Abstract
Objective
Infrainguinal autogenous vein grafts are especially prone to narrowing and failure, and both inflammatory and thrombotic pathways are implicated. Platelets and monocytes are the key thrombo-inflammatory cells that arrive first at sites of vascular injury. These cells have potent interactions that recruit and activate one another, propagating thrombotic and inflammatory responses within the vessel wall. We therefore hypothesized that elevated levels of platelet-monocyte aggregates might be associated with stenosis, and could possibly discriminate between patients with or without vein graft stenosis.
Design of Study
Thirty-six vascular surgery patients were studied, in a stable quiescent period after infrainguinal autogenous vein graft bypasses for occlusive disease. Eighteen patients had hemodynamically significant graft stenoses confirmed by imaging, and 18 were free from stenosis.
The level of platelet-monocyte aggregates (PMA) in whole blood was quantified after blood draw using 2-color flow cytometry. Three measurements were made per sample: the basal, in-vivo level of aggregates (Baseline PMA); the predisposition to spontaneously generate PMA (Spontaneous PMA); and PMA generation by the addition of exogenous thrombin receptor activating peptide (Stimulated PMA). The baseline, in-vivo level of PMA was estimated by immediate flow analysis. The predisposition to spontaneously generate PMA was measured after in-vitro incubation. Responsiveness to thrombin stimulation of the blood was quantified by the in vitro dose response to an exogenous thrombin receptor activating peptide (sfllrn).
Results
Baseline PMA levels were similar in patients with vein graft stenosis vs. non-stenosis (14.8% ±3.2 versus 10.1% ±1.5 respectively, mean ±sem). However, patients with stenosis showed higher Spontaneous PMA levels (58.5% ±4.5 vs. 28.3 % ±4.3, P< .01), and higher Stimulated PMA levels (P< .001, ANOVA). Covariables of smoking, diabetes, statin or antithrombotic therapy could not account for these differences.
Conclusions
Platelet-monocyte reactivity may play a role in the development of vein graft stenoses. Those with/without stenosis differed primarily in their threshold, or predisposition to form aggregates (Spontaneous PMA), while their basal circulating levels of PMA (Baseline PMA) were similar. These measurements may unmask pathologic differences in thrombo-inflammatory responsiveness that are not apparent in basal measurements. Understanding the causes and mechanisms leading to abnormal platelet-monocyte responses may improve approaches to predicting or preventing vein graft stenosis.
I. Introduction
Vein graft stenosis and pathologic vascular wall thickening are critical problems in vascular surgery, affecting 15–30% of infrainguinal grafts within the first year after surgery.1–5 Graft stenosis is a leading cause of reoperation, graft failure and limb loss,6, 7 and yet the contributory factors are poorly understood. Most authorities consider vein graft stenosis, fibrosis, and anastomotic intimal hyperplastic lesions as a spectrum of related pathological processes.8–10 Although there is significant variability among different patient’s clinical responses to vascular injury, little is known about what accounts for these differences, especially patients with peripheral arterial disease.
Research suggests that the processes of inflammation and thrombosis, with their extensive biological crosstalk, underlie the pathological response to vascular injury. Blood platelets and monocytes are among the first inflammatory cell types to arrive at sites of vascular injury.11, 12 Through cell-cell adhesion and co-stimulation they initiate both thrombotic and inflammatory responses, propagating activation to the endothelium and vascular smooth muscle cells. A broad range of basic and clinical studies have shown a close association between platelet and monocyte activity, derangements of vascular healing, and cardiovascular clinical outcomes.13, 14 In particular, the measurement of circulating platelet-monocyte aggregates (PMA) in the blood has become a powerful new tool to assess this systemic thrombotic and inflammatory state.15, 16 Elevated platelet-monocyte aggregates have been closely associated with myocardial infarction, unstable coronary syndrome, percutaneous coronary intervention, restenosis, and smoking.15–24 Recently Burdess et al. has shown that platelet-monocyte aggregates are elevated in subjects with peripheral arterial disease and critical limb ischemia.25
We hypothesized that differences in platelet-monocyte activation, as measured by the formation of PMA, might account in part for the differences in biological healing of autogenous vein grafts. We also wished to learn exactly what kinds of measurements of platelet-monocyte aggregate formation might better discriminate between patients with different thrombotic/inflammatory phenotypes, and clinical outcomes. Therefore, we conducted this pilot study to elucidate the feasibility and utility of measuring PMA formation in patients with peripheral arterial disease with vein grafts, and to determine if assessments of platelet monocyte interaction might be associated with vein graft stenosis.
