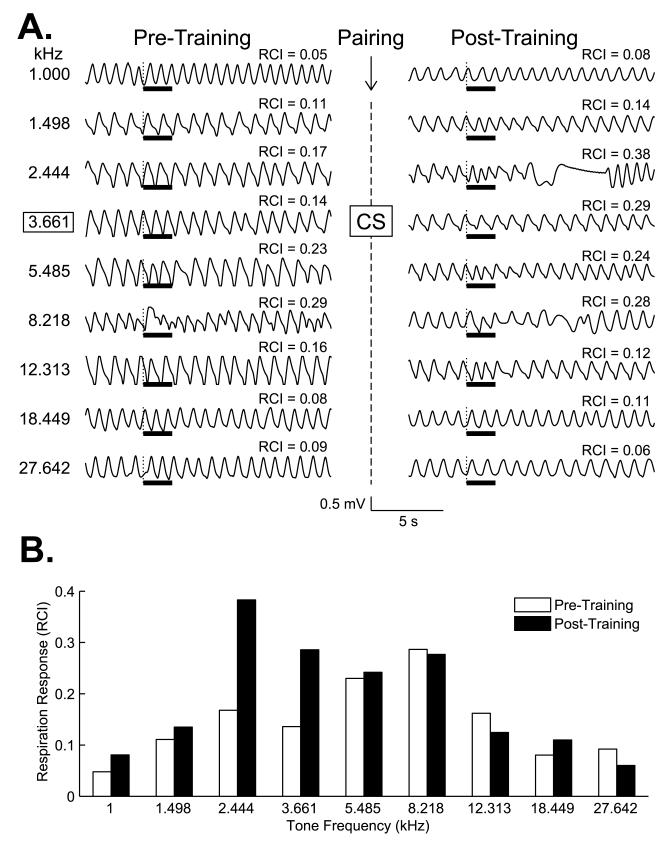
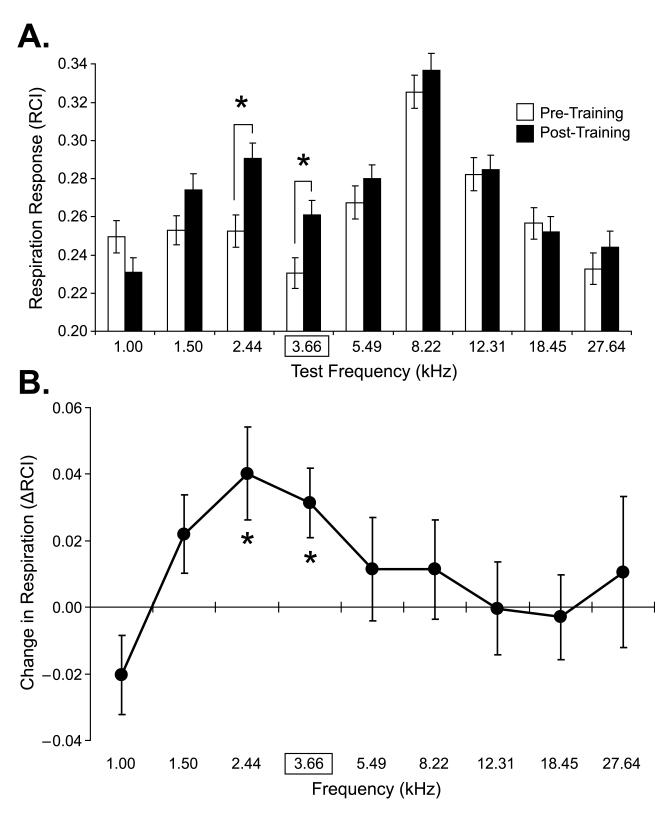
Following pairing of 3.66 kHz with NB stimulation on Days 3–5, responses increased significantly at the CS frequency (t(15) = 2.980, p < 0.01), and also for its adjacent lower frequency of 2.44 kHz (t(15) = 2.871, p < 0.02). Fig. 4 presents examples of responses for one animal on Day 2 vs. Day 6. Note that there is little interruption of respiration on Day 2 (“Pre-Training”). However, clear respiration responses to certain tones are seen on Day 6 (“Post-Training”). Moreover, the maximum response is not at the CS frequency itself but rather at its adjacent lower frequency of 2.44 kHz.
Fig. 4.
Examples of respiration responses for one subject. (A) Sample respiratory waveforms for one trial for each of the nine test tone frequencies. The CS was 3.66 kHz (outlined by frame). Shown are responses on pre-training Day 2 and 24 h post-training. RCI values are the quantified values for each record (within 13-s epoch, as shown in Fig. 3). Note the post-training large disruption of respiration at the CS (3.66 kHz, RCI = 0.29 post-training vs. 0.14 pre-training) and a larger disruption at the adjacent lower frequency of 2.44 kHz post-training (RCI = 0.38 vs. 0.17 pre-training). The thick horizontal bars indicate tone presentation. (B) Plot of RCI values for trials presented above. Note the frequency gradients pre- and post-training. The largest response, and the largest difference between pre- and post-training responses, was not at the CS frequency but at 2.44 kHz.
Group data are presented in Fig. 5. Mean pre- and post-training responses to all tones show that the only significant changes were increased responses to the CS frequency and its lower adjacent tone (Fig. 5A). The difference in response (post-minus pre-training, ΔFGG) is presented in Fig. 5B. It shows that the response peak is found not at the CS of 3.66 kHz but at the adjacent frequency of 2.44 kHz. This constitutes a peak shift. To determine if the peak at 2.44 kHz was significantly greater than the CS, a doubly multivariate repeated measures model ANOVA (2 × 2 design, with time (pre vs. post-training) and frequency (2.44 kHz, peak frequency vs. 3.66 kHz, CS frequency) was run. It yielded significant differences for both time and frequency: F(1, 15) = 12.612, p = 0.003 and F(1, 15) = 13.491, p = 0.002, respectively. The interaction was not significant (F(1, 15) = 0.375, p = 0.549). Thus, while respiration responses to the CS were significantly increased after training, so too were responses to 2.44 kHz, the peak of the ΔFGG, which was significantly greater than responses at the CS.
