Abstract
Young patients with congenital aortic valve disease are at risk for left ventricular (LV) diastolic dysfunction (DD). We evaluated LV remodeling and prevalence of and risk factors for DD in patients with aortic stenosis (AS), pure aortic regurgitation (AR), and AS+AR. Patients age 8–39 years with congenital AS (n=103), AR (n=36), or AS+AR (n=107) were identified. Cross-sectional assessment of LV remodeling pattern and diastolic function was performed. A diastolic function score (DFS) (0–4) was assigned to each patient with 1 point for an abnormal value in each of 4 categories: mitral inflow (E:A and E-wave deceleration time), tissue Doppler E′, E/E′, and left atrial volume. Patients with DFS ≥2 were compared to those with a score <2. Concentric hypertrophy was the most common remodeling pattern in AS (51%), while mixed/physiologic hypertrophy in AS+AR (48%) and eccentric hypertrophy in AR (49%) predominated. In the entire cohort, 91 patients (37%) had DFS ≥ 2. Patients with AS or AS+AR had higher DFS than pure AR patients (p<0.001). In multivariable analysis, higher LV mass z-score and prior aortic valve balloon dilation were associated with DFS ≥2. In patients with catheterization data (n=65), E/E′ correlated with LV end-diastolic pressure. Those with DFS ≥2 had higher LV end-diastolic pressure and mean pulmonary artery pressure than those with DFS <2. In conclusion, DD is common in young patients with AS and AS+AR, but not in pure AR patients. Higher LV mass and prior aortic valve dilation were associated with DD.
Keywords: aortic valve disease, diastolic function, congenital heart disease
Background
The effect of chronic pressure and volume loading due to aortic valve disease on left ventricular (LV) remodeling and compliance has been well described in adults. Chronic pressure loading leads to LV remodeling with development of concentric hypertrophy1–3. Early in the disease course, concentric hypertrophy allows wall stress to remain normal and for preservation of systolic function. Later, the deleterious effects of concentric hypertrophy and associated myocardial fibrosis become apparent with the development of systolic and diastolic dysfunction (DD)4, 5. Myocardial response to chronic pressure load due to congenital aortic valve disease in children is also characterized by concentric hypertrophy, myocardial fibrosis and impaired diastolic function6, 7. The time course and risk factors for progression of DD may be different in younger patients with congenital aortic stenosis (AS)8. The effect of LV volume load due to aortic regurgitation (AR) on diastolic function is less clear with most adult data showing a high incidence of DD9–11, but experimental and pediatric data showing less of an effect of volume loading on diastolic function12. The effect of chronic combined pressure and volume load due to AS+AR on diastolic function in younger patients has not been described. In this cross-sectional study of children and young adults with congenital aortic valve disease, we describe LV remodeling pattern, prevalence of and risk factors for DD in patients with AS, pure AR, and AS+AR.
Methods
The records of all patients age 8–39 years evaluated at our institution from January 2005–May 2011 with ≥ moderate congenital AS and/or > mild AR were retrospectively reviewed. Exclusion criteria included congenital heart disease (with the exception of bicommissural aortic valve and aortic coarctation), prior cardiac surgery with cardiopulmonary bypass, residual aortic arch obstruction (gradient > 20 mm Hg), systemic hypertension, chronic renal disease, acquired valve disease, orthotopic heart transplant, history of diseases or therapies known to affect diastolic function (coronary artery disease, chemotherapy, Kawasaki disease). Baseline demographics, clinical characteristics, and clinical course including cardiac interventions were collected.
Patients were classified into one of three groups based on the predominant aortic valve disease: AS, pure AR, or AR+AR. The AS group included patients with both ≥ moderate AS and ≤ mild AR. The AS+AR cohort consisted of patients with both > mild AR and ≥ moderate AS. The pure AR cohort included patients with > mild AR and no AS (AS gradient < 15 mm Hg and no prior history of BAVP for congenital AS). We defined ≥ moderate AS by a Doppler gradient ≥ 36 mm Hg using the higher value of the maximum instantaneous gradient from the apical imaging window or mean gradient form the suprasternal notch window and/or prior history of balloon aortic valvuloplasty (BAVP)13. AR qualitatively grading in our echocardiographic laboratory is performed on a 4 point ordinal scale (0=none, 1=trivial, 2=mild, 3= moderate, 4=severe) by ½ unit increments and is based on a combination of previously published criteria14, 15. AR was considered > mild if at least one of the following criteria were met: pan-diastolic flow reversal in the descending abdominal aorta, vena contract width/body surface area > 3.1 mm/m2, LV end-diastolic volume (EDV) z-score >2. The Committee for Clinical Investigation at Children’s Hospital Boston approved the use of patient medical records for this review.
