Abstract
Mechanics means relating to or caused by movement or physical forces. In this paper, I shall contend that OA is almost always caused by increased physical forces causing damage to a joint. While examples of joint injury causing osteoarthritis are numerous, I shall contend that most or almost all osteoarthritis is caused in part by mechanically induced injury to joint tissues. Further, once joint pathology has developed, as is the case for almost all clinical osteoarthritis, pathomechanics overwhelms all other factors in causing disease progression. Treatments which correct the pathomechanics have long lasting favorable effects on pain and joint function compared with treatments that suppress inflammation which have only temporary effects. I shall lastly contend that the mechanically induced joint injury leads to variable inflammatory responses but that the role of this inflammation in worsening structural damage in an already osteoarthritic joint has not yet been proven.
Keywords: osteoarthritis, biomechanics, inflammation
Introduction
The Oxford English dictionary defines mechanics as relating to or caused by movement or physical forces. I shall contend that OA is almost always caused by increased physical forces causing damage to a joint. The hypothesis that I will entertain is that OA is caused by increased forces across a local area of a joint either from a) abnormal anatomy (congenital or acquired) leading to increased focal stress with the overall load across the joint being normal; or b) excess overall load either acutely or chronically such as might occur with an injury during sports or with obesity chronically or c) a combination of anatomy and excess load. The latter might occur in a situation where an obese person has a slightly deformed joint, perhaps on a congenital basis, and develops OA in that joint.
Proving abnormal stresses across the joint causes OA is not a challenging proposition. Animal models of OA almost all rely on joint injury to induce disease. Further, it has been known for at least 60 years1 that meniscal tears and meniscectomies done after tears lead to an extremely high risk of OA, suggesting that abnormal focally increased forces across the knee certainly cause OA. I will attempt to prove a more challenging hypothesis, that abnormal mechanical forces cause most or even all OA. I note further that causes do not work alone to cause disease. Diseases are often the result of an interplay between causes, a fact evident in OA. A major injury (one cause) when combined with older age at the time of injury2 is more likely to produce OA than a major injury alone. Further, overweight young persons have only a modest risk of OA but older, overweight persons have a very high risk of knee OA. And the proportion of those with knee OA increases further when the person is not only obese and older but also female. In considering causes of OA, I will contend that mechanical forces play a role in almost all OA but that they do not necessarily act by themselves in causing disease.
Causation is difficult to prove for complex noninfectious human diseases. Generally, proving causation requires an intervention in which a putative causal agent is added or removed and the organism followed to see if they develop disease. Ideally, animal models provide this opportunity, but in osteoarthritis, animal models test causal agents by inducing injury in a normal non-diseased joint and evaluate whether a causal or preventive agent prevents joint damage in the face of this injury. This is not a model for most human osteoarthritis in which symptoms occur and patients present for treatment only after considerable joint pathology exists. It is ethically impossible to test many causal agents in human osteoarthritis, so this review will infer causation when there has been a consistently found and temporally appropriate relationship between a risk factor (e.g. meniscal tear) and later onset of disease (e.g. osteoarthritis) and when that relationship has biological plausibility (for details of criteria for causality, see Chapter 2 in Rothman and Greenland3.
To demonstrate the pivotal role of abnormal mechanics in causing OA, I shall make three arguments. First, abnormal mechanics cause OA both in animals and humans. Second, once OA has developed, abnormal mechanics overwhelms all other factors in terms of leading to disease worsening. And third, inflammation in OA is mostly a consequence of abnormal mechanics and is almost never primary. This is not a systematic review but rather draws from selected research findings to make certain points. Further, except when specified, it focuses on structural disease as opposed to pain. Pain is often affected by psychosocial and other factors, making it harder to determine its causes.
