Abstract
A small library of boron cluster and metallacarborane cluster-based ligands was designed, prepared and tested for isoform-selective activation or inhibition of the three nitric oxide synthase isoforms. Based on the concept of creating a hydrophobic analog of a natural substrate, a stable and non-toxic basic boron cluster system, previously used for boron neutron capture therapy, was modified by the addition of positively charged moieties to its periphery, providing hydrophobic and non-classical hydrogen bonding interactions with the protein. Several of these compounds show efficacy for inhibition of NO synthesis with differential effects on the various nitric oxide synthase isoforms.
Keywords: Nitric oxide synthase, Isoform-specific affectors, Boron clusters, Metallacarboranes, Cobalt bis(dicarbollide)
Introduction
Nitric oxide (NO) is a biological messenger, produced by the nitric oxide synthases (NOSs), which is involved in many physiological processes serving a variety of regulatory and immunological functions. The mammalian NOS family consists of three isoforms, neuronal NOS (nNOS or NOSI), inducible NOS (iNOS or NOSII), and endothelial cell NOS (eNOS or NOSIII). All isoforms are homodimers that catalyze the NADPH-dependent oxidation of L-arginine to L-citruline and nitric oxide. The three NOS isoforms play unique, separate, and characteristic roles in various tissues and cell types; the NO produced has been implicated in such varied processes as hemodynamic control, neurotransmission, and the immune response (for review, see refs. [1–8]).
Overproduction of NO by nNOS and iNOS has been implicated in ischemic injury following strokes, migraine headaches, autoimmune diseases such as rheumatoid arthritis and ulcerative colitis, Parkinson’s and Alzheimer’s diseases, liver cirrhosis, inflammation, and hypotension and vascular leakage during septic shock. Underproduction of NO by eNOS has been implicated in hypertension, atherosclerosis, and endothelial dysfunction. Clearly, the ability to selectively inhibit nNOS and iNOS, but not eNOS, has great potential in developing treatments for these diseases.
Regulation of NO production, with an emphasis on inhibition, is one of the leading themes in NOS biochemistry and structural biology. To date, many NOS inhibitors have been described, but from a structural point of view, only several tectons have been used for their construction, i.e. arginine and several other amino acid derivatives, amidines, pyridines, urea and thiourea derivatives, indazole derivatives and oligopeptides [9–20]. Applied structural motifs for the construction of NOS inhibitors have recently been reviewed [21].
From a physiological point of view, the final goal is to achieve isoform-specific inhibition; the challenge is to come up with a design for a protein-specific compound and the synthetic methodology to create it. Based on x-ray-structural analysis of individual NOS isoforms, it is evident that the design and the construction of isoform-specific inhibitors should rely on the molecular recognition of a region of the protein proximate to the binding site of its natural substrate. A search for other types of unconventional chemical structures that would fit into the NOS binding site, be biologically stable, and enable facile chemical modification identified several groups of inorganic compounds, icosahedral boranes, carboranes and metallacarboranes, as promising frameworks for a novel class of non-peptide protein inhibitors [22, 23]. These boron clusters are polyhedra based on a three-dimensional skeleton with triangular facets [22, 23]. Boron cluster derivatives (boranes, carboranes, metallacarboranes) are of interest as they have been rigorously studied because of their use in boron neutron capture therapy [24–29] and in radioimaging [28–31]. There are now a few examples in the literature of the use of carboranes as stable hydrophobic pharmacophores [22, 23, 32], namely enzyme inhibitors (HIV-1 protease [33–35], cyclooxygenase [36, 37], serine protease [38] or protein kinase C [28, 39,40]). Structurally, the variety of the known types of boranes, heteraboranes and metallacarboranes provide an interesting alternative to organic compounds, particularly aromatics. The icosahedral cage is slightly larger than the space occupied by a rotating phenyl ring; metallacarboranes consisting of two eleven vertex dicarbollide sub-clusters sandwiching the central metal atom [41] occupy approximately the same volume as a rotating anthracene ring. Among metallacarboranes, the cobalt bis(1,2-dicarbollide) ion is unique due to its synthetic availability, wide possibilities of exo-skeletal modifications, high stability, charge delocalization, low nucleophilicity, strong acidity of conjugated acids, high hydrophobicity, unique solution properties, and ion-pairing behavior [41–52].
The great potential seen with boron clusters as pharmacophores promoted our venture to this area in search of NOS inhibitors. Many new molecular systems based on two or more hydrophobic anions covalently bound together by simple or more sophisticated organic bridging moieties have been prepared and tested over the last several years.
Here we report a novel approach towards the development of isoform-specific inhibitors of NOS based on the application of properly substituted boron clusters. This non-natural, resistant-to-catabolism, non-toxic class of compounds represents a very interesting scaffold for the construction of a novel class of NOS inhibitors.
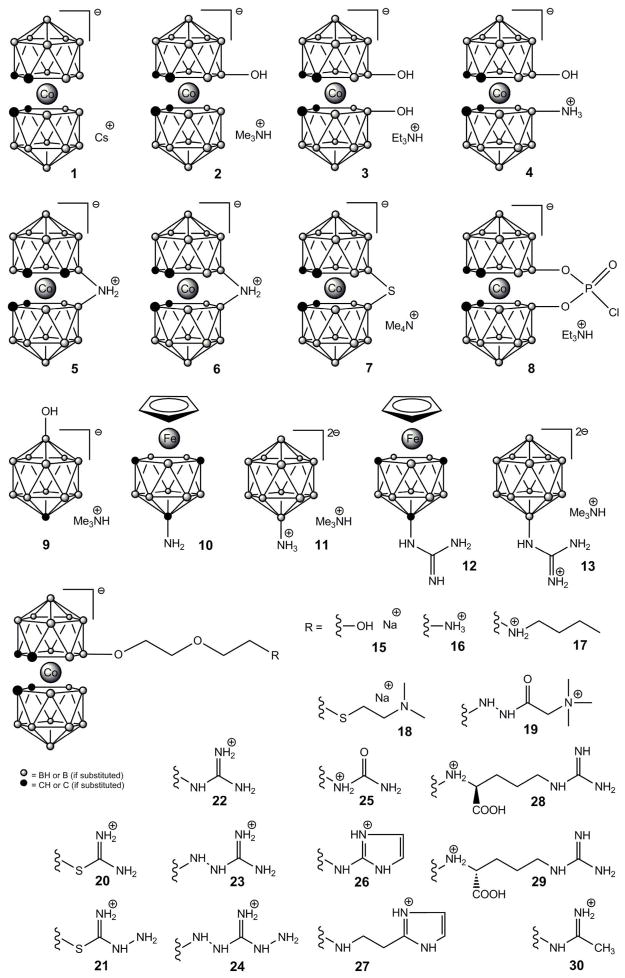
The structures of the designed inhibitors are shown in Fig. 1. This design allows variation of size, shape and proper peripheral structural modification for the construction of novel compounds for specific NOS interaction. The intention was to attach known NOS binding motifs, like guanidine, urea, thiourea, aminoguanidine, imidazole or substituted amine binding groups to the boron cluster periphery, allowing for creation of variety of compounds with the potential to inhibit NOS. The compounds generated were variations of the boron cluster type, metallacarborane clusters, with a positive charge on the periphery (protonated amino group, quaternary ammonium, guanidinium, aminoguanidinium, thiouronium salts) with various lengths of spacer between the positively charged peripheral functionality (primary interaction site) and the 3D boron cluster.