The most recent complete echocardiogram including full interrogation of diastolic function was included. AS gradient and AR grade were collected from reports produced at the time of the study. The following LV parameters were recorded: EDV, mass, mass:volume, and ejection fraction and the z-scores for these variables. LV EDV was calculated using the 5/6 area-length formula and LV mass using volumetric 2D measurements16. The pattern of LV remodeling was classified based on previously established criteria1 as: normal ventricle (normal mass, volume and mass:volume), concentric remodeling/hypertrophy (normal LV volume, high LV mass and/or mass:volume), eccentric remodeling/hypertrophy (high volume, normal mass and low or normal mass:volume) or mixed/physiologic hypertrophy (high mass, high volume with normal or high mass:volume).
All measurements of diastolic variables were retrospectively remeasured by a single echocardiographer from images obtained at the time of the echocardiogram (KF). Standard mitral valve inflow pulsed-Doppler indices of diastolic function, including peak early (E) and late (A) diastolic transmitral velocities, E:A, and E-wave deceleration time were measured. Pulsed wave tissue Doppler (TDI) velocities were obtained from the lateral mitral annulus and the interventricular septum from the apical 4-chamber view. Only tracings that demonstrated a clear E′ were used. Each TDI velocity was measured on 3 consecutive cardiac cycles and the average value was used. Peak early mitral inflow velocity/early mitral TDI velocity (E/E′) was calculated. Left atrial volumes (LA) were calculated using the prolate-ellipse formula17. LA volume ≥ 32 mL/m2 was considered abnormal18. For all other diastolic function variables, z-scores derived from normative data at our institution by previously described technique19 were used and z-score > 2 or < −2 was considered abnormal. Examinations were performed using commercially available ultrasound equipment (Philips iE33, Koninklijke Philips Electronics, N.V).
Diastolic parameters were grouped into 1 of 4 categories for analysis: 1). pulsed-wave Doppler mitral inflow (E:A, E wave deceleration time) 2). TDI velocities (mitral annular and septal E′) 3). E/E′ 4). LA volume. Patients were assigned a diastolic function score (DFS) between 0 and 4, with 1 point for an abnormal value in each category.
For patients who underwent catheterization within 3 months of the echocardiogram, hemodynamic data were collected from reports produced at the time of the catheterization (n=65). For cases in which interventions were performed (e.g. balloon aortic valvuloplasty), pre-intervention hemodynamic data were included in the analysis.
Demographics, clinical and testing data are reported as counts for categorical variables and as median (interquartile range) for continuous variables. Comparisons of demographic, clinical, and echocardiographic data between AS, AS+AR, and AR patients were made using Fisher’s exact test for categorical variables and Krusak-Wallis test for continuous variables. To evaluate risk factors for DD, patients with DFS < 2 were compared to those with DFS ≥ 2. Associations between demographic, clinical, and echocardiographic risk factors and DFS ≥ 2 were assessed. Multivariable analysis with stepwise logistic regression was used to assess for factors associated with DFS ≥ 2.
For the subset of patients with catheterization data, associations between echocardiographic markers of left heart filling pressures (E/E′) and invasively measured hemodynamic data were evaluated using Pearson correlation coefficients. Receiver-operator curves were constructed to assess the ability of E/E′ to predict elevated LV end diastolic pressure. All statistical analysis were 2-sided and type I error was controlled at a level of 0.05. Analyses were performed with SPSS (version 16.0, SPSS Inc, Chicago, Ill).
Results
The cohort consisted of 246 patients: 103 with AS, 107 with AS+AR, and 36 with pure AR patients. Patients with AS and AS+AR were older (p=0.003) and were more likely to have undergone BAVP than those with pure AR (p<0.001) (Table 1).
Table 1.