Fig. 5.
Group peak shift. (A) Group mean respiration responses (RCI) (mean ± se) for all test tone frequencies for the group of 16 subjects pre- and post-training. Note the significant pre-to post-training augmentation of behavioral responses to the CS (3.66 kHz, p = 0.009) and at the adjacent lower frequency (2.44 kHz, p = 0.012; post-hoc Univariate tests, Doubly Multivariate Repeated Measures Model). (B) Peak shift: the group ΔFGG (post-minus pre-training) showed that following training the group developed significant increases of respiratory response at the CS but a larger response at the immediately adjacent lower frequency of 2.44 kHz. Statistically significant paired comparisons are indicated by asterisks.
3.2. Cortical gradient of evoked potential response decrement
Given the behavioral peak shift in the frequency generalization gradient, we sought signs of a possible inhibitory neural gradient. Such a gradient was unlikely to form during training trials as they consisted of simple pairing rather than two-tone discrimination, i.e., there was no unreinforced CS−. Therefore, if present, the gradient most likely would have developed during the pre-training period, which consisted of the random repetition of nine test tones on Days 1 and 2. This procedure could have produced response decrement because repeated presentation of sounds is accompanied by decreased responses in A1 (Condon & Weinberger, 1991; Marsh & Worden, 1964; Wickelgren, 1968). To determine if tone repetition had produced a decrement in cortical response, evoked potentials from Day 1 were subtracted from those of Day 2.
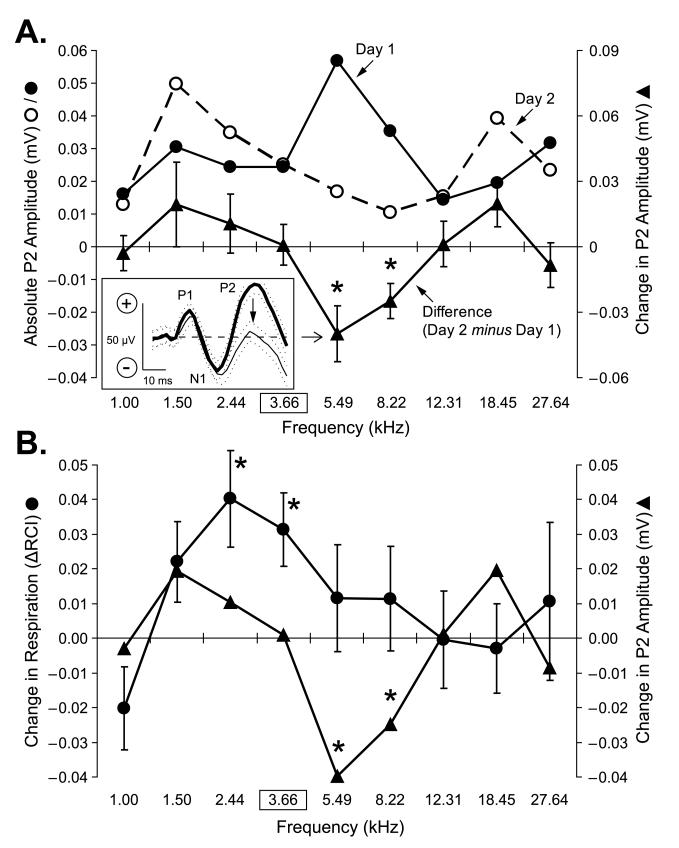
Reliable evoked potentials exhibiting tuning across the spectrum of test tones (1.00– 27.64 kHz) could be obtained from 7/16 subjects. (This subgroup had the same behavioral peak shift to 2.44 kHz as the entire group.) The evoked potentials consisted of three components, as explained in Methods: P1, N1 and P2. Fig. 6 presents the results for P2, which was the only component to show systematic changes. Fig. 6A shows that from Day 1 to Day 2, EPs decreased at 5.49 kHz and to a lesser extent at 8.22 kHz. They increased (to a lesser extent) at two lower (1.50 kHz and 2.44 kHz) frequencies and one higher (18.45 kHz) frequency. Overall, these changes constitute a neural gradient, with a prominent decremental component that for convenience is referred to as an “inhibitory gradient”.
Fig. 6.
Changes in pre-training auditory evoked potentials yield an “inverted” Gabor function. Inset: Group average epidural recorded evoked potential (5.49 kHz) exhibiting P1, N1 and P2 components (see Methods). Note the decrease in the P2 component from Day 1 (thick line) to Day 2 (thin line). Data in figure are for the subset of animals (n = 7) that yielded consistent evoked potentials. (The sequence of different components of evoked potentials [P1, N1 and P2] in this group is the same described in detail previously [Miasnikov et al., 2006].) (A) Dynamics of changes in P2 amplitude of group average evoked potentials for all test tones during pre-training. Day 1 (●): the maximum response was in the middle of the test frequency range at 5.49 kHz. Day 2 (◯): maximum responses were at low (1.50 kHz) and high (18.45 kHz) and the minimum was near the middle of the frequency range (8.22 kHz). The difference between days resembled an “inverted Gabor function” [▴, mean ± se] with the trough falling in the middle of the frequency range and smaller or no decrements, and even some increments, at lower and higher frequencies. The changes showed significant individual decreases in the mid-spectral range, at 5.49 kHz (t(5) = 3.08, p < 0.03) and 8.22 kHz (t(6) = 3.04, p < 0.03). Such response dynamics may suggest that when multiple tone frequencies are repeatedly presented at long inter-stimulus intervals, responses may “habituate” in a non-uniform manner, as if they are the components of one broad-spectrum acoustic stimulus producing maximum habituation at the center of the given spectrum. (Measures of variability were omitted from the response gradients obtained during Day 1 and 2, as they are reflected in the measure of variability shown on the difference function.) (B) Overlay, for ease of comparison, of the peak shifted group ΔFGG (Fig. 5B) and the neural function (Fig. 6A). Left y-axis: change in respiration (ΔFGG); right y-axis: change in P2 amplitude (neural gradient). Note that decremental portion of the neural function is located at 5.49 kHz and 8.22 kHz; both are at frequencies higher than that of the CS (3.66 kHz). This conforms to Spence’s model, which could explain peak shift, at least in part (see text). Statistically significant paired comparisons (Univariate tests) are indicated by asterisks.