Figure 1.
Structures of tested boron cluster derivatives
Synthetic results will be discussed along with spectroscopic results, solubility and other physicochemical properties of the new compounds. The results obtained indicate high efficacy of several lead structure types as inhibitors of NOS, with IC50 values from in vitro experiments lying in the 1–5 μM range.
Results and Discussion
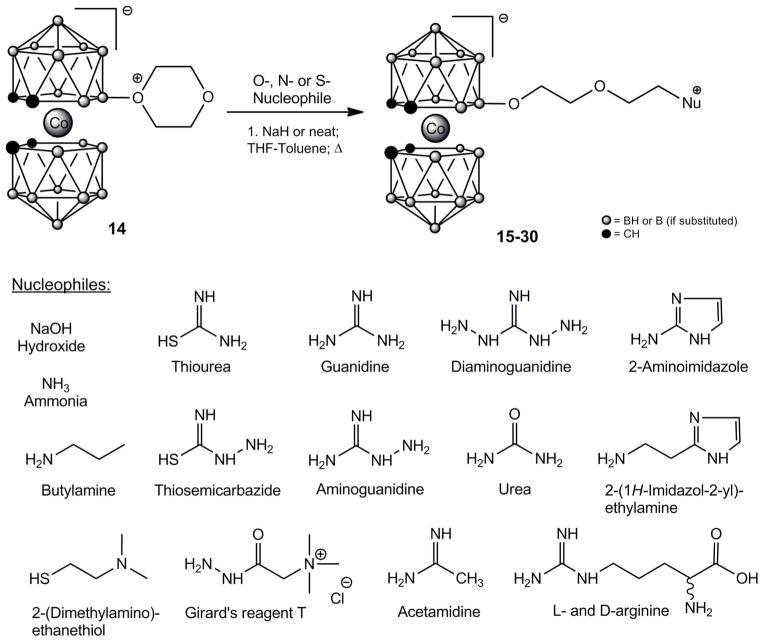
Compounds 15–30 were prepared by nucleophilic dioxane ring opening reaction of 8-dioxane-3-cobalt bis(dicarbollide) (14) with various nucleophiles. This general procedure has already become a routine method for attachment of the cobalt bis(dicarbollide) moiety to substance via a diethylene glycol spacer [33–35, 43,48–52]. If the nucleophile is anion, the reaction produced anionic derivative (compounds 15 and 18); in the case of uncharged or thiourea-type nucleophiles betain-type zwitterion are formed (compounds 16, 17, 19–30). Synthesis of compounds 15–30 are shown on Scheme 1.
Scheme 1.
Preparation of derivatives 15–30 via nucleopholic dioxane ring-opening reaction of 14
Recent quantum chemical studies showed that boron clusters exhibit specific and well-characterized non-classical dihydrogen bonding interactions with peptide backbones and functionalities [32, 53]. Metallacarborane derivatives usually display very low aqueous solubility as well as spontaneous self-assembling that are caused by their hydrophobicity. Self-assembling of metallacarboranes in aqueous solution is time-dependent. Fortunately, self-assembly can be easily suppressed by use of suitable biocompatible excipients or by dilution of their DMSO solution, which can also serve as solubility enhancing agents [51,52]. For this reason, solutions of metallacarboranes were used immediately after preparation. We also used their mixtures with heptakis(2,6-di-O-methyl)-β-cyclodextrin (DIMEB), which are stable in time, and boron cluster do not aggregate and/or precipitate [52]. In biological system, serum albumin can serve as an excipient [52].
The high degree of similarity between the arginine-binding sites of the NOS isoforms [54–58] makes design of highly selective inhibitors targeted to this region difficult. Our approach is based on the concept of affinity variations in the NOS isoforms outside of the binding cavity, where x-ray structure analysis reveals the most differences, rather than within the substrate-binding site. To this end, a library of compounds was generated, where the same basic binding motif targeted to the arginine binding site is combined with a variety of boron cluster structural motifs based on borane, carborane and metallacarborane compounds that would presumably interact with regions in close proximity to, but outside of, the substrate-binding site, where real differences between the NOS isoforms may exist. The same or modified binding groups as in the natural substrate were used, but arginine was avoided as the core structure in most of the compounds to prevent unwanted interactions with other arginine-utilizing enzymes. Arginine transport or metabolizing proteins, like dimethylarginine dimethylaminohydrolase (DDAH), arg-gly amidiniotransferase, arginase, argininosuccinate synthase and peptide deiminases, might also recognize inhibitors with close structural similarity to arginine.
NO synthesis assays were performed under direct, competitive or non-competitive conditions without benefit of pre-incubation. This experimental protocol was designed to detect interactions with the heme- and/or flavin-binding domains. The parent compounds 10 and 11 (structures shown on Fig. 1) displayed no inhibition of NOS activity; when conjugated with a guanidine group (compounds 12 and 13) as a substrate analog, however, these compounds (at 10 RM) completely inhibited all NOS activity, albeit in a non-specific manner. Thus, preliminary exploration of these structures has produced compounds that show potential for being modified even further to produce potent, highly specific inhibitors of the NOS isoforms. The IC50s of several of these compounds containing such further modifications obtained with nNOS, eNOS and iNOS using the hemoglobin capture assay to measure NO production are listed in Table 1 (for compounds 20, 22, 23 and 26). Other compounds (1–11, 15–19, 21, 24, 25 and 27–30) display no significant inhibition of NOS activity.
Table 1.
Inhibition of NO synthesis by 20, 22, 23 and 26: IC50 values (RM)
| iNOS | nNOS | eNOS | eNOS/iNOS | eNOS/nNOS | |
|---|---|---|---|---|---|
| 20 | 4.6 ± 0.33 | 34.6 ± 9.9 | 39.2 ± 9.2 | 8.5 | 1.1 |
| 22 | 1.8 ± 0.18 | 19.9 ± 7.6 | 9.1 ± 3.8 | 5.1 | 0.46 |
| 23 | 2.6 ± 0.18 | 13.7 ± 3.3 | 22.6 ± 3.6 | 8.7 | 1.6 |
| 26 | 4.7 ± 0.70 | 13.8 ± 2.1 | 38.2 ± 10.6 | 8.1 | 2.8 |
The reported IC50 values are +/− SE; selectivity ratios of eNOS to the other isoforms are also reported.
These data clearly show the differential effects of compounds 20 and 23 on the catalysis of NO formation by the three NOS isoforms. As can be seen by comparison with the unsubstituted boron-cage compounds, these substituted structures hold promise for inhibition of NOS activity as appropriate linkers and functional groups (in these cases, thiourea and aminoguanidine groups, respectively) are added to the basic cages.