Demographic and Patient Characteristics
| All Patients (n=246) | AS (n=103) | AS+AR (n=107) | AR (n=36) | p value | |
|---|---|---|---|---|---|
| Age (years, IQR) | 17 (14–21) | 18 (14–23) | 16.4 (14–21) | 14.7 (10–18) | 0.003† |
| Age >20 years (n, %) | 171 (70%) | 68 (66%) | 74 (69%) | 7 (19%) | 0.025† |
| Weight (kg) | 61 (45–76) | 61 (48–73) | 65 (46–81) | 51 (36–65) | 0.022† |
| BSA (m2) | 1.7 (1.4–1.9) | 1.7 (1.5–1.9) | 1.8 (1.4–2.0) | 1.5 (1.2–1.8) | 0.93 |
| Male sex | 191 (78%) | 73 (71%) | 89 (83%) | 29 (81%) | 0.068* |
| Prior Balloon Aortic Valvuloplasty | 111 (45%) | 49 (48%) | 62 (58%) | 0 (0%) | <0.001† |
| Intervention within the 1st year of life | 44 (18%) | 21 (20%) | 23 (21%) | 0 (0%) | 0.011† |
| Repeat Aortic Valvuloplasty | 27 (11%) | 9 (9%) | 18 (17%) | 0 (0%) | 0.096† |
| Unicommissural Aortic Valve | 71 (29%) | 34 (33%) | 31 (32%) | 6 (17%) | 0.10 |
| Systolic blood pressure | 111 (101–121) | 108 (99–118) | 114 (105–122) | 107 (102–122) | 0.14 |
| Diastolic blood pressure | 64 (56–73) | 67 (59–77) | 63 (53–69) | 58 (54–63) | <0.001*† |
AS, aortic stenosis; AR, aortic regurgitation
Significant difference between aortic stenosis and mixed valve disease groups (p<0.05)
Significant difference between mixed valve disease and aortic regurgitation groups (p<0.05)
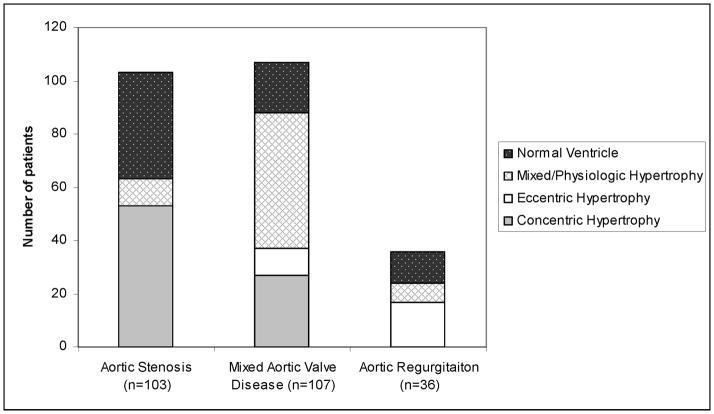
Most AS patients had concentric hypertrophy (51%) or normal ventricle (39%) (Figure 1). AS+AR disease patients most commonly had mixed/physiologic hypertrophy (48%) or concentric hypertrophy (25%), while in pure AR eccentric hypertrophy (49%) and normal ventricle (32%) predominated.
Figure 1.
Bar chart showing remodeling pattern for aortic stenosis, mixed aortic valve disease and aortic regurgitation patients.
LV EDV was higher in AS+AR and AR patients than in AS patients (p<0.001) (Table 2). LV mass z-score was highest in AS+AR patients followed by AR and then AS (p<0.001), whereas LV mass:volume was highest in patients with AS, intermediate in AS+AR patients and lowest in AR patients (p<0.001).
Table 2.
Echocardiographic Parameters
| All Patients (n=246) | AS (n=103) | AS+AR (n=107) | AR (n=36) | p value | |
|---|---|---|---|---|---|
| LV end-diastolic volume z-score | 1.2 (−0.3 to 3.2) | −0.3 (−1.1 to 0.9) | 2.4 (0.6 to 4.1) | 3.2 (1.5 to7.1) | <0.001*† |
| LV mass z-score | 1.9 (0.8 to 3.4) | 1.1 (0.1 to 2.15) | 2.9 (1.4 to 4.2) | 1.8 (0.9 to 3.9) | <0.001* |
| LV mass: volume | 1.0 (0.8 to 1.2) | 1.1 (0.9 to 1.3) | 0.9 (0.8 to 1.0) | 0.8 (0.7 to 0.9) | <0.001*† |
| LV mass: volume z-score | 1.0 (−0.5 to 2.2) | 1.7 (0.3 to 3.5) | 0.7 (−0.7 to 1.5) | −0.82 (−1.8 to 0.1) | <0.001*† |
| Aortic regurgitation grade | 2.5 (2 to 3) | 2 (0 to 2) | 3 (2.5 to 3.0) | 3 (2.5 to 4) | <0.001* |
| AS Gradient (mm Hg) | 36 (25 to 52) | 45 (30 to 57) | 40 (30 to 50) | 12 (0 to 17) | <0.001† |
| LV Ejection Fraction (%) | 67 (62 to 71) | 66 (62 to 71) | 67 (61 to 72) | 66 (61 to 73) | 0.06 |
AS, aortic stenosis; AR, aortic regurgitation; LV, left ventricle
Significant difference between aortic stenosis and mixed valve disease groups (p<0.01)
Significant difference between mixed valve disease and aortic regurgitation groups (p<0.01)
Values are median (IQR)
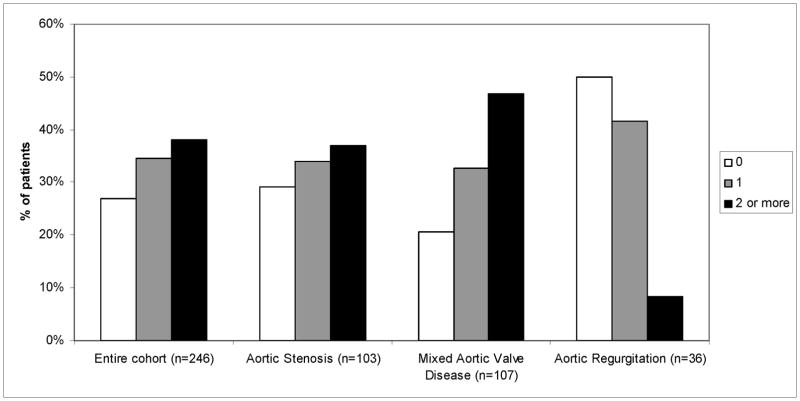
In the entire cohort, 186 patients (73%) had DFS ≥1 and 91 (37%) had DFS ≥ 2 (Figure 2). The percentage of patients with abnormal diastolic indices was similar between AS+AR and AS and both groups had higher DFS than the AR group (p<0.001 for DFS ≥1 and ≥2).
