Abstract
Dopamine contributes to corticostriatal plasticity and motor learning. Dopamine denervation profoundly alters motor performance, as in Parkinson’s disease; however, the extent to which these symptoms reflect impaired motor learning is unknown. Here we demonstrate a D2 receptor blockade induced aberrant learning that impedes future motor performance when dopamine signaling is restored, an effect diminished by co-administration of adenosine antagonists during blockade. We hypothesize that an inappropriate corticostriatal potentiation in striatopallidal cells of the indirect pathway underlies aberrant learning. Here, we demonstrate synaptic potentiation in striatopallidal neurons induced by D2 blockade and diminished by application of an adenosine antagonist, consistent with behaviaoral observations. A neurocomputational model of the basal ganglia recapitulates the behavioral pattern and further links aberrant learning to plasticity in the indirect pathway. Thus, D2-mediated aberrant learning may contribute to motor deficits in PD and suggest new avenues for the development of therapeutics.
INTRODUCTION
Corticostriatal plasticity has been directly linked to motor learning and performance (Costa et al., 2004; Yin et al., 2009; Jin and Costa, 2010). The dorsolateral striatum (posterior putamen in primates)—the region most prominently affected in Parkinson’s disease (PD) (Bernheimer et al., 1973; Hornykiewicz, 2001)—has been associated with the automization of behavior (Miyachi et al., 2002; Costa et al., 2004; Poldrack et al., 2005; Puttemans and Wenderoth, 2005; Doyon et al., 2009; Yin et al., 2009; Jin and Costa, 2010) and habit (Bernheimer et al., 1973; Hornykiewicz, 2001; Tang et al., 2007; Graybiel, 2008; Yin et al., 2009; Balleine and O’Doherty, 2010), providing a substrate for generating rapid and efficient behavioral responses without cognitive deliberation and planning.
Dopamine denervation induces abnormal corticostriatal plasticity (Calabresi et al., 1997; Picconi et al., 2003; Kreitzer and Malenka, 2007; Shen et al., 2008; Peterson et al., 2012), though the role this plays in the symptoms, progression and treatment of the PD has not been established. We recently proposed that altered plasticity, specifically inappropriate LTP in striatopallidal medium-spiny neurons (MSNs), gives rise to an aberrant learning process that contributes to the symptoms and progression of PD by inverting basal ganglia optimization of behavior (Wiecki and Frank, 2010; Beeler, 2011). Computational models suggest an interaction between dopamine’s effects on MSN activity and corticostriatal synaptic plasticity—performance and learning, respectively—within striatal D1- and D2-expressing cells of the direct and indirect pathways (Frank et al., 2004; Bódi et al., 2009; Palminteri et al., 2009; Wiecki and Frank, 2010). To the degree that the mechanisms of abnormal corticostriatal plasticity are dissociable from those mediating dopamine’s direct performance effects, they represent a target for novel therapeutics. Remediating abnormal plasticity and aberrant learning may be a significant but unrecognized component of current drug therapies and underlie the poorly understood but important long-duration response (LDR) observed in L-DOPA treatment (Beeler et al., 2010; Beeler, 2011).
Both the aberrant learning hypothesis and neurocomputational models point to critical interactive effects between dopamine-mediated performance and learning and make specific predictions:
Dopamine depletion or receptor blockade, in addition to direct performance effects, will result in inhibitory learning in the indirect pathway that will impair future performance and learning even when dopamine signaling is restored.
In animals that acquired the task under healthy dopamine conditions, dopamine blockade should induce a progressive decline in performance, reflecting an aberrant learning process that will impair future recovery.
If the above effects are due to induction of aberrant potentiation in the D2 pathway, dopamine blockade should induce potentiation in striatopallidal MSNs that is reversed by agents known to disrupt LTP in this pathway.
Disrupting LTP in the indirect pathway should be protective when administered during dopamine blockade by preventing aberrant learning but impede recovery when administered after aberrant learning by impairing relearning.
Here, we test these predictions in a mouse model of motor learning and concurrently test whether the empirically observed effects will emerge in an a priori computational model of basal ganglia function.
METHODS AND MATERIALS
Animals
Mice were housed in standard conditions on a 06:00 to 18:00 light cycle with ad libitum food and water. Experiments were carried out during the light cycle. Animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Chicago. All mice were C57BL/6 wild-type mice between 8-12 weeks of age. For the in-vitro electrophysiology, adult (4-12 week old) transgenic mice hemizygous for Drd2-enhanced green fluorescent protein (EGFP) bacterial artificial chromosome (BAC) of both sexes were used in all experiments. Drd2-EGFP homozygotes were identified from test crosses and then crossed with C57BLK6 mice to produce hemizygotes.
Behavior Tests
A computer-controlled rotarod apparatus (Rotamex-5, Columbus Instruments, Columbus, OH) with a rat rod (7cm diameter) was set to accelerate from 4 to 40 revolutions per minute (RPM) over 300 seconds, and recorded time to fall. Mice received 5 consecutive trials per session, one session per day. (~ 30s between trials). Open Field chambers were 40 × 40 cm (Med Associates, St. Albans, VT) with lighting at 21 lux. Infrared beams recorded the animals’ locomotor activity and rearing movements (vertical activity). Data were collected in 5-minute bins during 45 min sessions for 3 days. For the treadmill performance, a plexiglass enclosure was placed over the treadmill to force the mice to remain on the track during the trials (enclosed treadmill space, 5 × 20 cm). Mice were provided 3 20s trials per day for 3 days at incrementing speeds (10,15,20; 15,15,20; 15,2020 cm/s, days 1-3, respectively). Their performance was assessed on the final day at 15 and 20 cm/s by recording the amount of time the animals remained in the forward two-thirds of the track vs. falling back into the back third.
Drug administration
All injections were intraperitoneal (i.p.) at 0.01 ml/gram of body weight. SCH23390, eticlopride and theophylline (Sigma-Aldrich, St. Louis, MO) were administered 30 min prior to sessions at the specified doses prepared in 0.9% saline.
In-vitro Electrophysiology
After isoflurane anesthesia, animals were decapitated and their brains removed to ice-cold sucrose aCSF (in mM: sucrose 125, KCl 2.5, MgCl2 1, CaCl2 2.5, glucose 20, NaH2PO4 1, NaHCO3 25, ascorbic acid 10; bubbled with 95% O2/5% CO2). Coronal slices were cut (μM; VT1000S, Leica) containing the dorsolateral striatum (+0.4mm-+1.0mm from bregma) and removed to a holding chamber perfused with normal aCSF (in mM: NaCl 125, KCl 2.5, MgCl2 1, CaCl2 2.5, glucose 20, NaH2PO4 1, NaHCO3 25, ascorbic acid 1; bubbled with 95% O2/5% CO2), 20 mL/min at 34°C. For recording, the slices were superfused with normal aCSF without ascorbic acid at 2 mL/min, 32 °C. In the dorsolateral striatum D2-GFP MSN’s were visualized using fluorescence illumination on an upright microscope (Axioskop, Zeiss). Whole-cell voltage clamp recordings used an Axopatch 200B amplifier, a Digidata 1200 interface, and pCLAMP 8 (Molecular Devices). All recordings were filtered at 1 kHz and digitized at 5kHz, Vm = −70mV. For all recordings we used borosilicate electrodes (3-7 MΩ) containing (in mM), K-gluconate 154, KCl 1, EGTA 1, HEPES 10, glucose 10, and ATP 5 (pH 7.4 with KOH). Only cells with series resistance was <20 MΩ were included. For stimulation of corticostriatal inputs, a bipolar tungsten electrode with a 500 μm tip separation was placed inside the cortical border of the dorsolateral striatum. After establishing a consistent evoked EPSC amplitude once every 30s for 5 min, sulpiride (20μM) was bath applied either on its own or with theophylline (1μM). Recording was continued in the presence of drug for a further 15-20min after which most of the recordings were terminated. Sulpiride and theophylline were purchased from Sigma-Aldrich (St. Louis, MO).