Abnormal mechanics causes OA
Among the best examples that abnormal mechanics can cause OA is the widespread use of surgically induced injuries that cause OA in animal models of disease. These include ACL transection and meniscal injury models in numerous strains of animals. An intriguing example demonstrating the effect of focal areas of overload was produced by David Wu and colleagues many years ago4 in which they induced cartilage degradation in rabbits by creating 10° varus malalignment and not entering the knee jo int. These knees were then compared to the contralateral unoperated knee and for each of the rabbits, histologic changes of cartilage degradation were far greater in the operated than in the unoperated limbs, suggesting that abnormal loading caused disease.
In humans there are many examples of major joint injuries or abnormally shaped joints producing high levels of focal stress across the joint causing OA. First it has been known for at least 60 years1 that meniscal tears and meniscal removal lead to increased focal stress across the joint and subsequently high rates of OA. Menisci serve as washers to increase stability within the joint and to distribute load so that when the meniscus is intact, focal stress is kept at low levels5. When menisci are removed (or even partially removed), injury to areas of the joint where the meniscus was removed is far more likely with one large follow-up study estimating that half the knees that underwent meniscectomy during young adulthood had evidence of radiographic osteoarthritis 21 years later (vs. 7% in knees without meniscectomy) (Odds Ratio for OA development 14.0 (95% CI 3.5, 121.2)6). In fact, in joints where the meniscus has been removed and most of it is gone, the only preserved area of cartilage is the small area of the joint where the meniscus remains7. Tears of the anterior cruciate ligament are also associated with high rates of OA for reasons that are likely to do with increased compressive stress across the medial compartment of the knee where most of the disease in ACL tear patients occurs8,9. ACL tears are especially likely to lead to OA when accompanied by meniscal tears8.
While it has long been known that traumatic major tears of the meniscus in young athletes lead to high rates of later knee OA, recent evidence suggests that meniscal tears occurring in middle aged and older persons may be a common precipitant of disease. Englund et al10 showed in a population-based sample recruited without reference to knee pain that 30–60% of adults aged 50 and over had incidental meniscal tears. Many of these persons did not recall any injury to their knees. Following those with incidental meniscal tears in a later cohort study, Englund et al then demonstrated that persons with tears and no other cartilage damage were at marked increased risk of developing cartilage damage and subsequent radiographic OA11. In fact, among knees with only incidental meniscal tears, the risk of developing OA within 30 months was increased 10-fold compared to those without such tears12. Chang et al.13 demonstrated that meniscal tears did not just precede OA but they increased the risk of cartilage loss adjacent to the meniscal tear, not just cartilage loss throughout the joint. A posterior medial meniscal tear increased the risk of only posterior cartilage loss, and a tear in the body of the medial meniscus increased the risk of only adjacent cartilage loss, suggesting that meniscal tear per se increased focal stress on the underlying cartilage in that small limited region.
Meniscal tears therefore appear to be a consequence of major trauma at a young age often as an injury during sports participation but occur with minor trauma in older years. Regardless of when those tears occur, they appear to markedly increase the risk of OA by increasing focal loading or stress across adjacent areas of cartilage, leading to cartilage breakdown and subsequent changes of OA. These tears are common and confer an extremely high risk of later OA. Given the high prevalence and risk conferred, meniscal tears may account for as much as 40–50% of human knee OA. Meniscal tears serve as one of the major pieces of evidence that abnormal mechanics causes OA.
Meniscal tears are not the only common risk factor of mechanical basis that leads to high rates of knee OA. In recent work, Sharma et al.14, working with data from the MOST study, showed that knees without any cartilage damage that were from varus limbs were at high risk of subsequent cartilage loss. After adjusting for age, gender, body mass index and lateral laxity, varus knees had 3.5-fold increased odds of development of cartilage loss compared to knees without any varus deformity. This suggests, like the rabbit studies from Wu and colleagues, that malalignment causes increased stress across a focal area of the joint leading to damage there and subsequent disease. Indeed, some incidence studies looking at malalignment have shown that varus malalignment is associated not just with cartilage loss but with high rates of radiographic OA and even symptomatic disease later15,16.
