Abstract
Objective
Although some studies have suggested a relationship between MS exacerbations and psychological distress, methodological weaknesses limit their conclusions. This study was aimed to determine whether pseudo- and confirmed MS exacerbations are preceded by or concurrent with increased anxiety or depressive symptoms.
Methods
This was a secondary analysis of 121 patients with MS who were followed for 48 weeks during a randomized controlled trial. Participants completed monthly self-reports on depressive and anxiety symptoms. Patient-reported exacerbations were assessed through a phone-administered symptom checklist and neurological exam.
Results
Both pseudo-exacerbations and confirmed exacerbations were associated with concurrent somatic depressive, β = .16 and β = .33, respectively, ps < .05, affective depressive, β = .17, p = .02 and β = .12, p = .06, and anxiety symptoms, β = .24 and β = .20, ps < .01, controlling for baseline symptoms. Preexisting somatic and affective depressive symptoms predicted amplified relationships between concurrent confirmed exacerbations and these symptoms, β = .19 and β = .20, respectively, ps < .01. A standard deviation increase in anxiety symptoms relative to baseline predicted subsequent onset of pseudo-exacerbations, odds ratio [OR] = 1.54, p = .02, while increased somatic depressive symptoms predicted confirmed exacerbations, OR = 1.59, p = .01.
Conclusion
Patients with MS experiencing pseudo- or confirmed exacerbations should be assessed and monitored for depressive and anxiety symptoms, and confirmed exacerbations are particularly concerning in patients with a history of depression. The psychological or psychiatric antecedents of MS exacerbations generate new hypotheses on etiologies of confirmed and pseudo-exacerbations.
Trial Registration
ClinicalTrials.gov (NCT00147446).
Keywords: Multiple Sclerosis, Relapsing/remitting, Exacerbations, Depression, Anxiety, Illness Behavior
Individuals with Multiple Sclerosis (MS) are at high risk for depressive and anxiety disorders (1–3), which are linked to other areas of concern in MS (e.g. suicidality, non-adherence to MS disease-modifying medications) (4, 5). The most common forms of MS are relapsing and involve clinical exacerbations, which are characterized by rapid onset of new or increased symptoms. Untreated exacerbations can persist for weeks or months. It has long been noted that patients with MS who are currently in exacerbation report elevated distress (6–8) relative to those in remission.
Other researchers have examined the relationship between exacerbations and subsequent anxiety and depressive symptoms in patients with MS or clinically isolated syndrome, with evidence of a relationship but disagreement as to whether anxiety versus depressive symptoms are affected (9, 10). Conversely, there is evidence of greater anxiety symptoms and general psychological distress in the month(s) preceding exacerbations (11, 12). Another study failed to find a relationship between exacerbations and previous or subsequent depression or anxiety (13, 14); however, incomplete follow-up and attrition rates were high, particularly for participants who experienced depression or exacerbations (15).
Although the preponderance of evidence suggests a relationship between MS exacerbations and psychological distress during, after, and even before the exacerbation, many of these studies were cross-sectional, lacked physician confirmation of exacerbations, and/or relied on retrospective self-reports of exacerbations or prior psychological or psychiatric symptoms. The lag between exacerbations and assessment of distress was sometimes long and/or unclearly defined. Some studies measured only general psychological distress, while others examined depression or anxiety but not both. No studies examined depressive symptom clusters, such as somatic and affective symptoms, separately. Finally, no work examined potential risk factors for anxious or depressive responses to exacerbations, such as underlying or chronic preexisting anxiety or depressive symptoms.
Patients also sometimes present with a sudden onset of new or worsening symptoms resembling those of an MS exacerbation, but which, upon examination, is not confirmed as an MS exacerbation. Termed “pseudo-exacerbations,” these symptoms can have a variety of causes, such as bacterial infection or premenstrual syndrome, and have also been ascribed to acute stress (16). The relationship between pseudo-exacerbations and symptoms of depression and anxiety has not been carefully studied.
Differentiation between psychological symptom clusters, and pseudo- versus confirmed exacerbations, may generate hypotheses regarding underlying mechanisms. Though their etiology differs, both confirmed exacerbations and pseudo-exacerbations may heighten anxiety or depressive symptoms related to psychological factors (17). Anxiety, specifically, may also precede pseudo-exacerbations, as anxiety and panic symptoms (e.g. paresthesias, dizziness, trembling, reduced concentration, difficulty swallowing, subjective unsteadiness, fatigue) might be mistaken for MS exacerbations. Finally, inflammatory cytokines cause “sickness behaviors” (e.g., poor appetite, sleep difficulties, fatigue, lethargy, cognitive difficulties, social withdrawal) (18–20) that mimic somatic depressive symptoms. Thus, confirmed exacerbations may exhibit unique relationships with somatic depressive symptoms. Although somatic and affective depressive symptoms overlap, these clusters have demonstrated meaningful differences in relation to inflammatory processes (20, 21).
