Summary
o-Benzynes (arynes) are among the most versatile of all reactive (short-lived) intermediates in organic chemistry. These species can be trapped to give products that are valuable from the perspective of both fine (pharmaceuticals) and commodity (agrochemicals, dyes, polymers, etc.) chemicals. Here we show a fundamentally new strategy that unites a de novo generation of benzynes, through the title hexadehydro-Diels–Alder (HDDA) reaction, with their in situ elaboration into structurally complex benzenoid products. In the HDDA reaction a 1,3-diyne is engaged in a [4+2] cycloisomerization with a third (pendant) alkyne–the diynophile–to produce the highly reactive benzyne intermediate. The metal- and reagent-free reaction conditions for this simple, thermal transformation are notable. The subsequent and highly efficient trapping reactions increase the power of the overall process. Finally, we provide examples of how this de novo benzyne generation approach allows new modes of intrinsic reactivity to be revealed.
o-Benzyne [1,2-didehydrobenzene (1), Fig. 1a] is one of the oldest,1 most interesting, most useful, and most well-studied of all reactive intermediates in chemistry. The multifaceted and often remarkably efficient reactions of benzynes with suitable trapping reagents (cf. 1 to 3) have long been employed in the service of synthetic chemistry. Even by 1967 many such reactions were known2. Nonetheless, newly discovered benzyne reaction motifs continue to emerge, especially so within the last decade3–10. This renaissance attests to the additional versatility and heralds even greater potential of this intermediate. Currently, all practical methods for producing benzynes involve the removal of two adjacent atoms or substituents (cf. “G” and “X”) from benzenoid precursors 2. These protocols typically require the use of a strong base and/or access to a 1,2-disubstituted arene substrate. A complementary general method for benzyne/aryne generation would considerably expand the preparative utility of these remarkable intermediates.
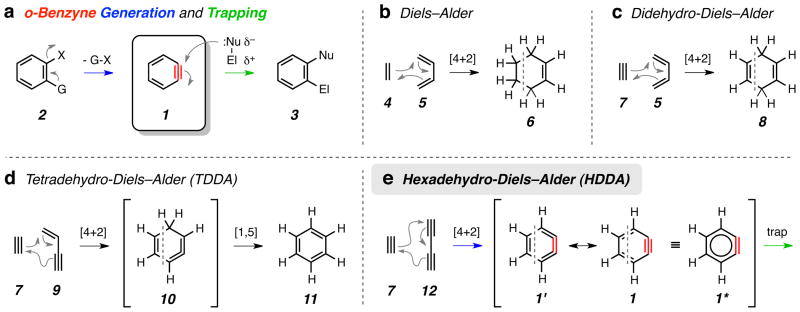
Figure 1. Diels-Alder reactions of varying oxidation states.
a, Generic benzyne generation (2 to 1) and trapping (1 to 3). Most commonly, G/X = H/halogen, CO2−/N2+, halogen/halogen, or TMS/OTf. b–e, Prototypes of Diels–Alder reactions differing in the oxidation levels of the reactant pairs and products: classic Diels–Alder (b), didehydro-Diels–Alder (c), tetradehydro-Diels–Alder (TDDA) (d), and hexadehydro-Diels–Alder (HDDA, this work, e) reactions. [TMS = trimethylsilyl, OTf = trifluoromethanesulfonate, Nu = nucleophile, El = electrophile]
The Diels–Alder [4+2] cycloaddition reaction11 is arguably the most revered of all chemical reactions12,13. The prototypical event (Fig. 1b), found in every introductory organic chemistry textbook, is the combination of 1,3-butadiene (5) as the 4π-component with ethylene (4) as the dienophile to give cyclohexene (6)–a product at the tetrahydrobenzene oxidation state. If, instead, an alkyne like ethyne (7) is the dienophile, a 1,4-cyclohexadiene [1,4-dihydrobenzene (8)] results (Fig. 1c); we suggest this be viewed as a didehydro-Diels–Alder reaction. Another known variant (Fig. 1d) involves engagement of a (yet more highly oxidized) 1,3-enyne (e.g., 9) as the 4π-component with an alkyne (e.g, 7). It is interesting to note that the first example of this process (the thermal dimerization of phenylpropiolic acid)14 was described 30 years prior to the initial report of Diels and Alder11. The intermediate cyclic allene 10 rapidly rearranges via a [1,5] hydrogen atom shift to benzene (11). This process has heretofore been called, generically, the dehydro-Diels–Alder (DDA) reaction15. In light of the generality of the results we present in this report, we suggest amending the moniker for the transformation in Fig. 1d to tetradehydro-Diels–Alder (TDDA) reaction.