Statistical analysis
Behavior
In all rotarod studies, the data were tested for significance using ANOVA (R statistical software (R version 2.12.1 2010-12-16); The R Foundation for Statistical Computing, http://www.r-project.org). Dose effects were modeled as continuous; comparison between drugs (including drug/dose comparisons) were modeled as factors with a level for either each drug or each drug/dose combination. As we used repeated measures (5 trials/session, multiple sessions across experiment), session was always included as a continuous independent variable with an error term of mouse/session for repeated measures, for example:
Electrophysiology
All data are reported as mean ± SEM and expressed as the normalized value of the baseline (5mins) before drug application. For analysis of drug effects on EPSC amplitude we used a two-way repeated measures ANOVA followed by a Tukey post-hoc test on the EPSC amplitudes collected 5 min before drug exposure and 15-20 min after that time. Statistical significance was determined by p<0.05, all statistical tests were performed using Sigmastat (Systat software).
RESULTS
Blockade of D1 and D2 signaling induce aberrant learning independent of direct effects on performance
To reversibly mimic dopamine denervation, we administered a cocktail of D1 and D2 dopamine receptor antagonists to block dopamine signalling in the direct and indirect pathways. The cocktail was administered either during the initial acquisition or after the establishment of a striatal-dependent motor skill. When a dopamine antagonist cocktail is administered during initial training, the cocktail dramatically impaired rotarod performance (treatment main effect, F(1,12) = 34.5, p < .001) with no apparent evidence of learning across the five training days (Fig. 1A, left). However, when the mice returned to the rotarod 72 hours after the last cocktail administration, their performance remains degraded and improves only gradually over 10 days (Fig. 1A, treatment × session interaction, F(1,12) = 23.6, p < .001), which contrasts markedly with the 1-2 days required for naïve mice to reach asymptotic performance (Fig. 1A, left; comparing initial 3 days naive vs relearning, treatment × session interaction, F(1,12) = 11.29, p < .01). The dramatically retarded re-acquisition following initial training under cocktail suggest that an aberrant learning process occurred during the cocktail training. This was not a residual effect of the cocktail treatment itself: mice administered the cocktail on the same schedule as the trained mice but without rotarod training showed no impairment in subsequent acquisition (Fig. 1A, right), indicating the diminished performance of cocktail trained mice is experience-dependent.
Figure 1. Dissociating learning and performance effects of dopamine receptor blockade on acquisition and maintenance of a motor skill.
To dissociate learning and performance effects we used a multi-phase rotarod design in which initial learning under either a dopamine-normal or dopamine-impaired condition is paired with a subsequent testing phase in the opposite condition. A two and three phase design (shown above graphs) was used to assess the effects of dopamine receptor blockade on initial acquisition (two-phase design) or continued performance of an established skill (three-phase design), respectively. Each daily session consisted of 5 trials. In all figures, session means averaged across the 5 trials are reported. Graphs show latency to fall in wild-type C57BL/6 mice administered either a cocktail of dopamine antagonists (0.16 mg/kg eticlopride + 0.1 mg/kg SCH23390, filled gray triangles) or saline (filled red circles) during either (A) initial acquisition of rotarod performance or (B) after performance is established through 12 days prior training. A control group was administered the antagonist cocktail and returned to their homecage without rotarod training and then subsequently tested without drug (A, open gray squares). Each point represents the mean of 5 trials during daily sessions. Between each treatment phase, there was a 72 hour break without training. (C) distance traveled in the open field (OF) for three 45 min sessions on 3 consecutive days for a group that previously received cocktail administration and rotarod training (open triangles), a naive group (red circles) and a group administered 0.16 mg/kg eticlopride 30 min prior to testing. (D) Average rearing/vertical time across all three OF sessions. (F) Average percent of time mice (same groups as D/E) remained in the forward two-thirds of track on treadmill during two 20s test trials at 15 and 20 cm/s. A, N= 7 (homecage controls, open square, N=5); B, N=6; C/D, N=7; E, N=8. Error bars, s.e.m.
We further tested if cocktail together with rotarod training impaired the animals on other tasks. In a new group we administered cocktail and provided rotarod training but following the 72 hour break, tested their open field (OF) activity and treadmill performance instead of rotarod. We compared these mice to a naive control group and a group administered eticlopride acutely as a reference for impaired D2 function. While decreased D2 activation (ie., eticlopride) greatly reduces OF activity, the cocktail/rotarod group do not show decreased activity (Fig 1C, F(2,18) = 39.12, p < .001). On the first two days, they show increased activity compared the naive mice. The reason for this is unclear, but inconsistent with decreased D2 function. Notably, the cocktail/rotarod group had a week of handling and exercise which may have increased their comfort level and decreased behavioral inhibition. Consistent with this possibility, they show greater rearing/vertical time, consistent with greater exploratory behavior (Fig 1D, F(2,18) = 16.46, p < .001). In a second motor performance test, performance on the treadmill was indistinguishable between naive and cocktail/rotarod groups, in contrast to greatly reduced performance as a consequence of reduced D2 signaling (Fig 1E, group, F(1,22) = 28.3, p < .001; naive vs cocktail trained, F(1,14) = 2.25, p = .15). Together these data suggest that the training under D1/D2 blockade resulted in an experience-dependent aberrant motor learning that task-specifically impaired subsequent performance and learning.
When the antagonist cocktail was administered after the motor skill was established (in a separate group of mice), we observed an aberrant learning effect as well. The cocktail results in an immediate impairment of performance (treatment main effect, F(1,10) = 58.7, p < .001), but also a further decline across the initial couple of sessions (Fig. 1B, middle; treatment group only, session effect, F(1,5) = 7.2, p = .013). Upon cessation of cocktail treatment, the mice did not return immediately to their prior asymptotic performance. Instead, they showed a gradual return to previous levels of performance (Fig. 1B, right; during recovery phase: prior treatment F(1,10) = 7.2, p = .023, prior treatment × session, F(1,10) = 5.8, p = .036). These data suggest that the cocktail not only impairs performance but when combined with experience on the motor task, also alters the underlying learning that supported the previously established skill, necessitating a relearning phase.
The cocktail blocks both the D1 and D2 receptors. Previous reports suggest that aberrant learning occurs primarily in the D2 pathway (Wiecki et al., 2009; Beeler et al., 2010). To independently test the contribution of each receptor subtype, we tested the behavioral consequences of administering eticlopride (D2 selective antagonist) or SCH23390 (D1 selective antagonist) either during acquisition or after asymptotic performance, as above.
Blockade of D2 but not D1 receptors during acquisition induces aberrant learning that impairs subsequent recovery
Both the D1 and D2 antagonists impair initial acquisition (Fig. 2A/B, left; acquisition only, treatment effect, F(7,23) = 7.78, p < .001) in a dose-dependent manner (SCH23390 dose, F(1,18) = 19.87, p < .001; eticlopride dose, F(1,13) = 5.39, p < .05) though not to the same degree as the cocktail (Fig. 1A, left). At higher doses, D1 and D2 antagonists impaired performance to a similar degree. However, a significantly different pattern of recovery emerged during the drug-free phase depending on whether D1 or D2 receptors were blocked during acquisition (drug × session during recovery, F(1,25) = 16.09, p < .001). Blockade of D1 has a weak effect on subsequent recovery (Fig. 2A/C; SCH23390 group during recovery, dose, F(1,18) = 4.14, p = .056). Indeed, we observed an immediate, discontinuous jump to better performance post-treatment in D1 treated mice with little subsequent improvement (Fig. 2A/C; SCH23390 recovery, sessions F(1,18) = .79, p = .38). For highest doses of D1 blockade during acquisition, some residual learning appears to have occurred during recovery (sessions × dose, F(1,18) = 3.37, p = .082). In contrast, D2 blockade during initial acquisition results in poor initial performance during recovery and only gradual relearning and improvement (Fig. 2B/D, right; eticlopride group during recovery, sessions F(1,13) = 21.4, p < .001; dose, N.S.; dose × session, F(1,13) = 3.36, p = .08). The mild relearning observed at high doses of D1 blockade may reflect a reduction in receptor selectivity at higher doses or indicate that sufficient interference with D1 can also induce mild aberrant learning. Nonetheless, in general D1 blockade impaired performance with minimal effects on subsequent performance, suggesting a limited role in aberrant learning. In contrast, D2 blockade significantly impairs future performance and appears to require a gradual relearning process. These data suggest that blockade of D2 induces an aberrant learning during initial acquisition that delays or hinders subsequent appropriate learning.
Figure 2. Effect of D1 or D2 antagonism on acquisition and subsequent drug-free recovery.