In the current study, we prospectively examined pseudo- and confirmed exacerbations and their relationships with previous and concurrent anxiety, somatic depressive, and affective depressive symptoms. We also investigated whether individuals with preexisting vulnerability to depression or anxiety were at higher risk for psychological distress during exacerbations. Against this background, we hypothesized that:
-
H1
Pseudo- and confirmed exacerbations would be associated with concurrent anxiety and affective depressive symptoms, controlling for baseline symptoms. Only confirmed exacerbations would be associated with somatic depressive symptoms that are consistent with cytokine-driven symptoms.
-
H2
Exacerbations would be associated with greater concurrent anxiety and depressive symptoms for individuals with greater baseline anxiety and depressive symptoms.
-
H3
Increased anxiety symptoms relative to baseline would predict onset of subsequent new pseudo-exacerbations, while changes in anxiety and depressive symptoms would not predict onset of subsequent confirmed exacerbations.
METHOD
Study Design
This is a secondary analysis of individuals with MS who were followed for 48 weeks during a Phase II multi-site, randomized controlled trial (22) comparing cognitive behavioral stress management therapy (23, 24) to a waitlist control. Participants provided consent in MS specialty clinics at three sites (University of California, San Francisco, Evergreen Hospital Medical Center in Seattle, and Feinberg School of Medicine at Northwestern University in Chicago) and through local chapters of the National MS Society. Institutional Review Boards at all sites approved this study, and a Data Safety Monitoring Board monitored the trial. Recruitment occurred from May 2005 through January 2008, and follow-up evaluations were completed in January 2009.
Participants
Enrollment criteria were designed to ensure participants had active disease. Inclusion criteria were: 1) MS diagnosis (25); 2) documentation of a clinical exacerbation or gadolinium enhancing lesion in the past year; 3) 18+ years of age; 4) ability to speak and read English; and 5) a score of 6.5 or less on the Expanded Disability Status Scale (EDSS) (26), indicating participants were ambulatory. Exclusion criteria were: 1) current exacerbation (patients could enroll after the exacerbation had been resolved for 28+ days); 2) current use of interferon medication with absence of use during the month prior to the qualifying lesion or exacerbation, or current use of glatiramer acetate with absence of use during the 6 months prior to the lesion or exacerbation; 3) corticosteroid use in the past 28 days; 4) current use of a cytotoxic agent or natalizumab; 5) diagnosis of another autoimmune or endocrine disorder; 6) inability to undergo MRI; 7) current or planned pregnancy; 8) severe psychiatric condition per the MINI-International Neuropsychiatric Interview (MINI) (27); 9) current or planned participation in psychotherapy; and 10) dementia, per scores below the 5th percentile on three or more neuropsychological tests (i.e., Symbol Digit Modalities, Digit Span, Hopkins Verbal Learning Test, Controlled Word Association Test, Similarities, and the 10/36) (28).
Measures
Interview measures were administered via telephone by evaluators unaware of treatment assignment. Participants completed self-report measures in-person at baseline and 48 weeks, and through the web or mail at all other time points.
Exacerbations
Participants completed weekly questionnaires and monthly phone interviews that queried whether the participant believed they had experienced a new MS exacerbation (defined as a sudden occurrence of new or increased symptoms within 24 hours, persisting beyond 48 hours). Participants were also asked to call research staff and report new exacerbations as soon as possible. Staff followed up self-reported exacerbations with a phone evaluation. The staff member recorded the reported start date of the exacerbation (typically within the previous seven days) and, with the patient, completed a symptom checklist adapted from a previous study (29).
If criteria for suspected exacerbation were met, the participant was asked to return to the study site for a neurological exam by a study neurologist, trained MS nurse practitioner, or clinical evaluator who was trained and supervised by the study neurologist. Evaluators were trained by the study neurologist in the administration of the EDSS with non-study MS patients until the evaluators demonstrated competence. Competence included both a determination that examination procedures were performed correctly and that the evaluator’s EDDS scores were identical to those of the neurologist. All EDSS evaluations performed by evaluators were videotaped. The study neurologist provided supervision based on those videotapes, initially on every evaluation, dropping in frequency as the evaluators demonstrated sustained competence.
Exacerbations were confirmed when symptoms were accompanied by corresponding abnormalities on the EDSS. Some patients declined to attend the study exam, seeing only their personal neurologist. In such cases, study staff obtained confirmation or non-confirmation of exacerbation and any prescribed medication from the patient’s neurologist. Self-reported exacerbations were considered pseudo-exacerbations if results from the neurological exam were inconsistent with MS exacerbation, or if the participant’s symptoms did not meet minimal criteria for referral for neurological evaluation (e.g., duration < 48 hours).
Exacerbations were considered concurrent with the first monthly administration of the anxiety and depressive symptom measures occurring on or after the exacerbation start date, unless the exacerbation was known to have resolved prior to that administration. Self-reported exacerbations that could not be verified as confirmed or pseudo-exacerbations (e.g., the participant refused neurological evaluation) were removed from analyses, along with concurrent measures of anxiety and depression. Additive effects were not modeled; in rare cases where more than one exacerbation of a given type (i.e., pseudo- or confirmed) was considered concurrent with a psychological assessment, the assessment was coded as concurrent with one exacerbation of that type. If an assessment was concurrent with both a pseudo- and a confirmed exacerbation, the confirmed exacerbation received precedence. In other words, the assessment was considered concurrent only with the confirmed exacerbation.