II. Materials and Methods
Measurement of platelet-monocyte aggregates in whole blood
For all assays, blood was collected into vacutainer tubes containing 3.2% sodium citrate (BD Biosciences) by clean, flawless venipuncture using a modification of the two-syringe technique and a void volume of at least 3 ml. From the single venous blood sample, one aliquot was immediately treated with EDTA (5 mM). This halts further aggregation ex vivo immediately after it is drawn. We call this the “Baseline PMA” because it is before we subject the blood to various conditions of incubation. A second aliquot was incubated with phosphate buffered saline (PBS). During this incubation, platelets and monocytes continue to aggregate together spontaneously. We call this the “Spontaneous PMA” level. A final series of aliquots was exposed to increasing concentrations of thrombin receptor activating peptide (TRAP, peptide SFLLRN, 1–5 μMolar). This stimulates the platelets and monocytes to aggregate and reflects thrombin sensitivity. We call the TRAP stimulated incubation the “Stimulated PMA” level.
Samples were incubated 15 minutes with mouse anti-human CD-41 conjugated PE antibody (platelet label), and mouse anti-human CD 14 conjugated FITC antibody (monocyte labeled): both antibodies were obtained from Dako Cytomation. Controls used to determine background fluorescence were non-specific antibodies matched for isotype, fluorophore, concentration, and F:P ratio (BD BioSciences). The cells were then fixed with formalin (1.3X Hanks/0.5% formaldehyde, final concentration) for 10 minutes and then lyzed with 6 volumes of water. All incubation steps were performed at room temperature in the dark. Samples were kept at 4°C until analyzed.
Flow cytometric quantification of platelet-monocyte heterotypic aggregates was performed on a Becton-Dickinson FACSCalibur, using the standard methods of Michelson et al.16, 27 Samples were run on a low flow setting, collecting light-scatter data in linear mode, with the threshold set on forward light scatter. A minimum of 1,000 CD14-positive monocytes are quantified. Within the dot plot of side scatter vs. log FITC-CD14, the monocyte region of interest is chosen, from which histograms of cell count vs. log (phycoerythrin-CD41 fluorescence) are developed. The positive marker is set just above the isotype-negative histogram, to include less than 1% of the total fluorescence. The parameter of interest is then measured as the percentage of monocytes positive for phycoerythrin staining platelets (% PMA).
As reported by Michelson and others, we found in control experiments that after cell fixation, sample results are stable and reproducible for at least 24 hours of refrigeration. We also compared the natural agonist, human alpha-thrombin, to the thrombin receptor activating peptide (TRAP), using established methods.27 TRAP was used as the thrombin agonist, because comparable results were obtained with both agonists. During the development of our methods, we also measured the formation of platelet-polymorphonuclear cell aggregates, and found that those levels closely paralleled those of PMA. We chose to focus on the monocyte, because of its unique inflammatory potential, and its proven role in impaired vascular healing.
Patient Selection Criteria
The population under study included those patients with established infrainguinal vein grafts undergoing routine postoperative follow up at the VA Puget Sound Health Care System and the University of Washington Medical Center. This included patients undergoing elective infrainguinal peripheral bypass grafting (redo and primary bypasses) with autogenous vein graft. Indications for surgery included claudication, rest pain, and tissue loss without active infection. Those with indication for tissue loss had healed wounds at the time of blood draw. The subjects were recruited at their follow up duplex visits. All vein graft patients undergo regular duplex ultrasound surveillance at 3, 6, 12, and 18 months and then yearly. Because this was a pilot study, eligible subjects were recruited as they arose, over a two-year period, and those without stenosis were not matched for co-morbid conditions.
Inclusion criteria were: 1) a patent, autogenous vein infrainguinal bypass graft performed for atherosclerotic occlusive vascular disease. Patency was determined post-operatively in the operating room by either ultrasound duplex or angiography; 2) either the presence or absence of a vein graft stenosis. All subjects had single segment autogenous vein conduits. Stenotic patients were included if they had a hemodynamically significant stenosis (greater than 75% or velocity ratio greater than 3.5)28 as detected by duplex ultrasound and confirmed with complementary vascular imaging when equivocal. Subjects with complete occlusion were excluded from the study.
