SUMMARY
Sustained canonical Wnt signaling requires inhibition of Glycogen Synthase Kinase 3 (GSK3) activity through its sequestration inside multivesicular endosomes (MVEs). Here we show that Wnt signaling is increased by the lysosomal inhibitor Chloroquine, which causes accumulation of MVEs. A similar MVE expansion and increased Wnt responsiveness was found in cells deficient in Presenilin, a protein associated with Alzheimer's disease. The Wnt-enhancing effects were entirely dependent on functional endosomal sorting complex required for transport (ESCRT), which are needed for formation of intraluminal vesicles in MVEs. We suggest that accumulation of late endosomal structures leads to enhanced canonical Wnt signaling through increased Wnt-receptor/GSK3 sequestration. The decrease in GSK3 cytosolic activity stabilized cytoplasmic GSK3 substrates such as β-Catenin, the microtubule associated protein Tau and other proteins. These results underscore the importance of the endosomal pathway in canonical Wnt signaling and reveal a new mechanism for regulation of Wnt signaling by Presenilin deficiency.
INTRODUCTION
Canonical Wnt signaling is essential for embryonic development, stem cell and tissue homeostasis, and regeneration in the adult (MacDonald et al., 2009; Angers and Moon, 2009). Aberrant Wnt signaling has been associated with human diseases such as cancer, bone disorders and neurodegeneration (Clevers and Nusse, 2012; Boonen et al., 2008). In the absence of Wnt ligands, the adaptor protein and transcription co-factor β-Catenin is phosphorylated by GSK3 in the destruction complex consisting of the tumor suppressor Adenomatous Polyposis Coli (APC), Axin, Casein Kinase 1 (CK1) and the E3-polyubiquitin ligase βTrCP (Cadigan and Peifer, 2009; Li et al., 2012). Phosphorylations by GSK3 target β-Catenin and other proteins for polyubiquitinylation and degradation in the proteasome (Kim et al., 2009; Taelman et al., 2010; Clevers and Nusse, 2012). Binding of Wnt ligands to their receptors Frizzled (Fz) and LDL-receptor related protein 5/6 (LRP5/6) triggers recruitment of Dishevelled (Dvl), Axin, and GSK3 to the plasma membrane (Bilic et al., 2007; Zeng et al., 2008). GSK3 is first recruited by the binding of Axin to LRP6, and then becomes engaged in the phosphorylation of LRP6, Fz, Dvl, Axin and β-Catenin, which contain multiple GSK3 sites, explaining the requirement of an intact GSK3 catalytic site for its relocalization (Taelman et al, 2010). Wnt receptor complexes, containing Axin and GSK3, are then internalized into the cell by endocytosis (Blitzer and Nusse, 2006; Yamamoto et al., 2006) and subsequently sequestered by incorporation into the intraluminal vesicles (ILVs) of late endosomes that are produced by invagination and scission from the endosomal limiting membrane (Taelman et al., 20120; Dobrowolski and De Robertis, 2012). Sequestration of active GSK3 inside MVEs leads to the sustained stabilization of the half-life of many GSK3 protein substrates (Taelman et al., 2010), principal among which is newly-synthesized β-Catenin which enters the nucleus to co-activate Wnt target genes.
The integration of cell signaling and endocytosis is critical for signal transduction outcomes (Sorkin and von Zastrow, 2009; Dobrowolski and De Robertis, 2011). While most receptor complexes are negatively regulated by endocytosis (Katzman et al., 2002), Wnt signal transduction requires the function of the endolysosomal pathway (Blitzer and Nusse, 2006). Inhibition of ILV formation in MVEs (also referred to in the literature as multivesicular bodies or MVBs) by interfering with components of the endosomal sorting complex required for transport (ESCRT) (Katzman et al., 2002; Wollert and Hurley, 2010) prevents canonical Wnt signaling (Taelman et al., 2010). Since endolysosomal function is essential for Wnt signaling, we decided to investigate the effect of inhibitors of lysosomal function on Wnt signaling. It was recently reported by Nixon's group that Presenilin 1 (PS1), an intramembrane protease mutated in early-onset Familial Alzheimer's disease (FAD), is required for proper autophagosome digestion (Lee et al., 2010). These authors found that the acidification of lysosomes was impaired in PS1-deficient cells, and proposed a model in which Presenilins are required for lysosomal maturation. An extensive literature linking autophagy defects and neurodegeneration exists (Nixon et al., 2008). Furthermore, toxic amyloid precursor protein (APP) peptides accumulate intracellularly specifically in MVBs in early stage Alzheimer's disease (Takahashi et al., 2002), and certain polymorphisms in the lysosomal protease Cathepsin D increase risk for Alzheimer's disease (Nixon and Yang, 2011). Taken together, these observations motivated us to investigate whether Presenilin deficiency might affect Wnt signaling through functional changes in the endolysosomal system.
In this study we report that canonical Wnt signaling activity was significantly increased when lysosomal function was inhibited by Chloroquine (CQ), a drug that raises lysosomal pH. Depletion of Presenilin 1 or 2 also resulted in a significant increase of Wnt transcriptional activity in TCF/β-Catenin reporter gene assays. In both cases, lysosomal function was inhibited downstream of ILV formation. Indeed, the ESCRT machinery that generates ILVs and the small GTPase Rab7 involved in MVE/late endosome formation (Bucci et al., 2000; Huotari and Helenius, 2011) required for the increase in Wnt signaling. In electron microscopy analyses, Chloroquine-treated or Presenilin-deficient cells displayed a striking accumulation of late endosomal vacuoles containing intraluminal vesicles. Immunofluorescence microscopy studies indicated that these MVEs were positive for Rab7 and the ILV membrane marker CD63 (Escola et al., 1998). The expanded late endosomes sequestered more GSK3/Wnt-receptor complexes than wild type cells when cells were treated with Wnt. Several GSK3 phosphorylation protein substrates became more stabilized by Wnt in PS1-depleted cells, such as β-Catenin, the previously developed GFP-GSK3-protein stability biosensor (Taelman et al., 2010), and the microtubule-associated protein Tau-GFP. The results presented highlight the key role played by the endolysosomal pathway in Wnt signal transduction.
RESULTS
Chloroquine increases Wnt signaling via MVEs
To investigate the role of the endolysosomal system in Wnt signaling, we used Chloroquine (CQ), an anti-malarial drug. CQ is a weak base that accumulates in lysosomes, causing their alkalinization and the inhibition of the activity of acidic hydrolases (Wibo and Poole, 1974). Sensitive transcriptional reporters of Wnt activity exist, in which multiple T-cell factor (TCF) binding sites (called SuperTopFlash and BAR reporters) activate transcription when β-Catenin accumulates in the nucleus (Veeman et al., 2003; Biechele and Moon 2008). As shown in Figure 1A and 1B, Wnt signaling was stimulated up to 7-fold by CQ treatment in HEK293T cells (brackets in left). Increases in Wnt signaling were also observed using the lysosomal protease inhibitors Leupeptin or E64 (increases of 2.6 and 2.1 fold, respectively, Supplementary Figure S1A). Therefore, interference with lysosomal maturation and function potentiates Wnt signaling.
