Cilia and flagella play important roles in human health by contributing to cellular motility as well as sensing and responding to environmental cues. Motile cilia (or flagella) provide both motion and sensing functions, for example to move mucus along the trachea in respiratory epithelial cells (Figure 1), to provide left-right asymmetry in the embryonic node, or for sperm motility. Non-motile cilia (otherwise known as primary cilia, Figure 1) house multiple signaling pathways necessary for normal cellular homeostasis and development [1, 2]. These signaling molecules allow for efficient olfaction in olfactory neurons, photoreception in photoreceptor cells, mechanosensing of fluid flow in kidney epithelial cells, and response to various extracellular signals including Hedgehog, Wnt and platelet-derived growth factor (PDGF) ligands. Due to their many roles in cell and developmental biology, defects in ciliary assembly and/or function can lead to a range of human diseases, known collectively as the ciliopathies, including polycystic kidney, liver, and pancreatic diseases, sterility, obesity, situs inversus, hydrocephalus, and retinal degeneration [2, 3]. A basic understanding of how cilia form and function is essential for deciphering ciliopathies and generating therapeutic treatments.
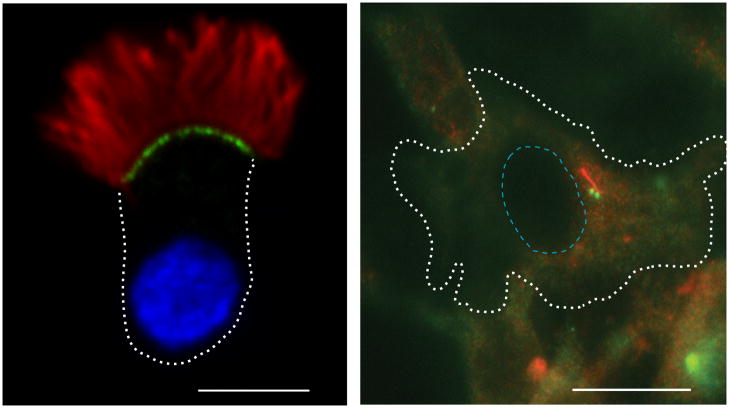
Figure 1. Motile and primary cilia.
Trachea epithelial cells contain multiple motile cilia (left panel) whereas cultured fibroblast cells contain a single primary cilium (right panel). Rat trachea epithelial cells (left) and cultured mouse NIH3T3 cells (right) were fixed and stained with antibodies to acetylated α-tubulin (red) to mark the axonemal microtubules and γ-tubulin (green) to mark the centrioles. White dashed line indicates the periphery of the cell. The nucleus is indicated by DAPI staining (blue, left panel) or a blue dotted line (right panel). Scale bars, 5 μm (left) and 10 μm (right).
The cilium is a unique compartment that contains a distinct complement of protein and lipid [4]. However, the molecular mechanisms by which soluble and membrane protein components are targeted to and trafficked into the cilium are not well understood. Cilia have no protein synthesis machinery, therefore all protein components for the cilium have to be synthesized within the cytosol and then imported into the cilium. Early biochemical and genetic work, largely carried out in the unicellular green alga Chlamydomonas reinhardtii, focused on the building blocks of axonemal microtubules and led to the identification of axonemal dynein as the first microtubule-based motor. Recent work has used proteomic, transcriptomic and genomic approaches to identify gene products that play critical roles in the formation and function of cilia across organisms. A database of cilia/flagella and basal body components identified in these studies can be found at the Ciliary Proteome Web Server at http://www.ciliaproteome.org.
Cilium structure
Cilia are microtubule-based organelles whose membrane is continuous with that of the plasma membrane [5]. The core axoneme is comprised of nine outer doublet microtubules (9 + 0) that emanate from the triplet microtubules of the mother centriole in the basal body (Figure 2). Motile cilia also contain a central pair of single microtubules (9 + 2) and protein complexes necessary for ciliary beating such as axonemal dynein arms and radial spokes. During cilia assembly, tubulin subunits and other axonemal components are incorporated onto the distal tip of the growing axoneme, suggesting a transport mechanism from the cell body to the distal end. Once assembled, the cilium remains highly dynamic as new tubulin subunits are continually incorporated into the tip of the axoneme [5].
Figure 2.