Non-stenotic patients were included if they had consistently normal duplex graft surveillance studies throughout their follow up. Exclusion criteria eliminated patients with known hypercoagulable states, or intercurrent thrombotic or inflammatory conditions that might affect a patient’s usual, chronic state of platelet and monocyte reactivity, namely: recent surgery or arteriography; active infection; or other transient medical condition that might influence the measurements (e.g. myocardial infarction, stroke). Patients with immediate occlusion or occlusion before 6 months post operatively were excluded.
Normal healthy individuals who were free of platelet-active or anti-inflammatory drugs were also recruited for study as normal controls. These normal healthy subjects were used as our control group to the subjects with peripheral vascular disease. All subjects were recruited and studied in an outpatient setting, after informed consent. The study and its protocols were approved by the Institutional Review Board of the University of Washington and the VA Puget Sound Health Care System, and all subjects gave informed consent.
Data Analysis
The significance of between-group differences in continuous variables (e.g. platelet monocyte aggregate levels) was determined by t-test or analysis of variance, using SigmaStat software. The proportions of dichotomous variables were tested by chi-square analysis or Fisher’s Exact test. Means with standard error of the mean are presented, and two-tailed P values < .05 were considered significant.
III. Results
Patient Characteristics
A total of 36 patients were studied. All of the 18 with stenosis had either a hemodynamically critical single graft stenosis awaiting surgical correction, or had a history of previous surgically corrected graft stenosis plus a new, uncorrected stenosis. Eighteen patients were studied who never had any stenosis of their vein grafts, which were widely patent throughout their surveillance follow-up. All patients were recruited at least 6 months from their primary surgery, and at least 3 months from any surgical procedures. Eighteen of the 36 patients studied underwent duplicate sampling at different times, and the values averaged. The clinical characteristics of the 36 patients are listed in Table 1. All patients were of the Caucasian race. Thirty-four healthy volunteer subjects were also studied, of whom 10 were female, and all Caucasian. These individuals were studied as a healthy control group to establish normal benchmarks, and were not intended to be a matched clinical cohort for the vein graft patients. Repeated measurements taken from the same subject on different weeks showed consistent, reproducible results. The coefficient of repeatability (Bland Altman) for this was 10%.
Table 1. Frequency and Distribution of Clinical Characteristics in the Subject Population.
Five patients were taking both an antiplatelet drug and warfarin. All of these clinical characteristics were evenly distributed (Chi-square or Fisher’s Exact test) between both groups, except aspirin rx (P<.03). A multivariate analysis of PBS values, looking at covariables of aspirin therapy, diabetes, and smoking were not significant, and had no interactions with the factor of stenosis.
| Clinical Characteristic | Stenosis n=18 | No Stenosis n=18 | All Patients n=36 |
|---|---|---|---|
| Mean Age (range) | 65.5 (41–84) | 68.7 (55–90) | 67.1 (41–90) |
| Female Gender | 6 (33%) | 3 (17%) | 9 (25%) |
| Diabetic | 7 (39%) | 6 (33%) | 13 (36%) |
| Statin Rx | 13 (72%) | 15 (83%) | 28 (78%) |
| Warfarin Rx | 9 (50%) | 3 (17%) | 12 (33%) |
| Aspirin Rx | 9 (50%) | 16 (89%) | 25 (69%) |
| Clopidogrel Rx | 3 (17%) | 1 (6%) | 4 (11%) |
| Active Smoker | 9 (50%) | 10 (56%) | 19 (53%) |
Rx denotes “therapy”, and percentages are rounded.