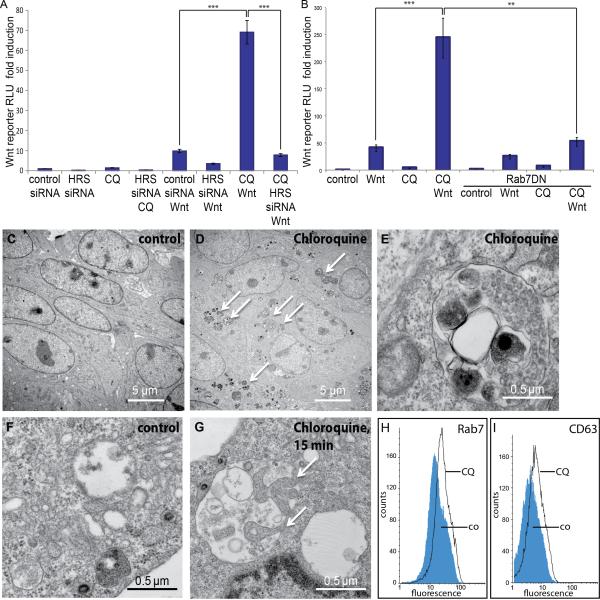
Figure 1. Lysosomal Inhibition by Chloroquine Increases Wnt Signaling and Expands the MVE Compartment.
(A) CQ (50 μM, Sigma) significantly increases the Wnt responsiveness of HEK293T cells in a Wnt reporter (SuperTopFlash) assay (left bracket, 11.2 ± 2.1 fold increase relative Luciferase Renilla units, RLU, in Wnt treated control siRNA cells vs. 69 ± 8.8 in Wnt treated CQ cells). HRS ESCRT protein is required for this stimulation (left bracket).
(B) Chloroquine stimulation of Wnt activity (BAR Wnt reporter) can be blocked by dominant-negative Rab7 co-transfection (cells treated with Wnt and CQ had a 243 ± 39 fold induction and 55 ± 8.9 RLU after co-expression of Rab7DN).
(C-E) Treatment of 3T3 fibroblasts with CQ for 12 h leads to the formation of numerous endosomal and autolysosomal vesicles bounded by a single membrane, containing intraluminal vesicles (ILVs) of about 50 nm characteristic of MVEs, and electron dense material representing digested cytoplasmic components taken in by microautophagy.
(F and G) After CQ treatment for 15 min, MVEs frequently showed membrane invaginations containing ribosomes and aggregated cytoplasmic components (arrows), reflecting the process of microautophagy.
(H and I) Flow cytometry analyses showing an increase of the late endosomal markers Rab7 and CD63 in cells treated with CQ for 8h.
All histograms are presented as mean ± SEM (standard error of the mean).
To test whether the amplification of Wnt signaling by CQ required formation of intraluminal vesicles in MVEs, we used siRNA knockdown of hepatocyte growth factor regulated Tyrosine kinase substrate (HRS), also known as Vacuolar protein sorting 27 (Vps27), which is required for early stages of ILV formation (Katzmann et al., 2002; Taelman et al., 2010). HRS/Vps27 was required for the stimulation of Wnt signaling by CQ (Figure 1A, right bracket). Vps4, another ESCRT component required for ILV formation was also required, as was Rab7, a protein required for late endosomal maturation (Figure 1B, right bracket, and data not shown).
To determine whether CQ causes the accumulation of MVEs, we examined 3T3 cells treated overnight (Figure 1C-1E) or L-cells treated with CQ for 6 h, 1 h or 15 min (Figure 1F and 1G) by transmission electron microscopy. In both cell lines Chloroquine treatment caused a striking increase in autolysosomes containing accumulations of electron-dense cytoplasmic materials, such as aggregated proteins, as well as small intraluminal vesicles of about 50 nm characteristic of MVEs (Figure 1D and E). Doubled-membraned macroautophagic vacuoles were not seen, even at early time points. However, the MVEs were clearly engaged in microautophagy (Sahu et al., 2011) since already after 15 minutes of CQ treatment many showed large invaginations of the limiting membrane (Figure 1G, arrows) enclosing regions of cytoplasm containing fine electron-dense granules corresponding to ribosomes. These invaginations, upon pinching off the limiting membrane, generate the electron dense deposits in late autolysosomes, which are enveloped by single or multilaminar membranes, depending on the stage of the individual autolysosomes (Figure 1E).
Chloroquine treatment causes the rapid accumulation of endosomes marked in their outer membrane by Rab7 (Figure S1B-S1C”’ and Movie S1). These vacuoles correspond to MVEs because they colocalize with the tetraspanin protein CD63, a marker for ILV membranes (Figure S1D-S1E”; Escola et al., 1998). Flow cytometry confirmed that CQ-treated cells had increased levels of Rab7 (by 25.2%) and of CD63 (by 28.5%) antigens in the cell population as a whole (Figure 1H and 1I).
We conclude that the inhibition of lysosomal function caused by Chloroquine does not prevent the formation of endosomal intraluminal vesicles but instead enhances it. The MVE expansion would enhance the sensitivity of cellular responses to Wnt, which are entirely dependent on the ESCRT machinery.
Dose-dependent Response of Wnt Signaling to Chloroquine and Bafilomycin
We next investigated the effects of inhibiting lysosomal function on Wnt signaling with different doses of CQ or the specific vacuolar ATPase (v-ATPase) inhibitor Bafilomycin A (Figure 2A and 2B). The v-ATPase enzyme acidifies the entire endosomal pathway as vesicles traffic from the plasma membrane to lysosomes. We found that low concentrations of CQ or Bafilomycin increased Wnt3a responses in LSL cells stably transfected with a TCF reporter, while at higher concentrations (>250 μM for CQ and >50 nM for Bafilomycin) both drugs inhibited Wnt signaling, presumably by alkalinizing also the early endosomal compartment (Figure 2A and 2B). Since the generation of ILVs within the expanded endosomes can still proceed in the presence of CQ (Figure 1E), the accumulation of intraluminal vesicles in MVEs may provide the basis for the increased sensitivity to Wnt signals after CQ treatment. At high CQ concentrations, the more extensive alkalinization of the entire endolysosomal pathway may actually inhibit ILV formation or receptor activation. Our results agree with a previous study by Cruciat et al. (2010) who, using HEK293T cells, found that Bafilomycin inhibited Wnt signaling, since LRP6 phosphorylation requires acidification of early endosomes/signalosomes. We now extend their findings by showing that at low doses v-ATPase inhibition increases Wnt signaling, presumably reflecting a higher requirement for the proton pump in the more acidic late endosomal compartment. These results highlight the link between Wnt signal transduction and membrane trafficking.