Ciliary structure and IFT. An axoneme consists of nine doublet microtubules that project from the triplet microtubules of the mother centriole. Motile cilia also contain a central pair of single microtubules (not shown). In addition to their role in ciliary structure and beating, axonemal microtubules serve as tracks for intraflagellar transport (IFT), the movement of IFT particles and ciliary proteins by kinesin and dyenin motors. Kinesin motors drive anterograde IFT (base to tip) whereas cytoplasmic dynein drives retrograde IFT (back to the base). Entry into the ciliary compartment is thought to be gated at the transition zone which may form a pore that regulates entry of ciliary proteins into the organelle.
The transition zone is the proximal region of the cilium where the microtubule structure changes from centriolar triplets to axonemal doublets and several microtubule-to-membrane connections are found [6]. Transitional fibers attach the distal end of the mother centriole to the plasma membrane whereas Y-shaped links connect the doublet microtubules to the ciliary membrane and organize rows of membrane-associated protein particles termed the ciliary necklace. Proteins that localize to the transition zone are thought to regulate entry of proteins into the cilium [7–9]. As the mother and daughter centrioles nucleate the mitotic spindle, the centriole-membrane links must be broken and the cilium must be disassembled prior to mitosis, suggesting an intimate link between cilia and cell cycle progression [10].
Intraflagellar transport
A landmark finding for cilia research was the discovery of intraflagellar transport (IFT) nearly 20 years ago. Using video-enhanced differential interference contrast (DIC) microscopy to observe Chlamydomonas flagella, Rosenbaum and colleagues observed IFT particles moving bidirectionally between the base and tips of flagella [11] (Figure 2). Indeed, axonemal microtubules serve as tracks for transport driven by two families of molecular motors, kinesins and cytoplasmic dyneins, in additional to their structural role. Kinesins are a superfamily of enzymes that have in common a core kinesin motor domain (~ 350 amino acids) for ATP-dependent movement along the surface of microtubules. Outside of the core motor domain, each kinesin family contains unique sequences for binding specific cellular cargoes and regulating motor activity [12, 13]. In general, kinesin motors use the energy of ATP hydrolysis to undergo directed motility to the plus (fast growing) ends of microtubules whereas cytoplasmic dynein uses the energy of ATP hydrolysis to drive motility toward the minus (anchored) ends of microtubules.
Work in the 1990s using genetic screens for flagellar assembly mutants (fla) in Chlamydomonas identified the heterotrimeric kinesin-2 motor as responsible for anterograde IFT (fla10, fla8/fla1, fla3 in Chlamydomonas; KIF3A, KIF3B, KAP in mammals) and cytoplasmic dynein 1b (DHC2 in mammals) as responsible for retrograde IFT. Parallel work identified heterotrimeric kinesin-2 as the anterograde IFT motor in sea urchin embryos and early vertebrate embryonic node. That the heterotrimeric kinesin-2 motor is critical for anterograde IFT has since been demonstrated in every organism tested [14–16].
The current model for IFT (Figure 2) proposes that heterotrimeric kinesin-2 motors assemble with IFT particles and associated cargoes (e.g., axoneme and ciliary membrane precursors, retrograde motors, signaling molecules, etc.) and drive anterograde motility along axonemal microtubules. At the distal tip, axonemal precursors and other cargo are unloaded via unknown mechanisms. Cytoplasmic dynein is then activated and drives retrograde transport of IFT particles and kinesin-2 motors back to the basal body.
IFT kinesins
The heterotrimeric kinesin-2 motor appears to be the “core” IFT motor for both generation and maintenance of cilia structure as disruption of kinesin-2 function leads to the loss of cilia in Chlamydomonas, Tetrahymena, Drosophila, Xenopus, sea urchin, trypanosomes, and mouse [14–16]. In C. elegans, however, the non-motile cilia at the dendritic endings of amphid chemosensory neurons are maintained in single mutants of the heterotrimeric kinesin-2 genes (klp-11, klp-20, kap-1). In this case, loss of heterotrimeric kinesin-2 function can be compensated for by another member of the kinesin-2 family, the homodimeric motor OSM-3 (KIF17 in mammals) [17–19]. The model that emerged from these and other observations describes heterotrimeric kinesin-2 and homodimeric OSM-3 as mechanically coordinated and working cooperatively and redundantly in the middle region of ASH/ASI channel and AWC wing cilia whereas OSM-3 works alone to build the distal region [16].