The most highly oxidized Diels-Alder variant (Fig. 1e) is the cycloaddition between a 1,3-diyne like 12 and an alkyne diynophile like 7, which generates o-benzyne (cf. 1′, 1, 1*). This hexadehydro-Diels–Alder (HDDA) reaction is the subject of this report. Given the efficiency, ease of precursor access, versatility, and mild reaction conditions revealed by the examples we present here, it is remarkable that this reaction has remained essentially unexploited16–19. It is interesting to speculate that this may be due in part to the fact that the most common depiction of benzyne–the resonance contributor 1 (Fig. 1e)–obscures its potential construction via a [4+2] cycloaddition reaction. It is the alternative, but rarely encountered, Kekulé depiction 1′ that reveals the opportunity for assembly via an HDDA process.
We report below the broad scope of a strategy that combines the versatile and efficient generation of benzynes via the HDDA reaction with various trapping reactions to yield structurally complex benzenoid products. Each substrate is a readily accessible conjugated diyne containing a remote alkynyl diynophile. These cycloisomerize in (a highly exergonic) [4+2] fashion to produce the reactive aryne intermediate. The examples demonstrate that the tandem benzyne forming/trapping sequence can be designed to proceed with excellent efficiency.
The HDDA reaction revealed
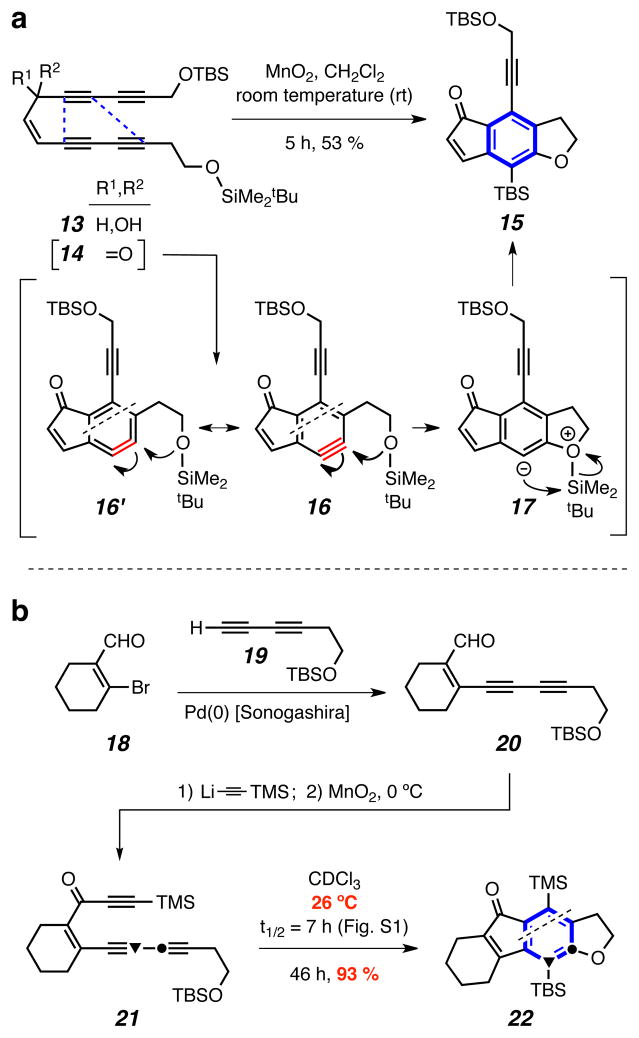
In the course of an otherwise unrelated study, we attempted to prepare the ketotetrayne 14 by oxidation of the precursor alcohol 13 with manganese dioxide (Fig. 2a). To our surprise, the major product from this experiment, formed in ca. 5 hours, was the (hexasubstituted) benzene derivative 15 (53% yield after purification). We quickly postulated that the benzyne intermediate 16′/16 was being both readily formed and efficiently trapped by the nucleophilic oxygen atom in the fortuitously poised β-siloxyethyl group. Migration of the silyl group from O to C within zwitterion 17, a retro-Brook rearrangement, accounts for formation of 15. This constitutes an unprecedented mode of benzyne trapping reaction. Additionally, the process is attended by a substantial increase in structural complexity. The potential power of this transformation was immediately apparent. We surmised that the modest yield observed in this reaction of tetrayne 14 reflected the fact that two competitive modes of [4+2] cyclization are possible. We were also keen to learn the feasibility of cyclization of analogous triynes. We therefore designed and synthesized a substrate–the ketotriyne 21 (Fig. 2b)–that could only undergo a HDDA reaction with a single regiochemical outcome. Our efforts were rewarded by its smooth transformation at room temperature to the hexasubstituted, tetracyclic indenone derivative 22 in 93% yield after chromatographic purification.