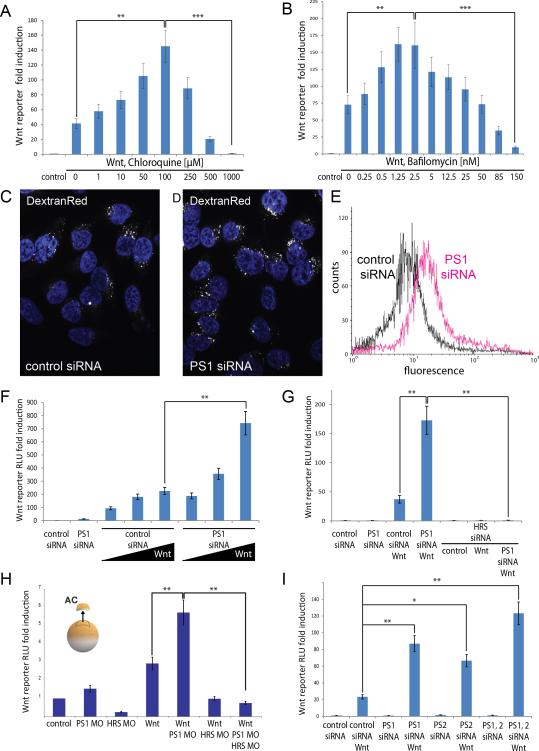
Figure 2. Wnt Sensitivity Increases when the Lysosomal Pathway Is Inhibited by CQ, low-dose Bafilomycin, or Presenilin Depletion.
(A and B) Concentration-dependent effects of Chloroquine and Bafilomycin A (Sigma) on Wnt Luciferase reporter assays in LSL-cells (SuperTopFlash reporter). Concentrations of 100 μM for CQ or 2.5 nM for Bafilomycin A caused maximal increases in Wnt signals (145 ± 23 or 162 ± 34 fold increase in RLUs, respectively). Higher concentrations of either drug lead to weaker increases and eventually inhibition of the signal. In the Bafilomycin experiment, all samples contained a final concentration of 0.1% ethanol, which was used as vehicle.
(C and D) PS1 depletion expands the endosomal fluid-phase compartment in DextranRed endocytosis assays in HeLa cells.
(E) Flow cytometry quantification confirms expansion of the endocytic compartment marked by DextranRed after PS1 depletion.
(F) Wnt signaling is increased in PS1 depleted HEK293T cells (BAR Luciferase assays). The highest concentration of Wnt3a used had a difference between PS1 siRNA and control siRNA of 3.3 times (743 ± 89 vs. 225 ± 27 fold induction, see bracket).
(G) The enhancing effect of Presenilin depletion on Wnt signaling (of 4.6 times in this experiment) is ESCRT dependent, for it is abolished when HRS is depleted by siRNA in HEK293T cells (173 ± 24 fold increase vs. 1.1 ± 0.2 RLU, right bracket).
(H) The Wnt-enhancing effect of Presenilin knockdown is also observed in Xenopus animal cap explant experiments. PS1 morpholino (MO) increased the Wnt signal by 2-fold (5.6 ± 0.8 vs. 2.8 ± 0.3 RLU, left bracket) and was inhibited when HRS MO was co-injected into early Xenopus laevis embryos (right bracket).
(I) Depletion of PS1, PS2, or both, led to increases of Wnt signaling in Wnt Luciferase reporter assays (BAR reporter) in HEK293T cells (24 ± 3.2 fold for Wnt in control siRNA cells, compared to 86 ± 9.2 for PS1, 69 ± 7.2 for PS2, and 124 ± 14.6 for both PS1 and PS2 siRNA).
All values in histograms are presented as mean ± SEM of three independent experiments.
See also Figure S2.
Presenilin deficiency Leads to Increased Sensitivity to Wnt Signals
A new function for Presenilin in the maturation of v-ATPase was recently reported (Lee et al., 2010), motivating us to explore the impact of lysosomal malfunction caused by Presenilin deficiency on Wnt signaling. We first confirmed that knock out or siRNA depletion of PS1 indeed generated less acidified endosomes, as was shown by reduced staining with LysoTracker which marks acidic organelles (Figure S2A-2E’). Efficient knockdown of PS1 was demonstrated with an immunoblot using a Flag-tagged PS1 construct (Figure S2F). We then extended the observations of Lee et al. (2010) by showing that the fluid-phase endosomal compartment, measured by endocytosis of DextranRed (Tetramethyl-Rhodamine Dextran), was significantly expanded by PS1 knockdown with siRNA (Figure 2C and 2D). Flow cytometry confirmed the increase in DextranRed accessible endosomes in the PS1-depleted cell population in a quantitative way (Figure 2E).
Endosomal expansion was accompanied by an increase in Wnt responsiveness in PS1-depleted HEK293T cells, particularly at higher Wnt3a doses (Figure 2F, bracket). The stimulation of Wnt signaling caused by PS1 siRNA-mediated depletion was entirely dependent on intraluminal vesicle formation in MVEs since, as shown in Figure 2G, HRS/Vps27 depletion strongly inhibited Wnt signaling in control siRNA or PS1 siRNA treated HEK293T cells. We extended these findings to the Xenopus animal cap system (Taelman et al., 2010). We designed a PS1 Morpholino (MO) oligo that reproducibly increased signaling by SuperTopFlash reporter in cells coinjected with xWnt8 mRNA (Figure 2H), and this effect was rescued by human Presenilin 1 coinjection (Figure S2G). This increase in Wnt signaling was blocked by HRS/Vps27 MO (Figure 2H, right bracket). We conclude that the ESCRT machinery is required for the effects of PS1 knockdown both in cultured cells and in the in vivo animal cap system.
We also tested whether Presenilin 2 (PS2) affected Wnt signaling using siRNA. Depletion of PS1 or PS2 led to increased responsiveness in Wnt Luciferase assays (Figure 2I), indicating that both proteins have functional overlap, at least in HEK293T and HeLa cells (see also Figure 3A-3D” below). Although the increase in Wnt signaling by PS1 siRNA was higher than that of PS2 siRNA, both together had additive effects (Figure 2I). The effects of PS2 siRNA on Wnt signaling, as well as those of PS1, could be rescued by overexpression of the human PS1 gene (Figure S2H). Interestingly, FAD-associated PS1 mutations (M146V, A246E or L392V) were much less effective at rescuing the Wnt signaling increased by PS1 depletion (Figure S2I). In contrast, PS1 mutations (D257A or D385A) in the aspartates required for proteolytic activity were as effective as wild-type PS1 in rescuing the effect on Wnt signaling (Figure S2I). This is in agreement with previous work showing that the effects on Presenilins on autolysosomes are independent of γ-secretase enzyme activity (Lee et al., 2010; Neely et al. 2011). The fact that FAD point mutations behave differently from protease-deficient PS1 could have relevance for disease.
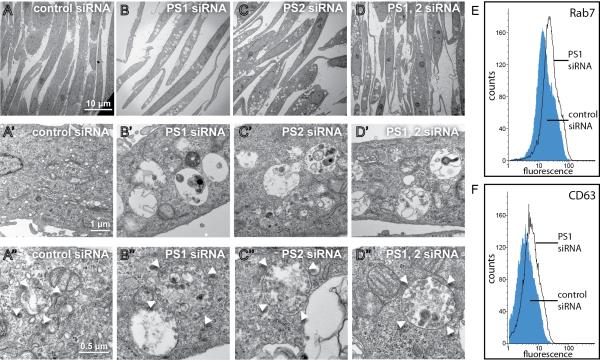
Figure 3. PS1 or PS2 Depleted Cells accumulate MVE/Autolysosomal vacuoles.