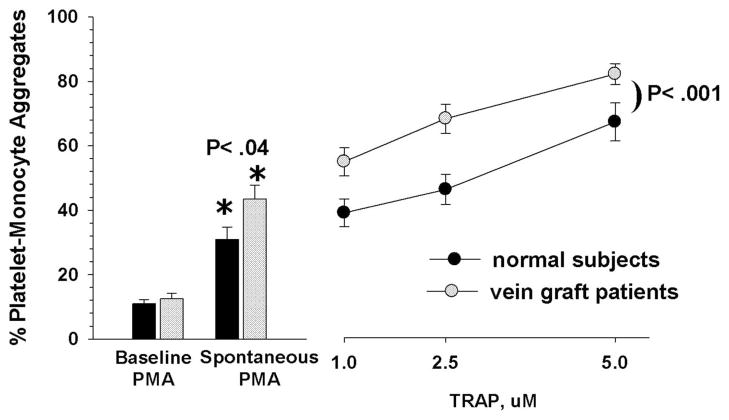
Platelet-Monocyte Aggregates in Healthy Volunteer Subjects compared to Vein Graft Patients
Three basic measures of platelet-monocyte reactivity were performed: 1) baseline PMA obtained by the immediate suppression of further aggregate formation by incubation with 5mM EDTA; 2) spontaneous PMA by incubation with PBS; and 3) stimulated PMA by incubating with increasing concentrations of TRAP. Figure 1 compares the healthy, normal subjects to the group of all vein graft patients. Baseline values of PMA were not significantly different between the PAD patients and healthy normals (12.5% ±1.8 vs 10.9% ±1.2, respectively). As anticipated, the patients with peripheral arterial disease had significantly higher spontaneous (43.4% ±4.3 vs. 31.8% ±4.1, P< .04), and stimulated formation of platelet monocyte aggregates compared with the healthy normals (P< .001, two-way analysis of variance).
Fig 1.
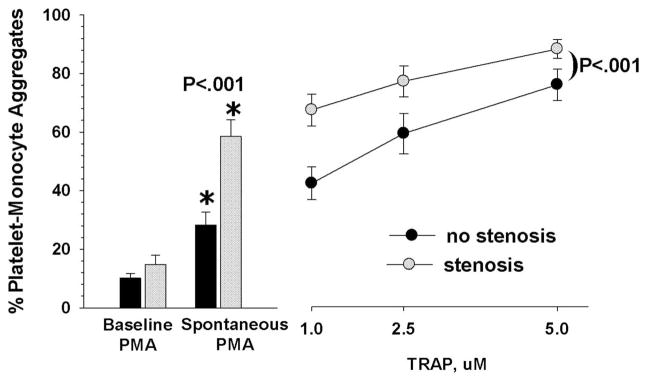
Association Between Platelet-Monocyte Aggregates and Graft Stenosis
Comparing the patients with stenosis to those without stenosis (figure 2), the baseline PMA values were also not significantly different (14.8% ±3.2 versus 10.1% + ±1.5, respectively). However, the spontaneous generation of PMA in the stenosis patients was significantly higher than in those without stenosis (58.5% ±4.5 versus 28.3% ±4.3, P < .016). Likewise, the differences in their responses to the thrombin agonist (stimulated) were highly significant (P < .001, two-way analysis of variance). Figure 2 illustrates the composite data from these three measures.
Fig 2.
These results indicate that patients with clinically severe vein graft stenosis had significantly higher spontaneous and stimulated formation of platelet-monocyte aggregates. We found that measurements of the spontaneous generation of PMA discriminated better than the baseline levels did. It distinguished normals from vein graft patients, and those with stenosis from those without stenosis. Likewise, the level of thrombin-stimulated PMA generated in whole blood was a more effective discriminator than baseline values.
Influence of Other Clinical Factors on Platelet-Monocyte Aggregates and Graft Stenosis
Although the clinical, systemic risk factors for vein graft stenosis are not known, potentially confounding factors could include conditions such as diabetes and smoking, which have been associated with increased PMA. Table I summarizes the clinical factors of the 36 patients. The clinical factors listed in Table I were evenly distributed between the two groups, except for aspirin therapy (P< .03). Although, three of the patients included were on both aspirin and warfarin therapy and all patients that were not on aspirin in both groups were on either warfarin, clopidogrel or both.
The average spontaneous PMA values were not significantly different among those patients with/without diabetes, an active smoking habit, and those on antiplatelet versus warfarin therapy (table 2). A multivariate analysis on PBS values, looking at covariables such as aspirin therapy, diabetes, and smoking were not significant and had no interactions with the factor of stenosis. Statin therapy bore no relation to PMA values (data not shown), although only 8 patients were not taking a statin. This does not rule out the possibility that any of them could be a confounding factor.
Table 2. Values of Spontaneous Platelet-Monocyte Aggregates in Patients with Different Clinical Characteristics.