Latency to fall in wild-type C57BL/6 mice administered an antagonist of either (A) D1 (SCH23390) or (B) D2 (Eticlopride) during 5 days of initial acquisition of rotarod performance and subsequent non-drug performance. Red traces indicate saline controls (shown in both plots). Bar graphs show the average of last three days of drug training (black bars) followed by the first 5 days of drug-free recovery averaged across all doses for (C) D1 and (D) D2 blockade. Each point represents the average of 5 trials during daily sessions. A 72 hour break occurred between treatment and non-treatment phases. N= 4/dose, ***, p < .001. Error bars, s.e.m.
When the antagonists are instead applied after asymptotic performance is established, a difference in the pattern of impairment and recovery emerges. D1 blockade results in an immediate, dose-dependent decrement in performance that is constant across drug administration sessions (Fig. 3A/C treatment phase; dose, F(1,18) = 27.1, p < .001, session and dose × session, N.S.). Upon cessation of the antagonist, performance in mice administered a D1 antagonist returns to asymptotic performance immediately, showing no dose-dependent effects or relearning (Fig. 3A/C, recovery phase; dose, F(1,18) = .81, p = .38, session and dose × session, N.S.). In contrast, D2 blockade, though it also causes an immediate performance decrement, also induces gradual deterioration across treatment sessions (treatment phase, dose main effect, F(1,14) = 5.9, p < .05, dose × session F(1,14) = 6.3, p < .05). During recovery from D2 blockade, unlike D1 blockade, we observe a continued effect of dose (Fig. 3B/D, recovery phase, dose, F(1,14) = 6.29, p < .05, session and dose × session, N.S.) and what appears to be a more gradual recovery compaired to recovery from D1 blockade, though this latter observation was not statistically significant (drug × session, F(1,15) = .77, p = .47).
Figure 3. Effect of D1 or D2 antagonism on established performance.
Latency to fall in wild-type C57BL/6 mice administered an antagonist of either (A) D1 (SCH23390) or (B) D2 (Eticlopride) following 5 days of initial training under non-drug conditions and subsequent recovery. Each point represents the average of 5 trials during daily sessions. (C/D) Average performance across sessions in each phase plotted by dose for (C) D1 and (D) D2 blockade. Bar graph insets show first three recovery days averaged across all doses of SCH23390 and for 0.16 and 0.64 mg/kg eticlopride. (E) Comparison on D1 and D2 blockade (SCH23390, 0.1 mg/kg and eticlorpide, 0.16 mg/kg, respecitvely) after 12 days of initial training. (F) Effect of initial training length on subsequent D2 blockade (eticlopride, 0.16 mg/kg) and recovery showing 12 (same data as E, 6 and 3 days of training). Throughout, red traces indicate saline controls. A 72 hour break occurred between treatment and drug-free recovery phases. N= 4/dose for A-D and N=6 for E-F, statistics reported in text. Error bars, s.e.m.
Though D2 blockade during initial acquisition clearly impairs recovery (Fig 2B/D), the degree to which D2 blockade of an established skill impairs subsequent recovery is less clear (Fig 3 B/D). This could arise because putative aberrant learning may depend on how established the skill is to begin with. Additionally, the effect of D2 blockade (Fig 3D) does not appear to titrate as clearly as D1 blockade (Fig 3C); the lowest dose had no apparent effect (comparing saline and .04, dose and dose × session, N.S.) and the 0.16 and 0.64 effect were similar (comparing .16 and .64, dose and dose × session, N.S.). Consequently, we conducted two additional experiments.
In the first, we provided a longer initial training period (12 days, similar to Fig 1B) and directly compare the effects of D1 and D2 blockade using the doses used in the cocktail (Fig 3E). The different treatments yield significantly different performance effects (treatment, F(1,15) = 13.9, p < .001; treatment × session, F(1,15) = 3.68, p < .05) and significantly different recoveries (recovery, prior treatment effect, F(1,15) = 9.53, p < .01). As observed previously, D1 blockade results in an immediate decrement that is constant across the treatment days (SCH23390 treatment effect, F(1,10) = 11.45, p < .01; session, N.S.; session × treatment, N.S.), with no ffects on subsequent drug-free recovery (Fig 3E, SCH23390 prior treatment effect, F(1,10) = .124, p = .73). In contrast, D2 blockade induces a gradual deterioration across treatment days (eticlopride treatment effect, F(1,10) = 20.2, p = < .01; treatment × session, F(1,10) = 4.71, p = .055) and impaired performance and slowed recovery during drug-free recovery (Fig 3E, eticlopride prior treatment effect, F(1,10) = 21.1, p < .001; session, F(1,5) = 5.6, p = .06).
In the second experiment, we varied the number of training days prior to administering the D2 blockade (Fig 3F, 12 day group same as 3E). We observe the same pattern as in Figure 3E with no significant difference in either treatment or recovery as a consequence of different initial training lengths (treatment phase, F(1,16) = 3.02, p = .1; recovery phase, F(1,16) = .53, p = .47). The impairment and slow relearning induced subsequent to D2 blockade during recovery is more apparent with longer training periods as a consequence of having achieved higher performance during initial training; however, training under D2 blockade impairs subsequent performance independent of prior training (recovery, training length, F(1,16) = .53, p = .47; training length × session, F(1,16) = .06, p = .80) suggesting that the putatitve aberrant learning that is induced is dependent on experience during the D2 blockade and not prior skill level.
Together, the data in Figures 2-3 show a dissociation between the immediate, performance degrading effects of dopamine blockade and the subsequent, drug-free performance. These effects on subsequent behavior reflect learning and, presumably, synaptic plasticity that occurs during training under the dopamine blockade. Blockade of D1 appears to primarily induce a performance decrement with immediate recovery upon cessation of drug. In contrast, D2 blockade appears to induce an aberrant learning process that results in persistent impairment and slowed recovery in the drug-free condition.
We hypothesized that these differential effects of D1 vs D2 blockade could arise as a function of their different effects on activity and plasticity within the direct and indirect corticostriatal pathways, respective. Specifically, D1 blockade would reduce striatonigral MSN activity in the direct “GO” pathway, associated with selection of the correct actions, thus impairing performance; however, this overall decrease in activity may also protect against aberrant learning by decreasing the probability of Hebbian plasticity in the first place. In contrast, D2 blockade enhances excitability of striatopallidal MSNs in the indirect, “NOGO” pathway, increasing inhibitory activity that impairs performance. In this case, however, the increased activity may increase the probability of Hebbian plasticity (for review, Wiecki and Frank, 2010; Beeler, 2011), thus inducing aberrant learning in the striatopallidal, NOGO pathway.
Adenosine antagonists mitigate aberrant learning
While D2 activation is believed to be critical for the induction of synaptic depression (LTD) in D2-expressing MSNs, activation of A2A facilitates potentiation at these synapses (LTP) and A2A blockade prevents this potentiation (Shen et al., 2008; Lovinger, 2010; Peterson et al., 2012). We thus tested the effectiveness of A2A antagonists in mitigating the deficits induced by dopamine blockade as described above, by administering adenosine receptor antagonists during dopamine blockade.
We co-administered an A2A selective antagonist, SCH58261, with the dopamine antagonists cocktail during initial acquisition and tested recovery under drug-free conditions. SCH58261 did not rescue performance during cocktail administration (Fig 4A left; during acquisition, dose, F(1,21) = .025, p = .87, dose × session, F(1,21) = 1.34, p = .25). Performance during drug-free recovery, however, differed according to the dose of SCH58261 co-administered, though only a trend (Fig 4A/B; dose, F(1,21) = 3.01, p = .09; dose × session, F(1,21) = 1.28, p = .27). It is striking that co-administered SCH58261, despite lack of observable effects during its administration, appears to improve subsequent performance during the drug-free recovery phase; however, the response does not appear to be linearly related to dose (Fig 4B).
Figure 4. Effect of adenosine antagonists on impairment and recovery from dopamine antagonist cocktail administered during initial acquisition.