Exacerbations were considered concurrent only with the first psychological assessment that occurred after the exacerbation start date, unless the exacerbation was known to have continued after that first psychological assessment (i.e., the patient reported the continuing exacerbation on a date after the first psychological assessment). As additional assessments of anxiety and depressive symptoms could have been affected by these exacerbations, the continuation(s) were also treated as concurrent with these additional assessments in the statistical models of anxiety and depressive symptoms for H1–H2. Continuations were classified in the same manner as new exacerbations, according to findings from subsequent assessments of the exacerbation. For H3, however, each continuation, and the anxiety, affective depressive, and somatic depressive symptom measures from the previous administration, were removed from analyses because the outcome was onset of new exacerbations.
Depressive and Anxiety Symptoms
The Hospital Anxiety and Depression Scale anxiety subscale (HADS-A) (30), and the Center for Epidemiologic Studies Depression Scale (CES-D) (31), were administered monthly to measure the frequency of self-reported anxiety and depressive symptoms in the past week. A 6-item subscale of the CES-D was used to assess somatic depressive symptoms (e.g., restless sleep, difficulty “get[ing] going”), and a 7-item subscale was used to assess affective depressive symptoms (e.g., sadness, depressed mood) (32).
Depressive and Anxiety Disorders
The MINI (27) was administered at baseline to characterize the sample.
Control Variables
A demographics form was used to ascertain age, sex, years of education, marital status, and employment status. A study neurologist, or a nurse/evaluator supervised by a neurologist, assessed disease-related disability at baseline through neurological examination using the EDSS (26). The Energy subscale of the Multiple Sclerosis Quality of Life-54 (33) self-report was used to control for baseline fatigue. We also controlled for treatment assignment.
Data Analysis
H1–H2
Linear mixed models were used to predict anxiety and depressive symptoms. Analyses were run using SAS 9.2 (34) with restricted maximum likelihood methods. Per likelihood-ratio tests, a first-order autoregressive moving-average structure produced the best fit for all three models, and was thus used.
We fit separate models for the anxiety and depressive symptom clusters, which were assessed at approximately 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, and 48 weeks. Covariates were: the baseline score on the outcome variable (e.g., baseline anxiety symptoms for the anxiety symptom model), two binary variables indicating whether the patient was in a confirmed (n = 0) or pseudo-exacerbation (n = 4) at baseline, treatment assignment, baseline EDSS score, baseline energy level, and demographics. The demographic variables were age, sex, years of education, marital status (coded 1 if the participant was married or cohabitating with a partner, and 0 otherwise), and employment status (categorized as employed, unemployed, receiving disability benefits, or other [e.g., retired]). Variables relevant to the hypotheses were time varying and comprised of: 1) main effects of concurrent pseudo- and confirmed exacerbations; and 2) interactions of concurrent pseudo- and confirmed exacerbations with the baseline score on the outcome measure for that model. Significant interactions would suggest preexisting anxiety or depressive symptoms moderate the level of acute distress during exacerbations. Main effects of exacerbations indicated whether exacerbations were associated with concurrent anxiety and depressive symptoms, among participants with the mean level of these symptoms at baseline.
H3
Generalized estimating equations logistic regression for repeated measures was used to predict pseudo- and confirmed exacerbations with onset after baseline. For confirmed exacerbations, the Toeplitz covariance structure produced the lowest Quasi-Likelihood Information Criterion and was used in the H3 analyses.
Variables relevant to the hypotheses were anxiety, affective depressive, and somatic depressive symptoms reported at the previous month’s assessment. Covariates were: a binary variable indicating whether or not that type of exacerbation occurred at baseline, baseline depressive and anxiety symptoms, treatment assignment, baseline EDSS, baseline energy, and demographics. The 4 week time points were removed from the H3 analyses to eliminate redundancy, as for these cases the previous month’s anxiety and depressive symptoms were also the baseline symptoms. Significant effects of baseline symptoms would suggest individuals with higher absolute levels of anxiety or depression are generally more prone to exacerbations. Significant effects of anxiety or depressive symptoms at the previous assessment would indicate that recent changes in anxiety or depressive symptoms relative to baseline predict subsequent exacerbations.
RESULTS
Sample Characteristics
Baseline characteristics of the participants (N = 121) are displayed in Table 1.
Table 1.