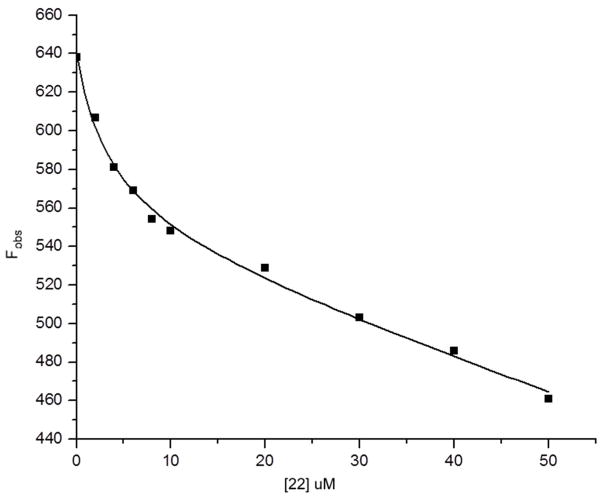
To confirm that binding of these metallacarborane compounds was actually occurring with binding affinities approximating the catalytic IC50 values, fluorescence binding experiments were performed. The representative data shown in Fig. 2 illustrate that binding of 22 by iNOS is biphasic and confirm that it is in the same concentration range as that obtained in the IC50 determinations, 3.66 and 1.8 μM, respectively (data fit to Equation 1 in Experimental Methods).
Figure 2.
Quenching of the intrinsic fluorescence of iNOS by 22.
The [22] was varied from 0–50 RM. The experimental conditions were as follows: excitation = 280 nm; emission = 330 nm; [TrisHCl] = 50 mM; NaCl = 100 mM, pH = 7.5 at 25°C. The fitted line passing through the data is from Eqn. 1 as described in the Experimental Methods. Other reaction conditions are also described.
These studies are preliminary and these “proof-of-principle” experiments show promise for leading to efficacious differential inhibitors. We have demonstrated that boron clusters represent convenient building blocks that can create important interactions with hydrophobic patches of the NOS binding site. Exo-skeletal substitution of the parent metallacarborane compounds introduces additional non-covalent interactions leading to dramatic improvement in inhibition efficacy and selectivity. The combination of the hydrophobic interactions of the scaffold with substitutions allowing for specific dihydrogen bonding and coulombic interactions might further increase the potency of this novel class of non-peptide NOS inhibitors based on an inorganic framework. As these compounds are very stable, exhibit low toxicity, and enable the introduction of modified side groups, they are extremely attractive prospects for development into much more specific inhibitors of the NOS isoforms.
Conclusions
We have identified metallacarboranes (cobalt bis(dicarbollide) derivatives) as a novel structural motif for the construction of isoform-specific NOS activators and/or inhibitors. The potential of these compounds relies on their stability, easy exo-skeletal substitution and spectroscopic properties. The boron cluster compounds thus represent a novel class of NOS activators and/or inhibitors depending on peripheral substitution of a boron cluster compound, which differ substantially from the organic ones in their constructions and structures.
Experimental Methods
Chemicals
Chemical used are as follows: (6R)-5,6,7,8-tetrahydrobiopterin was from Research Biochemicals International (Natick, MA). Sodium chloride was from EMD Chemicals, Inc. (Darmstadt, Germany). 8-dioxane-3-cobalt bis(1,2-dicarbollide) (14) were purchased from Katchem Ltd, Czech Rep. All other chemicals and solvents were purchased from Sigma-Aldrich and were of the highest grade available.
Characterization of compounds
1H and 11B NMR spectroscopy were performed at Varian Gemini 500 HC instrument using DMSO-d6 as solvent and tetramethyl silane as internal standard for 1H NMR and BF3*Et2O as external standard for 11B NMR, respectively. Chemical shifts are quoted in parts per million (δ scale; s = singlet, d = doublet, m = multiplet), coupling constants J in Hz. Mass spectrometry measurements were performed on a Bruker Esquire 3000 instrument using Electrospray Ionization (MS-ESI). Negative ions were detected. The purity of the prepared compounds was determined by elemental analysis and liquid chromatography (coupled with MS-ESI). All compounds have purity ≥97%.
Enzymes
The neuronal, endothelial, and inducible isoforms of NOS were expressed and purified as previously described [59–62]. Calmodulin was prepared by the method of Zhang and Vogel [63].
Inhibition assays
The generation of nitric oxide was measured at 23°C by the Hb capture method [64] on a Molecular Dynamics VERSAmax microplate reader in flat bottom 96 well plates with a path length of 0.72 cm. Inhibitors (10 RM) plus 100 to 200 nM nNOS, iNOS, or eNOS were assayed in 8 RM oxy Hb, 25 RM L-arginine, 50 RM Ca++, 1 RM CaM, 5 RM BH4, and 100 RM NADPH, in 50 mM TRIS/HCl, 100 mM NaCl, pH 7.5, in a total volume of 0.3 ml. A mixture of oxy Hb, L-arginine, Ca++, and CaM was added to each well with a multichannel pipette, while inhibitor, NOS, and BH4 were added individually from a single container in an effort to prevent degradation. The reaction was started with the addition of 100 μM NADPH, and the change in aborbance at 401 nm was measured every 3 seconds for 1 minute. The extinction coefficient used to calculate the rates was 60 mM−1. Only compounds that showed a differential inhibition of NOS isoforms were chosen for determination of IC50 values
Fluorescence study
The fluorescence spectra of iNOS were recorded at 25°C using a Fluorolog-3 (Jobin Yvon-Spex, Edison, NJ) spectrofluorometer. Excitation was at 280 nm (path length = 0.5 mm; slit = 2 nm band pass) and emission was recorded in the range 250–450 nm (path length 0.2 mm; 2 nm band pass). Buffer (50 mM TrisHCl, 100 mM NaCl, pH 7.5 at 25 °C) was filtered using 0.2 micron Corning filters and degassed. Intensities at the emission maximum of 330nm did not show any observable shifts as the concentration of the compound was increased by titrating in Rl volumes of concentrated stock solution into the protein solution. The final volume of the added solution was very small and did not cause any significant changes in fluorescence intensities.
After trying a variety of models for binding constants [65], the data fitted best to a two-part equation (Eqn. 2), with the first term similar to a Hill type equation for binding constants for specific binding and the second part consisting of a straight line.
| Equation 1 |
Where, Fobs are the observed fluorescence intensities of iNOS in the presence of varying concentrations of the compound (0–50 RM range); Fmin is the theoretical minimum of the observed fluorescence intensity; S is the compound concentration; n is the Hill Coefficient related to the number of binding sites; and K is the apparent binding constant.
Chemical syntheses
Derivatives 1–11, 15, 20, 22 and 25 (structures shown in Fig. 1) were prepared as described in literature: 2 and 3 [45], 4 [66], 5 [67], 6 [68], 7 [69], 8 [46], 9 [70], 10 [71], 11 [72, 73], 15 [74], 20, 22 and 25 [51]. Guanidinium derivatives 12 and 13 were prepared from corresponding aminoderivatives (10 and 11 resp.) by standard guanylation reactions [75–77].
The synthetic protocol for the preparation of the library of boron cluster inhibitors is shown in Scheme 1, where 8-dioxane-3-cobalt bis(dicarbollide) (14) is opened by series of S- and N-nucleophiles. All compounds were verified by elementary analysis, mass spectrometry, 1H NMR and 11B NMR spectroscopy.