(A) Latency to fall across consecutive days/sessions for mice co-administered SCH 58261 during cocktail training (red trace, cocktail only; gray darkens with increasing SCH58261) during the initial drug treatment phase (TX) and the drug-free recovery phase (NO TX), with a 72 hour break between phases. (B) Summary of dose-dependent effects of SCH58261 co-administered during initial acquisition on subsequent drug-free recovery. Mean latency to fall averaged across sessions during the drug-free recovery phase plotted by SCH58261 dose (red bar, cocktail only). (C) Mean latency to fall averaged across all drug-free recovery sessions showing dose response for MSX-3 and theophylline co-administered with dopamine antagonists cocktail during initial acquisition (red bar, mice administered cocktail only; darker gray shades represent increasing doses. MSX-3, 1, 2.5, 5 mg/kg; SCH58261, 0.6, 1.2, 2.5, 5 10 mg/kg; theophylline, 10, 30, 60 mg/kg). (D) Summary of the average latency to fall averaged across doses and sessions during initial acquisition (black bars, dopamine antagonists cocktail + adenosine antagonist) and the subsequent drug-free recovery (gray bars) for mice co-administered either MSX-3, SCH59261 or theophylline with a cocktail of SCH23390 (0.1 mg/kg) and eticlopride (0.16 mg/kg) during initial acquisition. Latency to fall in wild-type C57BL/6 mice administered the adenosine antagonist theophylline at the specified dose either (E) during initial acquisition under a cocktail of dopamine antagonists or (F) during the recovery phase subsequent to initial training under cocktail. Red traces represent control mice receiving no theophylline. Each point represents the average of 5 trials during daily sessions with a 72 hour break between phases. (G/H) Mean performance across sessions during the recovery phase plotted by theophylline dose for mice administered theophylline during either (G) initial training under cocktail or (H) during the recovery phase. N= 4/dose, statistics reported in text. *** p < .001. Error bars, s.e.m.
We next screened two additional drugs, MSX-3, another selective A2A antagonist and theophylline, a non-selective adenosine receptor antagonist. Both drugs co-administered during training under dopamine antagonists cocktail appear to improve subsequent drug-free recovery (Fig 4C), though only theophylline is significantly different from cocktail alone (MSX-3 dose, F(1,14) = 1.65, p = .21; theophylline dose, F(1,14) = 19.65, p < .001).
In the remainder of the studies, we use the non-specific adenosine antagonist theophylline over SCH58261 for several reasons. First, although not selective between A1 and A2A, numerous studies have demonstrated that motor enhancing effects of non-selective adenosine antagonists are mediated through A2A and not A1 (Yacoubi et al., 2000; Kelsey et al., 2008; Hsu et al., 2010) Moreover, in a study using 6-OHDA lesion animal models, both the non-specific adenosine antagonist caffeine and the A2A selective SCH58261 enhanced motor function and L-DOPA efficacy following the lesion while the A1 selective antagonist CPT did not, again demonstrating that it is the A2A specific actions of caffeine (and by extension, theophylline) that are relevant (Kelsey et al., 2008). Second, theophylline is a compound that has been in clinical use for decades and there is clinical evidence that theophylline has therapeutic efficacy in PD (Mally and Stone, 1996; Kostic et al., 1999; Kulisevsky et al., 2002), making it more relevant to potential clinical studies. Finally, theophylline is water-soluble and does not require DMSO vehicle. In some cases, we administer treatments over a period of weeks and wanted to avoid the potential confound of chronic injections of DMSO. Thus, we chose theophylline to investigate the amelioration of aberrant learning.
Theophylline diminishes aberrant learning during dopamine blockade but impairs performance when administered during recovery
During initial acquisition, theophylline had little effect on the performance impairment induced by the D1/D2 antagonist cocktail (Fig. 4E, left; dose main effect, F(1,22) = .90, p = .35). However, during the drug-free recovery phase (neither cocktail nor theophylline administered), a dose-dependent improvement in recovery was observed (Fig. 4E/G; dose main effect, F(1,22) = 3.4, p < .07) similar to that observed with SCH58261 (Fig 4A), suggesting that theophylline diminished the putative aberrant learning that occurred during acquisition under conditions of dopamine blockade. In contrast, theophylline administered during the recovery phase, with the exception of the lowest dose, impairs recovery (Fig. 4F; dose main effect, F(1,22) = 10.4, p < .01), showing a dose-response curve that reflects almost the inverse mirror (Fig. 4H) of that observed when theophylline is administered during acquisition (Fig. 4G).
Theophylline protects established skills
We then tested whether theophylline modified the effects of dopamine receptor blockade on established motor skills by first training mice to asymptotic performance under normal conditions (ie., no dopamine manipulation). Then eticlopride was administered to induce a degradation in performance and putative aberrant learning (as in Fig. 3B/E/F) either with or without co-administration of theophylline. In mice that received only eticlopride, we observe the same gradual deterioration in performance and gradual recovery observed above (Fig. 5A). In contrast, in mice that received co-administered theophylline, the performance impairment induced by D2 blockade did not show gradual deterioration but rather an immediate decrement that remained constant across sessions (Fig. 5A; during treatment, all groups, session × dose, F(1,22) = 13.1, p < .01; each group separately, session was only significant for saline group, F(1,4) = 12.33, p = .039, all SCH doses, session N.S.). Critically, upon discontinuation of the eticlopride and theophylline, the mice that had received theophylline showed no subsequent impairment in performance (Fig. 5A/C; dose main effect on recovery, all groups, F(1,22) = 8.18, p < .01; theophylline treated only, F(1,17) = 1.58, p = .227;), indicating that theophylline, though not eliminating the direct performance impairment induced by eticlopride, did effectively block aberrant learning protecting established skills.
Figure 5. Effect of adenosine antagonist theophylline on eticlopride induced aberrant learning and recovery of an established skill.
(A) Latency to fall in wild-type C57BL/6 mice co-administered the A2A antagonist theophylline at the specified dose together with the D2 antagonist eticlopride (0.16 mg/kg) after initial drug free training to asymptotic performance (ie., established skill). (B) Latency to fall in mice administered either theophylline at 80 mg/kg or saline for 5 days initial acquisition. (C) Mean latency to fall across sessions during the different phases of the experiment. N= 4/dose, statistics reported in text. Error bars, s.e.m.
Adenosine antagonism has no effect on initial acquisition under normal conditions
When applied during initial acquisition without dopamine blockade, theophylline has no effect on learning and performance (Fig. 5B). These data suggests a potential reduction in striatopallidal LTP arising from A2A blockade does not impair initial acquisition. Learning in the striatonigral pathway may compensate for reduced LTP in the indirect pathway in de novo learning; however, once aberrant learning is established, a full range of plasticity is apparently required to ‘unlearn’ it and implement appropriate learning.
D2 blockade induces potentiation in striatopallidal MSNs that is diminished by theophylline
Corticostriatal LTD, believed to be critical for behavior and motor execution, is facilitated by activation of D2 receptors (Gerdeman et al., 2002; Calabresi et al., 2007; Kreitzer and Malenka, 2008; Shen et al., 2008; Lovinger, 2010). Loss of D2 activation, such as occurs with dopamine denervation or depletion, blocks high-frequency stimulation induced LTD (Calabresi et al., 1997; Gerdeman et al., 2002; Kreitzer and Malenka, 2007; Bagetta et al., 2011). However, increasing evidence suggest that dopamine denervation not only impairs LTD but inverts it such that conditions that would normally induce LTD (ie., high-frequency stimulation or spike-timing dependent LTD) instead induce LTP (Calabresi et al., 1997; Picconi et al., 2003; Shen et al., 2008; Peterson et al., 2012). These studies provide compelling evidence that loss of dopamine signaling at D2 receptors can invert corticostriatal plasticity in the striatopalidal pathway, which we propose underlies aberrant learning. Therefore, we tested the effects of D2 blockade on the strength of excitatory cortical inputs to stratopallidal MSNs.