Baseline demographics and diagnoses (N = 121)
| Age† | 42.7 | (9.8) |
| Years of Education† | 15.7 | (2.3) |
| Expanded Disability Status Scale† | 3.1 | (1.5) |
| MS Diagnostic Status | ||
| Relapsing-Remitting | 118 | (98%) |
| Secondary-Progressive | 2 | (2%) |
| Women | 101 | (83%) |
| Ethnicity | ||
| White | 100 | (83%) |
| Hispanic/Latino | 6 | (5%) |
| Native American | 3 | (3%) |
| African American | 2 | (2%) |
| Asian/Pacific Islander | 1 | (1%) |
| Mixed/Other | 9 | (7%) |
| Employment Status | ||
| Working | 65 | (54%) |
| Unemployed | 20 | (17%) |
| Receiving Disability Benefits | 22 | (18%) |
| Other (e.g., retired) | 14 | (12%) |
| Marital Status | ||
| Married | 75 | (62%) |
| Single | 26 | (22%) |
| Divorced | 11 | (9%) |
| Cohabitating with Partner | 7 | (6%) |
| Widowed or Separated | 2 | (2%) |
| Current Anxiety and Depressive Disorder Diagnoses | ||
| Generalized Anxiety Disorder | 22 | (18%) |
| Major Depressive Disorder | 16 | (13%) |
| Agoraphobia without History of Panic Disorder | 13 | (11%) |
| Dysthymic Disorder | 8 | (7%) |
| Posttraumatic Stress Disorder | 4 | (3%) |
| Social Phobia | 3 | (3%) |
| Obsessive Compulsive Disorder | 3 | (3%) |
| Panic Disorder (with or without Agoraphobia) | 2 | (2%) |
Note. Data are provided as number (percentage) unless otherwise noted. Percentages may not total 100 due to rounding.
Data are provided as mean (SD).
Over the course of 48 weeks, patients self-reported 165 new exacerbations. Eighteen (10.9%) could not be verified, and were therefore excluded from analyses. Of the 18 unverifiable self-reported exacerbations, ten were due to the patient being unable or unwilling to come in for neurological evaluation, five could not be verified because symptoms had resolved or were resolving before the patient could come in for an evaluation, and in three cases the reasons for non-evaluation were unclear. There were also two new exacerbations that were excluded because they did not align with concurrent or previous self-reports of depression and anxiety and one new pseudo-exacerbation that was excluded because the patient experienced a confirmed exacerbation during the same time window. Excluding the four pseudo-exacerbations occurring at baseline, this left 64 pseudo- and 76 confirmed exacerbations. Exacerbations started a mean of 15.98 days before the next assessment of depressive and anxiety symptoms (SD = 13.38, range = 0–85). For H3 analyses, exacerbations started a mean of 15.40 days after the previous anxiety and depressive symptom assessment (SD = 10.11, range = 1–38).
Regarding the stability of depressive and anxiety symptoms over the trial, we calculated the range of the symptom scores for each of the participants over time by subtracting their minimum score from their maximum score, provided they had completed these assessments at a minimum of two time points. The mean range was 7.26 (SD = 3.58) for the HADS-A, 6.99 (SD = 3.12) for the CES-D somatic scale, and 7.52 (SD = 4.21) for the CES-D affective scale, suggesting there were substantial fluctuations over time.
Five participants did not complete any depression or anxiety assessments after baseline, and 4 were missing data for one or more baseline variables. These participants were thus excluded from analyses for H1–H3. For H3, one additional participant was excluded because they lacked any time points with data for the previous assessment of depression and anxiety.
H1
Table 2 displays results for H1 and H2. Consistent with H1, pseudo-exacerbations were associated with concurrent affective depressive symptoms, t(1072) = 2.36, p = .02, and anxiety symptoms, t(1072) = 3.19, p = .001, controlling for baseline symptoms. Inconsistent with H1, pseudo-exacerbations were also associated with somatic depressive symptoms, t(1071) = 2.29, p = .02. There was a significant main effect of confirmed exacerbations on somatic depressive, t(1071) = 5.05, p < .001, and anxiety symptoms, t(1072) = 2.93, p = .004, and there was a non-significant trend for a main effect of confirmed exacerbations on affective depressive symptoms, p = .06.
Table 2.
Standardized parameter estimates for the linear mixed models of depressive and anxiety symptoms (N = 112)
| CES-D1 | CES-D | HADS2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Affective Subscale | Somatic Subscale | Anxiety Subscale | |||||||
| β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | |
| Baseline Score3 | .46 | .34, .58 | <.001** | .28 | .14, .43 | <.001** | .47 | .34, .60 | <.001** |
| Pseudo-Exacerbation | .17 | .03, .31 | .02* | .16 | .02, .30 | .02* | .24 | .09, .39 | .001** |
| Confirmed Exacerbation | .12 | −.01, .25 | .06 | .33 | .20, .46 | <.001** | .20 | .07, .34 | .004** |
| Pseudo Exacerbation * Baseline Score | −.05 | −.19, .10 | .53 | .07 | −.05, .19 | .25 | .13 | −.01, .26 | .06 |
| Confirmed Exacerbation * Baseline Score | .20 | .08, .31 | <.001** | .19 | .05, .32 | .008** | .13 | −.01, .27 | .06 |
To determine whether these results may differ between the treatment and control groups, we added an interaction between treatment assignment and the presence of a current confirmed exacerbation, as well as an interaction between treatment and current pseudo-exacerbation. These interactions were non-significant in the models of anxiety, affective depressive, and somatic depressive symptoms (ps > .15), and were thus dropped from the models.