If mechanical causes of knee OA are common, hip OA may serve as the best example whereby mechanical load or abnormal stresses cause almost all disease. There are at least two anatomic abnormalities that occur often in childhood that predispose to high rates of OA. On the one hand, dysplasia which can occur congenitally puts increased focal stress on a small area of the acetabulum which provides insufficient coverage for the femur. Congenital dysplasia when severe is recognized often in infancy and corrected. When modest, it is uncorrected and increases markedly the risk of hip OA occurring at a young adult age. Further, Lane and colleagues17 have shown that mild dysplasia even present in adulthood increases the risk of later life hip OA, a finding corroborated by other longitudinal studies.
A potentially more prevalent cause of increased focal stress across the hip is femoroacetabular impingement (FAI). FAI consists of a variety of anatomic abnormalities, but the most common are cam and pincer deformities, which appear to be highly prevalent in young adults18. At work presented at the 2012 OARSI meetings, groups in the Netherlands19 and investigators from the Chingford Study20,21 convincingly showed that femoroacetabular impingement seen on x-ray in these studies markedly increases the risk of later clinical hip OA, of radiographic disease, and even of the likelihood of hip replacement. Thus, evidence is quickly accumulating that anatomic abnormalities associated with femoroacetabular impingement are major risk factors predisposing to later-life hip OA, suggesting once again that mechanical abnormalities overwhelm others as causes of this disease.
If there is remaining doubt, one excellent example pointing to the importance of hip shape abnormalities is our understanding of why Chinese populations are only rarely affected by hip OA. In the Beijing OA Study, a population-based study of older adults from Beijing22, only one case of symptomatic hip OA was found among 1800 older subjects drawn from the city of Beijing. Over 25 such cases would be expected if rates in Beijing were similar to rates in Western populations. In a study of non-diseased hips drawn from Beijing and from Western populations in which morphometry was assessed, Dudda et al.23 reported that anatomical changes suggesting femoroacetabular impingement were far more common in Caucasian than in Chinese populations, the latter of which tended to have purely spherical femoral heads. Surprisingly, evidence of mild dysplasia was, if anything, more common among Chinese. Further, Chinese populations actually had higher rates of knee OA than did Western populations, suggesting that the low rate of OA in the hips was not a function of low generalized OA rates.
Knee and hip OA are not unique in being strongly related to injury and mechanics. In joints rarely affected by OA such as the ankle, major injury accounts for almost all cases of disease24.
While it is obvious that some mechanical abnormalities such as ACL and meniscal tears cause a subset of knee OA, the bigger question is whether mechanical factors account for almost all knee OA as they appear to do for hip OA. Major risk factors for knee OA according to recent reviews include: older age, female gender, obesity, knee injury and occupational overuse25,26. Other than older age and female gender which increase the vulnerability of structures within the knee to injury, all of the factors that have been identified consistently represent types for mechanical overload. For knees, obesity represents chronic excess loading, whereas knee injury produces focal increased stress. The risk of OA in joints in which there has been stereotyped repetitive use patterns typical of occupations has been well documented and represents another type of chronic excess load. For example, cotton workers have a high rate of OA in their finger joints25. Miners have a high rate in their knees and spines, jackhammer operators experience excess rates of OA in joints that are very rarely affected by disease such as elbows, wrists and metacarpalphalangeal joints. Farmers get high rates of OA in their hips and knees.
One factor that is not on the list of causes of knee or other OA is inflammation. Even though isolated studies have reported that elevated C-Reactive Protein (CRP) levels are associated with certain phenotypes of OA, large-scale studies evaluating this question have been consistently negative. For example, data from the Framingham Study, Health ABC, and Johnston County have all shown that elevated CRP levels are not associated with OA in any joint27,28.