Figure 2.
Bar graph showing diastolic function score (0, 1 or ≥2) for the entire cohort, aortic stensois, mixed aortic valve disease and aortic regurgitation patients.
* Diastolic function score between 0 and 4 was calculated with 1 point for an abnormal value in each of the following 4 categories: 1. pulsed-Doppler mitral inflow (E:A, E wave deceleration time) 2. tissue Doppler velocities (mitral and septal E′) 3. E/E′ 4. left atrial volume
LA volume and pulsed-Doppler mitral inflow parameters did not vary between groups, while significant differences in TDI value and E/E′ were present (Table 3). Mitral annular and septal E′ were similar between AS and AS+AR patients and were lower than AR patients (p<0.001). E/E′ values and the percentage of patients with E/E′ z-score ≥ 2 were similar between AS and AS+AR patients and higher than in AR patients (p<0.001).
Table 3.
Diastolic Function Parameters
| All patients (n=246) | AS (n=103) | AS+AR (n=107) | AR (n=36) | p value | |
|---|---|---|---|---|---|
| Mitral Inflow E:A | 2.0 (1.6 to 2.5) | 1.9 (1.5 to 2.4) | 2.1 (1.6 to 2.6) | 2.1 (1.7 to 2.6) | 0.29 |
| Mitral inflow E:A z-score | −0.4 (−1.2 to 0.3) | −0.6 (−1.4 to 0.1) | −0.5 (−1.2 to 0.4) | −0.1 (−0.9 to 0.5) | 0.12 |
| Mitral inflow DT (msces) | 153 (127 to 176) | 154 (128 to 181) | 148 (120 to 174) | 153 (131 to 186) | 0.34 |
| Mitral inflow DT z-score | −0.5 (−1.1 to 0.3) | −0.5 (1.0 to 0.1) | −0.5 (−1.3 to 0.6) | −0.1 (−0.7 to 0.5) | 0.12 |
| Mitral annular E′ (cm/sec) | 13.5 (11.1 to 16.0) | 13.0 (10.5 to15.5) | 13.1 (10.7 to 15.2) | 16.6 (13.9 to 17.9) | <0.001† |
| Mitral annular E′ z-score | −1.8 (−2.6 to −1.0) | −2.0 (−4.9 to 1.3) | −2.1 (−2.8 to −1.2) | −0.6 (−1.4 to −0.03) | <0.001† |
| Mitral Annular E′ z-score ≤−2 | 107 (44%) | 48 (46%) | 58 (54%) | 1 (3%) | <0.001† |
| Septal E′ (cm/sec) | 10.0 (8.7 to11.7) | 10.2 (9.0 to 11.7) | 9.7 (8.2 to 11.0) | 12.2 (10.8 to 13.3) | <0.001† |
| Septal E′ z-score | −1.7 (−2.3 to −1.0) | −1.7 (−2.2 to −1.0) | −2.0 (−2.5 to −1.3) | −0.6 −1.3 to −0.1) | <0.001† |
| Septal E′ z-score ≤-2 | 92 (37%) | 37 (36%) | 52 (49%) | 3 (8%) | <0.001† |
| E/E′ | 8.2 (6.7 to 10.0) | 8.6 (7.2 to 10.0) | 8.7 (7.0 to 10.5) | 6.3 (5.5 to 6.9) | <0.001† |
| E/E′ z-score ≥ 2 | 69 (28%) | 29 (28%) | 38 (36%) | 2 (6%) | 0.003† |
| Left atrial volume (ml/m2) | 20 (16–24) | 21 (15–25) | 20 (16–24) | 19 (16–23) | 0.62 |
| Left atrial volume >32 ml/m2 | 54 (22%) | 19 (18%) | 28 (26%) | 7 (19%) | 0.75 |
| 4-point diastolic score ≥1 | 176 (72%) | 73 (71%) | 85 (80%) | 18 (50%) | 0.006† |
| 4-point diastolic score ≥ 2 | 91 (37%) | 38 (37%) | 50 (47%) | 3 (8%) | <0.001† |
AS, aortic stenosis; AR, aortic regurgitation
No significant differences between aortic stenosis and mixed aortic valve disease groups
Significant difference between mixed aortic valve disease and aortic regurgitation groups (p<0.05)
Values are counts (percentage) or median (IQR)
In the AS group, concentric hypertrophy/remodeling was associated with DFS ≥2 compared to mixed/physiologic hypertrophy or normal ventricle (50%, 30% and 15% respectively, p<0.001). In AS+AR and AR remodeling pattern was not associated with DFS. Subgroup analysis in AS patients comparing patients with ≤ mild residual AS (n=37) to those with > residual mild AS (n=66) showed lower LV mass z-score (0.84 (−1.8 to 3.8) vs. 1.52 (−0.8 to 10.2), p=0.026) and lower LV mass:volume (0.9 (0.7–1.6) vs. 1.2 (0.8–1.8) p=0.001) in patients with lower gradients, but no difference in demographics, cardiac interventions, or diastolic function parameters.