(A-D) Low-power electron micrographs of HeLa cells transfected with control siRNA, or depleted of PS1, PS2, or PS1 and PS2. Note the accumulation of MVEs and autolysosomes containing undigested electron dense material.
(A'-D') Higher magnification views of the same cells showing autolysosomes containing electron dense material, multilaminar membranes, and intraluminal vesicles (ILVs).
(A'-D') Even higher power views of endosomes and endolysosomal vesicles containing large numbers of intraluminal vesicles (white arrowheads indicate limiting membranes).
(E and F) Flow cytometry showing that PS1 depletion increases fluorescence intensity of the late endosome limiting membrane marker Rab7 (mean increase of 30.9%) or the ILV membrane protein CD63 (mean increase of 35.7%) in comparison to control siRNA.
See also Figure S3.
Taken together, these data suggest that Presenilin depletion increases the capacity of cells to respond to Wnt by promoting the generation of ESCRT-dependent ILVs in the endosomal pathway.
Late Endosomes Accumulate in Presenilin 1 and 2 Deficient Cells
PS depletion had a dramatic impact on the endolysosomal system. In electron microscopy studies, depletion of PS1, PS2, or the simultaneous depletion of both gene products, resulted in an increase in number and size of single-membrane-bounded vacuolar structures in HeLa cells (compare Figures 3A to 3B-3D). These structures were autolysosomes and multivesicular endosomes, as they contain a variable number of small and uniform intraluminal vesicles (Figure 3B’-3D’) as well as electron dense undigested cytoplasmic material. Double-membrane macroautophagic vesicles were not observed. Some of the most striking MVEs are shown in Figure 3A”-3D”. Immunofluorescence analyses showed increased staining for the endosomal pathway markers Rab7 and LAMP1, as well as the ILV marker CD63 when PS1 or PS1/2 were depleted with siRNA (Figure S3 and data not shown). Flow cytometry showed increased levels of Rab7 (by 30.9%) and CD63 (by 35.7%) in the entire cell population after PS1 knock down (Figure 3E and 3F).
We conclude that PS1 and PS2 play a fundamental role in endolysosomal biogenesis and function and, as in the case of Chloroquine, Presenilin deficiency blocks the pathway downstream of intraluminal vesicle formation.
Presenilin Depletion Leads to Increased phospho-LRP6 and nuclear β-Catenin upon Wnt signaling
Given the expansion of the MVE compartment in PS1 depleted cells, we next asked whether trafficking of the activated Wnt co-receptor LRP6 was affected upon Wnt ligand binding. Phosphorylation of LRP6 marks the initial activation step in the Wnt signaling cascade (Niehrs and Shen, 2010). HEK293T cells stably expressing LRP6-GFP (Kategaya et al., 2009) were transfected with control or PS1 siRNAs, treated with high concentrations of Wnt3a overnight, and activation of LRP6 detected with a phospho-specific anti p-LRP6 antibody (Figure 4A-4F). Phospho-LRP6 immunostaining in vesicle-like cytoplasmic puncta (Bilic et al., 2007) was detected only in cells treated with Wnt (arrows), and was significantly higher in cells lacking PS1 (compare Figure 4D to 4B). Only a subset of cells displayed strong responses to Wnt, probably due to the cell cycle dependence of Wnt signaling (Davidson et al., 2009; Niehrs and Acebron, 2012). Immunoblot analyses confirmed that PS1 knockdown caused a reproducible increase in LRP6 phosphorylation in the cell population as a whole, while total levels of non-phosphorylated LRP6-GFP were not affected (Figure 4E and 4E’). Accordingly, the ratio of pLRP6/LRP6-GFP was increased by Wnt in PS1-depleted cells (Figure 4F).
Figure 4. Presenilin Depletion Leads to Accumulation of phosphorylated-LRP6 receptor and nuclear β-Catenin upon Wnt signaling.
(A-D) The Wnt co-receptor LRP6 is phosphorylated specifically upon Wnt addition, and accumulates to a greater extent in PS1-depleted HEK293T cells stably expressing LRP6-GFP. Arrows indicate accumulation of phospho-LRP6 staining in cytoplasmic endosomes (called LRP6-Signalosomes by Bilic et al., 2007).
(E-E’) Phosphorylation of LRP6 increases after Wnt3a treatment of HEK293T cells while total LRP6-GFP levels remain unaffected.
(F) The ratio of pLRP6/LRP6-GFP (normalized to α-Tubulin) was increased by Wnt3a treatment in control siRNA and PS1-depleted cells. Quantitative evaluation from 3 independent western blot experiments using ImageJ software.
(G-J') Total β-Catenin increased with Wnt3a treatment and accumulated in the plasma membrane, cytoplasm, and inside the nucleus in HeLa cells. In the absence of Wnt3a addition, PS1-depleted cells showed a slight increase of β-Catenin in the cytoplasm, but β-Catenin was excluded from the nucleus. Wnt3a treatment of PS1 depleted cells led to a strong accumulation of β-Catenin inside the nucleus and in cytoplasmic puncta. Nuclear localization is indicated by white arrows and is counterstained with DAPI.
(K and K') Immunoblot analyses showing significantly increased β-Catenin levels in Wnt and PS1 siRNA treated cells (lanes 1, 2 and 4, 1.97 ± 0.12 fold and 2.59 ± 0.16 fold, respectively). Resulting mean values presented were obtained from 5 independent western blot experiments.
Accumulation of β-Catenin in the nucleus constitutes one of the hallmarks of the Wnt signaling cascade. Nuclear accumulation of β-Catenin was detectable only after Wnt treatment in HeLa cells (Figure 4G-4J’, arrows indicate location of individual nuclei). The increase in both nuclear and cytoplasmic β-Catenin was highest in PS1 depleted cells treated with Wnt (Figure 4J). The increase in β-Catenin levels was quantified in immunoblot analyses (Figure 4K, 4K’). A weak but significant increase in β-Catenin was also observed in PS1 deficient cells even in the absence of the Wnt ligand (1.38 ± 0.08 fold increase over control, Figure 4K, lanes 1 and 3). However, this increase of β-Catenin in PS1-depleted cells (see also Soriano et al. 2001 and Kang et al., 2002) did not generate a transcriptional Wnt signal (see Figure 2F, 2G and 2I). This is explained by the observed accumulation in the cytoplasm but not in the nucleus in the absence of Wnt (compare Figure 4I to 4J).
We conclude that PS1 depletion causes increased levels of activated phospho-LRP6 receptor in cytoplasmic puncta (presumably corresponding to endosomal vesicles of the type shown in Figure 3) when cells are exposed to Wnt, and that this correlates with increased nuclear β-Catenin accumulation.