Recent work has suggested that the coordination of kinesin-2 and OSM-3 is not a general feature of IFT across cell types or organisms. Indeed, the roles of kinesin-2 and OSM-3 appear to be different even in other chemosensory neurons of the C. elegans amphid organ. In the AWB wing cilia, OSM-3 moves partly independently of kinesin-2 in the middle region and is not essential for building or maintaining the distal region [20]. In cultured MDCK kidney epithelial cells, dominant negative interference of kinesin-2 function results in a loss of cilia that cannot be compensated for by KIF17. Rather, KIF17 is required for delivery of cyclic nucleotide-gated (CNG) channels to the cilium [21]. Finally, in zebrafish, loss of KIF17 function by either morpholino-mediated knockdown or expression of dominant negative constructs results in a loss or disorganization of outer segments in photoreceptor cells but has no effect on the formation of motile cilia in the pronephros [22, 23]. It thus appears that the heterotrimeric kinesin-2 motor comprises the core IFT machinery that functions in all cilia whereas accessory kinesins function in a cell-specific manner to generate diversity in ciliary shape and function.
Consistent with this model is recent work showing that cilia contain members of other kinesin families that can function as accessory motors. A kinesin-3 family member in C. elegans male-specific sensory neurons, KLP-6, is required not for cilia formation but for the ciliary targeting of polycystins, mechanosensory ion channels [24]. Members of the kinesin-4 family, Costal2 (Cos2) in flies and the related KIF7 and KIF27 motors in mammals, have been identified as critical regulators of Hedgehog signaling during patterning of fly and vertebrate embryos. In flies, Hedgehog signaling occurs in unciliated cells and Cos2 appears to function as a microtubule tether [25, 26]. In mouse embryos, KIF7 and KIF27 play important roles in the ciliary trafficking and signaling response of Hedgehog pathway components [27–30]. Whether Cos2, KIF7 and KIF27 are bone fide kinesin motors has been contentious [31] and the possibility remains that they function more as scaffolding proteins than as ATP-dependent transporters.
Non-IFT kinesins
Although kinesin motors are best known as cargo transporters, some kinesin families participate in other microtubule-related activities such as microtubule destabilization or even movement to the minus ends of microtubules [12, 13]. Thus, it is not surprising that kinesin motors appear to contribute to ciliary structure and function in ways other than transport of ciliary components along axonemal microtubules. Members of the kinesin-9 family are exclusively expressed in tissues and organisms with motile cilia and flagella. Yet despite having domain organization typical of cargo transporters, a Chlamydomonas kinesin-9 motor, Klp1, localizes to the central pair microtubules and regulates axonemal dynein-dependent beat frequency [32]. Thus, kinesin-9 motors may play a unique role in force generation that is conserved across species and cilia types.
Kinesin-13 family members have their kinesin motor domain located in the middle of the polypeptide and facilitate the removal of tubulin subunits from the plus ends, thereby destabilizing microtubules. In Giardia and Leishmania, a kinesin-13 localizes to the distal tips of flagella where it may depolymerize axonemal microtubules and cooperate with the IFT machinery to control the length of the cilium [33, 34]. In Chlamydomonas, a kinesin-13 contributes to flagellar growth as disruption of its activity leads to defects in ciliogenesis and flagella regeneration [35]. Finally, phylogenetic analyses have implicated two new families of kinesin motors in ciliary functions, namely kinesin-16 and -17, but their biological roles have so far not been reported [36].
Ciliary gating
While kinesin and dynein motors play established roles in the formation and function of all cilia, how motors and their associated ciliary cargoes are targeted to the ciliary compartment is largely unknown. Targeting of proteins to the ciliary compartment is likely to be multi-faceted. Current research indicates that membrane proteins are targeted to the ciliary compartment via vesicle transport from the Golgi complex [4]. Some ciliary membrane proteins possess sequence motifs that specify their localization to the ciliary compartment perhaps via incorporation into cilia-destined transport vesicles [21, 37–39]. Lateral transport between the plasma and ciliary membranes appears to be limited by a specialized membrane domain at the base of the cilium [40] although lateral movement may occur under specific circumstances such as Hedgehog-induced movement of Smoothened [41]. Lateral movement of membrane proteins is also regulated by the filament-forming small Gprotein Septin2 [42]. In addition, non-ciliary proteins can be prevented from entering the ciliary compartment by retention in the apical membrane via interactions with the ezrin/radixin/moesin (ERM)-actin network [43].