Another piece of evidence that mechanical forces induce all or almost all human OA consists of data from genetic studies. While the heritability of OA is moderate, much of it is joint-specific. As documented by MacGregor and colleagues29, the genetic influence on radiographic OA is site-specific at hand, hip and knee, a finding that has been confirmed also in the Framingham Study30. Specifically, MacGregor et al. reported that once environmental correlations were removed from family data on OA at multiple joints, the correlations between the occurrence of OA in the distal interphalangeal joints of the hands and that of the knee was actually a r = −.008 suggesting a trivial but inverse correlation of knee OA with distal interphalangeal joint (DIP) OA. The relation of DIP joint OA with hip OA was remarkably small (r = .036) and DIP OA was not even strongly associated from a genetics perspective with OA in the adjacent thumb base. Thus, even the genetics of OA suggest that there is no systemic predisposition but rather that the genetics of OA as a joint-specific disorder is likely due to inherited joint shape predisposing to aberrantly elevated stresses across local areas of joint leading to cartilage breakdown and other changes of OA.
Once OA has developed, pathomechanics overwhelms all other factors
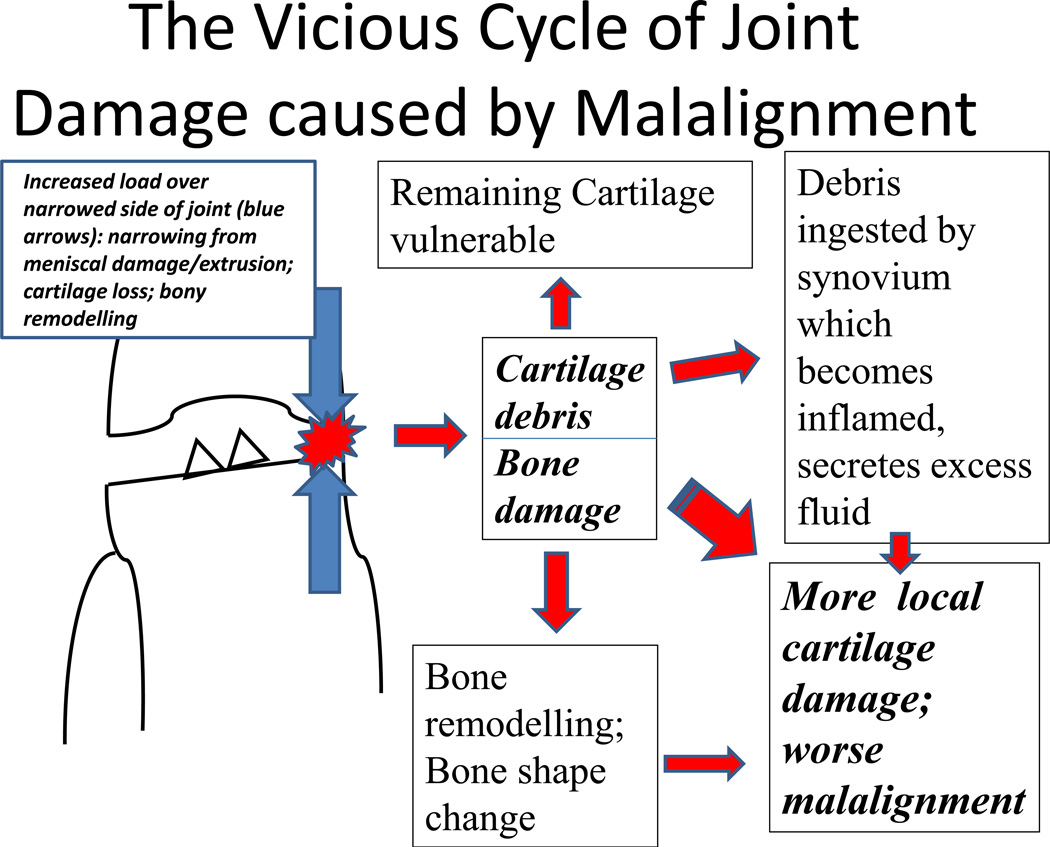
In the knee the onset of OA is accompanied by the development of either varus or valgus malalignment (depending on whether the disease develops predominantly in the medial or lateral compartment respectively). Malalignment causes a vicious circle of joint damage (see Figure 1). The narrowed area in a malaligned joint is subjected to increased load bearing that leads to increased cartilage damage, releasing debris into the joint space which then gets ingested by synovium, which becomes secondarily inflamed, secreting excess fluid. In addition to cartilage being damaged, the underlying bone undergoes remodeling and damage. The bone cortical envelope may remodel, creating more malalignment. The loss of cartilage, the damage to the meniscus which often becomes extruded, and the change in bone shape create an environment where malalignment, if anything, gets more severe. This then leads to more focal stress across the narrowed area of the joint, leading to more damage. A vicious cycle ensues.