The average measurements of spontaneous PMA (± sem) are shown for patients with or without the listed clinical factors. Antiplatelet Rx includes all patients exclusively taking aspirin and/or clopidogrel, but not warfarin. Warfarin Rx includes only those on warfarin.
| Clinical Factor | Present (n) | Absent (n) | P value |
|---|---|---|---|
| Graft Stenosis | 58.5% ± 5.7 (18) | 28.3% ± 4.3 (18) | <.001 |
| Diabetes | 58.7% ± 8.3 (13) | 42.8% ± 5.1 (23) | NS |
| Antiplatelet Rx | 37.4% ± 5.6 (24) | NS vs. warfarin | |
| Warfarin Rx | 63.9% ± 9.1 (7) | ||
| Smoking | 44.9% ± 6.3 (19) | 41.5% ± 5.9 (17) | NS |
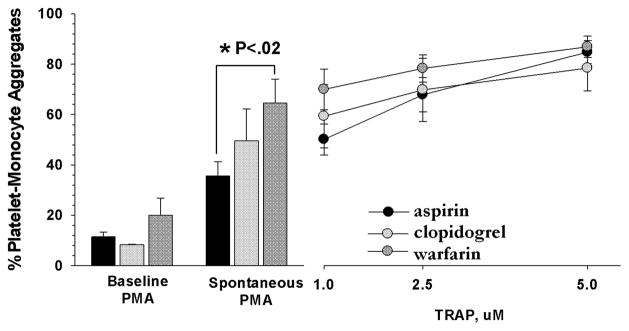
Many patients were receiving diverse combinations of antiplatelet and anticoagulant drugs, so the numbers were too small to conduct a multivariate analysis of the effects of all the different combinations of antithrombotic drugs. However, we did compare the PMA values from those patients who were exclusively treated with a single antithrombotic drug (Figure 3). The only significant differences in baseline, spontaneous, and stimulated PMA levels were between the spontaneous PMA levels between the aspirin only group and the warfarin only group (P< .02).
Fig 3.
Discussion
There were several purposes to this pilot study. First, we wished to test the association between platelet-monocyte aggregates and the presence of pathological vein graft stenosis. Second, we wished to determine which types of measurements of platelet-monocyte interactions might best discriminate among patients with different patterns of vascular healing (i.e. stenosis). Burdess et al. measured PMA in a variety of patients with peripheral arterial disease.25 Using their slightly different method of blood collection and processing, they reported PMA levels of ~ 30–40%, which is quite close to the value of ~40% spontaneous PMA that we measured in our overall group of patients with peripheral arterial disease.
This was not a prospective study in which medications or other clinical factors might be controlled or randomized, and as such it has several limitations. Although the consistency and accuracy of our techniques for measuring PMA were sufficient to discriminate between those with/without stenosis, we were not able to quantitatively assess the individual contributions of other clinical factors, perhaps because of the limited number of subjects.
Platelets and monocytes are at the root of the primordial cellular responses that lead to both thrombosis and inflammation at sites of vascular injury.18, 29–32 It has been well-established that patients with peripheral arterial disease suffer from increased platelet reactivity and aggregability.33 Michelson and others have persuasively argued that platelet-monocyte aggregates are one of the most sensitive measures of platelet activation.16 However, there is also extensive evidence that both cell types are intimately involved in the pathological vascular inflammation that is found in atherosclerosis as well as restenosis.34
Crosstalk between activated platelets and monocytes operates through at least four major pathways: the P-selectin/PSGL-1 receptor/counter-receptor system found on platelets and monocytes respectively;30 the CD40/CD40L axis;35, 36 monocyte integrin Mac-1 (αMβ2, cd11b/cd18) binding to the platelet von Willebrand receptor, GpIb,37–39 and finally, platelet integrin αIIbβ3 and the monocyte integrin αMβ2 can share the same ligand, fibrinogen. It is reasonable to speculate that platelets and monocytes, working through their extensive co-stimulatory pathways, may contribute significantly to the deranged vascular remodeling that causes vein graft stenosis.
A larger scale, prospective clinical study will be needed to resolve whether the observed increases in PMA formation are the cause of stenosis or the result of turbulence caused by the stenosis. Anecdotally, we have not observed a significant drop in PMA levels after the repair of stenoses in several patients. To address this question of which comes first, we are now measuring PMA before and after bypass surgery, and prospectively monitoring the development of vein graft stenosis. A larger scale study should also provide sufficient data to help elucidate the fundamental mechanisms underlying the elevated platelet and monocyte reactivity in certain patients with peripheral arterial disease.