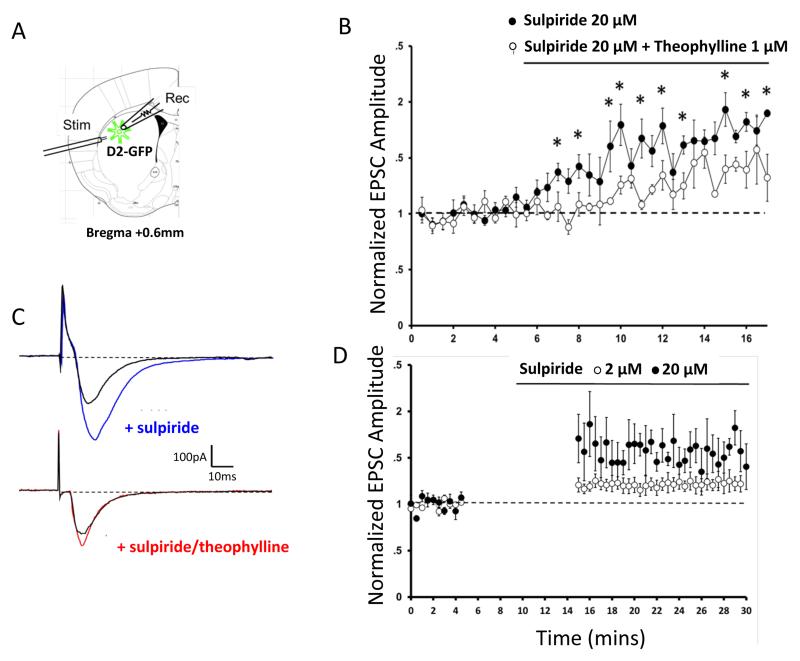
In the presence of bath applied sulpride (20 μM), we observed a gradual increase in the strength of glutamatergic input to D2-expressing MSNs, without any stimulation protocol (Fig 6B and C). As the measurement of EPSCs itself constitutes a low-frequency stimulation, for a subset of cells we witheld stimulation for 10 min during the sulpiride administration and applied two doses of sulpiride, 2 and 20 μM. In this subset of cells, we observe dose-dependent potentiation in the absence of any exogenously applied stimulation (Fig 6D). Previous reports demonstrate that LTP in the striatopallidal pathway is facilitated by activation of the A2A adenosine receptor and can be blocked by A2A antagonism (Schiffmann et al., 2003; Shen et al., 2008; Peterson et al., 2012). We then tested whether D2 blockade induced potentiation is sensitive to A2A antagonism. Bath application of theophylline (1 μM) in combination with sulpiride reduced the potentiation seen with sulpiride alone (Fig 6B and C).
Figure 6. D2 blockade potentiates excitatory inputs to striatopallidal MSNs, which is reduced by theophylline.
A. D2-GFP medium spiny neurons in the dorsolateral striatum were held in voltage clamp (Vm=−70mV; Rec = Recording electrode). Stimulating electrodes (Stim) were placed near the corpus callosum, which allowed stimulation of corticostriatal evoked excitatory synaptic currents (EPSCs) at 30s intervals. B. After baseline EPSC amplitude was established, bath application of the D2 receptor antagonist sulpiride potentiated evoked EPSC’s (20 μM; filled symbols; N=4). Co-application of the adenosine antagonist theophylline (1 μM) significantly attenuated the effects of sulpiride on EPSC amplitude (open symbols; N=6). C. Example traces from each recording condition. D. In a separate set of experiments, bath application of sulpiride still potentiated evoked EPSC’s in the absence of any stimulation (solid symbols, N=5). Similar effects were observed with administration of 2 μM sulpiride (open symbols, N=3). *p<0.05. Error bars, s.e.m.
These data suggest that D2 blockade induces potentiation in striatopallidal MSNs, a process that may contribute to the increased responsiveness observed in indirect pathway MSNs after dopamine denervation (Mallet et al., 2005; Gertler et al., 2008; Peterson et al., 2012). These data are consistent with previous reports showing that dopamine denervation or loss of D2 signaling can induce LTP instead of LTD (Calabresi et al., 1997; Picconi et al., 2003; Shen et al., 2008; Peterson et al., 2012), suggesting that dopamine denervation and subsequent decreases in D2 signaling favor LTP in the corticostriatal synapses in the indirect pathway and increase inhibitory tone modulating cortical activity. Enhanced excitatory drive onto striatopalidal MSNs under these conditions is consistent with the proposed mechanism underlying the aberrant learning observed behaviorally. The decrease in D2-blockade induced potentiation observed with application of theophylline, together with reports that A2A antagonism can impede striatopallidal potentiation (Schiffmann et al., 2003; Shen et al., 2008; Peterson et al., 2012), is a likely explanation for the partial protection from aberrant learning observed in our behavioral studies.
Modeling rotarod skill learning
Adapting a previously published basal ganglia (BG) model (Frank, 2005; Wiecki et al., 2009), we simulated the demands of the rotarod task by assuming that the mice have to select between four possible motor outputs (R1-R4, e.g., which paw to move forward) depending on the sensory state. Motor tasks like the rotarod are dynamic and integrative, such that the correct action needed in response to a particular sensory state (e.g., position on the rod) may depend on the identity of another variable (e.g., proprioceptive input). We simulated this type of task by including two sets of inputs, each having two possible stimuli (ie., SA1 SA2; SB1, SB2). The “correct” motor actions (i.e. those that would prevent the animal from falling off the rod) were dependent on conjunctive combinations of the two sets. For example, if SA1 is present, then SB1 should be associated with R1 and SB2 with R2. If SA2 is present, then SB1 should be associated with R3 and SB2 with R4. We adopted this input-output structure to capture the integrative stimulus-response learning attributed to the dorsal striatum. The different sets of inputs to be integrated could potentially represent context and discrete stimuli, information from different sensory modalities (visual, proprioceptive, vestibular), different coordinate systems (eg., position on rod as medial/lateral and forward/backward) and so on. The requirement to integrate on-going stimuli to determine a complex state and the appropriate response more realistically reflects the sensorimotor integration required in the rotarod task. To simulate the acceleration of the rotarod, we restricted the amount of time that the model had to select a response, such that with each correct action the time limit was decreased, and then reset with each. The model is described briefly in Fig. 7F and detailed description and equations are provided in Supplemental Materials.
Figure 7. Model performance under conditions recapitulating mouse experiments.
(A) shows percentage of correct responses by the model intact (open red symbols) and with dopamine blockade (filled gray triangles) and subsequent recovery with dopamine activity restored (open gray triangles). Each point represents the average of four trials. (B) shows the effect of blocking either the D1/GO layer (filled triangles) or the D2/NOGO layer (filled squares) subsequent to initial intact learning and subsequent recovery when dopamine function is restored (open symbols). (C) the model was trained under dopamine blockade with a reduced learning rate in the D2/NOGO layer (learning set to ½, light gray, set to 0, dark gray) to simulate A2A antagonism either during initial acquisition under dopamine blockade (triangles) or during dopamine restored recovery (squares). (D) Initial model learning and performance under normal dopamine function with (gray symbols) and without (red symbols) D2/NOGO learning rate reduced to 0 to simulate A2A antagonism. (E) Bar graph showing average performance across epochs during the recovery phase grouped by time (during acquisition or recovery) and degree of reduction in D2/NOGO learning rate (ie., ‘theophylline’). (F) Schematic of basal ganglia neurocomputational model. The basal ganglia model includes layers incorporating the direct (GO) and indirect (NOGO) pathways from cortex (2 input layers) through the striatum (GO and NOGO units), to the globus pallidus externa (GPe), the substantia nigra reticulata/globus pallidus interna (GPi), the thalamus, the premotor cortex (PMC) to the output, or motor cortex. SNc dopamine neurons project to both the GO and NOGO layers of the striatum simulating projections to D1 and D2 MSNs (GO/NOGO, respectively, see Supplemental Materials for detailed description). Fast-spiking inhibitory interneurons (not shown) regulate activity in both striatal populations via feed-forward inhibition. At each trial, the network is presented input from each of two input layers (raised cylinders represent example unit activity). Premotor cortical (PMC) units representing the four candidate responses then become noisily activated. Under baseline conditions, the thalamus is inhibited, but a response is selected once a thalamic unit becomes disinhibited and amplifies activity in the corresponding motor units. The role of the BG is to modulate activity in the thalamus according to whether the responses are adaptive in the current sensory state. Each phase of the experiment (ie., analogous to with or without administered drugs, as in the mouse studies) consisted of 20 epochs, equivalent to sessions. Each epoch contained four trials. Model performance is reported as percentage of correct responses. Each data point is the average performance of 20 models initialized with different random weights (ie., N = 20).