A comparison of the neural gradient with the behavioral peak shift is presented in Fig. 6B. Note that the greatest amount of cortical decrement is at the tone adjacent on the high frequency side of the CS, 5.49 kHz, while the peak of the behavioral ΔFGG is at 2.44 kHz, the tone adjacent on the low frequency side of the CS. Therefore, the cortical inhibition might account, at least in part, for the behavioral peak shift.
Our general approach to estimate the extent to which the neural gradient might be able to account for the peak shift was to subtract its effect from the ΔFGG. This procedure would be expected to “compensate” for the shift and thus move the peak of the ΔFGG back toward the CS frequency. If the neural gradient could account for the entire shift, then the result would be a compensated ΔFGG whose peak would be at or beyond the CS frequency.
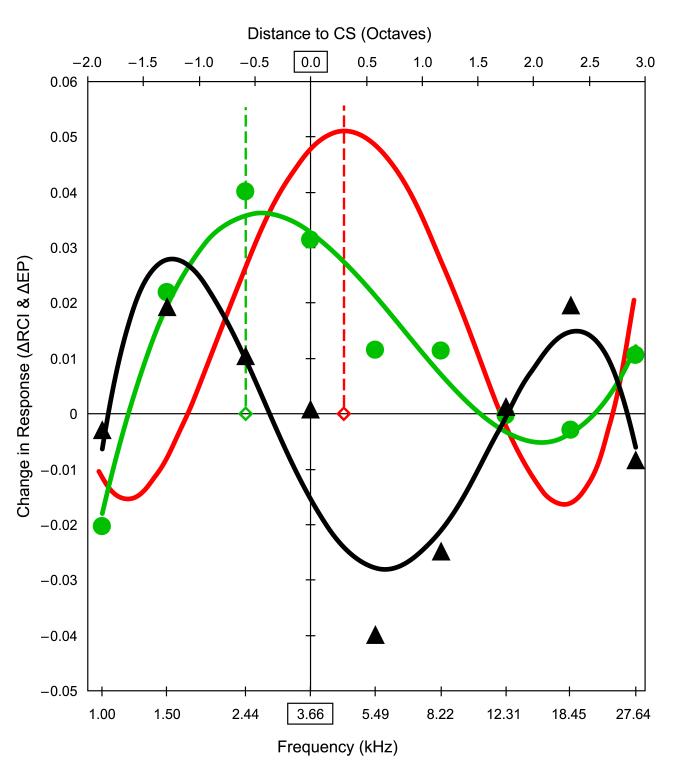
As the actual data points constituting both the behavioral and neural gradients can be considered to represent underlying functional processes, we first determined best-fit polynomial functions. As the neural gradient resembled a Gabor function (peak with side-band troughs) that was inverted in the case of induced response decrement (trough with side band increased responses) (Fig. 6A,B), it was best fit with a fourth order polynomial (p < 0.001). The behavioral ΔFGG (Fig. 5B) was best fit with a third order polynomial (p < 0.001). The neural function could not be directly subtracted from the peak shift function because they represent different processes lacking a common scale of magnitude. Therefore, each function was normalized to its amount of change, by determining the range of change (minimum to maximum values) and assigning it a value of 100% of change, and then scaling each function to percent change. Finally, the value of the normalized neural function (calculated at 0.05 octave frequency resolution) was algebraically subtracted from the normalized behavioral ΔFGG function (calculated at the same frequency resolution) to yield the respiration response corrected for the neural gradient.
The resulting function has its peak on the high frequency side of the CS frequency (0.30 octaves beyond the CS) (Fig. 7). That is, the inhibitory gradient was capable of accounting for 100% of the behavioral peak shift. Its “overshoot” of the CS frequency is considered later (see 4.4. Inhibitory cortical gradient as a mechanism of behavioral peak shift).
Fig. 7.
Inhibitory gradient as a mechanism of behavioral peak shift. A normalized linear algebraic summation model was used to estimate the degree to which the neural gradient might be able to account for the behavioral peak shift. The ΔFGG for the peak shift (Figs. 5B, 6B) was best fit with a 3rd order polynomial (green curve): y = 0.0052x3 − 0.0125x2 − 0.0144x + 0.0327; r = 0.97, p(ν=7) < 0.001. The neural gradient was best fit as a Gabor function with a 4th order polynomial (black curve): y = −0.0046x4 + 0.011x3 + 0.02x2 − 0.0358x − 0.0154; r = 0.91, p(ν=7) < 0.001. To estimate the extent to which the neural gradient could account for the peak shift, it was subtracted from the behavioral function. As direct subtraction could not be done because the two functions had no common scale, values were normalized to percent of change based on the range of values within each function, which was assigned a value of 100%. (The behavioral data (ΔRCI) range was maximum around 2.44 kHz and minimum around 18.45 kHz. The neural data (difference in P2 amplitude Day 2 minus Day 1) range was maximum around 18.50 Hz and minimum around 5.49 kHz). The neural function was then algebraically subtracted from the peak shift function to remove its effect on the latter. Finally, the corrected behavioral function was re-coded into actual units of ΔRCI, to yield an estimate of the behavioral gradient that would have been shifted by the neural gradient: y = 0.0045x4 − 0.0056x3 − 0.0322x2 + 0.0208x + 0.0478 (red curve). Note that this completely eliminated the peak shift. In fact, the compensation for the neural gradient actually moved the peak-shifted function beyond the CS frequency (vertical line). The peak-shift had been displaced from the CS by 0.58 octaves. (vertical green dashed line). Compensating for the neural gradient eliminated this and moved the peak of the estimated ΔFGG beyond the CS by 0.30 octaves (vertical red dashed line). See text.