General method of preparation of compounds 15–30
To a stirred slurry of sodium hydride (60% susp. in oil) in anhydrous THF was added S-; O- or salt of N-nucleophile. Reaction mixture was stirred at r.t. for 30–60 min. Then was added a solution of 8-dioxane-3-cobalt bis(dicarbollide) (14) in toluene-THF mixture (3:1 v/v) (molar ratio nucleophile : 14 = 2:1). In the case of neat N-nucleophiles, solution of 14 was added directly to the solution of N-nucleophile. The reaction mixture was heated to 70 °C for 15–60 min (TLC monitoring; eluent: dichloromethane). After cooling down, water was added and aqueous phase was extracted with dichloromethane (5x 50 mL). The combined organic layers were washed with water (20 mL) and brine (20 mL) and dried (MgSO4). After evaporation, the resulting glassy solid was dissolved in CH2Cl2 (5 ml) and precipitated by addition of hexane (30 ml) or purified by column chromatography on silica (eluent: dichloromethane followed by dichloromethane/acetone 3:1 to 1:1 v/v). Orange solid product was dried in vacuum at 50°C.
1H and 11B NMR spectra of compounds 15–30 are similar
1H NMR (DMSO-d6) δ: 0.90–4.15 (m; BH and OCH2, NCH2, SCH2(if present), CH2(if present) and NH); 4.14 (s; CHcarborane); compound 26 additional signal: 6.98 (s; 2H; imidazole-H), compound 18 additional signal: 2.32 (s; 6H; NMe2), compound 19 additional signal: 3.31 (s; 9H; N+Me3), compound 27 additional signal: 7.11 (s; 2H; imidazole-H).
11B NMR (DMSO-d6): δ = −28.3 (d; 1B; B6; 1J(B,H) = 140 Hz); −21.6 (d; 1B; B6'; 1J(B,H) = 171 Hz); −20.4 (d; 2B; B5, B11; 1J(B,H) = 153 Hz); −17.3 (d; 2B; B5', B11'; 1J(B,H) = 145 Hz); −7.8 – −8.2 (m; 6B; B4, B7, B9, B12, B9', B12'), −4.3 (d; 2B; B4', B7'; 1J(B,H) = 153 Hz); −2.5 (d; 1B; B10; 1J(B,H = 143 Hz); 0.4 (d; 1B; B10'; 1J(B,H) = 141 Hz); 3.9 (d; 1B; B8'; 1J(B,H) = 142 Hz); 23.3 (s; 1B; B8)
Compound 18
NaH (48 mg, 60% in oil, 1.2 mmol), 2-(dimethylamino)ethanethiol hydrochloride (85 mg, 0.6 mmol), THF (4 mL), then 14 (165 mg, 0.4 mmol), toluene-THF (3:1 v/v, 6 mL). Yield of 18: 204 mg (95%). Anal. Calcd for C12H39B18CoNNaO2S: C, 26.79; H, 7.31; N, 2.60. Found: C, 26.70; H, 7.40; N, 2.55. MS-ESI m/e: 538.37 (100.0%), 537.38 (100.0%), 539.37 (90.2%), 536.38 (63.3%), 540.36 (34.6%), 535.38 (33.6%), 540.37 (15.4%), 534.39 (15.0%), 538.38 (12.7%), 541.36 (11.4%), 541.37 (6.2%), 533.39 (4.9%), 536.39 (4.8%), 542.36 (2.7%), 535.39 (2.3%), 539.38 (2.2%), 532.39 (1.3%), 540.38 (1.3%), 542.37 (1.0%)
Compound 19
Acethydrazide trimethylammonium chloride (Girard's reagent T; 135 mg, 0.8 mmol), 14 (165 mg, 0.4 mmol), THF (12 mL). Yield of 19: 201 mg (93%). Anal. Calcd for C13H42B18CoN3O3: C, 28.81; H, 7.81; N, 7.75. Found: C, 28.75; H, 7.90; N, 7.73. MS-ESI m/e: 541.44 (100.0%), 542.43 (98.1%), 543.43 (74.4%), 540.44 (63.3%), 544.43 (46.1%), 539.45 (36.1%), 543.44 (15.8%), 538.45 (14.5%), 542.44 (13.4%), 545.42 (8.1%), 545.43 (5.7%), 540.45 (5.1%), 537.45 (4.9%), 544.44 (1.9%), 546.43 (1.7%), 536.46 (1.4%), 542.45 (1.1%)
Compound 20
NaH (16 mg, 60% in oil, 0.4 mmol), thiourea (31 mg, 0.4 mmol), THF (2 mL), then 14 (83 mg, 0.2 mmol), toluene-THF (3:1 v/v, 3 mL). Yield of 20: 80 mg (82%). Anal. Calcd for C9H33B18CoN2O2S: C, 22.20; H, 6.83; N, 5.75. Found: C, 22.14; H, 6.79; N, 5.77. MS-ESI m/e: 487.34 (100.0%), 486.34 (84.4%), 488.33 (67.2%), 485.35 (61.2%), 489.33 (36.5%), 484.35 (31.2%), 488.34 (13.8%), 490.33 (13.7%), 483.35 (13.1%), 489.34 (8.0%), 486.35 (6.1%), 482.36 (4.6%), 491.33 (2.7%), 484.36 (1.4%), 481.36 (1.2%), 490.34 (1.2%), 487.35 (1.1%)
Compound 21
NaH (16 mg, 60% in oil, 0.4 mmol), thiosemicarbazide (37 mg, 0.4 mmol), THF (2 mL), then 14 (83 mg, 0.2 mmol), toluene-THF (3:1 v/v, 3 mL). Yield of 21: 93 mg (92%). Anal. Calcd for C9H34B18CoN3O2S: C, 21.53; H, 6.83; N, 8.37. Found: C, 21.55; H, 6.80; N, 8.35. MS-ESI m/e: 502.35 (100.0%), 501.35 (89.6%), 503.35 (87.4%), 500.36 (66.4%), 504.34 (39.8%), 499.36 (33.8%), 505.34 (14.9%), 498.36 (14.2%), 502.36 (9.9%), 501.36 (8.6%), 504.35 (8.4%), 497.37 (5.0%), 506.34 (2.8%), 503.36 (1.6%), 499.37 (1.5%), 505.35 (1.4%), 496.37 (1.3%)
Compound 22
NaH (400 mg, 60% in oil, 10 mmol), guanidine hydrochloride (955 mg, 10 mmol), THF (10 mL), then 14 (410 mg, 1 mmol), toluene-THF (3:1 v/v, 3 mL). Yield of 22: 452 mg (95%).