H2
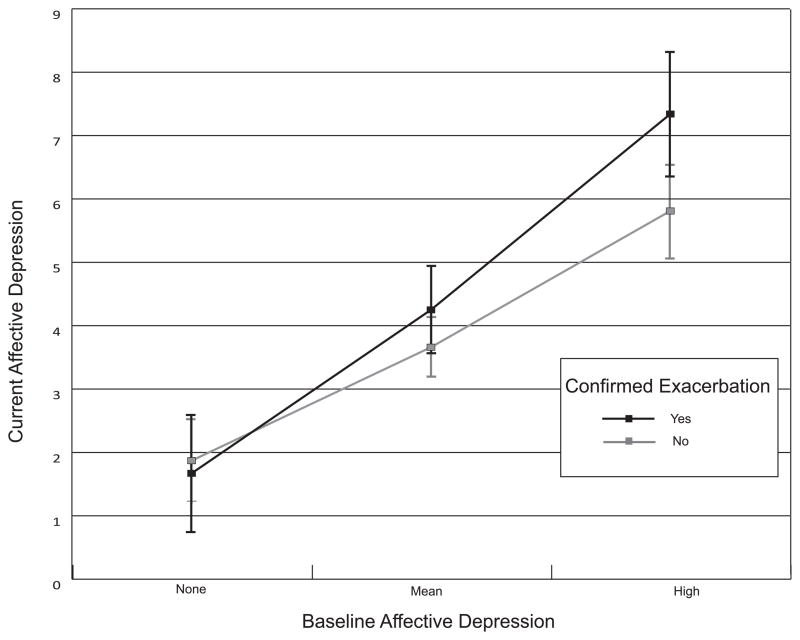
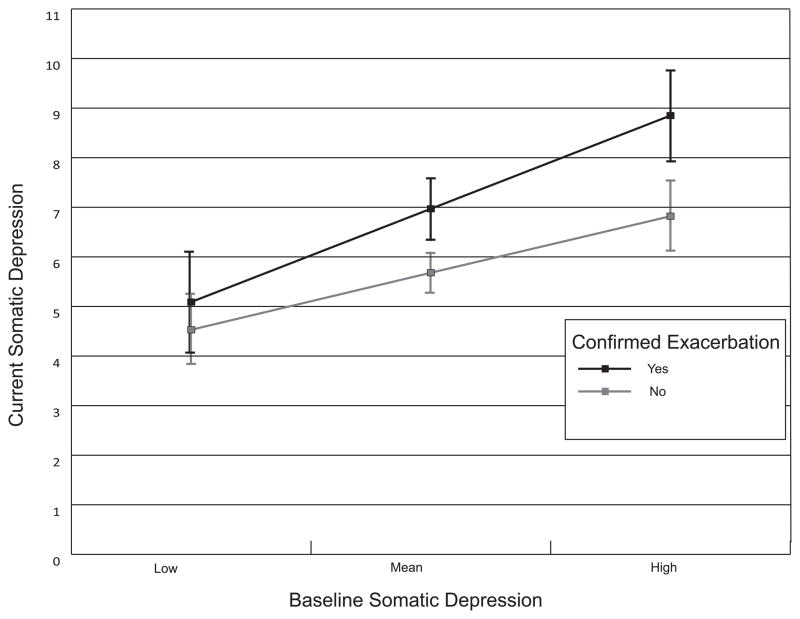
Inconsistent with H2, the relationships of pseudo-exacerbations to concurrent depressive symptoms, both affective, p = .53 and somatic, p = .25, were not moderated by baseline levels of these depressive symptoms. For anxiety symptoms, there were non-significant trending moderation effects for both pseudo-, t(1072) = 1.85, p = .06, and confirmed exacerbations, t(1072) = 1.87, p = .06. Consistent with H2, a stronger relationship between confirmed exacerbations and concurrent affective, t(1072) = 3.40, p < .001, as well as somatic, t(1071) = 2.68, p = .008, depressive symptoms was observed for patients with greater baseline affective or somatic depressive symptoms, respectively (see Figures 1–2, created using graphical software) (35).
Figure 1.
Relationship between confirmed exacerbations and concurrent affective depressive symptoms, moderated by baseline affective depressive symptoms. The high level of baseline symptoms is +1 SD from the mean (36). As the −1 SD point was below zero, the low level is set to zero (i.e., no symptoms). Error bars denote the 95% CI around each estimated mean. The error bars overlapped at all levels of baseline affective depressive symptoms. This suggests that although the moderation effect was significant overall, we cannot be confident that confirmed exacerbations were associated with affective depressive symptoms at any of the discrete levels of baseline symptoms conveyed by the graph.
Figure 2.
Relationship between confirmed exacerbations and concurrent somatic depressive symptoms, moderated by baseline somatic depressive symptoms. High and low levels of baseline symptoms are scores ± 1 SD from the mean (36). Error bars denote the 95% CI around each estimated mean. The pattern of overlap and non-overlap of the error bars at particular levels of baseline symptoms indicates that confirmed exacerbations were only associated with somatic depressive symptoms if the severity of the patient’s baseline somatic depressive symptoms was close to the mean or greater.