GSK3 Translocates to Endosomes in Presenilin Deficient Cells upon Wnt signaling
To investigate whether the increase in Wnt signaling observed in CQ treated or PS1 depleted cells could be explained by the GSK3 sequestration mechanism, we used HeLa cells transiently expressing GSK3-RFP. Overnight exposure of these cells to Wnt conditioned medium led to a relocalization of GSK3-RFP into cytoplasmic puncta (compare Figure 5A to 5B), which could be visualized when cells were washed with the mild detergent Saponin to reveal the endocytic compartment prior to fixation (see Extended Experimental Procedures). This re-localization of GSK3 to cytoplasmic puncta upon Wnt signaling was strongly enhanced when cells were treated with Chloroquine (Figure 5C and S4A). These Wnt-induced GSK3 puncta colocalized with the late endosome marker Rab7 (Figure 5B” and 5C”).
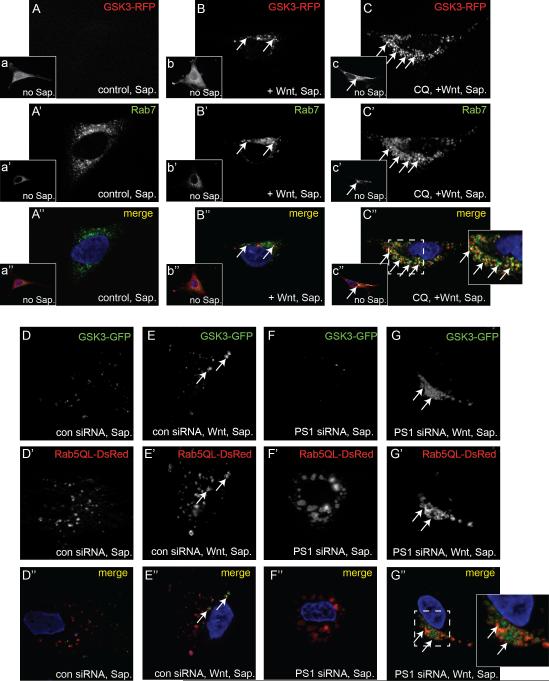
Figure 5. Wnt-Induced Translocation of GSK3 is increased by Chloroquine or Presenilin siRNA Treatment.
(A-C’) GSK3-RFP expressing HeLa cells were treated with Saponin to visualize endosomal GSK3 and endogenous Rab7 staining. GSK3 signal without Saponin treatment is shown in insets labeled a’-c”. Note that virtually no endosomal GSK3 staining was detected in control cells; endosomal GSK3 staining was detected when cells were exposed to Wnt3a overnight, and was strongly increased when cells were treated with Chloroquine and Wnt. Magnification of an area in C’ shows partial co-localization of GSK3-RFP with Rab7 antigen (arrows).
(D-G’) GSK3-GFP re-localized to Rab5QL-DsRed MVBs in Wnt-treated cells, and this co-localization was strongest when PS1 is depleted (arrows). Experiments carried out with Saponin-treated HeLa cells.
See also Figure S4.
We next used an activated form of Rab5 (Rab5QL-DsRed), which induces formation of large MVBs (Wegener et al., 2010), to show that GSK3-GFP is translocated to endosomes. HeLa cells were transfected with either control or PS1 siRNAs, treated with Wnt3a conditioned medium overnight, and treated with Saponin before fixation. We observed an accumulation of GSK3-GFP puncta inside and in the periphery of Rab5QL MVBs, specifically when PS1-depleted cells were treated with Wnt (compare Figure 5F-F” to 5G-G”; see also S4B). We also found that Wnt treatment led to the translocation of GSK3-GFP to MVBs in control siRNA cells (Figure 5D-D” and 5E-E”; see also S4B), although to a lesser degree.
These results suggest that depletion of PS1 with siRNA or inhibition of lysosome function with Chloroquine enhanced the translocation of GSK3 to late endosomes/MVBs that is normally triggered by Wnt.
GSK3 Protein Substrates are Stabilized by Wnt in PS1-depleted cells
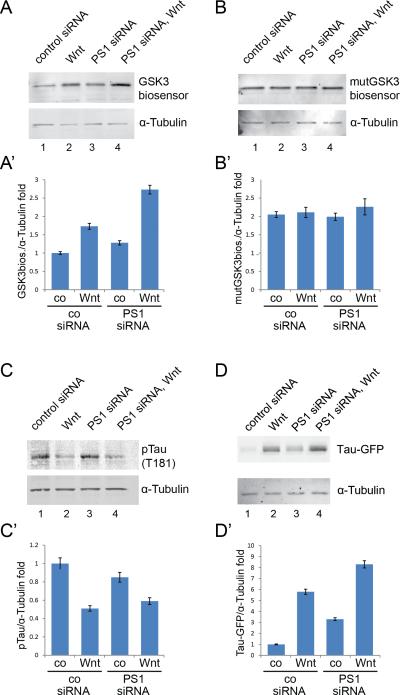
Since GSK3 is more efficiently sequestered in MVEs of PS1-deficient cells, we investigated whether GSK3 phosphorylation substrates would be more stable in these cells. Consistent with the GSK3 sequestration model, we observed that cytosolic GSK3 substrates such as β-Catenin (Figure 4K) and a GFP-GSK3 protein stability biosensor primed by a MAPK site (Figure 6A; Taelman et al., 2010) were more efficiently stabilized in Presenilin knockdown cells upon receiving a Wnt signal (Figure 6A). As a control, we used a mutated GFP biosensor construct in which the three GSK3 phosphorylation sites were mutated into alanines. It was found that the GSK3-resistant biosensor became stabilized and its levels not affected by Wnt or PS1 siRNA (Figure 6B and 6B’, compare to 6A and 6A’). This indicates that the effects of Wnt and PS1 siRNA are exerted at the level of protein half-life via GSK3 sites.
Figure 6. Presenilin Depletion Causes Increased Stability of Wnt-regulated GSK3 Substrates.
(A and A') The GSK3-GFP biosensor consists of a Flag-tagged GFP containing MAPK-primed GSK3 phosphorylation sites (Taelman et al., 2010), and provides a measure of cytosolic GSK3 activity. Wnt treatment stabilized a GSK3-biosensor in control conditions (lane 2, 1.8 ± 0.08 fold increase over control siRNA) and was more efficiently stabilized in PS1-depleted cells when treated with Wnt (lane 4, 2.6 ± 0.16 fold increase over control).
(B and B') The control GFP biosensor (mutGSK3 biosensor) lacking GSK3 phosphorylation sites showed a higher stability than the GSK3-GFP biosensor protein, but no significant changes in stability were detected when cells were treated with Wnt or PS1 siRNA. This control shows that the stabilization of protein half-life by PS1 depletion and Wnt is mediated by the GSK3 sites.
(C and C') GSK3-specific Tau phosphorylation was decreased by Wnt treatment in control or PS1-depleted cells (decrease to 0.5 ± 0.03 in control or 0.59 ± 0.04 in PS1 siRNA lysates). Specific GSK3 phosphorylation of the endogenous microtubule associated protein phospho-Tau was tested in immunoblots using the pTau (T181) antibody.
(D and D') Total Tau stability determined in Tau-GFP expressing HeLa cells. Tau-GFP was stabilized in control siRNA transfected cells by overnight treatment with Wnt3a (lane 2, 5.9 ± 0.23 fold increase over control). Accumulation of Tau without Wnt was also detected in PS1-depleted cells (lane 3, 3.2 ± 0.13 fold increase over control). The stability of Tau was further increased when PS1 siRNA cells were treated with Wnt (lane 4, 8.3 ± 0.33 fold increase).