4. Discussion
4.1. Peak shift behavior
Stimulus generalization was first noted by Pavlov (1927). After conditioning with one conditioned stimulus, later testing with many stimuli along the same sensory dimension revealed conditioned responses whose magnitude was inversely related to their distance from the CS. Quantified studies of generalization carried out many years later confirmed Pavlov’s observations and also demonstrated that the CS did elicit maximal CRs, i.e., was at the peak of the generalization gradient (Hovland, 1937). Subsequent research replicated and extended the fact that in simple conditioning (one CS), stimulus generalization gradients reliably identified the most behaviorally relevant stimulus (peak response) as that of the conditioned stimulus (Moore, 1972). Auditory generalization continues to be an area of active research (Wright & Zhang, 2009). In contrast, studies of stimulus discrimination revealed the unexpected finding that the peak of the generalization gradient was not at the CS+ (or S+ for instrumental conditioning), but rather was shifted away from the value of the non-reinforced stimulus (Farthing & Hearst, 1968; Hanson, 1959; Miller & Grace, 2003; Purtle, 1973).
However, peak shift developed in the present study in the absence of a CS−, i.e., without discrimination training, as it did in prior studies (e.g., Miasnikov et al., 2006, 2011). Rather, training consisted of simple classical conditioning, albeit with NB stimulation used to “by-pass” standard unconditioned stimuli and their otherwise unavoidable arousal and motivational effects.
It could be argued that the present findings are due to the use of brain stimulation as an “unconditioned stimulus”, and therefore are not applicable to natural learning. However, this explanation is not likely to be correct. First, as noted in the Introduction, memory implanted by pairing a tone with NBstm has the same major characteristics as natural associative memory: associativity, specificity, consolidation and long-term retention (Weinberger, 2003; Weinberger et al., 2006). Second, NB stimulation apparently does not act like an unconditioned stimulus because it is motivationally neutral, serving neither as a reward nor as a punishment (Miasnikov et al., 2008a). Third, it might be argued that NBstm has sensory properties and therefore could serve as the second of two paired sounds, leading to “sensory pre-conditioning” (Thompson, 1972). However, NBstm has well-established neuromodulatory functions that can account for implantation of auditory memory. For example, NBstm releases acetylcholine into the cortical mantle where ACh can activate the cortex (Metherate, Cox, & Ashe, 1992) and facilitate auditory (Aramakis, Bandrowski, & Ashe, 1997; Bandrowski, Aramakis, Moore, & Ashe, 2001; Metherate & Ashe, 1993) and other sensory (Nuñez, Dominguez, Buno, & Fernández de Sevilla, 2012) evoked activity. Moreover, NB stimulation facilitates responses to the acoustic parameters of the particular sounds with which it is paired, whether they are pure tones, modulation rates or complex acoustic stimuli (Kilgard, Pandya, Engineer, & Moucha, 2002). Thus, it seems unlikely that the release of ACh in A1 has its own auditory perceptual properties.
Rather, NB activation and its cortical release of ACh are more likely to be normal processes that occur during learning because NB neurons respond to sensory stimuli that signal reward or punishment (Wilson & Rolls, 1990). Therefore, current and past studies that demonstrate the implantation of specific behavioral memory by pairing a neutral tone with NB stimulation would appear to reveal behavioral consequences of cholinergic facilitation of sensory processing in the auditory cortex. However, it would be quite premature to conclude that the peak shift observed in this study is within the domain of peak shifts observed in previous natural learning studies. Without extensive further research, the differences in training protocol (e.g., one tone vs. discrimination) and means of inducing specific memory (NB stimulation vs. natural positive or negative reinforcers) are simply too great to allow for any such conclusions.
This brings us to two major questions. First, how could peak shift develop in the absence of discrimination training? Second, what is the evidence that the inhibitory gradient in A1 can account, at least in part, for the observed behavioral peak shift?
4.2. Peak shift without discrimination training
Regarding the first point, the protocol used may have engendered implicit frequency discrimination training. Pre-training frequency gradients were obtained in the present study and all prior studies that produced peak shifts. All tonal frequencies were presented repeatedly (randomly) on Days 1–2, preceding training on Days 3–5 (Fig. 3). Perhaps repeated pre-training presentation of various frequencies set up a “non-reinforced frequency context” that served as an implicit CS−. Then, subsequent training may have “reversed” such learning only for the CS frequency. This hypothesis is supported by the lack of a peak shift in the only NB behavioral study that did not present pre-training tones (McLin et al., 2002). However, this explanation would have difficulty explaining why the peak shift was to a lower frequency rather than to a higher frequency relative to the CS, because all tones were not reinforced during the pre-training period. One possible explanation may be that there were five tones higher than the CS frequency but only three tones that were lower (Fig. 5A). Thus, unreinforced presentation of all tones might have produced greater cortical inhibition for higher frequencies than for lower frequencies, resulting in a shift away from higher frequencies.
An alternative explanation is that discrimination learning of any sort is not necessary for peak shift. It happened, historically, that peak shift was discovered during investigations of discrimination training (Hanson, 1959). However, peak shift may simply require that an inhibitory gradient be formed. While discrimination training may produce this gradient, repeated presentation of tone alone (“habituation”) also appears capable of producing such a gradient. But standard approaches to learning and memory do not employ extensive pre-training unreinforced stimulus presentation unless its effects on subsequent learning are themselves specifically under investigation, as in the case with latent inhibition (Lubow & Moore, 1959). Therefore, in prior non-discrimination behavioral studies, a pre-training inhibitory gradient either would not have been formed, or if formed, not been detected.