Figure 1.
Bone marrow lesions are present underneath the cortical surface of malaligned joints. If there is varus malalignment, there is an increasing risk for medial bone marrow lesions both in the tibia and the femur. If there is valgus malalignment, bone marrow lesions tend to occur on the lateral side of the joint31. Bone marrow lesions show evidence of bone trauma with healing microfractures, adjacent osteoblasts and osteoclasts, and bone marrow necrosis. There are reversal lines in bone, suggesting microcracks32. Ironically, even though on MRI the lesions would appear to contain water, there is little edema on histology in these lesions and there are no inflammatory infiltrates. Bone marrow lesions are the structural equivalent of malalignment in the knee.
Before developing OA, knees are mostly neutrally aligned with normal mechanical axis ranging from −1 (varus) to +1 (valgus). When knees develop OA they become more malaligned by a median of 1.7° either varus or valgus (per unpublished data from MOST Study in which there are repeated long limb films on subjects). Among knees with radiographic tibiofemoral OA, only 18% of osteoarthritic knees are neutral with 82% of them being clinically malaligned33. There are more knees that are varus than valgus malaligned, and up to 20% of OA knees have severe varus malalignment of >7°. The 82% figure of the prevalence of malalignment in osteoarthritic knees does not count the substantial number of knees with patellofemoral malalignment, nor does this figure take into account the possibility of dynamic malalignment (during walking) occurring in knees which look neutral statically. Therefore, it is probable that roughly 80–90% of knees with radiographic OA have substantial degrees of tibiofemoral and/or patellofemoral malalignment which predispose these knees to increased focal load and further damage. If we assume that 82% of osteoarthritic knees have tibiofemoral malalignment and also that there is an increased risk of progression according to studies by Sharma et al34 and Felson et al.31, then the proportion of tibiofemoral disease progression due to malalignment is roughly 60%. This does not necessarily include dynamic malalignment when walking which has also been shown to markedly increase the risk of disease progression35. It also does not count other causes of progression that are also mechanically driven such as meniscal tears or extrusion or possible sources of dynamic laxity in the knee. Thus, the preponderance of progression of extant knee OA is mechanically driven.
One way of evaluating whether mechanical factors or inflammatory factors are more important in determining the course of OA is to evaluate the effects of correcting each of these abnormalities. High tibial osteotomy (HTO) surgery corrects malalignment without entering the knee. In a classic study of the effects of high tibial osteotomy, Prodromos et al36 reported that of patients who underwent HTOs and who had reduction in their dynamic varus momen37t, there were no clinical failures based on Hospital for Special Surgery rating scale38 over an average of 3.2 years follow-up. In contrast, among patients who underwent this surgery but whose varus moment was not reduced, only 50% had satisfactory results.
When we compared this correction of mechanical abnormalities with correction of the inflammation that occurs in OA, we find stark differences. Among the most potent anti-inflammatories available for treatment are corticosteroids which can be injected intra-articularly. In studies evaluating the efficacy of intra-articular steroid vs. placebo injection, there has been a consistent finding39 that steroids work better than placebo but that this effect is not durable. The efficacy of steroids exceeds placebo for 1–2 weeks after the injection and then wears off. Admittedly steroids are not supposed to be a permanent cure, but a substantial minority of patients do not have even temporary responses to intraarticular steroids, suggesting that at least in them, inflammation plays at best a minor role in their symptoms. In terms of structural disease, Myers et al37 showed that continuous steroid treatment of dogs with induced OA did not have any effect on long term structural changes in their OA. Therefore, treatment to correct inflammation vs. mechanics provides us with stark information about what the real cause of OA is. Correcting abnormal mechanics corrects and alleviates the problem for many years. Correcting the increased inflammation has only a transient effect on disease because inflammation is not central to OA pathogenesis.