Figure 2. Mechanistic rationale, substrate synthesis, and mild conditions for our initial two HDDA reactions.
a, Serendipitous observation of the HDDA reaction: cyclization of ketone 14 to putative benzyne (red) intermediate 16/16′ and subsequent trapping by the pendant silyl ether gave a hexasubstituted benzenoid (blue), the indenone 15. b, Synthesis of 21 (via convergent coupling of 18 with 19, addition of an ethynyl unit to 20, and oxidation) and its facile, high-yielding conversion to the tetracyclic benzenoid (blue) 22. [TBS = tert-butyldimethylsilyl]
Intramolecular Trapping
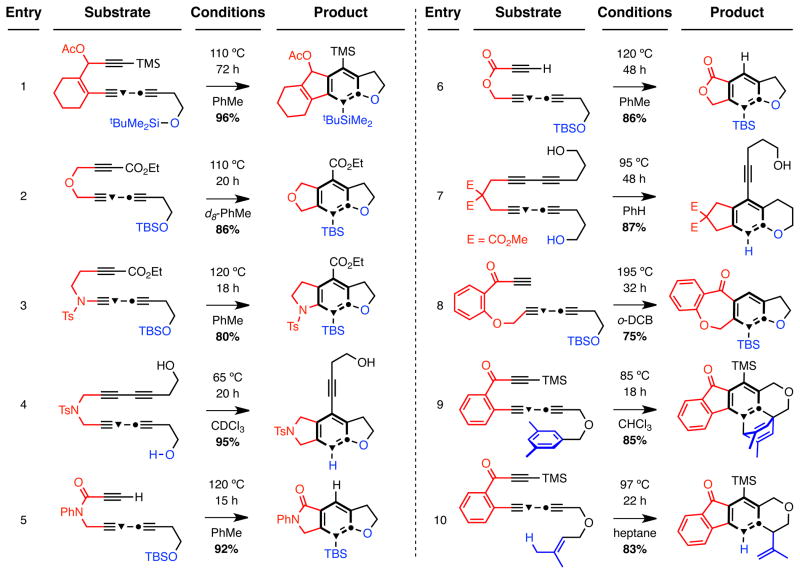
As the examples presented in Fig. 3 clearly show, the HDDA-initiated cascade has considerable scope with respect to both the cycloisomerization and the intramolecular trapping events. Each substrate is readily accessible by a convergent coupling strategy (cf. Fig. 2b). All yields of purified products were ≥75%, and (with the exception of entry 8) all reactions occurred between temperatures of 20–120 °C. Highlights include: i] the presence of an electron withdrawing substituent on the diynophile enhances substrate reactivity [cf. conditions for 21 to 22 (Fig. 2b) vs. entry 1]; ii] the activating carbonyl group can be a distal (carboalkoxy) rather than a tethering substituent (entries 2 and 3); iii] many classical methods of aryne generation are not compatible with electron-withdrawing groups in the substrate;20 iv] carbonyl activation is not a necessity (entries 1, 4, and 7); v] products having nitrogen-containing heterocycles annulated to the new arene ring can be prepared (entries 3–5); vi] an ester tether (entry 6) cyclizes more slowly than its N-phenyl amide analog (entry 5), consistent with the lower concentration of the s-cis conformation required for ring closure; vii] our observations are consistent with the absence of radical character in both the cycloaddition and trapping phases of the process; e.g., reactions performed in chloroform solvent, an excellent hydrogen atom donor, have shown no evidence of hydrogen atom transfer (entries 4 and 9); viii] the new silyl ether trapping reaction has considerable generality (entries 1–3, 5, 6, and 8 and Fig. 2); ix] other efficient internal benzyne traps include tethered alcohols (entries 4 and 7), aryl rings ([4+2] cycloaddition in entry 9), or alkenes (ene reaction in entry 10); x] seven-membered ring formation is feasible (entry 8), and the robust nature of the substrate and product at the high temperature required for this slower cyclization are noteworthy; and xi] the silyl substituents in many of the products provide handles for subsequent elaboration through protonative, oxidative, or halogenative desilylation or cross-coupling reactions21.