For statistical evaluation, signals from 3 or more immunoblot analyses were quantified using ImageJ.
Using the same experimental design, we found that transfected Tau-GFP (Kwan and Kirschner, 2005) was stabilized by Wnt treatment and that this stabilization was more marked when PS1 was depleted (Figure 6D and 6D’). Tau is a microtubule associated protein that plays an important role in Alzheimer's disease and contains multiple GSK3 phosphorylation sites. When Tau phosphorylation by GSK3 was measured in immunoblots using an anti phospho-Tau (T181) antibody, Wnt signaling decreased phosphorylation in both control and PS1-depleted cells (Figure 6C and 6C’). These results support the hypothesis that Wnt signaling removes active GSK3 enzyme from the cytosol, decreasing protein phosphorylations that target GSK3 protein substrates for degradation. The increased protein stabilization caused by Wnt in PS1-deficient cells, in particular in the case of Tau, could provide a new link between Wnt signaling, protein stabilization and neurodegeneration.
DISCUSSION
Late Endosomes are required for Wnt Signaling
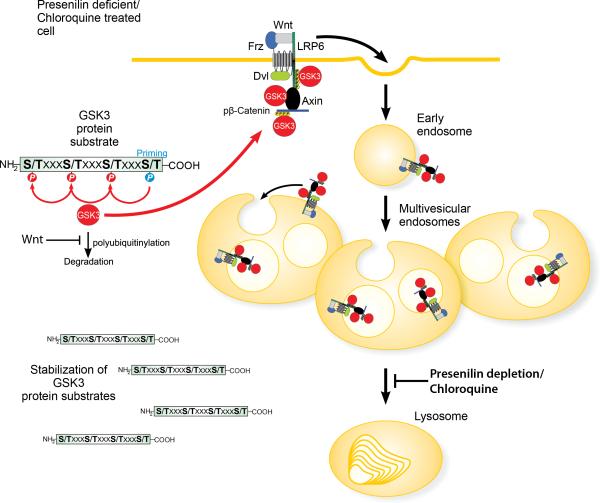
Recently we proposed a model for sustained Wnt signaling through sequestration of GSK3 in multivesicular endosomes (Taelman et al., 2010). In this study we investigated how Wnt signal transduction is affected when the function of the endolysosomal pathway is altered by inhibiting lysosomal function with Chloroquine, low doses of Bafilomycin A, the lysosomal protease inhibitors Leupeptin or E64, or Presenilin 1 depletion. We found that lysosomal inhibition caused an expansion of the late endosomal compartment, leading to a more efficient sequestration of GSK3 and the generation of an enhanced Wnt signal. The Chloroquine effect had an absolute requirement for the ESCRT machinery, which is essential for the formation of intraluminal vesicles in the endosomal compartment. The enhanced Wnt signaling caused by Presenilin depletion required Hrs/Vps27 not only in cell culture experiments but also in vivo in explants from Xenopus PS1 morpholino-injected embryos. Figure 7 shows a proposed model of how lysosomal inhibition increases Wnt/GSK3 signaling and protein stabilization.
Figure 7. Model of how Chloroquine or Presenilin depletion affects Wnt signaling by expanding the late endosomal compartment.
Lysosomal inhibition by Chloroquine or depletion of Presenilin leads to an accumulation of late endosomal vesicles which, upon Wnt signaling, cause increased sequestration of GSK3 in multivesicular endosomes. Lower levels of active GSK3 in the cytosol during Wnt signaling result in increased stabilization of the half-life of GSK3 protein substrates. Note that the activated Wnt receptor complex consists of multiple GSK3 substrates – such as LRP6, Frizzled (Fz), Dishevelled (Dvl), Axin, and phospho-β-Catenin – leading to sequestration of active GSK3 enzyme molecules bound to its substrates.
The anti-malarial drug Chloroquine, which alkalinizes lysosomes but still allows the formation of ILVs, caused a great expansion of the late endosomal compartment. Not all signaling pathways were increased by Chloroquine treatment; in the case of Sonic Hedgehog (Shh) signaling by a Luciferase reporter was inhibited by Chloroquine, while Hrs-siRNA increased signaling instead of inhibiting signaling as in the case of Wnt (data not shown). An increase in the fluid-phase of the endosomal compartment (marked by endocytosed DextranRed) was observed after siRNA-mediated depletion of Presenilin 1. This gene has been linked to Alzheimer's disease (AD) and recently found to be critical for lysosomal maturation (Lee et al., 2010; Neely et al., 2011; Zhang et al., 2012). PS1 and PS2 are intramembrane proteases that form the catalytic core of the γ-Secretase complex that cleaves the transmembrane domains of a large number of proteins. Presenilins are found in most cellular membranes, including in the ILVs of MVEs (De Strooper and Annaert, 2010). In the experiments reported here, lysosomal inhibition or PS depletion was shown to increase Wnt signaling in an ESCRT-dependent manner, but did not generate a signal in the absence of Wnt ligand. This effect could be due to an expanded surface of the limiting membrane of MVEs, or perhaps to changes in pH within the endosome that could increase the sensitivity of the endosomal membrane to undergo invagination to generate ILVs.
Recently published work by Li et al. (2012) described the formation of active Wnt receptor complexes containing most of the components of the β-Catenin destruction complex, except for the E3 ligase βTrCP. In agreement with that report, we observed that βTrCP does not translocate into LRP6 endosomes, while β-Catenin phosphorylated by GSK3 is sequestered inside these Wnt-specific endosomes (Taelman et al., 2010 and unpublished results). These findings underscore the deep relationship between endosomal trafficking and the Wnt signaling pathway.
Depletion of Presenilin Increases Wnt Signaling
Presenilins have been proposed to be involved in maturation of lysosomes through N-glycosylation of the V0a1 subunit of the v-ATPase complex needed for endosomal acidification (Lee et al., 2010). This mechanism has most recently been questioned (Zhang et al., 2012), and it has been suggested instead that Presenilins are involved in the regulation of the gene network associated with lysosomal biogenesis (Sardiello et al., 2009) affecting autophagy/lysosomal proteolysis independently of lysosomal acidification (Neely et al., 2011; Zhang et al., 2012). Lee et al. (2010) had observed a decrease of endosomal acidification in LysoTracker staining of PS1 knockout cells and siRNA knockdowns, and we were able to confirm their observations. We also found significant increases of MVE markers like CD63, Rab7 and endocytosed DextranRed in cells depleted of PS1. The effects on lysosomal maturation were independent of γ-Secretase activity (Lee et al., 2010; Neely et. al., 2011; Figure S2I), excluding an effect on Notch or other γ-Secretase-dependent pathways.