Modeling dopamine effects on performance and learning
Dopamine modulates the balance of activity and plasticity in the direct (GO) and indirect (NOGO) pathways. Increased dopamine excites the D1-expressing GO pathway and inhibits the D2-expressing NOGO pathway, whereas decreased dopamine has the opposite effect. Dopamine also modulates learning. Following a correct response, phasic increases modulate plasticity differently in the direct, striatonigral GO and the indirect, striatopallidal NOGO pathways. Increased phasic dopamine activates D1 and D2, increasing (LTP) and decreasing (LTD) synaptic weights in the direct and indirect pathways, respectively, in proportion to their activity, thereby facilitating (GO LTP) and disinhibiting (NOGO LTD) that response in future presentations of the same stimuli. The net effect is to drive activity-dependent plasticity, so that weights from the active inputs to GO units associated with rewarding actions are increased while diminishing the NOGO activity associated with those same inputs. When the network selects an erroneous response, phasic dips of dopamine induce the reverse learning process such that the weights in the indirect, striatopallidal NOGO pathway are strengthened (LTP) reducing the likelihood of repeating the error in the future. .
These performance and learning effects are interactive. For example, D2 blockade enhances the excitability of NOGO units, and hence their propensity for activity-dependent plasticity. This effect manifests itself such that even if adaptive actions are selected, the greater NOGO activity arising from D2 blockade drives inhibitory learning in response to the current sensory states but does so as if there had been a dip in phasic dopamine activity. Thus, even after D2 blockade is removed, NOGO activity is associated with correct responses and impairs performance.
Simulating pharmacological manipulations
Pharmacological blockade of D1 or D2 were directly and independently mimicked in the model by reducing the efficacy with which dopamine modulates the excitatory D1-GO projections or the inhibitory D2-NOGO projections to approximately 65% of the intact values (Supplemental Materials). As the partial protection against aberrant learning observed above is hypothesized to arise from an A2A blockade that diminishes plasticity in the indirect, D2-expressing pathway, we examined whether the effects of theophylline could be recapitulated soley by reducing or eliminating plasticity in the corticostriatal projections to D2 units. The model, then, tests whether plasticity in the indirect pathway alone can account for the behavioral findings.
Model recapitulates effects of dopamine blockade on learning and performance
Figure 7A shows that the intact model can robustly learn the conjunctive associations and select correct actions R1-R4. As expected, simulated D1/D2 blockade results in the same severe performance deficit observed in the mouse studies (Fig. 7A, left). However, we hypothesized that it would also drive aberrant learning as described above. Indeed, upon removal of the blockade, recovery is gradual (slower than that for intact models during initial acquisition) and does not fully recover (Fig. 7A, middle).
We next examined whether the model can account for performance if dopamine blockade is applied after asymptotic performance, independently for D1 and D2 receptors (Fig. 7B). We observed that initial application of D2 blockade after learning was associated with relatively preserved motor performance that then gradually declined, as observed with mice. With D1 blockade, there is less impairment and, unlike in the mice, it does show a small gradual decline, though not as pronounced as D2 blockade. However, upon removal of D1 blockade, performance returned immediately to prior asymptotic levels whereas with D2 blockade a gradual relearning was required, consistent with behavioral observations.
The model recapitulates the pharmacological data because D2 blockade enhances activity in the NOGO units and induces synaptic potentiation (LTP) even following correct responses that would normally induce depression (LTD), driving aberrant learning. Once indirect pathway inhibition has been learned through inappropriate LTP, it needs to be unlearned for recovery to occur even when dopamine is restored. In contrast, D1 blockade, although it prevents the network from selecting the correct response due to reduced facilitation from GO units, does not significantly induce an aberrant learning process because D1 blockade reduces activity and lowers the propensity for activity-dependent plasticity, thus minimizing aberrant learning. Thus, in contrast to D2 blockade, removal of D1 blockade allows the network to express its previously learned adaptive weights.
Can blocking plasticity in the D2 pathway mimic theophylline administration?
Finally, we tested whether plasticity in the indirect, NOGO pathway can account for the differential effects of theophylline when the drug is applied during acquisition or recovery. Specifically, as A2A antagonism can diminish striatopallidal LTP under at least some conditions (Schiffmann et al., 2003; Shen et al., 2008; Peterson et al., 2012), we modeled theophylline as a reduction of plasticity in the striatopallidal NOGO units.
Consistent with behavioral observations, simulated A2A antagonism (blocking corticostriatal NOGO plasticity) during initial acquisition did not rescue performance during simultaneous dopamine blockade (Fig. 7C, left). However, blockade of NOGO plasticity did improve subsequent recovery when dopamine blockade was removed, reducing aberrant learning (Fig. 7C/E). Moreover, subsequent recovery speed was now in the same range as that of intact models during initial learning (compare with previous Fig. 7A) and better than recovery without NOGO blockade of plasticity (Fig. 7C).
In the mouse studies, theophylline applied during the recovery phase rather than during acquisition impaired rather than improved recovery. The same effect was observed in the model (Fig. 7C/E). Reducing or blocking plasticity in the NOGO pathway during recovery impairs the model’s ability to (a) learn which actions should be suppressed because they are maladaptive and (b) ‘unlearn’ inhibitory, NOGO weights inappropriated associated with correct actions. Finally, as with the mice, blocking plasticity in the NOGO pathway had little effect during initial acquisition under normal conditions (ie., no dopamine blockade, Fig. 7D), suggesting in the naive state, correct stimulus-action associations can be learned in the GO pathway, suggesting that aberrant learning and plasticity in the indirect pathway (ie., inappropriate LTP) is more deleterious than a simple deficit of plasticity.
Unlike experiments with animals and humans, where it is difficult to isolate potential mechanisms and substrates, in a computational model we have complete control. Using an a priori model of the basal ganglia that has been applied to various human and animal datasets, we show here that the aberrant learning observed in mice can be accounted for specifically by alterations in plasticity in the indirect, striatopallidal pathway. As this model was not developed specifically for these studies nor to test the aberrant learning hypothesis, it is notable that the model recapitulates so closely the array of behavioral phenomena observed.
DISCUSSION
Corticostiatal throughput is modulated through two main pathways. Activity in the D1-expressing direct, striatonigral “GO” pathway favors disinhibition of cortical activity and facilitates behavioral throughput. Activity in the D2-expressing, indirect striatopallidal “NOGO” pathway, in contrast, favors inhibition of cortical activity and inhibits behavioral throughput (Albin et al., 1989; Alexander et al., 1990; Mink, 1996). Dopamine shifts the balance between these two pathways such that increased dopamine increases the responsiveness of the GO pathway via D1 activation while simultaneously decreasing the influence of the NOGO pathway via activation of D2. Conversely, diminished dopamine will favor the inhibitory NOGO pathway due to greater activity in D2-expressing MSNs as a consequence of less activation of D2. Much evidence supports this dual pathway model in controlling motor performance in rodents (Hikida et al., 2010; Kravitz et al., 2010).
Dopamine also modulates corticostiatal plasticity (Calabresi et al., 1992a; 1992b; Kreitzer and Malenka, 2008; Surmeier et al., 2009; Wickens, 2009; Lovinger, 2010) in both the striatonigral GO and striatopallidal NOGO pathways, further influencing motor performance through learning. Corticostriatal plasticity can enhance or diminish the responsiveness of either pathway to cortical input, selectively facilitating the expression or inhibition of specific responses and motor skills. Our previous studies suggest that this adaptive plasticity is altered in the D2 pathway under conditions of dopamine depletion, blockade or denervation (Wiecki et al., 2009; Beeler et al., 2010; Wiecki and Frank, 2010; Beeler, 2011), giving rise to an aberrant learning that selectively encodes inappropriate inhibition through experience-dependent synaptic changes that impede future motor responses even if dopamine is restored (ie., such as in L-DOPA treatment).