Univariable analysis of factors associated with DFS ≥ 2 is shown in Table 4. In multivariable analysis only higher LV mass z-score and prior BAV were associated with DFS ≥2.
Table 4.
Univariate and Multivariate Risk Factors for Diastolic Function Score ≥2
| Odds Ratio (Confidence Interval) | p value | |
|---|---|---|
|
Demographic Variables
| ||
| Male sex | 2.02 (1.09–3.63) | 0.03 |
| Age (median, IQR) | 1.01 (0.98–1.04) | 0.44 |
| Age ≥20 years | 2.10 (1.20–3.70) | 0.01 |
| Weight (kg) | 1.00 (0.99–1.01) | 0.91 |
|
| ||
|
Clinical Variables
| ||
| Balloon aortic valvuloplasty | 3.21 (1.87–5.49) | <0.001 |
| Balloon aortic valvuloplasty within the first year of life | 3.00 (1.50–5.87) | <0.001 |
| Repeat balloon aortic valvuloplasty | 2.31 (1.03–5.26) | 0.04 |
| Systolic blood pressure (mm Hg) | 0.99 (0.98–1.01) | 0.88 |
| Diastolic blood pressure (mm Hg) | 1.01 (0.99–1.04) | 0.37 |
|
| ||
|
Echocardiographic Variables
| ||
| Left ventricle end-diastolic volume z-score | 0.96 (0.88–1.06) | 0.45 |
| Left ventricle mass z-score | 1.21 (1.06–1.38) | 0.006 |
| Left ventricle mass:volume z-score | 1.18 (1.05–1.34) | 0.008 |
| Aortic regurgitation grade | 1.03 (0.76–1.38) | 0.87 |
| Aortic stenosis gradient (mm Hg) | 1.01 (1.00–1.03) | 0.02 |
|
| ||
|
Multivariate Risk Factors
| ||
| Left Ventricle mass z-score | 1.24 (1.03–1.41) | 0.02 |
| Prior balloon aortic valvuloplasty | 2.71 (1.52–4.94) | 0.001 |
| Age ≥ 20 years | 1.73 (0.88–3.21) | 0.12 |
| Male sex | 1.71 (0.89–3.52) | 0.12 |
| Aortic stenosis gradient | 1.01 (0.99–1.03) | 0.21 |
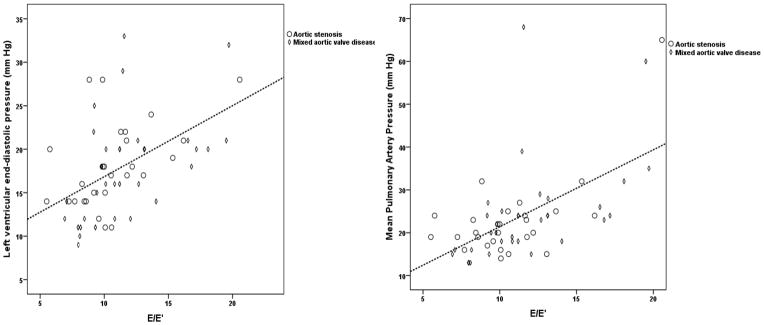
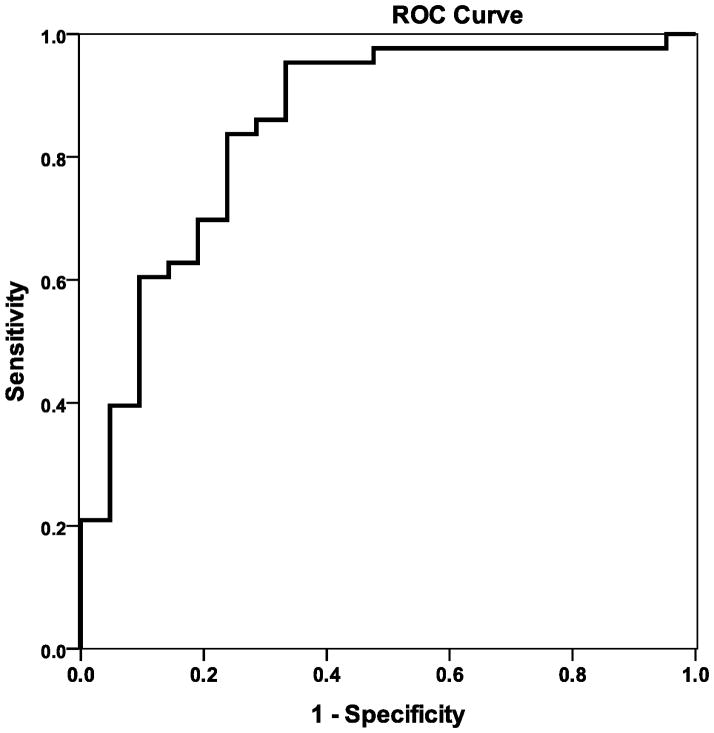
Catheterization data was available in 65 patients: 28 with AS and 37 with AS+AR. Median LV end-diastolic pressure (EDP) was 18 mm Hg (range 9–33) with 43 patients (66%) having LV EDP ≥ 15 mm Hg. Mean pulmonary artery pressure (PA) was ≥ 35 mm Hg in 5 patients (8%). Pulmonary vascular resistance was ≥2 Woods units in 19 patients (29%). E/E′ correlated with LV EDP (r=0.58, p<0.001) and mean PA pressure (r=0.63, p<0.001) (Figure 3). E/E′> 9.5 predicted LV EDP ≥ 15 mm Hg with 84% sensitivity and 76% specificity (Figure 4). Patients with DFS≥ 2 had higher LV EDP and mean PA pressure than those with those with DFS <2 (Table 5).
Figure 3.
Scatter-plot of LV end diastolic pressure (mm Hg) vs. E/E′ (Panel A) (r= 0.58, p<0.001 and mean pulmonary artery pressure (mm Hg) vs. E/E′ (Panel B) (r=0.63, p<0.001) for patients with catheterization data (n=65).
Figure 4.
ROC curve for E/E′ predicting LV end diastolic pressure≥15.
E/E′>9.5 is 84% sensitive and 76 % specific for LV end diastolic pressure≥15
Area under the curve=0.85
Table 5.
Catheterization Data
| Diastolic Function Score <2 n=34 | Diastolic Function Score ≥ 2 n=31 | p value | |
|---|---|---|---|
| LV end diastolic pressure (mmHg) | 14 (12–18) | 20 (16–22) | <0.001 |
| Pulmonary capillary wedge pressure (mm Hg) | 19 (12–18) | 20 (16–22) | <0.001 |
| Mean pulmonary artery pressure (mm Hg) | 18 (15–22) | 24 (22–28) | <0.001 |
| Pulmonary vascular resistance (Wood units) | 1.4 (1.1–2.0) | 1.7 (1.2–2.8) | 0.24 |
Values are median (range, IQR)
Discussion