Inflammation in osteoarthritis is mostly a consequence of pathomechanics
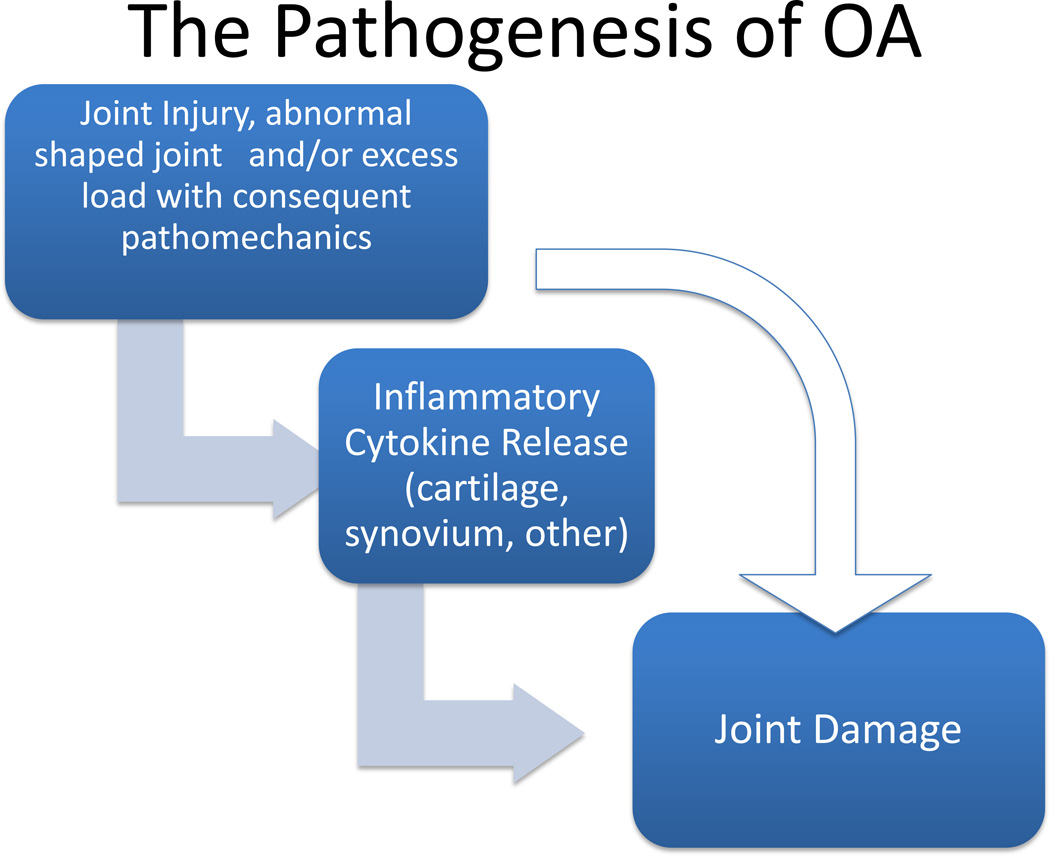
Many joints with OA have evidence of inflammation with synovitis or with inflammatory cytokines present in the cartilage matrix; generally, some inflammation on a microscopic level is present. The hypothesized role of inflammation is shown in Figure 2. Generally, joint injury produces injury into the joint and consequent pathomechanics. That injury can then lead to release of cytokines and even infiltration of inflammatory cells within the synovium. The joint injury may work on its own to cause joint damage without any involvement from the inflammation that has been produced or the inflammation can accelerate or magnify the injury that is produced by pathomechanics.
Figure 2.