We propose that the mechanism that underlies aberrant learning is altered corticostriatal plasticity in the indirect, striatopallidal pathway that favors synaptic potentiation (LTP) at the expense of synaptic depression (LTD), inappropriately increasing the responsiveness of striatopallidal MSNs to cortical input and pathologically increasing behavioral inhibition. Substantial evidence supports this proposed mechanism. In mice lacking D2 receptors, the same high-frequency stimulation protocol (HFS) that induces LTD instead induces LTP (Calabresi et al., 1997). In 6-OHDA lesioned rats, a model of PD, HFS also induces LTP rather than the normal LTD (Picconi et al., 2003), though Kreitzer and Malenka (Kreitzer and Malenka, 2007) in a similar study observed only a loss of LTD and not its inversion to LTP. This reason for this discrepancy is unclear, though subsequent reports confirm the inversion of plasticity under dopamine denervation and further suggest the abnormal LTP is dependent upon A2A activation (Shen et al., 2008; Peterson et al., 2012). Here, we show that in the absence of HFS, D2 blockade induces potentiation in striatopallidal synapses that is diminished by administration of an adenosine antagonist that blocks A2A, consistent with recent studies and with the observed ameliorative effects of theophylline administered during dopamine blockade. Taken together, the present data and published studies strongly support the hypothesis that D2 blockade (and dopamine denervation/depletion) shift striatopallidal plasticity to inappropriately favor LTP. By favoring and increasing synaptic potentiation at the expense of depression, dopamine blockade/denervation increases the learned responsiveness of striatopallidal neurons to afferent input, consequently enhancing inhibitory tone on cortical activity, as proposed to underlie aberrant learning (Wiecki et al., 2009; Beeler, 2011). In short, dopamine denervation is widely believe to induce an imbalance between the direct and indirect pathways; here we suggest that altered corticostriatal plasticity contributes a learned component to this imbalance: inappropriate inhibition structurally embedded as physical changes in synapses. Importantly, though pharmacological treatment may both reverse the imbalanace in activity between the direct and indirect pathways and restore normal plasticity, structural changes arising from aberrant learning can only be reversed through further structural changes; that is, relearning.
We attribute the partial protection against aberrant learning afforded by theophylline to its actions on postsynaptic A2A receptors. Though theophylline is non-selective adenosine antagonist, several studies have demonstrated the motor improving effects of non-selective adenosine antagonists are mediated through their actions on A2A, not A2A but not A1 selective compounds yield the same results (Yacoubi et al., 2000; Kelsey et al., 2008; Hsu et al., 2010). Moreover, published studies have demonstrated that A2A blockade can decrease potentiation in striatopallidal MSNs (Shen et al., 2008; Peterson et al., 2012), consistent witht the electrophysiological data obtained here. As A2A is expressed both postsynaptically on striatopallidal MSNs as well as presynaptically on glutamatergic terminals, it is possible that the effects of theophylline, even on A2A receptors, is mediated pre-rather than post-synaptically. Indeed, Calabresi and colleagues have demonstrated that A2A antagonist (though notably only in combination with D2 agonist) can reduce glutamatergic transmission through a pre-synaptic mechanism (Tozzi et al., 2007); however, Quiroz et al (Quiroz et al., 2009) have shown that the A2A receptor is expressed presynaptically only on cortical afferents synapsing on striatonigral MSNs, suggesting that effects of A2A antagonist on striatopallidal cells will be mediated exclusively through post-synaptic A2A receptors. Finally, the model suggests that reducing synaptic plasticity in the striatopallidal pathway can account for and recapitulate the theophylline effects, further supporting a striatopallidal, post-synaptic mechanism underlying the partial protection from aberrant learning conferred by theophylline. Nonetheless, it should be borne in mind that theophylline is not A2A selective, leaving open the possibility that other actions in addition to A2A antagonism may contribute to its ameliorative effects.
The pharmacological model used here captures one aspect of PD, the reduction in activation of D1 and D2 that occurs as a consequence of denervation. The simplicity of the model, the reversibility of pharmacological blockade together with ability to block D1 and D2 individually represents strengths that facilitated the present studies, which would have been intractable in the more traditional 6-OHDA model of PD. We show that impaired dopamine signaling induces aberrant learning. By necessity, our model induces acute, temporary decreases in dopamine signaling. The role such aberrant learning plays in PD is likely to be complex and complicated by adaptation to chronic denervation; however, in a previous study using PITx3-deficient mice that exhibit a 90% loss of dopamine denervation in the dorsal striatum from birth and show physiological adaptations characteristic of PD, we observe a similar aberrant learning (Beeler et al, 2010). We have advanced the hypothesis that the poorly understood long-duration response (LDR) to L-DOPA (Muenter and Tyce, 1971; Anderson and Nutt, 2011) arises as a correction of aberrant learning (Beeler et al., 2010; Beeler, 2011). In this view, under progressive dopamine denervation, patients take a ‘double hit’ in that declining dopamine induces direct motor performance deterioration but also, through abnormal corticostriatal plasticity and aberrant learning, unravels previously established learning, essentially inverting it to favor inhibition rather than facilitation of movement. The short-duration response (SDR) of L-DOPA, then, reflects the correction of direct motor performance deficits induced by dopamine depletion and lasts only as long as L-DOPA is present (Nutt et al., 1997). In contrast, by correcting underlying abnormal corticostriatal plasticity, L-DOPA restores normal learning and skill building. This corrective aspect of L-DOPA treatment is cumulative and retained as the appropriate calibration of millions of synaptic strengths that endures during trough periods of medication-- or even on discontinuation of treatment, until aberrant learning reverses this learning and inappropriate synaptic strengths again predominate.
This view suggests a novel therapeutic strategy of targeting pathways that mediate abnormal corticostriatal plasticity in the D2-expressing, indirect pathway; in essence, seeking to effect an LDR-like therapeutic independent of an SDR-like effect. The theophylline studies described here suggest this strategy may be feasible. Theophylline improved recovery in a dose-dependent manner when administered during putative aberrant learning under dopamine blockade, suggesting this intervention partially mitigated aberrant learning. In contrast, when administered subsequent to aberrant learning, it did not facilitate performance; indeed, at most doses it appeared to slow recovery. These data would suggest that A2A antagonists are likely to have limited therapeutic efficacy in ameliorating the direct effects of dopamine denervation on performance, ie., limited SDR-like actions. However, A2A antagonists may mitigate underlying abnormal corticostriatal plasticity in the D2-expressing indirect pathway and diminish aberrant learning, as observed here, inducing an LDR-like therapeutic efficacy. Importantly, from a clinical perspective, the A2A antagonism had no effect on learning under normal conditions (ie., without prior aberrant learning). These observations may suggest alternative perspectives on the clinical potential of A2A antagonists, including their use early in the disease process to preserve established skills and slow aberrant learning, though more investigation is necessary.
The present observations have implications for rehabilitative approaches to treating PD (Abbruzzese et al., 2009; Keus et al., 2009; Nieuwboer et al., 2009). Rehabilitation protocols are, at their core, based on repetition and practice. That dopamine depletion induces experience-dependent aberrant learning that impedes performance would suggests that any skill practice occurring during states of low medication (ie., medication troughs, holidays) might actually induce deterioration of skills. In contrast, practice occurring during peak medication would maximally engage corrected, optimizing learning mechanisms. In short, rehabilitative treatments may potentially enhance or diminish the LDR of L-DOPA treatment depending upon when they are administered. The ‘use it or lose it’ strategy (Archer et al., 2011) may depend critically on timing.
Evidence suggest that aberrant learning may precede frank motor symptoms of PD (see Beeler, 2011 for review). As different territories of the striatum are differentially affected as denervation progresses, it is possible that a process of denervation -> aberrant learning -> compensation -> failure of compensation is recapitulated across striatal territories at different time-courses. Thus, aberrant learning may play an important role in the development of cognitive impairments in PD (Wiecki and Frank, 2010). Consequently, an ‘LDR-like’ treatment targeting aberrant learning may be useful in ameliorating or delaying the development of cognitive symptoms.
Supplementary Material
Highlights.