In this study, we evaluated LV diastolic function in children and young adults with aortic valve disease and found that DD is common in patients with AS and AS+AR and uncommon in those with pure AR. DD was associated with higher LV mass and prior need for BAVP. These findings suggest that pressure load on the LV leads to concentric hypertrophy, likely myocardial fibrosis, and subsequent DD in AS and AS+AR, whereas volume loading in AR leads to eccentric hypertrophy and was rarely associated with DD. In AS patients, the pattern of LV remodeling, particularly presence of concentric hypertrophy, was associated with higher risk of DD. Concentric hypertrophy and associated myocardial fibrosis have been identified, along with impaired relaxation due to alterations in calcium handling, as the etiology of DD in older adults with AS1, 20–22.
Patients with pure AR had little evidence DD in our study, in contrast to the majority of previous studies of adults with AR which report that DD is common9, 11, 23. One possible explanation for this discrepancy is less severe and shorter duration of volume load in this cohort compared to previous literature, which has generally evaluated older adults undergoing aortic valve replacement. Another potential explanation is that the normal decrease in ventricular compliance with age may be accelerated in older patients with AR, who are likely to have additional co-morbidities that may affect diastolic function9, 11, 23. Previous literature on diastolic function in younger patients with AR is scant. Larger future studies evaluating DD inpatients with LV volume load are needed to clarify this issue.
Risk factors for DD in our study included higher LV mass and prior BAVP. These factors suggest diastolic function is worse in patients with longer duration and severity of LV pressure load. The cross-sectional design of the study and lack of longitudinal diastolic data limited our ability to directly assess the effect of duration and timing of pressure load on DD. Pressure and volume load at the time of latest follow-up were not associated with DD, but prior BAVP and higher LV mass were, likely indicating that cumulative pressure load is an important an factor in development of DD. This is also reflected in AS group where concentric hypertrophy, regardless of current gradient, was a risk factor for DD compared to other remodeling patterns. In the AS and AS+AR cohort, a substantial number of patients had DD despite having undergone previous BAVP and having low residual AS gradient. This suggests that LV pressure load at vulnerable periods, possibly in utero or neonatal, or peak pressure load may play a role in the development of DD in addition to cumulative pressure load8, 24, 25. Data from patients who have undergone in utero aortic valvuloplasty for evolving hypoplastic left heart syndrome support the concept that patients with LV pressure load in utero frequently develop DD even if pressure load is effectively reduced postnatally24. In adults undergoing aortic valve replacement, a subset of patients have ongoing progression of DD despite elimination of pressure load suggesting that myocardial changes, including fibrosis, can progress in the absence of ongoing pressure load26.