Figure 3. Examples of intramolecular trapping of HDDA-generated benzynes.
Benzenoid (bold black bonds) synthesis via the HDDA cycloaddition has considerable substrate scope with respect to the nature of i) the poly-yne tether (red) and ii) the intramolecular trapping moiety (blue). [Ac = acetyl, Ts = p-toluenesulfonyl, o-DCB = 1,2-dichlorobenzene]
Intermolecular trapping
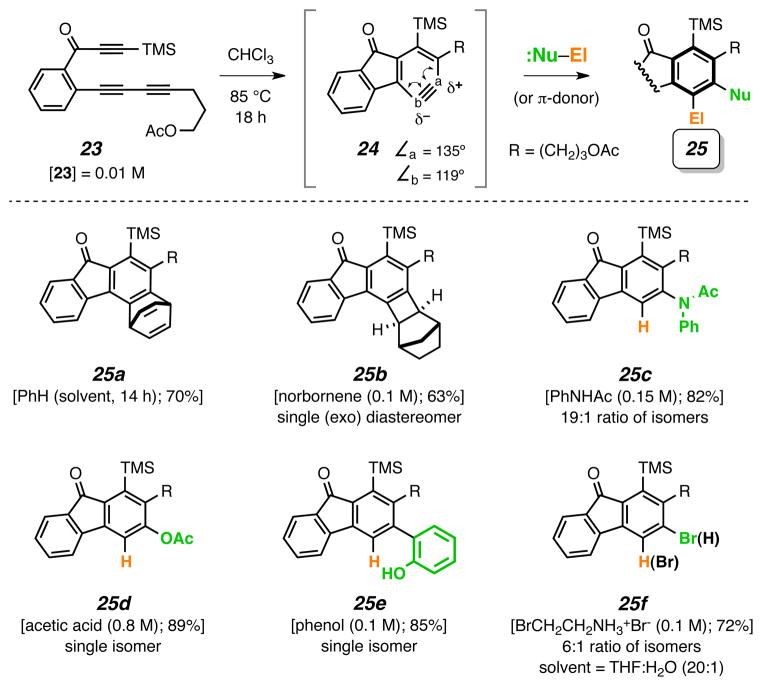
We were eager to validate the feasibility of bimolecular trapping of these thermally generated benzynes. Clearly, this would add considerable versatility and power to a HDDA-initiated transformation. The triyne 23 (Fig. 4) represents a substrate that bears an innocent (non-participating) side chain. We have successfully captured the derived benzyne 24 (formed from 23 in a cyclization reaction having a half-life of ca. 4 h at ca. 85 °C) by a variety of external reagents to give adducts 25. Highlights include: i] benzene as solvent forms the Diels–Alder adduct 25a (cf. Fig. 3, entry 9); although a precedented process22, because of the low reactivity of simple aromatics, rarely have they been trapped by benzynes in high yield; ii] this result also indicates that many intramolecular trapping events are faster than capture by the aromatic solvents used in earlier examples (entries 1–3 and 5–8 of Fig. 3); iii] the [2+2] cycloaddition of norbornene gives 25b in higher yield than has been observed for trapping of arynes formed by conventional methods2; iv] N-phenylacetamide gives 25c, demonstrating that a nitrogenous substituent can be conveniently installed; v] acetic acid22 or phenol traps 24 to cleanly provide adducts 25d or 25e, respectively, in processes that may share a mechanistic feature of transfer of a hydroxyl proton coincident with nucleophilic attack; vi] this mode of reaction with acetic acid or phenol is unique and complementary to that seen with benzynes generated by non-reagent-free methods23,24; vii] net trapping by hydrogen bromide was achieved using Br(CH2)2NH2•HBr in THF/H2O (20:1) as an HBr source to give 25f (6:1 mixture of isomers).