H3
Baseline depressive and anxiety symptoms were not significantly associated with risk for pseudo- or confirmed exacerbations, ps > .14 (see Table 3). Consistent with H3, recent increases in anxiety symptoms relative to baseline predicted subsequent onset of new pseudo-exacerbations, Z = 2.30, p = .02. For a 1 SD increase in recent anxiety symptoms, the odds ratio was 1.5 (95% CI = 1.1–2.2). Neither increased affective, p = .23, nor somatic depressive symptoms, p = .21, predicted onset of new pseudo-exacerbations, also consistent with H3. New confirmed exacerbations were not predicted by increases in anxiety, p = .88, or affective depressive symptoms, p = .64. Inconsistent with H3, however, worsening somatic depressive symptoms did predict subsequent onset of new confirmed exacerbations, Z = 2.55, p = .01, OR = 1.6 (95% CI = 1.1–2.3) for a 1 SD increase in somatic depressive symptoms.
Table 3.
Odds ratios for prediction of exacerbations from 1 SD increases in anxiety and depressive symptoms at baseline and the assessment prior to the exacerbation (N = 111)
| Pseudo-Exacerbation | Confirmed Exacerbation | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| CES-D1 Affective Subscale (Baseline) | 1.26 | .76, 2.10 | .37 | .80 | .45, 1.45 | .47 |
| CES-D Somatic Subscale(Baseline) | 1.41 | .83, 2.40 | .20 | 1.17 | .74, 1.84 | .50 |
| HADS2 Anxiety Subscale (Baseline) | .73 | .47, 1.12 | .15 | 1.04 | .59, 1.81 | .90 |
| CES-D Affective Subscale (Prior) | .77 | .50, 1.18 | .23 | .90 | .57, 1.41 | .64 |
| CES-D Somatic Subscale (Prior) | .73 | .44, 1.20 | .21 | 1.59 | 1.11, 2.28 | .01* |
| HADS Anxiety Subscale (Prior) | 1.54 | 1.07, 2.21 | .02* | .96 | .60, 1.54 | .88 |
To evaluate whether treatment may have affected the relationships between depressive and anxiety symptoms and subsequent exacerbations, we added interactions between recent anxiety and depressive symptoms and treatment assignment to the models. These three interactions were non-significant in the models for both confirmed and pseudo-exacerbations (ps > .48), and were thus dropped from the models.
DISCUSSION
Confirmed exacerbations were associated with concurrent anxiety and somatic depressive symptoms, controlling for baseline symptoms. There was a trend for an association with affective depression. These findings are generally consistent with existing research in this area. We extended the literature by demonstrating that pseudo-exacerbations were also associated with somatic depression, affective depression, and anxiety.
Patients who reported preexisting somatic and affective depressive symptoms were more vulnerable to these symptoms, respectively, during confirmed exacerbations. In contrast, pseudo-exacerbations were not associated with greater depression for patients with preexisting vulnerabilities to either cluster of depressive symptoms. This difference suggests a unique mechanism for the relationship between confirmed exacerbations and preexisting depressive symptoms, independent of a psychological reaction one would also expect to see in pseudo-exacerbations. Rather, pathogenic factors underlying exacerbation may aggravate underlying pathogenic factors related to depression. For example, there may be a lower threshold for MS inflammatory processes to amplify preexisting depressive symptoms relative to new depressive symptoms.
On the other hand, there were trends for both types of exacerbation to be more strongly associated with anxiety for individuals with greater preexisting anxiety symptoms. This suggests that if there is a relationship between preexisting anxiety and vulnerability to anxiety during exacerbations, it is not unique to confirmed exacerbations. Thus, the most parsimonious explanation is that these trends were due to psychological factors.
Baseline psychological symptoms were not predictive of subsequent pseudo- or confirmed exacerbations. Rather, pseudo-exacerbations were predicted by recent increases in anxiety symptoms relative to baseline. This is consistent with the notion that anxiety symptoms could be mistaken for symptoms of MS exacerbations. In contrast, only increases in somatic depressive symptoms preceded confirmed exacerbations. There are at least two possible explanations, which are not mutually exclusive. First, somatic depression or its biological etiologies may increase the probability that underlying pathogenic processes will progress to full clinical exacerbation. For instance, hypothalamic–pituitary–adrenal axis dysregulation may interfere with regulation of inflammation at the site of a potential lesion (37). A second explanation is that what appear to be somatic symptoms of depression may actually be early signs of exacerbation, reflecting cytokine-driven sickness behaviors.
To examine the effect sizes of the psychological symptoms for predicting exacerbations, we can use the effect of the most commonly used disease modifying medications for MS, the interferons; a meta-analysis of randomized controlled trials reported a mean relative risk of 0.80 for MS exacerbations (38). In the current study, the RR for confirmed exacerbations was 1.53 for an increase of 1 SD in somatic depressive symptoms. Inverted, this is equivalent to 0.65. The RR for pseudo-exacerbations was 1.49 for a 1 SD increase in anxiety symptoms (inverted RR = 0.67). While we cannot make absolute comparisons, consideration of these effect sizes suggests that the predictive value of anxiety and somatic depressive symptoms for subsequent exacerbation is in the range viewed by the field as clinically meaningful.