Our data show that non-invasive measures or LA pressure, primarily E/E′, correlate well with invasively measured LV EDP and mean PA pressure. While considerable data exist demonstrating TDI indices to be relatively load-independent measures of LV diastolic function and correlates of LV EDP in normal elderly patients and a variety of adult disease states27, this relationship has not been previously reported in children with aortic valve disease. Additionally, we show an association between elevation in E/E′ and elevated PA pressure, which has been reported in adults with AS in whom E/E′ has been shown to be the best non-invasive predictor of elevated PA pressure28. Recognition of the ability of non-invasive measures to predict LV EDP and PA pressure may add useful information in surgical timing and peri-operative management of younger patients undergoing aortic valve surgery.
Future studies are needed to evaluate the proposed mechanisms of DD, including myocardial fibrosis, in children and young adults. Quantification of fibrosis with cardiac magnetic resonance imaging and evaluation for biomarkers indicating fibrosis may be helpful in identifying reversible and irreversible myocardial damage and help with optimal timing of interventions20. Previous studies have shown non-invasive measures of elevated filling pressure are strongly predictive of reduced exercise capacity29. Long term, clinical, echocardiographic and exercise data are needed to determine to how frequently progressive symptoms attributable to diastolic heart failure or significantly decreased exercise capacity develop in this patient population.
Limitations of this study include the cross-sectional, retrospective design, which limits our ability to evaluate the effect of timing and duration of pressure and volume load on LV function. In order to avoid the confounding effect cardiopulmonary bypass may have on diastolic function, patients who have undergone cardiac surgery were excluded. Eliminating patients with history of aortic valve surgery may bias our cohort towards having less DD as patients who have undergone surgery may have more severe aortic valve disease than those who have not. Within the AS, AS+AR, and pure AR groups there was significant variation between patients in duration and severity of valve disease. Last, lack of a diastolic function grading system designed and validated for younger patients including patients with congenital heart disease is a major limitation of this study and for the field in general.
Acknowledgments
Dr. Friedman is supported by an NIH training grant (T32 HL 007572-28).
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol. 2011;58:1733–1740. doi: 10.1016/j.jacc.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 2.LINZBACH AJ. Heart failure from the point of view of quantitative anatomy. Am J Cardiol. 1960;5:370–382. doi: 10.1016/0002-9149(60)90084-9. [DOI] [PubMed] [Google Scholar]
- 3.Peterson KL, Tsuji J, Johnson A, DiDonna J, LeWinter M. Diastolic left ventricular pressure-volume and stress-strain relations in patients with valvular aortic stenosis and left ventricular hypertrophy. Circulation. 1978;58:77–89. doi: 10.1161/01.cir.58.1.77. [DOI] [PubMed] [Google Scholar]
- 4.Hess OM, Villari B, Krayenbuehl HP. Diastolic dysfunction in aortic stenosis. Circulation. 1987:IV73–IV76. [PubMed] [Google Scholar]
- 5.Stewart RA, Kerr AJ, Whalley GA, Legget ME, Zeng I, Williams MJ, Lainchbury J, Hamer A, Doughty R, Richards MA, White HD. Left ventricular systolic and diastolic function assessed by tissue Doppler imaging and outcome in asymptomatic aortic stenosis. Eur Heart J. 2010;31:2216–2222. doi: 10.1093/eurheartj/ehq159. [DOI] [PubMed] [Google Scholar]
- 6.de KE, Thijssen JM, Daniels O, de Korte CL, Kapusta L. Improvement of heart function after balloon dilation of congenital valvar aortic stenosis: a pilot study with ultrasound tissue Doppler and strain rate imaging. Ultrasound Med Biol. 2006;32:1123–1128. doi: 10.1016/j.ultrasmedbio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Fifer MA, Borow KM, Colan SD, Lorell BH. Early diastolic left ventricular function in children and adults with aortic stenosis. J Am Coll Cardiol. 1985;5:1147–1154. doi: 10.1016/s0735-1097(85)80017-6. [DOI] [PubMed] [Google Scholar]