Other local factors such as graft diameter and quality, velocity and turbulence of flow, compliance mismatch, and technical factors are believed to contribute to premature graft failure in some cases. Studies employing broader definitions of “graft failure” have also demonstrated associations with a patient’s inflammatory state.38, 39 Stent restenosis has also been correlated with inflammatory markers in peripheral arterial stenting and in the coronary circulation.22, 41–44
Regarding the methodology for measuring platelet-monocyte aggregates, we found that provocative measurements of PMA could more robustly differentiate between relatively small groups of patients with/without stenosis. Looking to the future of biomarkers to predict vein graft stenosis, we imagine that stimulatory tests, which measure a patient’s biological tendencies, may be more useful than baseline measurements.
Acknowledgments
This material is based upon work supported by a grant to Drs. Sobel, Kohler, and Hatsukami from the U.S. Department of Veterans Affairs, Office of Research and Development, Merit Review Agency.
Reference List
- 1.Mills JL, Wixon CL, James DCM, Devine J, Westerband A, Hughes JD. The natural history of intermediate and critical vein graft stenosis: Recommendations for continued surveillance or repair. J Vasc Surg. 2001;33:273–80. doi: 10.1067/mva.2001.112701. [DOI] [PubMed] [Google Scholar]
- 2.Gupta AK, Bandyk DF, Cheanvechai D, Johnson BL. Natural history of infrainguinal vein graft stenosis relative to bypass grafting technique. J Vasc Surg. 1997;25(2):211–20. doi: 10.1016/s0741-5214(97)70344-6. [DOI] [PubMed] [Google Scholar]
- 3.Visser K, Idu MM, Buth J, Engel GL, Hunink MG. Duplex scan surveillance during the first year after infrainguinal autologous vein bypass grafting surgery: costs and clinical outcomes compared with other surveillance programs. J Vasc Surg. 2001;33(1):123–30. doi: 10.1067/mva.2001.109745. [DOI] [PubMed] [Google Scholar]
- 4.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43(4):742–51. doi: 10.1016/j.jvs.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 5.Conte MS. Technical factors in lower-extremity vein bypass surgery: How can we improve outcomes? Semin Vasc Surg. 2009;22:227–33. doi: 10.1053/j.semvascsurg.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Seeger JM, Pretus HA, Carlton LC, Flynn TC, Ozaki CK, Huber TS. Potential predictors of outcome in patients with tissue loss who undergo infrainguinal vein bypass grafting. J Vasc Surg. 1999;30(3):427–35. doi: 10.1016/s0741-5214(99)70069-8. [DOI] [PubMed] [Google Scholar]
- 7.Monahan TS, Owens CD. Risk factors for lower-extremity vein graft failure. Semin Vasc Surg. 2009;22:216–26. doi: 10.1053/j.semvascsurg.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Davies MG, Hagen PO. Pathophysiology of vein graft failure: a review. Eur J Vasc Endovasc Surg. 1995;9(1):7–18. doi: 10.1016/s1078-5884(05)80218-7. [DOI] [PubMed] [Google Scholar]
- 9.Kraiss LW, Johansen K. Pharmacologic intervention to prevent graft failure. Surg Clin North Am. 1995;75(4):761–72. doi: 10.1016/s0039-6109(16)46697-1. [DOI] [PubMed] [Google Scholar]
- 10.Owens CD, Kim JM, Hevelone ND, Hamdan A, Raffetto JD, Creager MA, et al. Novel adipokines, high molecular weight adiponectin and resistin, are associated with outcomes following lower extremity revascularization with autogenous vein. J Vasc Surg. 2010;51(5):1152–9. doi: 10.1016/j.jvs.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linden MD, Jackson DE. Platelets: Pleiotropic roles in atherogenesis and atherothrombosis. Int J Biochem Cell Biol. 2010;42(11):1762–6. doi: 10.1016/j.biocel.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Shantsila E, Lip GY. The role of monocytes in thrombotic disorders. Thromb Haemost. 2009;102:916–24. doi: 10.1160/TH09-01-0023. [DOI] [PubMed] [Google Scholar]
- 13.Smout J, Dyker A, Cleanthis M, Ford G, Kesteven P, Stansby G. Platelet function following acute cerebral ischemia. Angiology. 2009;60(3):362–9. doi: 10.1177/0003319709332959. [DOI] [PubMed] [Google Scholar]