Our recent work on the kinesin-2 motor KIF17 motor suggests that the mechanisms of ciliary entry may be similar to those that regulate entry into the nuclear compartment. KIF17 contains a ciliary localization signal (CLS) that is identical to classical nuclear localization signals (NLS). Indeed, this sequence can function as a CLS in the context of full-length KIF17 and as an NLS for truncated forms of KIF17 [44]. Interestingly, other kinesin motors have been noted to shuttle between nuclear and ciliary compartments. First, nuclear targeting has been noted for a KIF17 splice variant that regulates nuclear shuttling of transcriptional regulators and RNA-binding proteins in male germ cells [45, 46]. Second, truncated forms of the kinesin-3 motor KLP-6 localize to the nucleus in C. elegans male-specific neurons [24]. Third, the heterotrimeric kinesin-2 subunit KAP has been shown to shuttle between nuclear and ciliary compartments during mitotic cycling in sea urchin embryos [47].
Parallels between ciliary and nuclear import extend beyond the targeting signals. Both the nucleus and the cilium contain high levels of the small G protein Ran bound to GTP (Ran-GTP) whereas the cytoplasm contains high levels of Ran-GDP. In the cytoplasm, NLS-containing proteins bind to importin receptors for transport across the nuclear pore complex (NPC) where high levels of Ran-GTP in the nuclear compartment dissociate the complex [48]. Also in the cytoplasm, the CLS-containing KIF17 binds to importin-β2 (transportin-1) for transport across the transition zone into the ciliary compartment where Ran-GTP dissociates the complex [44]. This is consistent with the proposal by Rosenbaum and Witman [14] that the transition fibers constitute a ciliary pore complex (CPC) that, like the NPC, functions as a regulated gate of entry. How ciliary versus nuclear import is regulated is currently unknown.
That importins and Ran-GTP play a general role in regulating ciliary entry is supported by recent work from Margolis and colleagues. First, a splice isoform of the membrane protein Crumbs3 localizes specifically to the ciliary compartment and requires an interaction with importin-β1 and Ran for this localization [49]. Second, the ciliary protein retinitis pigmentosa 2 (RP2) interacts with importin-β2 (transportin-1) via two NLS-like sequences and requires importin-β2 for ciliary localization [50]. Clearly, further work is required to understand the role of the various importin receptors in regulating ciliary entry. In addition the evidence to date has examined the ciliary entry of single proteins and it is unclear whether how large complexes such as IFT particles and/or the BBSome gain access to the ciliary compartment and whether this is dependent on importins and Ran. Finally, the Ran/importin system may also play a critical role in regulating ciliary assembly as Ran and importins play important roles in regulating microtubule assembly [51] and knockdown of importin-β1 abolished ciliogenesis [49].
Figure 3. Models for ciliary and nuclear import.
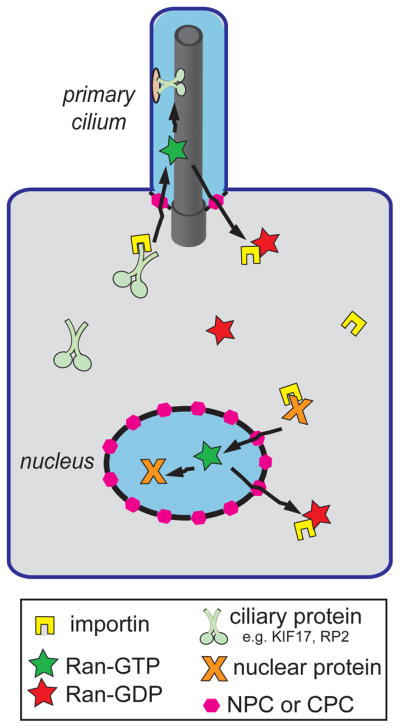
Importin receptors (yellow) bind to NLS-containing nuclear proteins (orange) and CLS-containing ciliary proteins (light green) in the cytoplasm and escort them across the NPC or CPC (pink hexagons), respectively. Ran-GTP (green stars) in the nuclear and ciliary compartments causes dissociation of importin-cargo complexes. Ran-GTP and the importin are recycled back to the cytoplasm where Ran is converted to Ran-GDP (red stars). Blue shading indicates regions of high Ran-GTP whereas gray shading indicates regions of high Ran- GDP.