Figure 4. Examples of intermolecular trapping of HDDA-generated benzynes.
Bimolecular trapping reactions of benzyne 24 to give adducts 25a–f [trapping agent (and amount); yield following purification]. [THF = tetrahydrofuran]
The sense of regioselectivity observed for formation of products 25c–f is consistent with the analyses of Cramer and Buszek25 and of Houk and Garg24 in which the relative magnitude of the computed internal bond angles of an unsymmetrical benzyne is correlated with the site of nucleophilic attack. Namely, the more obtuse angle corresponds to the more electron deficient (δ+) of the two benzyne carbon atoms. We computed the geometry for 24 [R = CH3; M06-2X/6-31+G(d, p)25] to have internal angles of 135° and 119° at atoms “a” and “b”, respectively. We are currently investigating additional substrates that should allow us to distinguish the relative impact of electronic vs. ring distortion effects on the site of attack by external nucleophiles.
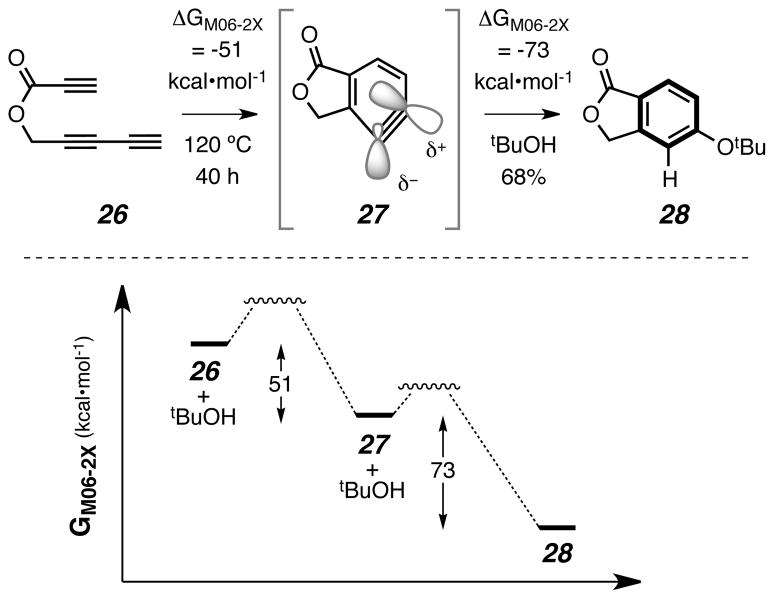
To gain understanding of some of the key thermodynamic features associated with the HDDA aryne-forming step, we turned to computational analysis of the reaction of ester 26 (Fig. 5). This simple triyne was cycloisomerized and the resulting benzyne trapped in tert-butanol (120 °C, closed tube) to produce 5-tert-butoxyphthalide (28) in 68% yield. Johnson and coworkers recently reported a density functional theory (DFT) approach to compute the energetics of the hypothetical HDDA reaction of 7 with 12 to produce o-benzyne (1, Fig. 1e).26 They concluded that this benzyne-forming reaction was downhill by 51.4 kcal•mol−1. Using similar DFT methodology we have computed the free energy of reaction for the conversion of triyne 26 to the aryne 27 and found it to be −51 kcal•mol−1. It is remarkable that highly reactive benzyne intermediates can be accessed by a thermal process that is ca. 50 kcal•mol−1 downhill. These very favorable free energies of reaction reflect the large amount of potential energy inherent in the (albeit kinetically protected) alkyne functional group. Finally, this point is further underscored by the computed free energy of reaction–namely, −73 kcal•mol−1 –for the trapping by tert-butanol of the strained alkyne27 in 27. Thus, the overall transformation of 26 to 28 is computed to be exothermic by >120 kcal•mol−1.
Figure 5. Computed free energy changes for a representative HDDA-initiated cascade.