- 14.Beijk MA, Klomp M, Verouden NJ, van GN, Koch KT, Henriques JP, et al. Genous™ endothelial progenitor cell capturing stent vs. the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomized, single-centre, pilot study. Eur Heart J. 2009;31(9):1055–1064. doi: 10.1093/eurheartj/ehp476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarma J, Laan CA, Alam S, Jha A, Fox KAA, Dransfield I. Increased platelet binding to circulating monocytes in acute coronary syndromes. Circulation. 2002;7;105(18):2166. doi: 10.1161/01.cir.0000015700.27754.6f. [DOI] [PubMed] [Google Scholar]
- 16.Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin - Studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104(13):1533–7. doi: 10.1161/hc3801.095588. [DOI] [PubMed] [Google Scholar]
- 17.Cipollone F, Ferri C, Desideri G, Paloscia L, Materazzo G, Mascellanti M, et al. Preprocedural level of soluble CD40L is predictive of enhanced inflammatory response and restenosis after coronary angioplasty. Circulation. 2003;108(22):2776–82. doi: 10.1161/01.CIR.0000103700.05109.0D. [DOI] [PubMed] [Google Scholar]
- 18.Freedman JE, Loscalzo J. Platelet-monocyte aggregates bridging thrombosis and inflammation. Circulation. 2002;105:2130–2. doi: 10.1161/01.cir.0000017140.26466.f5. [DOI] [PubMed] [Google Scholar]
- 19.Schober A, Manka D, von Hundelshausen P, Huo Y, Hanrath P, Sarembock IJ, et al. Deposition of platelet rantes triggering monocyte recruitment requires p-selectin and is involved in neointima formation after arterial injury. Circulation. 2002;106:1523–9. doi: 10.1161/01.cir.0000028590.02477.6f. [DOI] [PubMed] [Google Scholar]
- 20.Harding SA, Sarma J, Josephs DH, Cruden NL, Din JN, Twomey PJ, et al. Upregulation of the CD40/CD40 ligand dyad and platelet-monocyte aggregation in cigarette smokers. Circulation. 2004;109:1926–9. doi: 10.1161/01.CIR.0000127128.52679.E4. [DOI] [PubMed] [Google Scholar]
- 21.Weyrich AS, Prescott SM, Zimmerman GA. Platelets, endothelial cells, inflammatory chemokines, and restenosis. Circulation. 2002;106:1433–5. doi: 10.1161/01.cir.0000033634.60453.22. [DOI] [PubMed] [Google Scholar]
- 22.Lee MS, David EM, Makkar RR, Wilentz JR. Molecular and cellular basis of restenosis after percutaneous coronary intervention: the intertwining roles of platelets, leukocytes, and the coagulation-fibrinolysis system. J Pathol. 2004;203(4):861–70. doi: 10.1002/path.1598. [DOI] [PubMed] [Google Scholar]
- 23.Zhang SZ, Jin YP, Qin GM, Wang JH. Association of platelet-monocyte aggregates with platelet activation, systemic inflammation, and myocardial injury in patients with non-st elevation acute coronary syndromes. Clin Cardiol. 2007;30(1):26–31. doi: 10.1002/clc.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linder F, Furman MI, Frelinger AL, Fox ML, Barnard MR, Li Y, et al. Indicies of platelet activation and teh stability of coronary artery disease. J Thromb Haemost. 2007;5(4):761–5. doi: 10.1111/j.1538-7836.2007.02462.x. [DOI] [PubMed] [Google Scholar]
- 25.Burdess A, Nimmo AF, Campbell N, Harding SA, Garden OJ, Dawson AR, et al. Perioperative platelet and monocyte activation in patients with critical limb ischemia. J Vasc Surg. 2010;52(3):697–703. doi: 10.1016/j.jvs.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Furman MI, Kereiakes DJ, Krueger LA, Mueller MN, Pieper K, Broderick TM, et al. Leukocyte-platelet aggregation, platelet surface p-selectin and platelet surface glycoprotein IIIa after percutaneous coronary intervention: effects of dalteparin or unfractionated heparin in combination with abciximab. Am Heart J. 2001;142:790–8. doi: 10.1067/mhj.2001.119128. [DOI] [PubMed] [Google Scholar]
- 27.Michelson AD, Barnard MR, Krueger LA, Frelinger AL, Furman MI. Evaluation of platelet function by flow cytometry. Methods-A Companion To Methods In Enzymology. 2000;21(3):259–70. doi: 10.1006/meth.2000.1006. [DOI] [PubMed] [Google Scholar]