For guanidine carbonate (900 mg, 5 mmol), THF (20 mL), then 14 (821 mg, 2 mmol). Yield of 22: 549 mg (58%). Anal. Calcd for C9H34B18CoN3O2: C, 23.00; H, 7.29; N, 8.94. Found: C, 22.96; H, 7.32; N, 9.00. MS-ESI m/e: 470.38 (100.0%), 469.38 (85.2%), 471.37 (69.5%), 468.38 (59.1%), 472.37 (33.2%), 467.39 (33.1%), 466.39 (13.5%), 473.37 (10.9%), 471.38 (9.7%), 472.38 (7.9%), 469.39 (6.3%), 465.39 (4.6%), 468.39 (3.2%), 464.40 (1.3%)
Compound 23
NaH (240 mg, 60% in oil, 6 mmol), aminoguanidine hemisulphate (738 mg, 6 mmol), THF (10 mL), then 14 (410 mg, 1 mmol), toluene-THF (3:1 v/v, 10 mL). Yield of 23: 482 mg (98%). Anal. Calcd for C9H35B18CoN4O2: C, 22.29; H, 7.27; N, 11.55. Found: C, 22.31; H, 7.30; N, 11.56. MS-ESI m/e: 485.39 (100.0%), 484.39 (85.2%), 486.38 (69.7%), 483.39 (58.7%), 487.38 (33.4%), 482.40 (31.8%), 481.40 (13.5%), 488.38 (11.0%), 486.39 (9.4%), 487.39 (7.9%), 484.40 (6.2%), 480.41 (4.7%), 483.40 (3.7%), 482.41 (1.4%), 479.41 (1.3%), 485.40 (1.0%)
Compound 24
NaH (16 mg, 60% in oil, 0.4 mmol), N,N′-diaminoguanidine hydrochloride (50 mg, 0.4 mmol), THF (2 mL), then 14 (83 mg, 0.2 mmol), toluene-THF (3:1 v/v, 4 mL). Yield of 24: 94 mg (93%). Anal. Calcd for C9H36B18CoN5O2: C, 21.62; H, 7.26; N, 14.01. Found: C, 21.68; H, 7.33; N, 13.98. MS-ESI m/e: 500.40 (100.0%), 499.40 (92.8%), 501.39 (74.2%), 498.41 (67.7%), 502.39 (36.5%), 497.41 (34.6%), 496.41 (14.7%), 501.40 (12.0%), 503.39 (11.9%), 500.41 (10.0%), 502.40 (8.2%), 499.41 (6.7%), 495.42 (5.2%), 497.42 (1.5%), 494.42 (1.4%)
Compound 26
NaH (48 mg, 60% in oil, 1.2 mmol), 2-aminoimidazole hemisulphate (80 mg, 0.6 mmol), THF (4 mL), then 14 (165 mg, 0.4 mmol), toluene-THF (3:1 v/v, 6 mL). Yield of 26: 172 mg (87%). Anal. Calcd for C11H34B18CoN3O2: C, 26.75; H, 6.94; N, 8.51. Found: C, 26.68; H, 6.93; N, 8.45. MS-ESI m/e: 494.38 (100.0%), 493.38 (83.6%), 495.37 (68.2%), 492.38 (58.1%), 491.39 (32.8%), 496.37 (32.6%), 490.39 (13.3%), 495.38 (11.5%), 497.37 (11.4%), 496.38 (9.4%), 493.39 (7.5%), 489.39 (4.5%), 492.39 (3.9%), 488.40 (1.3%), 498.37 (1.1%)
Compound 27
NaH (48 mg, 60% in oil, 1.2 mmol), 2-(1H-imidazol-2-yl)-ethylamine hydrochloride (89 mg, 0.6 mmol), THF (4 mL), then 14 (165 mg, 0.4 mmol), toluene-THF (3:1 v/v, 8 mL). Yield of 27: 177 mg (85%). Anal. Calcd for C13H38B18CoN3O2: C, 29.91; H, 7.34; N, 8.05. Found: C, 29.83; H, 7.38; N, 7.43. MS-ESI m/e: 522.41 (100.0%), 521.41 (82.3%), 523.40 (66.1%), 520.42 (61.5%), 524.40 (32.0%), 519.42 (30.7%), 523.41 (13.9%), 518.42 (13.1%), 525.40 (11.9%), 524.41 (10.9%), 521.42 (8.5%), 517.43 (4.6%), 519.43 (2.0%), 522.42 (1.3%), 516.43 (1.2%), 526.40 (1.2%), 523.42 (1.2%), 525.41 (1.1%)
Compound 28
L-arginine (70 mg, 0.4 mmol) in THF (4 mL), then 14 (83 mg, 0.2 mmol), toluene-THF (3:1 v/v, 6 mL). Yield of 28: 110 mg (94%). Anal. Calcd for C15H45B18CoN4O4: C, 30.07; H, 7.57; N, 9.35. Found: C, 29.97; H, 7.64; N, 9.40. MS-ESI m/e: 585.44 (100.0%), 584.44 (92.8%), 586.44 (91.2%), 583.45 (69.7%), 587.43 (36.4%), 582.45 (34.6%), 585.45 (15.9%), 581.45 (14.7%), 587.44 (12.8%), 584.45 (10.2%), 588.43 (8.4%), 588.44 (7.2%), 580.46 (5.0%), 582.46 (2.4%), 586.45 (2.4%), 589.43 (1.4%), 579.46 (1.4%), 587.45 (1.3%)
Compound 29
D-arginine (52 mg, 0.3 mmol) in THF (3 mL), then 14 (109 mg, 0.26 mmol), toluene (4 mL). Yield of 29: 133 mg (86%). Anal. Calcd for C15H45B18CoN4O4: C, 30.07; H, 7.57; N, 9.35. Found: C, 29.96; H, 7.66; N, 9.36. MS-ESI m/e: 585.44 (100.0%), 584.44 (92.8%), 586.44 (91.2%), 583.45 (69.7%), 587.43 (36.4%), 582.45 (34.6%), 585.45 (15.9%), 581.45 (14.7%), 587.44 (12.8%), 584.45 (10.2%), 588.43 (8.4%), 588.44 (7.2%), 580.46 (5.0%), 582.46 (2.4%), 586.45 (2.4%), 589.43 (1.4%), 579.46 (1.4%), 587.45 (1.3%)
Compound 30
NaH (48 mg, 60% in oil, 1.2 mmol), acetamidine hydrochloride (57 mg, 0.6 mmol), THF (4 mL), then 14 (165 mg, 0.4 mmol), toluene-THF (3:1 v/v, 6 mL). Yield of 30: 166 mg (88%). Anal. Calcd for C10H35B18CoN2O2: C, 25.61; H, 7.52; N, 5.97. Found: C, 25.57; H, 7.59; N, 5.93. MS-ESI m/e: 468.39 (100.0%), 469.38 (99.7%), 470.38 (86.2%), 467.39 (64.4%), 471.37 (35.9%), 466.39 (34.7%), 465.40 (15.3%), 469.39 (10.7%), 471.38 (8.5%), 472.37 (8.2%), 464.40 (5.0%), 472.38 (4.8%), 467.40 (4.1%), 466.40 (1.7%), 470.39 (1.5%), 463.40 (1.4%), 471.39 (1.0%)
Acknowledgments
This work was supported by NIH Grant GM52419 (to LJR and BSSM), the Grant Agency of the Czech Republic (grant No. P303/11/1291), a MSMT grant (MSM0021620849), and grants from Charles University, Prague (PRVOUK P24/LF1/3 and UNCE 204011). BSSM holds the Robert A. Welch Distinguished Chair in Chemistry (AQ-0012).