Free energies of reaction [M06-2X/6-31+G(d, p)] for the aryne-generating (26 to 27) and tert-butanol (tBuOH) trapping (27 to 28) stages highlight the favorable thermodynamics associated with both the triyne cycloisomerization and aryne trapping events.
Discussion
Our results show that the HDDA reaction is a general method for generating benzynes under conditions amenable to a wide variety of intra- and intermolecular trapping events. This powerful and efficient domino sequence comprises a fundamentally new, arguably revolutionary, way to synthesize benzenoid compounds–especially those having high structural complexity. Because HDDA-derived benzynes are born in the absence of byproducts and external reagents, the intrinsic reactivity of a benzyne can be more fully explored and exploited. Even at this early stage of development, myriad additional lines of investigation have revealed themselves. These include studies of mechanistic issues (concerted vs. stepwise cycloaddition), substituent effects (electronic vs. steric), catalysis (Lewis acid or transition metal), new modes of benzyne trapping, the use of cleavable tethers, and the feasibility of bimolecular HDDA cycloadditions. This enabling technology is well-suited for preparing compound libraries, for accessing aromatics having substitution patterns that would be otherwise challenging to prepare, and for application to target molecule synthesis [e.g., drugs, natural products9,10, heteroaromatics, polyacenes]. Lessons of how imagery [i.e., 1(vs. 1′) from 7 + 12] can bias one’s perception are also embedded in the developments described here. Finally, our results serve as a reminder that even in the 21st century serendipitous discovery (“in the course of an otherwise unrelated study”) in science can still play a pivotal role.
METHODS SUMMARY
General Procedure
An oven dried vial containing the triyne precursor in the indicated solvent (0.05 M) was closed with a Teflon®-lined cap and heated at the indicated (external bath) temperature. After the indicated time the product was purified by chromatography on silica gel. Full procedural details and characterization data for all new compounds (substrates and products) and a detailed description of the computational protocols and results are provided in the Supplementary Information.
Supplementary Material
Acknowledgments
P.H.W. thanks the National Science Foundation for a Graduate Research Fellowship. Financial support from the National Institutes of Health (GM65597 and CA76497) is acknowledged. This work was carried out in part using hardware and software provided by the University of Minnesota Supercomputing Institute.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions B.B. performed the initial experiments. All authors designed the experiments, analyzed the data, and wrote the manuscript.
The authors declare no competing financial interest.
Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Wenk HH, Winkler M, Sander W. One century of aryne chemistry. Angew Chem Int Ed. 2003;42:502–528. doi: 10.1002/anie.200390151. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann RW. Organic Chemistry, A Series of Monographs. Vol. 11. Academic Press; 1967. Dehydrobenzene and Cycloalkynes. [Google Scholar]
- 3.Pellissier H, Santelli M. The use of arynes in organic synthesis. Tetrahedron. 2003;59:701–730. [Google Scholar]
- 4.Dyke AM, Hester AJ, Lloyd-Jones GC. Organometallic generation and capture of ortho-arynes. Synthesis. 2006:4093–4112. [Google Scholar]
- 5.Sanz R. Recent applications of aryne chemistry to organic synthesis. A review. Org Prep Proc Intl. 2008;40:215–291. [Google Scholar]
- 6.Gilchrist TL. In: Science of Synthesis. Hopf H, editor. Vol. 43. Georg Thieme Verlag KG; Stuttgart: 2008. pp. 151–215. [Google Scholar]
- 7.Chen Y, Larock RC. In: Modern Arylation Methods. Ackermann L, editor. Wiley-VCH; 2009. pp. 401–473. [Google Scholar]