Dopamine blockade induces aberrant learning impairing future motor performance
Aberrant learning is mediated by the D2-expressing striatopallidal pathway
A2A antagonism protects against aberrant learning but impairs recovery
D2 blockade induces potentiation at striatopallidal corticostriatal synapses
ACKNOWLEDGEMENTS
This work was support by NIDA, DA25875 (JB) and NINDS R21NS070269 (XZ). We would like to acknowledge the contribution of Zhen Fang Huang Cao, Jessica Koranda and Mari Murakami to developing the rotarod method and strategy and Carrie Swetlik for assistance with additional behavior testing. We thank Giselle Petzinger for insightful feedback on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbruzzese G, Trompetto C, Marinelli L. The rationale for motor learning in Parkinson’s disease. Eur J Phys Rehabil Med. 2009;45:209–214. [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Anderson E, Nutt J. The long-duration response to levodopa: phenomenology, potential mechanisms and clinical implications. Parkinsonism Relat Disord. 2011;17:587–592. doi: 10.1016/j.parkreldis.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Archer T, Fredriksson A, Johansson B. Exercise alleviates Parkinsonism: clinical and laboratory evidence. Acta Neurol Scand. 2011;123:73–84. doi: 10.1111/j.1600-0404.2010.01360.x. [DOI] [PubMed] [Google Scholar]
- Bagetta V, Picconi B, Marinucci S, Sgobio C, Pendolino V, Ghiglieri V, Fusco FR, Giampà C, Calabresi P. Dopamine-dependent long-term depression is expressed in striatal spiny neurons of both direct and indirect pathways: implications for Parkinson’s disease. J Neurosci. 2011;31:12513–12522. doi: 10.1523/JNEUROSCI.2236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA. Preservation of function in Parkinson“s disease: what”s learning got to do with it? Brain Res. 2011;1423:96–113. doi: 10.1016/j.brainres.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, Cao ZFH, Kheirbek MA, Ding Y, Koranda J, Murakami M, Kang UJ, Zhuang X. Dopamine-dependent motor learning: insight into levodopa’s long-duration response. Ann Neurol. 2010;67:639–647. doi: 10.1002/ana.21947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Bódi N, Keri S, Nagy H, Moustafa A, Myers CE, Daw N, Dibó G, Takáts A, Bereczki D, Gluck MA. Reward-learning and the novelty-seeking personality: a between- and within-subjects study of the effects of dopamine agonists on young Parkinson’s patients. Brain. 2009;132:2385–2395. doi: 10.1093/brain/awp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J. Neurosci. 1992a;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term Potentiation in the Striatum is Unmasked by Removing the Voltage-dependent Magnesium Block of NMDA Receptor Channels. Eur J Neurosci. 1992b;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Saiardi A, Pisani A, Baik JH, Centonze D, Mercuri NB, Bernardi G, Borrelli E. Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J. Neurosci. 1997;17:4536–4544. doi: 10.1523/JNEUROSCI.17-12-04536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Cohen D, Nicolelis MAL. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. 2004;14:1124–1134. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehéricy S, Benali H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cogn Neurosci. 2005;17:51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O’Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Chemical neuroanatomy of the basal ganglia--normal and in Parkinson’s disease. J Chem Neuroanat. 2001;22:3–12. doi: 10.1016/s0891-0618(01)00100-4. [DOI] [PubMed] [Google Scholar]
- Hsu CW, Wang CS, Chiu TH. Caffeine and a selective adenosine A2A receptor antagonist induce sensitization and cross-sensitization behavior associated with increased striatal dopamine in mice. J. Biomed. Sci. 2010;17:4. doi: 10.1186/1423-0127-17-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Costa RM. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature. 2010;466:457–462. doi: 10.1038/nature09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey JE, Langelier NA, Oriel BS, Reedy C. The effects of systemic, intrastriatal, and intrapallidal injections of caffeine and systemic injections of A2A and A1 antagonists on forepaw stepping in the unilateral 6-OHDA-lesioned rat. Psychopharmacology (Berl) 2008;201:529–539. doi: 10.1007/s00213-008-1319-0. [DOI] [PubMed] [Google Scholar]
- Keus SHJ, Munneke M, Nijkrake MJ, Kwakkel G, Bloem BR. Physical therapy in Parkinson’s disease: evolution and future challenges. Mov Disord. 2009;24:1–14. doi: 10.1002/mds.22141. [DOI] [PubMed] [Google Scholar]
- Kostic VS, Svetel M, Sternic N, Dragasevic N, Przedborski S. Theophylline increases “on” time in advanced parkinsonian patients. Neurology. 1999;52:1916. doi: 10.1212/wnl.52.9.1916. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulisevsky J, Barbanoj M, Gironell A, Antonijoan R, Casas M, Pascual-Sedano B. A double-blind crossover, placebo-controlled study of the adenosine A2A antagonist theophylline in Parkinson’s disease. Clin Neuropharmacol. 2002;25:25–31. doi: 10.1097/00002826-200201000-00005. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci. 2005;25:3857–3869. doi: 10.1523/JNEUROSCI.5027-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mally J, Stone TW. Potential role of adenosine antagonist therapy in pathological tremor disorders. Pharmacol Ther. 1996;72:243–250. doi: 10.1016/s0163-7258(96)00119-2. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res. 2002;146:122–126. doi: 10.1007/s00221-002-1213-7. [DOI] [PubMed] [Google Scholar]
- Muenter M, Tyce G. L-dopa therapy of Parkinson’s disease: plasma L-dopa concentration, therapeutic response, and side effects. Mayo Clinic Proceedings Mayo Clinic. 1971 [PubMed] [Google Scholar]
- Nieuwboer A, Rochester L, Müncks L, Swinnen SP. Motor learning in Parkinson’s disease: limitations and potential for rehabilitation. Parkinsonism Relat Disord. 2009;15(Suppl 3):S53–S58. doi: 10.1016/S1353-8020(09)70781-3. [DOI] [PubMed] [Google Scholar]
- Nutt J, Carter J, Van Houten L. Short- and long-duration responses to levodopa during the first year of levodopa therapy. Annals of …. 1997 doi: 10.1002/ana.410420311. [DOI] [PubMed] [Google Scholar]
- Palminteri S, Lebreton M, Worbe Y, Grabli D, Hartmann A, Pessiglione M. Pharmacological modulation of subliminal learning in Parkinson“s and Tourette”s syndromes. Proc Natl Acad Sci USA. 2009;106:19179–19184. doi: 10.1073/pnas.0904035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JD, Goldberg JA, Surmeier DJ. Adenosine A2a receptor antagonists attenuate striatal adaptations following dopamine depletion. Neurobiol Dis. 2012;45:409–416. doi: 10.1016/j.nbd.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Håkansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6:501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, Knowlton BJ. The neural correlates of motor skill automaticity. J Neurosci. 2005;25:5356–5364. doi: 10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttemans V, Wenderoth N. Changes in brain activation during the acquisition of a multifrequency bimanual coordination task: from the cognitive stage to advanced levels of automaticity. The Journal of …. 2005 doi: 10.1523/JNEUROSCI.3866-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz C, Luján R, Uchigashima M, Simoes AP, Lerner TN, Borycz J, Kachroo A, Canas PM, Orru M, Schwarzschild MA, et al. Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. Scientific World Journal. 2009;9:1321–1344. doi: 10.1100/tsw.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, Dassesse D, d’Alcantara P, Ledent C, Swillens S, Zoli M. A2A receptor and striatal cellular functions: regulation of gene expression, currents, and synaptic transmission. Neurology. 2003;61:S24–S29. doi: 10.1212/01.wnl.0000095207.66853.0d. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Plotkin J, Shen W. Dopamine and synaptic plasticity in dorsal striatal circuits controlling action selection. Curr Opin Neurobiol. 2009;19:621–628. doi: 10.1016/j.conb.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Pawlak AP, Prokopenko V, West MO. Changes in activity of the striatum during formation of a motor habit. Eur J Neurosci. 2007;25:1212–1227. doi: 10.1111/j.1460-9568.2007.05353.x. [DOI] [PubMed] [Google Scholar]
- Tozzi A, Tscherter A, Belcastro V, Tantucci M, Costa C, Picconi B, Centonze D, Calabresi P, Borsini F. Interaction of A2A adenosine and D2 dopamine receptors modulates corticostriatal glutamatergic transmission. Neuropharmacology. 2007;53:783–789. doi: 10.1016/j.neuropharm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Wickens JR. Synaptic plasticity in the basal ganglia. Behav Brain Res. 2009;199:119–128. doi: 10.1016/j.bbr.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ. Neurocomputational models of motor and cognitive deficits in Parkinson’s disease. Prog Brain Res. 2010;183:275–297. doi: 10.1016/S0079-6123(10)83014-6. [DOI] [PubMed] [Google Scholar]
- Wiecki TV, Riedinger K, Ameln-Mayerhofer, von A, Schmidt WJ, Frank MJ. A neurocomputational account of catalepsy sensitization induced by D2 receptor blockade in rats: context dependency, extinction, and renewal. Psychopharmacology (Berl) 2009;204:265–277. doi: 10.1007/s00213-008-1457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoubi, El M, Ledent C, Ménard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilário MRF, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.