Abbreviations: The abbreviations used are as follows
- NOS
nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- NO
nitric oxide
- CaM
calmodulin
References
- 1.Masters BS. In: Nitric Oxide, Biology and Pathobiology. Ignarro LJ, editor. Academic Press; New York: 2000. [Google Scholar]
- 2.Roman LJ, Martásek P, Masters BSS. Intrinsic and extrinsic modulation of nitric oxide synthase activity. Chem Rev. 2002;102(4):1179–1189. doi: 10.1021/cr000661e. [DOI] [PubMed] [Google Scholar]
- 3.Michel T, Feron O. Nitric oxide synthases: Which, where, how, and why? J Clin Invest. 1997;100(9):2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;279(35):36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 6.Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43(3):521–531. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 7.Li HY, Poulos TL. Structure-function studies on nitric oxide synthases. J Inorg Biochem. 2005;99(1):293–305. doi: 10.1016/j.jinorgbio.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Bruckdorfer R. The basics about nitric oxide. Mol Aspects Med. 2005;26(1–2):3–31. doi: 10.1016/j.mam.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Vidal JA, Martásek P, Roman LJ, Silverman RB. Potent and selective conformationally restricted neuronal nitric oxide synthase inhibitors. J Med Chem. 2004;47(3):703–710. doi: 10.1021/jm030297m. [DOI] [PubMed] [Google Scholar]
- 10.Hah JM, Martásek P, Roman LJ, Silverman RB. Aromatic reduced amide bond peptidomimetics as selective inhibitors of neuronal nitric oxide synthase. J Med Chem. 2003;46(9):1661–1669. doi: 10.1021/jm0202932. [DOI] [PubMed] [Google Scholar]
- 11.Hah JM, Roman LJ, Martásek P, Silverman RB. Reduced amide bond peptidomimetics. (4S)-N-(4-amino-5-[aminoalkyl]aminopentyl)-N'-nitroguanidines, potent and highly selective inhibitors of neuronal nitric oxide synthase. J Med Chem. 2001;44(16):2667–2670. doi: 10.1021/jm0101491. [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Martásek P, Roman LJ, Masters BSS, Silverman RB. N-omega-nitroarginine-containing dipeptide amides. Potent and highly selective inhibitors of neuronal nitric oxide synthase. J Med Chem. 1999;42(16):3147–3153. doi: 10.1021/jm990111c. [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Martásek P, Roman LJ, Silverman RB. Synthesis and evaluation of peptidomimetics as selective inhibitors and active site probes of nitric oxide synthases. J Med Chem. 2000;43(15):2938–2945. doi: 10.1021/jm000127z. [DOI] [PubMed] [Google Scholar]
- 14.Huang H, Martásek P, Roman LJ, Silverman RB. Synthesis and evaluation of dipeptide amides containing N-omega-nitroarginine and D-2,4-diaminobutyric acids as inhibitors of neuronal nitric oxide synthase. J Enzyme Inhib. 2001;16(3):233–239. doi: 10.1080/14756360109162371. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, Marletta MA, Martásek P, Roman LJ, Masters BSS, Silverman RB. Conformationally-restricted arginine analogues as alternative substrates and inhibitors of nitric oxide syntheses. Bioorg Med Chem. 1999;7(6):1097–1104. doi: 10.1016/s0968-0896(99)00029-2. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y, Martásek P, Roman LJ, Masters BSS, Silverman RB. Imidazole-containing amino acids as selective inhibitors of nitric oxide synthases. Bioorg Med Chem. 1999;7(9):1941–1951. doi: 10.1016/s0968-0896(99)00117-0. [DOI] [PubMed] [Google Scholar]
- 17.Silverman RB, Huang H, Marletta MA, Martásek P. Selective inhibition of neuronal nitric oxide synthase by N-omega-nitroarginine- and phenylalanine-containing dipeptides and dipeptide esters. J Med Chem. 1997;40(18):2813–2817. doi: 10.1021/jm970200u. [DOI] [PubMed] [Google Scholar]
- 18.Zhang HQ, Fast W, Marletta MA, Martásek P, Silverman RB. Potent and selective inhibition of neuronal nitric oxide synthase by N-omega-propyl-L-arginine. J Med Chem. 1997;40(24):3869–3870. doi: 10.1021/jm970550g. [DOI] [PubMed] [Google Scholar]
- 19.Silverman RB. Design of selective neuronal nitric oxide synthase inhibitors for the prevention and treatment of neurodegenerative diseases. Acc Chem Res. 2009;42(3):439–451. doi: 10.1021/ar800201v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji H, Tan S, Igarashi J, Li H, Derrick M, Martásek P, Roman LJ, Vásquez-Vivar J, Poulos TL, Silverman RB. Selective neuronal nitric oxide synthase inhibitors and the prevention of cerebral palsy. Ann Neurol. 2009;65(2):209–217. doi: 10.1002/ana.21555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdal EP, Litzinger EA, Seo JW, Zhu YQ, Ji HT, Silverman RB. Selective neuronal nitric oxide synthase inhibitors. Curr Top Med Chem. 2005;5(7):603–624. doi: 10.2174/1568026054679317. [DOI] [PubMed] [Google Scholar]
- 22.Issa F, Kassiou M, Rendina LM. Boron in Drug Discovery: Carboranes as Unique Pharmacophores in Biologically Active Compounds. Chem Rev. 2011;111(9):5701–5722. doi: 10.1021/cr2000866. [DOI] [PubMed] [Google Scholar]
- 23.Scholz M, Hey-Hawkins E. Carbaboranes as Pharmacophores: Properties, Synthesis, and Application Strategies. Chem Rev. 2011;111(11):7035–7062. doi: 10.1021/cr200038x. [DOI] [PubMed] [Google Scholar]
- 24.Hawthorne MF. The Role of Chemistry in the Development of Boron Neutron-Capture Therapy of Cancer. Angew Chem Int Ed Eng. 1993;32(7):950–984. [Google Scholar]
- 25.Tjarks W. The use of boron clusters in the rational design of boronated nucleosides for neutron capture therapy of cancer. J Organomet Chem. 2000;614:37–47. [Google Scholar]
- 26.Bregadze VI, Sivaev IB, Glazun SA. Polyhedral boron compounds as potential diagnostic and therapeutic antitumor agents. Anticancer Agents Med Chem. 2006;6(2):75–109. doi: 10.2174/187152006776119180. [DOI] [PubMed] [Google Scholar]
- 27.Crossley EL, Ziolkowski EJ, Coderre JA, Rendina LM. Boronated DNA-binding compounds as potential agents for boron neutron capture therapy. Mini Rev Med Chem. 2007;7(3):303–313. doi: 10.2174/138955707780059808. [DOI] [PubMed] [Google Scholar]
- 28.Valliant JF, Guenther KJ, King AS, Morel P, Schaffer P, Sogbein OO, Stephenson KA. The medicinal chemistry of carboranes. Coord Chem Rev. 2002;232(1–2):173–230. [Google Scholar]