In the present experiment, repeated unreinforced tones were presented to determine the behavioral baseline prior to pairing tone and NBstm, and compared to post-training tonal responses to determine if pairing had produced specific changes in response to or near to the CS frequency. This approach was possible because of the recording of a sensitive behavioral index, disruption of ongoing respiration. In summary, the critical factor for a peak shift appears to be the formation of an inhibitory gradient, rather than the means by which it is established.
4.3. Cortical inhibitory gradient
Although we cannot be certain why peak shift developed from the standpoint of behavior theories, a pre-training frequency specific inhibitory gradient did develop in the primary auditory cortex (Fig. 6A). This gradient was detected by comparing evoked potentials elicited by all 9 test frequencies on Day 2 with those on Day 1. Repeated presentation of all tones on two successive days might be expected to produce general habituation, i.e., decreased responses to all frequencies (Thompson, 2009). Prior studies of repeated acoustic stimulation have reported decreased evoked responses in A1 (e.g., Condon & Weinberger, 1991; Marsh & Worden, 1964; Westenberg, Paige, Golub, & Weinberger, 1976; Wickelgren, 1968). More recent studies of “stimulus specific adaptation” (SSA) have also found response decrements in auditory cortex to repeated sounds (Nelken & Ulanovsky, 2007; Szymanski, Garcia-Lazaro, & Schnupp, 2009; von der Behrens, Bäuerle, Kössl, & Gaese, 2009) and some authors consider that SSA demonstrates habituation (e.g., Netser, Zahar, & Gutfreund, 2011).
However, tone repetition in the present study did not yield a general decrease in the amplitude of auditory evoked potentials on Day 2 compared to Day 1. Rather, it produced an “inverted Gabor function”, consisting of a maximum decrement at 5.49 kHz, the center of the acoustic range, with side-band increments, before return to baseline at the lowest (1.00 kHz) and highest (27.64 kHz) frequencies (Fig. 6A).
Although it might be assumed that all frequencies should have developed decreased responses, this expectation is based on prior studies of repetition of a single stimulus. The differences in the results of repeated tone stimulation in the present study and prior studies probably reflect major differences in stimulus presentation. Previous experiments repeated a single sound at relatively short inter-stimulus levels (seconds). The current experiment presented nine different tones randomly at long ISIs (~1 minute). We could find no previous reports in which many different stimuli were repeatedly presented in a tone-alone context. Therefore, the absence of uniform decrement across the frequency spectrum in the present experiment appears to be novel, but it may be based on the effects of single tone habituatory decrements. Recall that the tone levels were 70 dB. At this moderately high intensity, the excitatory receptive fields of most A1 cells are broad and overlap considerably with neighboring RFs. When a repeated tone produces a response decrement, the resultant decrement (“inhibition”) would not necessarily be confined to the center of each RF but could also spread to neighboring cells. Across the frequency spectrum, the cumulative inhibition could be greatest for cells tuned toward the mid-range of the spectrum, as observed. On the other hand, neurons near the lower and higher extremes of the tonotopic organization would suffer least inhibition because they do not receive inhibition from cells at the lowest and highest extremes of the tonotopic axis (e.g., 500 Hz and 45 kHz), because those extreme frequencies were not presented. The small side-band facilitations (at 1.50 and 18.50 kHz) might reflect release from lateral inhibition when neurons tuned to mid-frequencies (5.49 and 8.22 kHz) developed marked reduction of excitation (Fig. 6). Overall, the result could be an inverted Gabor function, as found in this study. Such a function is the inhibitory complement of standard excitatory effects in which activated neurons exhibit increased responses while simultaneously reducing the responses of side-band frequencies via lateral inhibition, producing “Mexican Hat” (i.e., Gabor function) response profiles.
4.4. Inhibitory cortical gradient as a mechanism of behavioral peak shift
The group (n = 16) behavioral peak shift was to 2.44 kHz, the low frequency adjacent to the CS of 3.66 kHz (Fig. 5). Evoked potentials were obtained from 7/16 animals. Peak shift was also to 2.44 kHz, permitting the neural gradient to be used to help explain the group peak shift. What findings suggest that this peak shift could be accounted for, at least in part, by the inhibitory gradient?
First, Spence’s theory holds that the peak of the inhibitory gradient must differ from the peak of the excitatory gradient, simply because the S+ and the S− are different values along a sensory dimension (Spence, 1937, 1942; Fig. 1). In the current study, there was no built-in constraint on the location of the peak of the inhibitory gradient because there was no CS− stimulus; it could have been at the CS frequency. Nonetheless, its “peak” (trough) was not at the CS frequency. Therefore, the first criterion for a neural mechanism of peak shift is satisfied.
Second, Spence’s formulation asserts that the shift of the peak will be away, rather than toward, the CS− stimulus. In the present case, the behavioral peak was shifted to the low side of the CS while the inhibitory peak was on the high side of the CS. This satisfies the second criterion.
Finally, although Spence provided a graphical account of peak shift, rather than a quantitative estimate of the effect of the postulated inhibitory gradient on learning, we have attempted to move research in this direction. Using a normalized linear algebraic summation model, we calculated that the location and magnitude of the inhibitory gradient accounted for the entire peak shift. The amount of original behavioral peak shift was 0.58 octaves; subtracting the best-fit function of the neural gradient from the best-fit function of the behavioral peak shift (ΔFGG, Fig. 6B) “reversed” this amount of shift completely. That is, the neural gradient induced prior to training could explain the entire peak shift (Fig. 7).