The enhancement of Wnt signaling by Presenilin depletion discovered here was entirely dependent on ESCRT machinery (Figures 1A, 2G and 2H). Thus, formation of intraluminal vesicles of late endosomes is required for the enhanced effects of the Wnt ligand. Our observations differ from a report by Kang et al. (2002) who proposed that Presenilin functioned as an alternative scaffold for β-Catenin degradation. Importantly, they found that loss of PS1 stabilized β-Catenin in a Wnt-independent way, while in our experiments addition of Wnt protein was required to trigger Wnt transcriptional activity in cell lines with low levels of endogenous Wnt signaling (Figures 2F, 2G and 2I). We note, however, that in Xenopus explants PS1 MO did slightly increase β-Catenin signaling (Figure 2H). In addition, PS1 siRNA caused a small but reproducible increase of cytoplasmic β-Catenin levels in HeLa cells (Figure 4I and 4K’). We suggest that the reported Wnt-independent accumulation of β-Catenin (Kang et al., 2002) might be explained in part by an endogenous autocrine Wnt signal that became enhanced though MVE expansion in PS1 knockout cells.
Presenilin Depletion Increases Protein Stabilization by Wnt
Proteins normally degraded in lysosomes are taken up from the extracellular space by endocytosis, are membrane proteins, or are ingested from the cytosol via autophagy. Upon Wnt signaling, cells experience an additional effect, namely the endosomal sequestration of cytoplasmic GSK3 which is translocated into MVBs together with activated Wnt receptor complexes (Taelman et al., 2010; Dobrowolski and De Robertis, 2012). Here we provided evidence that three cytosolic GSK3 substrates were stabilized by Wnt signaling in PS1-depleted cells: the microtubule-associated protein Tau, β-Catenin, and a GFP-GSK3 biosensor.
The experiments suggest a new mechanism for Wnt signaling in Presenilin-deficient cells. We propose that upon Wnt signaling activated receptor complexes are internalized in endosomes and sequester GSK3 inside the expanded late endosomes together with the activated Wnt receptor complex, which is composed of multiple protein components phosphorylated by GSK3 (Figure 7). Once cytoplasmic levels of active GSK3 are sufficiently decreased, GSK3 protein substrates become less phosphorylated, their phosphodegron domains are not recognized by E3 polyubiquitin ligases and GSK3 target proteins become stabilized.
This new mechanism could play a role in Alzheimer's disease (AD), since Presenilin deficiency increases the stability of multiple GSK3 substrates upon Wnt signaling. Some of these substrates, such as the microtubule-associated protein Tau, play crucial roles in the pathogenesis of AD. An extensive literature exists linking Tau and GSK3 signaling (Hall et al., 2000; de Calignon, 2012). A role for Wnt signaling in prevalent neuropsychiatric diseases including AD, schizophrenia and autism has been proposed (De Ferrari and Moon, 2006), but studies performed to elucidate the function of Wnt in neurodegeneration have often generated contradictory results (summarized by Boonen et al., 2009). It is possible that Wnt signaling may result in different outcomes at different stages of AD progression. At early stages of AD, increased sequestration of GSK3 may stabilize many proteins, as shown here for Tau. However, chronic protein stabilization in PS1-deficient cells might eventually lead to failure of the endosomal pathway and Wnt signaling as the disease progresses. Once GSK3 ceases to be sequestered, elevated levels of cytosolic Tau might be more readily phosphorylated by GSK3, triggering AD pathology. It is possible that repeated Wnt stimulation through the course of a lifetime aging process, in combination with the accumulation of multivesicular endosomes caused by defective trafficking, could result in increased protein stabilization and the eventual accumulation of the neurotoxic Amyloid β42 (Aβ42) peptide (De Strooper and Annaert, 2010).
At early AD stages Aβ42 has been localized to MVBs (Takahashi et al., 2002) and is released from neurons via exosomes (Rajendran et al., 2006) to form extracellular amyloid deposits (Vella et al., 2008). According to the amyloid hypothesis for the onset of AD (Selkoe, 1991; Hardy and Higgins, 1992; Hardy 2009), extracellular amyloid plaque deposits would lead to altered kinase/phosphatase activities and hyperphosphorylation of Tau. Other recent studies suggest that phosphorylated Tau spreads in a prion-like manner through neuronal synaptic circuits (Liu et al., 2012; de Calignon, 2012; Kfoury et al., 2012). In addition, autophagy and lysosomal proteolysis defects have also been proposed to be involved in the pathogenesis of AD (Nixon et al., 2008; Lee et al., 2010).
The experiments presented here demonstrate that decreased Presenilin expression causes an increase in Wnt signaling. The results also show that PS1 depletion causes a considerable expansion of the endosomal system, which results from the inhibition of lysosomal function downstream of intraluminal vesicle formation. The new connection between Wnt signaling and increased stability of GSK3 phosphorylation protein targets in Presenilin-deficient cells could explain why AD neurodegeneration is most severe in the hippocampus, especially in its dentate gyrus, and cerebral pyramidal neurons (Brundin et al., 2010), for it is in these particular cells that physiological Wnt signaling in the adult brain is maximal (Maretto et al., 2003). We propose that, during the aging process defects in membrane trafficking may increase protein stabilization in the cytosol after repeated stimulation by Wnt signaling, contributing to the formation and deposition of intracellular protein aggregates. Whether the proposed mechanisms play a role in human pathology remains to be determined. In the meantime, the results presented here strengthen the intimate connection between Wnt signaling and the cell biology of intracellular membrane trafficking.
EXPERIMENTAL PROCEDURES
Cell Culture and Knock-Down Experiments
All cell lines used (HeLa, 3T3, HEK293T and L-cells) were cultured in DMEM complete medium containing 10% FBS, 1% Glutamine and 1% Pen/Strep. For knock-down experiments in cultured cells, siRNAs targeting human PS1, PS2, and HRS were ON-TARGETplus SMARTpool from Thermo Scientific #L-004998, #L-006018, #L-016835, respectively. The non-targeting control-siRNA pool was from the same company, #D-001810. For RNAi depletion experiments, cells were transfected with siRNAs 24 h prior to transfections with DNA. For the rescue experiments we introduced a full-length human PS1 gene (Open Biosystems, EHS1001-33034) into the pCS2+ expression plasmid and transfected 0.3 μg of this DNA into each well of a 12-well plate containing siRNA pretreated cells. Knock-down experiments in Xenopus laevis were conducted using Presenilin 1 morpholino (sequence: TTCACTGGTGTCATTCATATTAGCT, Gene Tools, LLC) and Hrs morpholino (sequence: TGCCGCTTCCTCTTCCCATTGCGAA , Gene Tools, LLC).
Immunostaining, Flow Cytometry, and Western Blots
The following primary antibodies were used for immunostainings and flow cytometry: anti-Rab7 rabbit monoclonal (Cell Signaling # 9367) at 1:350; anti-CD63 mouse monoclonal (BD Biosciences #556019) at 1:250; anti-β-Catenin rabbit (SantaCruz H-102) at 1:4000; anti-phospho-S1490-LRP6 rabbit (Cell Signaling # 2568) at 1:500.