- 29.Armstrong AF, Valliant JF. The bioinorganic and medicinal chemistry of carboranes: from new drug discovery to molecular imaging and therapy. Dalton Trans. 2007;(38):4240–4251. doi: 10.1039/b709843j. [DOI] [PubMed] [Google Scholar]
- 30.Hawthorne MF, Maderna A. Applications of radiolabeled boron clusters to the diagnosis and treatment of cancer. Chem Rev. 1999;99(12):3421–3434. doi: 10.1021/cr980442h. [DOI] [PubMed] [Google Scholar]
- 31.Soloway AH, Tjarks W, Barnum BA, Rong FG, Barth RF, Codogni IM, Wilson JG. The Chemistry of Neutron Capture Therapy. Chem Rev. 1998;98(4):1515–1562. doi: 10.1021/cr941195u. [DOI] [PubMed] [Google Scholar]
- 32.Lesnikowski ZJ. Boron units as pharmacophores - New applications and opportunities of boron cluster chemistry. Collect Czech Chem Commun. 2007;72(12):1646–1658. [Google Scholar]
- 33.Cígler P, Kožíšek M, Řezáaová P, Brynda J, Otwinowski Z, Pokorná J, Plešek J, Grüner B, Doleaková-Marešová L, Máša M, Sedláaek J, Bodem J, Kräusslich HG, Král V, Konvalinka J. From nonpeptide toward noncarbon protease inhibitors: Metallacarboranes as specific and potent inhibitors of HIV protease. Proc Natl Acad Sci U S A. 2005;102(43):15394–15399. doi: 10.1073/pnas.0507577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kožíšek M, Cígler P, Lepšík M, Fanfrlík J, Řezáaová P, Brynda J, Pokorná J, Plešek J, Grüner B, Šašková KG, Václavíková J, Král V, Konvalinka J. Inorganic polyhedral metallacarborane inhibitors of HIV protease: A new approach to overcoming antiviral resistance. J Med Chem. 2008;51(15):4839–4843. doi: 10.1021/jm8002334. [DOI] [PubMed] [Google Scholar]
- 35.Řezáaová P, Pokorná J, Brynda J, Kožíšek M, Cígler P, Lepšík M, Fanfrlík J, Rezáa J, Šašková KG, Sieglová I, Plešek J, Šícha V, Grüner B, Oberwinkler H, Sedláaek J, Kräusslich HG, Hobza P, Král V, Konvalinka J. Design of HIV Protease Inhibitors Based on Inorganic Polyhedral Metallacarboranes. J Med Chem. 2009;52(22):7132–7141. doi: 10.1021/jm9011388. [DOI] [PubMed] [Google Scholar]
- 36.Scholz M, Bensdorf K, Gust R, Hey-Hawkins E. Asborin: The Carbaborane Analogue of Aspirin. ChemMedChem. 2009;4(5):746–748. doi: 10.1002/cmdc.200900072. [DOI] [PubMed] [Google Scholar]
- 37.Scholz M, Kalueerovif GN, Kommera H, Paschke R, Will J, Sheldrick WS, Hey-Hawkins E. Carbaboranes as pharmacophores: Similarities and differences between aspirin and asborin. Eur J Med Chem. 2011;46(4):1131–1139. doi: 10.1016/j.ejmech.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 38.Page MFZ, Jalisatgi SS, Maderna A, Hawthorne MF. Design and synthesis of a candidate alpha-human thrombin irreversible inhibitor containing a hydrophobic carborane pharmacophore. Synthesis. 2008;(4):555–563. [Google Scholar]
- 39.Endo Y, Yoshimi T, Kimura K, Itai A. Protein kinase C modulators bearing dicarba-closo-dodecaborane as a hydrophobic pharmacophore. Bioorg Med Chem Lett. 1999;9(17):2561–2564. doi: 10.1016/s0960-894x(99)00436-9. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji M, Koiso Y, Takahashi H, Hashimoto Y, Endo Y. Modulators of tumor necrosis factor alpha production bearing dicarba-closo-dodecaborane as a hydrophobic pharmacophore. Biol Pharm Bull. 2000;23(4):513–516. doi: 10.1248/bpb.23.513. [DOI] [PubMed] [Google Scholar]
- 41.Hawthorne MF, Young DC, Wegner PA. Carbametallic Boron Hydride Derivatives .I. Apparent Analogs of Ferrocene and Ferricinium Ion. J Am Chem Soc. 1965;87(8):1818–1819. [Google Scholar]
- 42.Plešek J. Potential Applications of the Boron Cluster Compounds. Chem Rev. 1992;92(2):269–278. [Google Scholar]
- 43.Plešek J, Hemánek S, Franken A, Císaová I, Nachtigal C. Dimethyl sulfate induced nucleophilic substitution of the [bis(1,2-dicarbollido)-3-cobalt(1-)]ate ion. Syntheses, properties and structures of its 8,8'-mu-sulfato, 8-phenyl and 8-dioxane derivatives. Collect Czech Chem Commun. 1997;62(1):47–56. [Google Scholar]
- 44.Sivaev IB, Bregadze VI. Chemistry of cobalt bis(dicarbollides). A review. Collect Czech Chem Commun. 1999;64(5):783–805. [Google Scholar]
- 45.Plešek J, Grüner B, Báaa J, Fusek J, Císaová I. Syntheses of the B-(8)-hydroxy- and B((8),(8)')-dihydroxy-derivatives of the bis(1,2-dicarbollido)-3-colbalt(1-)ate ion by its reductive acetoxylation and hydroxylation: molecular structure of [8,8 '-mu-CH3C(O)(2)(1,2-C2B9H10)(2)-3-Co](0) zwitterion determined by X-ray diffraction analysis. J Organomet Chem. 2002;649(2):181–190. [Google Scholar]
- 46.Plešek J, Grüner B, Císaová I, Báaa J, Selucký P, Rais J. Functionalized cobalt bis(dicarbollide) ions as selective extraction reagents for removal of M2+ and M3+ cations from nuclear waste, crystal and molecular structures of the [8,8 '-mu-CIP(O)(O)(2)(1,2-C2B9H10)(2)-3,3 '-Co]HN(C2H5)(3) and [8,8 '-mu-Et2NP(O)(O)(2) (1,2-C2B9H10)(2)-3,3 '-Co](HN(CH3)(3)) J Organomet Chem. 2002;657(1–2):59–70. [Google Scholar]
- 47.Matijíaek P, Cígler P, Procházka K, Král V. Molecular assembly of metallacarboranes in water: Light scattering and microscopy study. Langmuir. 2006;22(2):575–581. doi: 10.1021/la052201s. [DOI] [PubMed] [Google Scholar]
- 48.Matijíaek P, Cígler P, Olejniczak AB, Andrysiak A, Wojtczak B, Procházka K, Lesnikowski ZJ. Aggregation behavior of nucleoside-boron cluster conjugates in aqueous solutions. Langmuir. 2008;24(6):2625–2630. doi: 10.1021/la702852e. [DOI] [PubMed] [Google Scholar]
- 49.Semioshkin AA, Sivaev IB, Bregadze VI. Cyclic oxonium derivatives of polyhedral boron hydrides and their synthetic applications. Dalton Trans. 2008;(8):977–992. doi: 10.1039/b715363e. [DOI] [PubMed] [Google Scholar]
- 50.Sivaev IB, Bregadze VV. Polyhedral Boranes for Medical Applications: Current Status and Perspectives. Eur J Inorg Chem. 2009;(11):1433–1450. [Google Scholar]
- 51.Rak J, Kaplánek R, Král V. Solubilization and deaggregation of cobalt bis(dicarbollide) derivatives in water by biocompatible excipients. Bioorg Med Chem Lett. 2010;20(3):1045–1048. doi: 10.1016/j.bmcl.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 52.Rak J, Jakubek M, Kaplánek R, Matijíaek P, Král V. Cobalt bis(dicarbollide) derivatives: Solubilization and self-assembly suppression. Eur J Med Chem. 2011;46(4):1140–1146. doi: 10.1016/j.ejmech.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 53.Fanfrlík J, Lepšik M, Horinek D, Havlas Z, Hobza P. Interaction of carboranes with biomolecules: Formation of dihydrogen bonds. ChemPhysChem. 2006;7(5):1100–1105. doi: 10.1002/cphc.200500648. [DOI] [PubMed] [Google Scholar]