However, the neural gradient further produced an “overshoot” of the peak shift, of 0.30 octaves (Fig. 7). What factors might explain this overly large effect? Recall that the neural data were obtained four days before the ΔFGG was obtained; the neural gradient was calculated by subtracting the average amplitude of the P2 evoked potential component on Day 1 from those on Day 2. Training was conducted on Days 3, 4 and 5. The peak shift was not revealed until post-training testing on Day 6. The long period between induction of the neural gradient at the end of Day 2 and subsequent training and testing might be responsible. The neural gradient probably reflects the effects of repeated presentation of unreinforced tones. In particular, the inhibitory part of the gradient (i.e., that for decreased responses at 5.49 and 8.42 kHz, Fig. 6) is likely to index a habituatory process. Habituation is known to exhibit some spontaneous recovery over time (Thompson & Spencer, 1966). Therefore, the inhibitory part of the neural gradient may have recovered to some extent (i.e., become reduced) over days. If so, then the Day 2 gradient (the only neural gradient available) was too large and thus would have produced the overshoot.
Although the inhibitory gradient can account for the peak shift, one should not assume that behavioral performance that reflects learning is necessarily completely controlled by processes in a single cortical site. Rather, the primary auditory cortex should be viewed as an important entry point into what is undoubtedly a complex set of circuits and networks, some of which ultimately determine the pattern and magnitude of behavior, i.e., effector action. That the inhibitory gradient accounts for the behavioral peak shift four days later is noteworthy, but there should be no expectation of a perfect match between a cortical neural gradient and the precise amount of peak shift in a behavioral generalization gradient. Even an imperfect match does not detract from the fact that the type of neural gradient uncovered in the present study appears to be a predictive correlate of the peak shift behavior.
4.5. Future directions and “false memory”
The present study introduces a line of inquiry rather than providing a definitive solution to the neural bases of stimulus misattribution as exemplified by peak shift. The finding of an inhibitory gradient was opportunistic and to some extent serendipitous. Recall that this experiment initially set out to determine if the use of a stronger, but still moderate, level of NBstm (100 μA instead of 65 μA) would produce an implanted memory in which the CS frequency was at the peak of the ΔFGG. When it became evident that peak shift still developed, we retrospectively analyzed auditory evoked potentials from tone repetition on Days 1 and 2 to determine if their presentation had induced an inhibitory gradient; such proved to be the case.
Future studies should test several aspects of the current account of peak shift, and also expand inquiry. Regarding the former, the quantitative relationship between the neural gradient and behavioral peak shift may depend upon the interval between pre-training and post-training determination of ΔFGGs. Would the fit between brain and behavior be better or worse if the interval were reduced? For example, testing could be conducted on Day 5, reducing the interval by 24 h. Or training could be restricted to Day 3 with testing later in the day, reducing the interval from four days to one day. It is possible that the neural gradient itself needs to “consolidate” before it can affect behavioral FGGs. Likewise, the longest interval for a brain– behavior match (e.g., weeks) needs to be determined in order to formulate a more general theory of how cortical dynamics influence a model case of learning and memory.
Another issue concerns the formation of the inhibitory gradient by pre-training tone repetition. If the gradient depends on the particular frequency range and amount of repetition, then it should be possible to predictably produce a desired gradient by manipulating the particular tonal frequencies used and the number of repetitions of selected tones. For example, repeating tones lower than a selected CS many times while higher tones are presented less often should produce greater lower frequency functional inhibition; the peak shift should then be toward higher frequencies. This possibility is concordant with prior studies in which pre-exposure to a non-reinforced stimulus (CS−) has a marked effect on subsequent learning. For example, pre-exposure to a non-reinforced taste facilitates later discrimination learning between this stimulus and the CS+ (Burch-Vernon & Riccio, 1997).
Perhaps the most important issue concerns the nature of the inhibitory gradient itself. Greater precision can be obtained by implanting an array of depth electrodes in A1, which would yield greater spatial specificity as well as provide unit activity in addition to evoked potentials. Additionally, the cellular bases for changes in pre-training responses to tones, especially decreased responses, need to be determined. What mechanisms underlie the cortical changes in response to the repetition of multiples tones? For example, in the best studied reductionistic approach to habituation, that of the Aplysia, response decrement was attributable to a pre-synaptic reduction in transmitter release (Castellucci & Kandel, 1974). It should be noted that the same neuronal questions need to be answered for behavioral peak shifts that develop in discrimination learning due to the use of a non-reinforced CS−.
Among the many aspects of the current study, one needs to not lose sight of the fact that the results provide support for Spence’s prescient theory. This consideration leads to the issue of learning-based behavioral misattribution. We suggest that peak shift, which is so well established in the behavioral literature, should be considered to be a type of false memory. In this, we follow Jasnow, Cullen, & Riccio (2012) who have pointed out that forgetting of stimulus attributes leads to generalization over time, i.e., responding to stimuli that had never previously been presented, much less reinforced. In the case of peak shift, subjects behave as though a stimulus that had not been paired with reinforcement was actually more important than the stimulus that was reinforced. This is clearly not an accurate reflection of the actual experience. However, unlike false memory studied in humans, and often associated with biased recollection in witness testimony, peak shift is probably not caused by inaccurate encoding, forgetting or confabulation in memory representations, or suggestions of events that never occurred (Loftus, 2005). The present findings also constitute a prime example of the effects of a predisposing bias upon subsequent learning, which advantageously can be subjected to neuro-behavioral analysis.
Peak shift may be caused by a systematic biasing of associative strength, due to the presence of a non-reinforced stimulus along the same dimension as the reinforced stimulus during learning. While there remains controversy in the behavioral literature about the sources of peak shift (Livesey & McLaren, 2009), theories of discrimination learning need not concern us here, especially as there was no CS− in the present study. The current observations indicate that how an inhibitory gradient is established is not critical. Instead of employing a CS− to do so, the current study found that mere tone repetition is sufficient to produce peak shift. Discrimination training appears to be unnecessary, as long as an appropriate neural gradient can be induced.