- 8.Kitamura T. Synthetic methods for the generation and preparative application of benzyne. Aust J Chem. 2010;63:987–1001. [Google Scholar]
- 9.Tadross PM, Stoltz BM. A comprehensive history of arynes in natural product total synthesis. Chem Rev. 2012;112:3550–3577. doi: 10.1021/cr200478h. [DOI] [PubMed] [Google Scholar]
- 10.Gampe CM, Carreira EM. Arynes and cyclohexyne in natural product synthesis. Angew Chem Int Ed. 2012;51:3766–3778. doi: 10.1002/anie.201107485. [DOI] [PubMed] [Google Scholar]
- 11.Diels O, Alder K. Syntheses in the hydroaromatic series [in German] J Liebigs Ann der Chem. 1928;460:98–122. [Google Scholar]
- 12.Onishchenko AS. Diene Synthesis. Israel Program for Scientific Translations Ltd; 1964. [Google Scholar]
- 13.Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. The Diels Alder reaction in total synthesis. Angew Chem Int Ed. 2002;41:1668–1698. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Michael A, Bucher JE. Über die Einwirkung von Eissigsäureanhydrid auf Phenylpropiolsäure [in German] Chem Zentrblt. 1898:731–733. [Google Scholar]
- 15.Wessig P, Müller G. The dehydro-Diels Alder reaction. Chem Rev. 2008;108:2051–2063. doi: 10.1021/cr0783986. [DOI] [PubMed] [Google Scholar]
- 16.Bradley AZ, Johnson RP. Thermolysis of 1,3,8-nonatriyne: Evidence for intramolecular [2+4] cycloaromatization to a benzyne intermediate. J Am Chem Soc. 1997;119:9917–9918. [Google Scholar]
- 17.Miyawaki K, Suzuki R, Kawano T, Ueda I. Cycloaromatization of a non-conjugated polyenyne system: Synthesis of 5H-benzo[d]fluoreno[3,2-b]pyrans via diradicals generated from 1-[2-{4-(2-alkoxymethylphenyl)butan-1,3-diynyl}]phenylpentan-2,4-diyn-l-ols and trapping evidence for the 1,2-didehydrobenzene diradical. Tetrahedron Lett. 1997;38:3943–3946. [Google Scholar]
- 18.Kimura H, Torikai K, Miyawaki K, Ueda I. Scope of the thermal cyclization of nonconjugated ene-yne-nitrile system: a facile synthesis of cyanofluorenol derivatives. Chem Lett. 2008;37:662–663. and references therein. [Google Scholar]
- 19.Tsui JA, Sterenberg BT. A metal-templated 4 + 2 cycloaddition reaction of an alkyne and a diyne to form a 1,2-aryne. Organometallics. 2009;28:4906–4908. [Google Scholar]
- 20.Uchiyama M, Miyoshi T, Kajihara Y, Sakamoto T, Otani Y, Ohwada T, Kondo Y. Generation of functionalized asymmetric benzynes with TMP-zincates. Effects of ligands on selectivity and reactivity of zincates. J Am Chem Soc. 2002;124:8514–8415. doi: 10.1021/ja0202199. [DOI] [PubMed] [Google Scholar]
- 21.Chang W-tT, Smith RC, Regens CS, Bailey AD, Werner NS, Denmark SE. Cross-coupling with organosilicon compounds. Org Reactions. 2011;75:213–745. [Google Scholar]
- 22.Stiles M, Miller RG, Burckhardt U. Reactions of benzyne intermediates in non-basic media. J Am Chem Soc. 1963;85:1792–1797. [Google Scholar]
- 23.Liu Z, Larock RC. Facile O-arylation of phenols and carboxylic acids. Org Lett. 2004;6:99–102. doi: 10.1021/ol0361406. [DOI] [PubMed] [Google Scholar]
- 24.Cheong PHY, Paton RS, Bronner SM, Im GJ, Garg NK, Houk KN. Indolyne and aryne distortions and nucleophilic regioselectivities. J Am Chem Soc. 2010;132:1267–1269. doi: 10.1021/ja9098643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garr AN, et al. Experimental and theoretical investigations into the unusual regioselectivity of 4,5-, 5,6-, and 6,7-indole aryne cycloadditions. Org Lett. 2010;12:96–99. doi: 10.1021/ol902415s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Truhlar DG. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two newfunctionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc. 2008;120:215–241. [Google Scholar]
- 27.Ajaz A, et al. Concerted vs. stepwise mechanisms in dehydro-Diels2Alder reactions. J Org Chem. 2011;76:9320–9328. doi: 10.1021/jo201567d. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RP, Daoust KJ. Interconversions of cyclobutyne, cyclopentyne, cyclohexyne, and their corresponding cycloalkylidene carbenes. J Am Chem Soc. 1995;117:362–367. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.