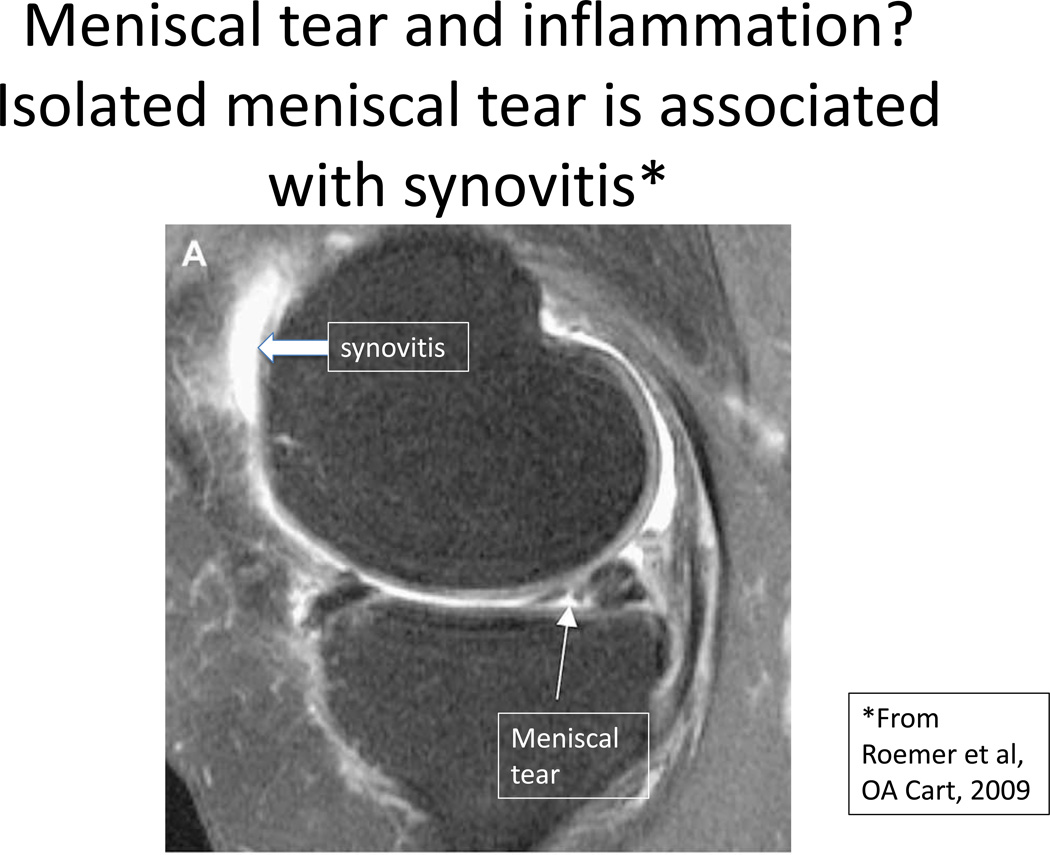
Evidence from multiple clinical studies already shows considerable evidence that injury to the joint can lead to secondary inflammation. For example, in a magnetic resonance imaging scan40 in a paper on the co-occurrence of meniscal tears and synovitis (Figure 3), Roemer et al showed even without any other lesions in the knee, that the presence of a meniscal tear was associated with isolated synovitis suggesting that the tear brought on that secondary synovitis. As Higuchi and colleagues noted41, most persons after an ACL tear develop high levels of interleukin 6 in their synovial fluids, levels far higher than are seen in normals.
Figure 3.