Understanding memory involves not only accounting for accurate storage and recall but also inaccurate mnemonic processes. Peak shift can serve as an animal model whose advantages include strong laboratory control, reliable behavior, and the ability to apply neurobiological approaches toward an understanding of why memory, and the behavior from which it is inferred, may fail to be veridical.
Highlights.
Peak shift in stimulus generalization is an experiential error, i.e., false memory.
We produced peak shift by giving many different tones before training with 1 tone.
Repeated tones induced an inhibitory neural gradient in primary auditory cortex.
This specific inhibitory neural gradient accounted for the behavioral peak shift.
An animal model to discover the neural bases of selected false memories is at hand.
Acknowledgements
We are most grateful to Jemmy C. Chen for help with data analysis and figures, Steven Clifford (CED) for specialized software development, Gabriel K. Hui for assistance with the manuscript and Jacquie Weinberger for citation research. This study was funded by (NIDCD/NIH) DC-02938 to NMW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aramakis VB, Bandrowski AE, Ashe JH. Activation of muscarinic receptors modulates NMDA receptor-mediated responses in auditory cortex. Experimental Brain Research. 1997;113(3):484–496. doi: 10.1007/pl00005601. [DOI] [PubMed] [Google Scholar]
- Bandrowski AE, Aramakis VB, Moore SL, Ashe JH. Metabotropic glutamate receptors modify ionotropic glutamate responses in neocortical pyramidal cells and interneurons. Experimental Brain Research. 2001;136(1):25–40. doi: 10.1007/s002210000556. [DOI] [PubMed] [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: A combined fluorescent tracer and acetylcholinesterase analysis. Brain Research Bulletin. 1982;8(6):727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Learning and behavior: A contemporary synthesis. Sinauer Associates; Sunderland, MA: 2007. [Google Scholar]
- Burch-Vernon AS, Riccio DC. The effects of CS− preexposure in conditioned taste aversion: Enhanced flavor discrimination. Learning and Motivation. 1997;28(2):170–187. [Google Scholar]
- Castellucci VF, Kandel ER. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proceedings of the National Academy of Sciences of the United States of America. 1974;71(12):5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celesia GG, Jasper HH. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966;16(11):1053–1063. doi: 10.1212/wnl.16.11.1053. [DOI] [PubMed] [Google Scholar]
- Condon CD, Weinberger NM. Habituation produces frequency-specific plasticity of receptive fields in the auditory cortex. Behavioral Neuroscience. 1991;105(3):416–430. doi: 10.1037//0735-7044.105.3.416. [DOI] [PubMed] [Google Scholar]
- Détári L, Rasmusson DD, Semba K. Phasic relationship between the activity of basal forebrain neurons and cortical EEG in urethane-anesthetized rat. Brain Research. 1997;759(1):112–121. doi: 10.1016/s0006-8993(97)00252-7. [DOI] [PubMed] [Google Scholar]
- Détári L, Rasmusson DD, Semba K. The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Progress in Neurobiology. 1999;58(3):249–277. doi: 10.1016/s0301-0082(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Duque A, Balatoni B, Détári L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. Journal of Neurophysiology. 2000;84(3):1627–1635. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- Farthing GW, Hearst E. Generalization gradients of inhibition after different amounts of training. Journal of the Experimental Analysis of Behavior. 1968;11(6):743–752. doi: 10.1901/jeab.1968.11-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Saito K, Miyakawa H, Ito K, Kato H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Research. 1991;555(1):112–122. doi: 10.1016/0006-8993(91)90867-u. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Rakovska A, Benton RS, Pazzagli M, Bianchi L, Pepeu G. Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience. 2001;106(1):43–53. doi: 10.1016/s0306-4522(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Hanson HM. Effects of discrimination training on stimulus generalization. Journal of Experimental Psychology. 1959;58(5):321–334. doi: 10.1037/h0042606. [DOI] [PubMed] [Google Scholar]
- Hovland CI. The generalization of conditioned responses: I. The sensory generalization of conditioned responses with varying frequencies of tone. Journal of General Psychology. 1937;17:125–148. [Google Scholar]
- Jasnow AM, Cullen PK, Riccio DC. Remembering another aspect of forgetting. Frontiers in Psychology. 2012;3(175):1–8. doi: 10.3389/fpsyg.2012.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172(3983):601–602. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Engineer ND, Moucha R. Cortical network reorganization guided by sensory input features. Biological Cybernetics. 2002;87(5–6):333–343. doi: 10.1007/s00422-002-0352-z. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Nagy JI, Atmadja S, Fibiger HC. The nucleus basalis magnocellularis: The origin of a cholinergic projection to the neocortex of the rat. Neuroscience. 1980;5(7):1161–1174. doi: 10.1016/0306-4522(80)90195-5. [DOI] [PubMed] [Google Scholar]
- Livesey EJ, McLaren IP. Discrimination and generalization along a simple dimension: Peak shift and rule-governed responding. Journal of Experimental Psychology: Animal Behavior Processes. 2009;35(4):554–565. doi: 10.1037/a0015524. [DOI] [PubMed] [Google Scholar]
- Loftus EF. Planting misinformation in the human mind: A 30-year investigation of the malleability of memory. Learning and Memory. 2005;12(4):361–366. doi: 10.1101/lm.94705. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: The effect of nonreinforced preexposure to the conditional stimulus. Journal of Comparative and Physiological Psychology. 1959;52(4):415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Marsh JT, Worden FG. Auditory potentials during acoustic habituation: Cochlear nucleus, cerebellum and auditory cortex. Electroencephalography and Clinical Neurophysiology. 1964;17(6):685–692. doi: 10.1016/0013-4694(64)90236-6. [DOI] [PubMed] [Google Scholar]