- 28.Tinder CN, Chavanpun JP, Bandyk DF, Armstrong PA, Back MR, Johnson BL, et al. Efficacy of duplex ultrasound surveillance after infrainguinal vein bypass may be enhanced by identification of characteristics predictive of graft stenosis development. J Vasc Surg. 2008;48(3):613–8. doi: 10.1016/j.jvs.2008.04.053. [DOI] [PubMed] [Google Scholar]
- 29.Johnson K, Choi Y, DeGroot E, Samuels I, Creasey A, Aarden L. Potential mechanisms for a proinflammatory vascular cytokine response to coagulation activation. J Immunol. 1998;160:5130–5. [PubMed] [Google Scholar]
- 30.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23:2131–7. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 31.McEver RP. P-selectin and PSGL-1: Exploiting connections between inflammation and venous thrombosis. Thromb Haemost. 2002;87(3):364–5. [PubMed] [Google Scholar]
- 32.Libby P, Simon DI. Inflammation and thrombosis: The clot thickens. Circulation. 2001;103(13):1718–20. doi: 10.1161/01.cir.103.13.1718. [DOI] [PubMed] [Google Scholar]
- 33.Sobel M. Platelets in peripheral vascular disease. In: Michelson AD, editor. Platelets. 2. San Diego: Academic Press; 2006. [Google Scholar]
- 34.Davis C, Fischer J, Ley K, Sarembock IJ. The role of inflammation in vascular injury and repair. J Thromb Haemost. 2003;1(8):1699–709. doi: 10.1046/j.1538-7836.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- 35.Donners MM, Beckers L, Lievens D, Munnix I, Heemskerk J, Janssen BJ, et al. The CD40-TRAF6 axis is the key regulator of the CD40/CD40L system in neointima formation and arterial remodeling. Blood. 2008;111(9):4596–604. doi: 10.1182/blood-2007-05-088906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutgens E, Lievens D, Beckers L, Donners M, Daemen M. CD40 and its ligand in atherosclerosis. Trends Cardiovasc Med. 2007;17(4):118–23. doi: 10.1016/j.tcm.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Kuijper PH, Torres HI, Houben LA, Lammers JJ, Zwaginga JJ, Koenderman L. P-selectin and Mac-1 mediate monocyte rolling and adhesion to ECM-bound platelets under flow conditions. J Leukoc Biol. 1998;64:467–73. doi: 10.1002/jlb.64.4.467. [DOI] [PubMed] [Google Scholar]
- 38.Simon DI, Chen ZP, Xu H, Li CQ, Dong JF, McIntire LV, et al. Platelet glycoprotein Ib alpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Of Exp Med. 2000;192(2):193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Sakuma M, Chen Z, Ustinov V, Shi C, Croce K, et al. Leukocyte engagement of platelet glycoprotein Ibalpha via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation. 2005;112(19):2993–3000. doi: 10.1161/CIRCULATIONAHA.105.571315. [DOI] [PubMed] [Google Scholar]
- 40.Esposito CJ, Popescu WM, Rinder HM, Schwartz JJ, Smith BR, Rinder CS. Increased leukocyte-platelet adhesion in patients with graft occlusion after peripheral vascular surgery. Thromb Haemost. 2003;90(6):1128–34. doi: 10.1160/TH03-04-0226. [DOI] [PubMed] [Google Scholar]
- 41.Owens CD, Ridker PM, Belkin M, Hamdan AD, Pomposelli F, Logerfo F, et al. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg. 2007;45(1):2–9. doi: 10.1016/j.jvs.2006.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szkodzinkski J, Blazelonis A, Wilczek K. The role of interleukin-6 and transforming growth factor-beta 1 in predicting restenosis within stented infarct-related artery. Int J Immunopathol Pharmacol. 2009;22(2):493–500. doi: 10.1177/039463200902200226. [DOI] [PubMed] [Google Scholar]
- 43.Schillinger M, Exner M, Mlekusch W, Rumpold H, Ahmadi R, Sabeti S, et al. Vascular Inflammation and percutaneous transluminal angioplasty of the femoropopliteal artery: association with restenosis. Radiology. 2002;225(1):21–6. doi: 10.1148/radiol.2251011809. [DOI] [PubMed] [Google Scholar]
- 44.McDermott MM, Lloyd-Jones DM. The role of biomarkers and genetics inperipheral arterial disease. J Am Coll Cardiol. 2009;54(14):1228–37. doi: 10.1016/j.jacc.2009.04.081. [DOI] [PubMed] [Google Scholar]