Clinical Implications
Results suggest patients experiencing both pseudo- and confirmed MS exacerbations should be carefully assessed for anxiety and depressive symptoms, and patients with a history of depression should be particularly closely monitored for psychiatric deterioration during confirmed exacerbations. The relationship between exacerbations and psychological distress did not differ by treatment group. Thus, the stress management therapy did not appear to buffer any stress-inducing effects of either type of exacerbation, suggesting a potential need for more targeted interventions for patients experiencing exacerbations. However, as original trial was not powered to examine these interactions, the absence of statistical significance does not necessarily imply the absence of an effect.
Physicians should also follow patients with MS who are not in exacerbation but report recent increases in somatic depressive symptoms, as such symptoms may forecast an upcoming clinical exacerbation. Finally, as anxiety predicted onset of pseudo-exacerbations, psychoeducation on physiological symptoms of anxiety and panic may help patients with MS to more clearly distinguish symptoms of exacerbation from pseudo-exacerbation. As the psychological antecedents of exacerbations did not differ by treatment assignment, a general stress management program may be insufficient to help individuals with MS make this distinction; again, however, studies with more statistical power are needed to resolve this question.
Limitations
We were unable to evaluate all suspected exacerbations, and those exacerbations had to be removed from analyses to allow differentiation between pseudo- and confirmed exacerbations. Further, as this was a secondary analysis, potential explanatory mechanisms such as proinflammatory cytokines were not assessed. We also relied on patients’ recall of exacerbation start dates. However, start dates were usually within approximately one week of reports, and patients were typically still in exacerbation at the time of report. Finally, there may have been cases where the exacerbation ended prior to subsequent administration of the anxiety and depression measures, but this would have attenuated rather than exaggerated effect sizes for H1–H2 analyses.
Conclusions
Confirmed and pseudo-exacerbations were associated with concurrent anxiety and depression symptoms, controlling for baseline symptoms. Patients with higher preexisting levels of depression were at greater risk for depression during confirmed exacerbations. Somatic depressive symptoms predicted subsequent confirmed exacerbations and may reflect or influence inflammatory processes occurring prior to the emergence of neurological symptoms of exacerbations. In contrast, increases in anxiety preceded pseudo-exacerbations, indicating patients with MS may have difficulty distinguishing anxiety symptoms from those of exacerbations.
Acknowledgments
Study Funding: NICHD grant R01-HD043323
Glossary
- CES-D
Center for Epidemiologic Studies Depression Scale
- EDSS
Expanded Disability Status Scale
- H1
Hypothesis 1
- H2
Hypothesis 2
- H3
Hypothesis 3
- HADS-A
Hospital Anxiety and Depression Scale anxiety subscale
- MINI
MINI-International Neuropsychiatric Interview
- MS
Multiple Sclerosis
Footnotes
None of the authors have conflicts of interest.
References
- 1.Sadovnick AD, Remick RA, Allen J, Swartz E, Yee IM, Eisen K, Farquhar R, Hashimoto SA, Hooge J, Kastrukoff LF, Morrison W, Nelson J, Oger J, Paty DW. Depression and multiple sclerosis. Neurol. 1996;46:628–32. doi: 10.1212/wnl.46.3.628. [DOI] [PubMed] [Google Scholar]
- 2.Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. 2007;13:67–72. doi: 10.1177/1352458506071161. [DOI] [PubMed] [Google Scholar]
- 3.Beiske AG, Svensson E, Sandanger I, Czujko B, Pedersen ED, Aarseth JH, Myhr KM. Depression and anxiety amongst multiple sclerosis patients. Eur J Neurol. 2008;15:239–45. doi: 10.1111/j.1468-1331.2007.02041.x. [DOI] [PubMed] [Google Scholar]
- 4.Mohr DC, Goodkin DE, Likosky W, Gatto N, Baumann KA, Rudick RA. Treatment of depression improves adherence to interferon beta-1b therapy for multiple sclerosis. Arch Neurol. 1997;54:531–3. doi: 10.1001/archneur.1997.00550170015009. [DOI] [PubMed] [Google Scholar]
- 5.Feinstein A. An examination of suicidal intent in patients with multiple sclerosis. Neurol. 2002;59:674–8. doi: 10.1212/wnl.59.5.674. [DOI] [PubMed] [Google Scholar]
- 6.Uguz F, Akpinar Z, Ozkan I, Tokgoz S. Mood and anxiety disorders in patients with multiple sclerosis. Int J Psychiatry Clin Pract. 2008;12:19–24. doi: 10.1080/13651500701330825. [DOI] [PubMed] [Google Scholar]
- 7.Dalos NP, Rabins PV, Brooks BR, O’Donnell P. Disease activity and emotional state in multiple sclerosis. Ann Neurol. 1983;13:573–7. doi: 10.1002/ana.410130517. [DOI] [PubMed] [Google Scholar]
- 8.Kroencke DC, Denney DR, Lynch SG. Depression during exacerbations in multiple sclerosis: the importance of uncertainty. Mult Scler. 2001;7:237–42. doi: 10.1177/135245850100700405. [DOI] [PubMed] [Google Scholar]