- McLin DE, 3rd, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: An overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983;10(4):1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14(2):132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: Modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. Journal of Neuroscience. 1992;12(12):4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Gross N, Poytress BS, Weinberger NM. Motivationally neutral stimulation of the nucleus basalis induces specific behavioral memory. Neurobiology of Learning and Memory. 2008a;90(1):125–137. doi: 10.1016/j.nlm.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Rapid induction of specific associative behavioral memory by stimulation of the nucleus basalis in the rat. Neurobiology of Learning and Memory. 2006;86(1):47–65. doi: 10.1016/j.nlm.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Specific auditory memory induced by nucleus basalis stimulation depends on intrinsic acetylcholine. Neurobiology of Learning and Memory. 2008b;90(2):443–454. doi: 10.1016/j.nlm.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Consolidation and long-term retention of an implanted behavioral memory. Neurobiology of Learning and Memory. 2011;95(3):286–295. doi: 10.1016/j.nlm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Grace RC. Conditioning and learning. In: Healy AF, Proctor RW, editors. Handbook of psychology: Experimental psychology. vol. 4. John Wiley; Hoboken, NJ: 2003. pp. 357–397. chap. 13. [Google Scholar]
- Moore JW. Stimulus control: Studies of auditory generalization in rabbits. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current theory and research. Appleton–Century–Crofts; New York: 1972. pp. 206–230. [Google Scholar]
- Moriizumi T, Hattori T. Separate neuronal populations of the rat globus pallidus projecting to the subthalamic nucleus, auditory cortex and pedunculopontine tegmental area. Neuroscience. 1992;46(3):701–710. doi: 10.1016/0306-4522(92)90156-v. [DOI] [PubMed] [Google Scholar]
- Nelken I, Ulanovsky N. Mismatch negativity and stimulus-specific adaptation in animal models. Journal of Psychophysiology. 2007;21(3–4):214–223. [Google Scholar]
- Netser S, Zahar Y, Gutfreund Y. Stimulus-specific adaptation: Can it be a neural correlate of behavioral habituation? Journal of Neuroscience. 2011;31(49):17811–17820. doi: 10.1523/JNEUROSCI.4790-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez A, Dominguez S, Buno W, Fernández de Sevilla D. Cholinergic-mediated response enhancement in barrel cortex layer V pyramidal neurons. Journal of Neurophysiology. 2012;108(6):1656–1668. doi: 10.1152/jn.00156.2012. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Oxford University Press; London: 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtle RB. Peak shift: A review. Psychological Bulletin. 1973;80(5):408–421. [Google Scholar]
- Sherrington CS. Experiments on the value of vascular and visceral factors for the genesis of emotion. Proceedings of the Royal Society of London. 1900;66:390–403. [Google Scholar]
- Simons-Weidenmaier NS, Weber M, Plappert CF, Pilz PK, Schmid S. Synaptic depression and short-term habituation are located in the sensory part of the mammalian startle pathway. BMC Neuroscience. 2006;7:38. doi: 10.1186/1471-2202-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence KW. The differential response in animals to stimuli varying within a single dimension. Psychological Review. 1937;44(5):430–444. [Google Scholar]
- Spence KW. The basis of solution by chimpanzees of the intermediate size problem. Journal of Experimental Psychology. 1942;31(4):257–271. [Google Scholar]
- Szymanski FD, Garcia-Lazaro JA, Schnupp JW. Current source density profiles of stimulus-specific adaptation in rat auditory cortex. Journal of Neurophysiology. 2009;102(3):1483–1490. doi: 10.1152/jn.00240.2009. [DOI] [PubMed] [Google Scholar]
- Thompson RF. Sensory preconditioning. In: Thompson RF, Voss JF, Brogden WJ, editors. Topics in Learning and Performance. Academic Press; New York: 1972. pp. 105–120. [Google Scholar]
- Thompson RF. Habituation: A history. Neurobiology of Learning and Memory. 2009;92(2):127–134. doi: 10.1016/j.nlm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;73(1):16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- von der Behrens W, Bäuerle P, Kössl M, Gaese BH. Correlating stimulus-specific adaptation of cortical neurons and local field potentials in the awake rat. Journal of Neuroscience. 2009;29(44):13837–13849. doi: 10.1523/JNEUROSCI.3475-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. The nucleus basalis and memory codes: Auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiology of Learning and Memory. 2003;80(3):268–284. doi: 10.1016/s1074-7427(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learning and Memory. 2007;14(1–2):1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Miasnikov AA, Chen JC. The level of cholinergic nucleus basalis activation controls the specificity of auditory associative memory. Neurobiology of Learning and Memory. 2006;86(3):270–285. doi: 10.1016/j.nlm.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Miasnikov AA, Chen JC. Sensory memory consolidation observed: Increased specificity of detail over days. Neurobiology of Learning and Memory. 2009;91(3):273–286. doi: 10.1016/j.nlm.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenberg IS, Paige G, Golub B, Weinberger NM. Evoked potential decrements in auditory cortex: I. Discrete-trial and continual stimulation. Electroencephalography and Clinical Neurophysiology. 1976;40(4):337–355. doi: 10.1016/0013-4694(76)90186-3. [DOI] [PubMed] [Google Scholar]
- Wickelgren WO. Effect of acoustic habituation on click-evoked responses in cats. Journal of Neurophysiology. 1968;31(5):777–784. doi: 10.1152/jn.1968.31.5.777. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Rolls ET. Neuronal responses related to reinforcement in the primate basal forebrain. Brain Research. 1990;509(2):213–231. doi: 10.1016/0006-8993(90)90546-n. [DOI] [PubMed] [Google Scholar]
- Wright BA, Zhang Y. A review of the generalization of auditory learning. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 2009;364(1515):301–311. doi: 10.1098/rstb.2008.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]