Acknowledgments
Funding
Work in KJ Verhey’s lab is funded by the National Institutes of Health [GM070862].
Abbreviations
- IFT
intraflagellar transport
- CLS
ciliary localization signal
- NLS
nuclear localization signal
- NPC
nuclear pore complex
- CPC
ciliary pore complex
References
- 1.Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–35. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veland IR, Awan A, Pedersen LB, Yoder BK, Christensen ST. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol. 2009;111:p39–53. doi: 10.1159/000208212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12:222–34. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 6.Silverman MA, Leroux MR. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 2009;19:306–16. doi: 10.1016/j.tcb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Craige B, Tsao CC, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190:927–40. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen DP, Koenekoop RK, Khanna H, Jenkins PM, Lopez I, Swaroop A, Martens JR. Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc Natl Acad Sci U S A. 2007;104:15917–22. doi: 10.1073/pnas.0704140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, Yoder BK, Leroux MR. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192:1023–41. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seeley ES, Nachury MV. The perennial organelle: assembly and disassembly of the primary cilium. J Cell Sci. 2010;123:511–8. doi: 10.1242/jcs.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A. 1993;90:5519–23. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–96. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 13.Verhey KJ, Hammond JW. Traffic control: regulation of kinesin motors. Nat Rev Mol Cell Biol. 2009;10:765–77. doi: 10.1038/nrm2782. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–25. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 15.Scholey JM. Intraflagellar transport. Annu Rev Cell Dev Biol. 2003;19:423–43. doi: 10.1146/annurev.cellbio.19.111401.091318. [DOI] [PubMed] [Google Scholar]
- 16.Scholey JM. Intraflagellar transport motors in cilia: moving along the cell’s antenna. J Cell Biol. 2008;180:23–9. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans JE, Snow JJ, Gunnarson AL, Ou G, Stahlberg H, McDonald KL, Scholey JM. Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans. J Cell Biol. 2006;172:663–9. doi: 10.1083/jcb.200509115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 2004;6:1109–13. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- 19.Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–7. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- 20.Mukhopadhyay S, Lu Y, Qin H, Lanjuin A, Shaham S, Sengupta P. Distinct IFT mechanisms contribute to the generation of ciliary structural diversity in C. elegans. EMBO J. 2007;26:2966–80. doi: 10.1038/sj.emboj.7601717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins PM, Hurd TW, Zhang L, McEwen DP, Brown RL, Margolis B, Verhey KJ, Martens JR. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr Biol. 2006;16:1211–6. doi: 10.1016/j.cub.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 22.Insinna C, Humby M, Sedmak T, Wolfrum U, Besharse JC. Different roles for KIF17 and kinesin II in photoreceptor development and maintenance. Dev Dyn. 2009;238:2211–22. doi: 10.1002/dvdy.21956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Insinna C, Pathak N, Perkins B, Drummond I, Besharse JC. The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev Biol. 2008;316:160–70. doi: 10.1016/j.ydbio.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peden EM, Barr MM. The KLP-6 kinesin is required for male mating behaviors and polycystin localization in Caenorhabditis elegans. Curr Biol. 2005;15:394–404. doi: 10.1016/j.cub.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 25.Robbins DJ, Nybakken KE, Kobayashi R, Sisson JC, Bishop JM, Therond PP. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell. 1997;90:225–34. doi: 10.1016/s0092-8674(00)80331-1. [DOI] [PubMed] [Google Scholar]
- 26.Sisson JC, Ho KS, Suyama K, Scott MP. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell. 1997;90:235–45. doi: 10.1016/s0092-8674(00)80332-3. [DOI] [PubMed] [Google Scholar]
- 27.Cheung HO, Zhang X, Ribeiro A, Mo R, Makino S, Puviindran V, Law KK, Briscoe J, Hui CC. The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian hedgehog signaling. Sci Signal. 2009;2:ra29. doi: 10.1126/scisignal.2000405. [DOI] [PubMed] [Google Scholar]