- 54.Li HY, Shimizu H, Flinspach M, Jamal J, Yang WP, Xian M, Cai TW, Wen EZ, Jia QA, Wang PG, Poulos TL. The novel binding mode of N-Alkyl-N '-hydroxyguanidine to neuronal nitric oxide synthase provides mechanistic insights into NO biosynthesis. Biochemistry. 2002;41(47):13868–13875. doi: 10.1021/bi020417c. [DOI] [PubMed] [Google Scholar]
- 55.Flinspach M, Li HY, Jamal J, Yang WP, Huang H, Silverman RB, Poulos TL. Structures of the neuronal and endothelial nitric oxide synthase heme domain with D-nitroarginine-containing dipeptide inhibitors bound. Biochemistry. 2004;43(18):5181–5187. doi: 10.1021/bi0361867. [DOI] [PubMed] [Google Scholar]
- 56.Raman CS, Li H, Martásek P, Král V, Masters BS, Poulos TL. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95(7):939–50. doi: 10.1016/s0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- 57.Fischmann TO, Hruza A, Niu XD, Fossetta JD, Lunn CA, Dolphin E, Prongay AJ, Reichert P, Lundell DJ, Narula SK, Weber PC. Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nature Struct Biol. 1999;6(3):233–242. doi: 10.1038/6675. [DOI] [PubMed] [Google Scholar]
- 58.Crane BR, Arvai AS, Ghosh DK, Wu CQ, Getzoff ED, Stuehr DJ, Tainer JA. Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science. 1998;279(5359):2121–2126. doi: 10.1126/science.279.5359.2121. [DOI] [PubMed] [Google Scholar]
- 59.Martásek P, Liu Q, Liu JW, Roman LJ, Gross SS, Sessa WC, Masters BSS. Characterization of bovine endothelial nitric oxide synthase expressed in E-coli. Biochem Biophys Res Commun. 1996;219(2):359–365. doi: 10.1006/bbrc.1996.0238. [DOI] [PubMed] [Google Scholar]
- 60.Roman LJ, Martásek P, Miller RT, Harris DE, de La Garza MA, Shea TM, Kim JJ, Masters BS. The C termini of constitutive nitric-oxide synthases control electron flow through the flavin and heme domains and affect modulation by calmodulin. J Biol Chem. 2000;275(38):29225–29232. doi: 10.1074/jbc.M004766200. [DOI] [PubMed] [Google Scholar]
- 61.Roman LJ, Miller RT, de la Garza MA, Kim JJP, Masters BSS. The C terminus of mouse macrophage inducible nitric-oxide synthase attenuates electron flow through the flavin domain. J Biol Chem. 2000;275(29):21914–21919. doi: 10.1074/jbc.M002449200. [DOI] [PubMed] [Google Scholar]
- 62.Roman LJ, Sheta EA, Martásek P, Gross SS, Liu Q, Masters BSS. High-Level Expression of Functional-Rat Neuronal Nitric-Oxide Synthase in Escherichia-Coli. Proc Natl Acad Sci U S A. 1995;92(18):8428–8432. doi: 10.1073/pnas.92.18.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang MJ, Vogel HJ. Characterization of the Calmodulin-Binding Domain of Rat Cerebellar Nitric-Oxide Synthase. J Biol Chem. 1994;269(2):981–985. [PubMed] [Google Scholar]
- 64.Hevel JM, Marletta MA. Nitric-oxide synthase assays. Methods Enzymol. 1994;233:250–258. doi: 10.1016/s0076-6879(94)33028-x. [DOI] [PubMed] [Google Scholar]
- 65.Connors KA. Binding Constants. John Wiley & Sons; New York: 1981. [Google Scholar]
- 66.Plešek J, Rajabi FH, Vangani V, Fusek J. Constitution and Properties of the 8,8(')-Mu-H2no (1,2-C2b9h10)2–3-Co Bridged Cobaltaborane. Collect Czech Chem Commun. 1994;59(6):1326–1336. [Google Scholar]
- 67.Plešek J, Grüner B, Hemánek S, Fusek J, Votavová H. Constitution and Hplc Resolution of Enantiomers of the [8,4'-Mu-R2n-Commo-(1,2-C2b9h10)2-3-Co] Complex - the 3rd Isomer of Nitrogen-Bridged Bisicosahedral Cobaltacarborane. Collect Czech Chem Commun. 1994;59(2):374–380. [Google Scholar]
- 68.Plešek J, Hemánek S, Baše K, Todd LJ, Wright WF. Zwitterionic compounds of 8,8'-X(C2B9H10)2Co series with monoatomic O, S, Se, Te, N bridges between carborane ligands. Collect Czech Chem Commun. 1976;41(12):3509–3515. [Google Scholar]
- 69.Janoušek Z, Plešek J, Hemánek S, Baše K, Todd LJ, Wright WF. Synthesis and characteristics of sulfur interligand bridge-derivatives and of some S-substituted compounds in the (C2B9H11)2Co-series - conformations of (C2B9H11)2Mx-metallocarboranes. Collect Czech Chem Commun. 1981;46(11):2818–2833. [Google Scholar]
- 70.Grüner B, Císaová I, jáslavský J, Bonnetot B, Cornu D. Synthesis of 12-hydroxy and 12-dioxane derivatives of the closo-1-carbadodecaborate(1-) ion. Variations on the Plesek's cobalt bis(dicarbollide) pattern. Collect Czech Chem Commun. 2002;67(7):953–964. [Google Scholar]
- 71.Holub J, Grüner B, Císaová I, Fusek J, Plzák Z, Teixidor F, Viñas C, Štíbr B. A series of the twelve-vertex ferratricarbollides [2-(eta(5)-C5H5)-9-X-closo-2,1,7,9-FeC3B8H10] (Where x = H2N, MeHN, Me2N, (BuHN)-H-t, Bu-t(Me)N). A highly stable metallatricarbaborane system with amine functions in the para position to the metal center. Inorg Chem. 1999;38(12):2775–2780. doi: 10.1021/ic981400e. [DOI] [PubMed] [Google Scholar]
- 72.Hertler WR, Raasch MS. Chemistry of Boranes .14. Amination of B10h10-2 + B12h12-2 with Hydroxylamine-O-Sulfonic Acid. J Am Chem Soc. 1964;86(18):3661–3668. [Google Scholar]
- 73.Grüner B, Bonnetot B, Mongeot H. Synthesis of N- and B-substituted derivatives of closo-amino-undecahydro-dodecaborate(1-) anion. Collect Czech Chem Commun. 1997;62(8):1185–1204. [Google Scholar]
- 74.Sivaev IB, Starikova ZA, Sjöberg S, Bregadze VI. Synthesis of functional derivatives of the [3,3 '-Co(1,2-C2B2H11)(2)](-) anion. J Organomet Chem. 2002;649(1–2):1–8. [Google Scholar]
- 75.Katritzky AR, Rogovoy BV. Recent developments in guanylating agents. Arkivoc. 2005:49–87. [Google Scholar]
- 76.Lee YB, Folk JE. Branched-chain and unsaturated 1,7-diaminoheptane derivatives as deoxyhypusine synthase inhibitors. Bioorg Med Chem. 1998;6(3):253–270. doi: 10.1016/s0968-0896(97)10030-x. [DOI] [PubMed] [Google Scholar]
- 77.Kraus T, Budešínský M, Závada J. Synthesis of per-6-guanidinylated cyclodextrins. Tetrahedron Lett. 2006;47(5):679–681. [Google Scholar]