- 9.McCabe MP. Mood and self-esteem of persons with multiple sclerosis following an exacerbation. J Psychosom Res. 2005;59:161–6. doi: 10.1016/j.jpsychores.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Di Legge S, Piattella MC, Pozzilli C, Pantano P, Caramia F, Pestalozza IF, Paolillo A, Lenzi GL. Longitudinal evaluation of depression and anxiety in patients with clinically isolated syndrome at high risk of developing early multiple sclerosis. Mult Scler. 2003;9:302–6. doi: 10.1191/1352458503ms921oa. [DOI] [PubMed] [Google Scholar]
- 11.Potagas C, Mitsonis C, Watier L, Dellatolas G, Retziou A, Mitropoulos PA, Sfagos C, Vassilopoulos D. Influence of anxiety and reported stressful life events on relapses in multiple sclerosis: a prospective study. Mult Scler. 2008;14:1262–8. doi: 10.1177/1352458508095331. [DOI] [PubMed] [Google Scholar]
- 12.Warren S, Warren KG, Cockerill R. Emotional stress and coping in multiple sclerosis (MS) exacerbations. J Psychosom Res. 1991;35:37–47. doi: 10.1016/0022-3999(91)90005-9. [DOI] [PubMed] [Google Scholar]
- 13.Brown RF, Tennant CC, Sharrock M, Hodgkinson S, Dunn SM, Pollard JD. Relationship between stress and relapse in multiple sclerosis: part II. Direct and indirect relationships. Mult Scler. 2006;12:465–75. doi: 10.1191/1352458506ms1296oa. [DOI] [PubMed] [Google Scholar]
- 14.Brown RF, Valpiani EM, Tennant CC, Dunn SM, Sharrock M, Hodgkinson S, Pollard JD. Longitudinal assessment of anxiety, depression, and fatigue in people with multiple sclerosis. Psychol Psychother-T. 2009;82:41–56. doi: 10.1348/147608308X345614. [DOI] [PubMed] [Google Scholar]
- 15.Brown RF, Tennant CC, Sharrock M, Hodgkinson S, Dunn SM, Pollard JD. Relationship between stress and relapse in multiple sclerosis: part I. Important features. Mult Scler. 2006;12:453–64. doi: 10.1191/1352458506ms1295oa. [DOI] [PubMed] [Google Scholar]
- 16.Poser CM. The role of trauma in the pathogenesis of multiple sclerosis: a review. Clin Neurol Neurosur. 1994;96:103–10. doi: 10.1016/0303-8467(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 17.Wingerchuk DM, Carter JL. Practical Consultations: Multiple Sclerosis. Semin Neurol. 2003;23:253,64. [Google Scholar]
- 18.Gold SM, Irwin MR. Depression and Immunity: Inflammation and Depressive Symptoms in Multiple Sclerosis. Immunol Allergy Clin. 2009;29:309–20. doi: 10.1016/j.iac.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharpley CF, Bitsika V. Joining the dots: neurobiological links in a functional analysis of depression. Behav Brain Funct. 2010;6:73. doi: 10.1186/1744-9081-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dantzer R, O’Connor JC, Castanon N, Lestage J, Kelley KW. Cytokines and depression: experimental evidence and intermediate mechanisms. In: Pariante CM, Nesse RM, Nutt D, Wolpert L, editors. Understanding Depression: A Translational Approach. New York: Oxford University Press Inc; 2009. pp. 123–38. [Google Scholar]
- 22.Mohr DC, Lovera J, Brown T, Cohen B, Neylan T, Henry R, Siddique J, Jin L, Daikh D, Pelletier D. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurol. doi: 10.1212/WNL.0b013e3182616ff9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohr DC. The Stress and Mood Management Program for Individuals with Multiple Sclerosis: Therapist Guide. New York: Oxford Press; 2010. [Google Scholar]
- 24.Mohr DC. The Stress and Mood Management Program for Individuals with Multiple Sclerosis: Workbook. New York: Oxford Press; 2010. [Google Scholar]
- 25.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2005 Revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–6. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 26.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurol. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 27.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- 28.Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69:942–9. [PubMed] [Google Scholar]
- 29.Verdier-Taillefer MH, Roullet E, Cesaro P, Alperovitch A. Validation of self-reported neurological disability in multiple sclerosis. Int J Epidemiol. 1994;23:148–54. doi: 10.1093/ije/23.1.148. [DOI] [PubMed] [Google Scholar]
- 30.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 31.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 32.Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62:123–46. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- 33.Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995;4:187–206. doi: 10.1007/BF02260859. [DOI] [PubMed] [Google Scholar]
- 34.SAS Institute Inc. SAS Version 9.2. Cary, NC: SAS Institute Inc; 2008. [Google Scholar]
- 35.Jose PE. ModGraph: A computer programme to graphically display moderation. Wellington, New Zealand: Victoria University of Wellington; 2002. Excel. [Google Scholar]
- 36.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage Publications; 1991. [Google Scholar]
- 37.Mohr DC, Pelletier D. A temporal framework for understanding the effects of stressful life events on inflammation in patients with multiple sclerosis. Brain Behav Immun. 2006;20:27–36. doi: 10.1016/j.bbi.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Rice GP, Incorvaia B, Munari L, Ebers G, Polman C, D’Amico R, Filippini G. Interferon in relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev. 2001:CD002002. doi: 10.1002/14651858.CD002002. [DOI] [PMC free article] [PubMed] [Google Scholar]