For Western blots, the following primary antibodies were used: anti-β-Catenin (Sigma #C2206) at 1:4000; anti-Flag monoclonal (Sigma #F1804) at 1:1500; anti-GFP (Molecular Probes, #A6455) at 1:500; anti-phospho-LRP6 (Cell Signaling #2568) at 1:500; anti-phospho-Tau (T181) (Cell Signaling #5383) at 1:500; anti-α-Tubulin monoclonal (Calbiochem #CP06) at 1:1500. Secondary antibodies coupled to Infra-Red Dyes (IRDye 680 and IRDye 800) at 1:3000 (LI-COR) were used and western blots were analyzed using a LI-COR Odyssey system.
Statistics
Results of three or more independent experiments are given as the mean ± standard error of the mean (SEM). Statistical analyses were performed with Excel (Microsoft Co) applying the two-tailed t test, as appropriate. Significant differences of means are indicated as *≤0.05, **≤0.01, ***≤0.005.
Additional Methods
Detailed methods for Luciferase assays, endolysosomal staining, in vivo time-lapse movies, and electron microscopy are provided in Extended Experimental Procedures.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. R. Nusse for LSL cells, R. Moon for HEK293T LRP6-GFP cells and the SuperTopFlash and BAR Wnt reporter plasmids, M. Kirschner for xTau-GFP, and B. van Deurs for Rab7-GFP constructs, and K. Ohmi for advice. Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility that is supported by National Institutes of Health awards CA-16042 and AI-28697, and the David Geffen School of Medicine at UCLA. We thank the Deutsche Forschungsgemeinschaft for supporting R.D. (DO1429/1-1) and P.V. (VI574/1-1), and the NIH (HD21502-25) for funding. E.M.D.R. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures, four figures and one movie.
REFERENCES
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Biechele TL, Moon RT. Wnt signaling, Assaying β-catenin/TCF transcription with β-catenin/TCF transcription-based reporter constructs. Methods Mol. Biol. 2008;468:99–110. doi: 10.1007/978-1-59745-249-6_8. [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 Signalosomes and promotes Dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Blitzer JT, Nusse R. A critical role for endocytosis in Wnt signaling. BMC Cell Biol. 2006;7:28. doi: 10.1186/1471-2121-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonen RA, van Tijn P, Zivkovic D. Wnt signaling in Alzheimer's disease: Up or down, that is the question. Age. Res. Rev. 2009;8:71–82. doi: 10.1016/j.arr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat. Rev. Mol. Cell. Biol. 2010;11:301–307. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosomes biogenesis. Mol. Biol. Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Peifer M. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb. Perspect. Biol. 2009;2:a002881. doi: 10.1101/cshperspect.a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/β-Catening signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of Prorenin receptor and Vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459–463. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- Davidson G, Shen J, Huang YL, Su Y, Karaulanov E, Bartscherer K, Hassler C, Stannek P, Boutros M, Niehrs C. Cell cycle control of Wnt receptor activation. Dev. Cell. 2009;17:788–799. doi: 10.1016/j.devcel.2009.11.006. [DOI] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suárez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari GV, Moon RT. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene. 2006;57:7545–7553. doi: 10.1038/sj.onc.1210064. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W. Novel research horizons for presenilins and γ-secreatases in cell biology and disease. Annu. Rev. Cell Dev. Biol. 2010;26:235–260. doi: 10.1146/annurev-cellbio-100109-104117. [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, De Robertis EM. Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles. Nat. Rev. Mol. Cell. Biol. 2012;13:53–60. doi: 10.1038/nrm3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escola J-M, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshiei O, Geuze HJ. Selective Enrichment of Tetraspan Proteins on the Internal Vesicles of Multivesicular Endosomes and on Exosomes Secreted by Human B-lymphocytes. J. Biol. Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J. Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, Koo EH. Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell. 2002;110:751–62. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- Kategaya LS, Changkakoty B, Biechele T, Conrad WH, Kaykas A, Dasgupta R, Moon RT. Bili inhibits Wnt/beta-catenin signalling by regulating the recruitment of axin to LRP6. PLoS One. 2009;4:e6129. doi: 10.1371/journal.pone.0006129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell. Biol. 2002;12:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular Propagation of Tau Aggregation by Fibrillar Species. J. Biol. Chem. 2012;287:19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NG, Xu C, Gumbiner BM. Identification of targets of the Wnt pathway destruction complex in addition to β-Catenin. Proc. Natl. Acad. Sci. USA. 2009;106:5165–5170. doi: 10.1073/pnas.0810185106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KM, Kirschner MW. A microtubule-binding Rho-GEF controls cell morphology during convergent extension of Xenopus laevis. Development. 2005;132:4599–4610. doi: 10.1242/dev.02041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, Mohammed S, Heck AJ, Maurice MM, Mahmoudi T, Clevers H. Wnt Signaling through Inhibition of β-Catenin Degradation in an Intact Axin1 Complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/β-Catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta Catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely KM, Green KN, LaFerla FM. Presenilin is necessary for efficient proteolysis through the autophagy-lysosome system in a γ-secretase-independent manner. J. Neurosci. 2011;31:2781–2791. doi: 10.1523/JNEUROSCI.5156-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C, Acebron SP. Mitotic and mitogenic Wnt signaling. EMBO J. 2012;31:2705–2713. doi: 10.1038/emboj.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C, Shen J. Regulation of Lrp6 phosphorylation. Cell. Mol. Life Sci. 2010;67:2551–2562. doi: 10.1007/s00018-010-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, Yang DS, Lee JH. Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy. 2008;5:590–599. doi: 10.4161/auto.6259. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Yang DS. Autophagy failure in Alzheimer's disease--locating the primary defect. Neurobiol. Dis. 2011;43:38–45. doi: 10.1016/j.nbd.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, Potolicchio I, Nieves E, Cuervo AM, Santambrogio L. Microautophagy of cytosolic proteins by late endosomes. Dev. Cell. 2011;20:131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Soriano S, Kang DE, Fu M, Pestell R, Chevallier N, Zheng H, Koo EH. Presenilin 1 negatively regulates beta-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of beta-amyloid precursor protein and notch processing. J. Cell. Biol. 2001;152:785–794. doi: 10.1083/jcb.152.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nature Rev. Mol. Cell Biol. 2009;1:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM. Wnt Signaling Requires the Sequestration of Glycogen Synthase Kinase 3 inside Multivesicular Endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella LJ, Sharples RA, Nisbet RM, Cappai R, Hill AF. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur. Biophys. J. 2008;37:323–332. doi: 10.1007/s00249-007-0246-z. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Wegener CS, Malerod L, Pedersen NM, Prodiga C, Bakke O, Stenmark H, Brech A. Ultrastructural characterization of giant endosomes induced by GTPase-deficient Rab5. Histochem. Cell Biol. 2010;133:41–55. doi: 10.1007/s00418-009-0643-8. [DOI] [PubMed] [Google Scholar]
- Wibo M, Poole B. Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J. Cell. Biol. 1974;63:430–440. doi: 10.1083/jcb.63.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev. Cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–75. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Garbett K, Veeraraghavalu K, Wilburn B, Gilmore R, Mirnics K, Sisodia SS. A Role for Presenilins in Autophagy Revisited: Normal Acidification of Lysosomes in Cells Lacking PSEN1 and PSEN2. J. Neurosci. 2012;32:8633–8648. doi: 10.1523/JNEUROSCI.0556-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.