- 28.Endoh-Yamagami S, Evangelista M, Wilson D, Wen X, Theunissen JW, Phamluong K, Davis M, Scales SJ, Solloway MJ, de Sauvage FJ, Peterson AS. The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Curr Biol. 2009;19:1320–6. doi: 10.1016/j.cub.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 29.Liem KF, Jr, He M, Ocbina PJ, Anderson KV. Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2009;106:13377–82. doi: 10.1073/pnas.0906944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson CW, Nguyen CT, Chen MH, Yang JH, Gacayan R, Huang J, Chen JN, Chuang PT. Fused has evolved divergent roles in vertebrate Hedgehog signalling and motile ciliogenesis. Nature. 2009;459:98–102. doi: 10.1038/nature07883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farzan SF, Ascano M, Jr, Ogden SK, Sanial M, Brigui A, Plessis A, Robbins DJ. Costal2 functions as a kinesin-like protein in the hedgehog signal transduction pathway. Curr Biol. 2008;18:1215–20. doi: 10.1016/j.cub.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokoyama R, O’Toole E, Ghosh S, Mitchell DR. Regulation of flagellar dynein activity by a central pair kinesin. Proc Natl Acad Sci U S A. 2004;101:17398–403. doi: 10.1073/pnas.0406817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blaineau C, Tessier M, Dubessay P, Tasse L, Crobu L, Pages M, Bastien P. A novel microtubule-depolymerizing kinesin involved in length control of a eukaryotic flagellum. Curr Biol. 2007;17:778–82. doi: 10.1016/j.cub.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 34.Dawson SC, Sagolla MS, Mancuso JJ, Woessner DJ, House SA, Fritz-Laylin L, Cande WZ. Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in Giardia intestinalis. Eukaryot Cell. 2007;6:2354–64. doi: 10.1128/EC.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piao T, Luo M, Wang L, Guo Y, Li D, Li P, Snell WJ, Pan J. A microtubule depolymerizing kinesin functions during both flagellar disassembly and flagellar assembly in Chlamydomonas. Proc Natl Acad Sci U S A. 2009;106:4713–8. doi: 10.1073/pnas.0808671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wickstead B, Gull K. A “holistic” kinesin phylogeny reveals new kinesin families and predicts protein functions. Mol Biol Cell. 2006;17:1734–43. doi: 10.1091/mbc.E05-11-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell. 2008;19:1540–7. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci. 2006;119:1383–95. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- 39.Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28:183–92. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci U S A. 2006;103:18556–61. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol. 2009;187:365–74. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–9. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Francis SS, Sfakianos J, Lo B, Mellman I. A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J Cell Biol. 2011;193:219–33. doi: 10.1083/jcb.201009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dishinger JF, Kee HL, Jenkins PM, Fan S, Hurd TW, Hammond JW, Truong YN, Margolis B, Martens JR, Verhey KJ. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nat Cell Biol. 2010;12:703–10. doi: 10.1038/ncb2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chennathukuzhi V, Morales CR, El-Alfy M, Hecht NB. The kinesin KIF17b and RNA-binding protein TB-RBP transport specific cAMP-responsive element modulator-regulated mRNAs in male germ cells. Proc Natl Acad Sci U S A. 2003;100:15566–71. doi: 10.1073/pnas.2536695100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macho B, Brancorsini S, Fimia GM, Setou M, Hirokawa N, Sassone-Corsi P. CREM-dependent transcription in male germ cells controlled by a kinesin. Science. 2002;298:2388–90. doi: 10.1126/science.1077265. [DOI] [PubMed] [Google Scholar]
- 47.Morris RL, English CN, Lou JE, Dufort FJ, Nordberg J, Terasaki M, Hinkle B. Redistribution of the kinesin-II subunit KAP from cilia to nuclei during the mitotic and ciliogenic cycles in sea urchin embryos. Dev Biol. 2004;274:56–69. doi: 10.1016/j.ydbio.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol. 2007;8:195–208. doi: 10.1038/nrm2114. [DOI] [PubMed] [Google Scholar]
- 49.Fan S, Fogg V, Wang Q, Chen XW, Liu CJ, Margolis B. A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin beta interactions. J Cell Biol. 2007;178:387–98. doi: 10.1083/jcb.200609096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurd TW, Fan S, Margolis BL. Localization of retinitis pigmentosa 2 to cilia is regulated by Importin beta2. J Cell Sci. 2011;124:718–26. doi: 10.1242/jcs.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalab P, Heald R. The RanGTP gradient - a GPS for the mitotic spindle. J Cell Sci. 2008;121:1577–86. doi: 10.1242/jcs.005959. [DOI] [PMC free article] [PubMed] [Google Scholar]