- 8.Robinson JD, del Nido PJ, Geggel RL, Perez-Atayde AR, Lock JE, Powell AJ. Left ventricular diastolic heart failure in teenagers who underwent balloon aortic valvuloplasty in early infancy. Am J Cardiol. 2010;106:426–429. doi: 10.1016/j.amjcard.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 9.Cayli M, Kanadasi M, Akpinar O, Usal A, Poyrazoglu H. Diastolic function predicts outcome after aortic valve replacement in patients with chronic severe aortic regurgitation. Clin Cardiol. 2009;32:E19–E23. doi: 10.1002/clc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamb HJ, Beyerbacht HP, de RA, van der LA, Vliegen HW, Leujes F, Bax JJ, van der Wall EE. Left ventricular remodeling early after aortic valve replacement: differential effects on diastolic function in aortic valve stenosis and aortic regurgitation. J Am Coll Cardiol. 2002;40:2182–2188. doi: 10.1016/s0735-1097(02)02604-9. [DOI] [PubMed] [Google Scholar]
- 11.Villari B, Hess OM, Kaufmann P, Krogmann ON, Grimm J, Krayenbuehl HP. Effect of aortic valve stenosis (pressure overload) and regurgitation (volume overload) on left ventricular systolic and diastolic function. Am J Cardiol. 1992;69:927–934. doi: 10.1016/0002-9149(92)90795-z. [DOI] [PubMed] [Google Scholar]
- 12.Spencer AU, Hart JP, Cabreriza SE, Rabkin DG, Weinberg AD, Spotnitz HM. Aortic regurgitation in the heterotopic rat heart transplant: effect on ventricular remodeling and diastolic function. J Heart Lung Transplant. 2003;22:937–945. doi: 10.1016/s1053-2498(02)00816-1. [DOI] [PubMed] [Google Scholar]
- 13.Vlahos AP, Marx GR, McElhinney D, Oneill S, Goudevenos I, Colan SD. Clinical utility of Doppler echocardiography in assessing aortic stenosis severity and predicting need for intervention in children. Pediatr Cardiol. 2008;29:507–514. doi: 10.1007/s00246-007-9169-9. [DOI] [PubMed] [Google Scholar]
- 14.Beroukhim RS, Graham DA, Margossian R, Brown DW, Geva T, Colan SD. An echocardiographic model predicting severity of aortic regurgitation in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:542–549. doi: 10.1161/CIRCIMAGING.110.957175. [DOI] [PubMed] [Google Scholar]
- 15.Chin CH, Chen CH, Chen CC, Chen TH, Chang ML, Chiou HC. Prediction of severity of isolated aortic regurgitation by echocardiography: an aortic regurgitation index study. J Am Soc Echocardiogr. 2005;18:1007–1013. doi: 10.1016/j.echo.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–495. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Jiamsripong P, Honda T, Reuss CS, Hurst RT, Chaliki HP, Grill DE, Schneck SL, Tyler R, Khandheria BK, Lester SJ. Three methods for evaluation of left atrial volume. Eur J Echocardiogr. 2008;9:351–355. doi: 10.1016/j.euje.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 19.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–457. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 20.Weidemann F, Herrmann S, Stork S, Niemann M, Frantz S, Lange V, Beer M, Gattenlohner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 21.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 22.Zile MR, Gaasch WH. Abnormal calcium homeostasis: one mechanism in diastolic heart failure. J Am Coll Cardiol. 2011;58:155–157. doi: 10.1016/j.jacc.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 23.Villari B, Sossalla S, Ciampi Q, Petruzziello B, Turina J, Schneider J, Turina M, Hess OM. Persistent diastolic dysfunction late after valve replacement in severe aortic regurgitation. Circulation. 2009;120:2386–2392. doi: 10.1161/CIRCULATIONAHA.108.812685. [DOI] [PubMed] [Google Scholar]
- 24.Friedman KG, Margossian R, Graham DA, Harrild DM, Emani SM, Wilkins-Haug LE, McElhinney DB, Tworetzky W. Postnatal left ventricular diastolic function after fetal aortic valvuloplasty. Am J Cardiol. 2011;108:556–560. doi: 10.1016/j.amjcard.2011.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McElhinney DB, Vogel M, Benson CB, Marshall AC, Wilkins-Haug LE, Silva V, Tworetzky W. Assessment of left ventricular endocardial fibroelastosis in fetuses with aortic stenosis and evolving hypoplastic left heart syndrome. Am J Cardiol. 2010;106:1792–1797. doi: 10.1016/j.amjcard.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Gjertsson P, Caidahl K, Bech-Hanssen O. Left ventricular diastolic dysfunction late after aortic valve replacement in patients with aortic stenosis. Am J Cardiol. 2005;96:722–727. doi: 10.1016/j.amjcard.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 27.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 28.Casaclang-Verzosa G, Nkomo VT, Sarano ME, Malouf JF, Miller FA, Jr, Oh JK. E/Ea is the major determinant of pulmonary artery pressure in moderate to severe aortic stenosis. J Am Soc Echocardiogr. 2008;21:824–827. doi: 10.1016/j.echo.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Grewal J, McCully RB, Kane GC, Lam C, Pellikka PA. Left ventricular function and exercise capacity. JAMA. 2009;301:286–294. doi: 10.1001/jama.2008.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]