The issue with respect to inflammation is not whether inflammation is present within osteoarthritic joints. It is to a variable extent. Rather the issue is how much and whether inflammation contributes to the joint damage experienced as a consequence of pathomechanics. While in animal models and in vitro studies, inflammatory cytokines accelerate the degradation of cartilage when it is subjected to damage from mechanical forces, it is not clear whether and how much these inflammatory mediators affect the human osteoarthritic joint or whether they play a major role in joint damage. Animal models of OA replicate mostly posttraumatic OA, occurring on the substrate of a normal joint. This is true even of the remarkable MRL/MpJ mice that despite intraarticular fractures do not get OA42. Osteoarthritic joints in humans are joints that, as noted above, have already sustained damage and where pathomechanics predominate. The role of inflammation in contributing to the further destruction of the human osteoarthritic joint is likely, but has not been proven. The strikingly focal nature of damage in OA and our ability to explain this focal injury by invoking mechanical explanations without any inflammation suggests that abnormal mechanics is still the basis for human OA.
Acknowledgements
We appreciate the technical assistance of Anne Plunkett
Supported by NIH AR47785
Role of Funding source: The funder of this work played no role in its creation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper is derived from a debate at the 2012 OARSI meetings in Barcelona on whether osteoarthritis is a disease of mechanics or an inflammatory disease.
Contributions: Dr. Felson was responsible for conceptualization and writing this paper.
Competing Interests: There are no competing interests.
References
- 1.Fairbank TJ. Knee joint changes after meniscectomy. Journal of Bone and Joint Surgery British Volume. 1948;30B:664–670. [PubMed] [Google Scholar]
- 2.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3:261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 3.Rothman KJ, Greenland S. Causation and causal inference. In: Rothman KJ, Greenland S, editors. Modern Epidemiology. Chapter 2. Philadelphia: 1998. pp. 7–28. [Google Scholar]
- 4.Wu DD, Burr DB, Boyd RD, Radin EL. Bone and cartilage changes following experimental varus or valgus tibial angulation. Journal of Orthopaedic Research. 1990;8:572–585. doi: 10.1002/jor.1100080414. [DOI] [PubMed] [Google Scholar]
- 5.Fu FF, Thompson WO. Motion of the meniscus during knee flexion. In: Mow VC, Arnoczky SP, Jackson DW, editors. Knee Meniscus: Basic and Clinical Foundations. Chapter 6. New York: 1992. pp. 75–89. [Google Scholar]
- 6.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis and Rheumatism. 1998;41:687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Fahmy NR, Williams EA, Noble J. Meniscal pathology and osteoarthritis of the knee. Journal of Bone and Joint Surgery British Volume. 1983;65:24–28. doi: 10.1302/0301-620X.65B1.6687393. [DOI] [PubMed] [Google Scholar]
- 8.Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. American Journal of Sports Medicine. 2009;37:1434–1443. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Medicine and Science in Sports and Exercise. 2008;40:215–222. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- 10.Englund M, Guermazi A, Gale D, Felson DT. Incidental meniscal findings on knee MRI in middle aged and elderly persons in the United States. New England Journal of Medicine. 2008;359:1108–1115. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Englund M, Guermazi A, Roemer F, Felson DT. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons. The MOST Study. Arthritis Rheum. 2009;60:831–839. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Englund M, Guermazi A, Lohmander LS. The meniscus in knee osteoarthritis. Rheumatic Diseases Clinics of North America. 2009;35:579–590. doi: 10.1016/j.rdc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Chang A, Moisio K, Chmiel JS, Eckstein F, Guermazi A, Almagor O, et al. Subregional effects of meniscal tears on cartilage loss over 2 years in knee osteoarthritis. Annals of the Rheumatic Diseases. 2011;70:74–79. doi: 10.1136/ard.2010.130278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma L, Chmiel JS, Almagor O, Felson D, Guermazi A, Roemer F, et al. The role of varus and valgus alignment in the initial development of knee cartilage damage by MRI: the MOST study. Annals of the Rheumatic Diseases. 2012 doi: 10.1136/annrheumdis-2011-201070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma L, Song J, Dunlop D, Felson D, Lewis CE, Segal N, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Annals of the Rheumatic Diseases. 2010;69:1940–1945. doi: 10.1136/ard.2010.129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouwer GM, van Tol AW, Bergink AP, Belo JN, Bernsen RM, Reijman M, et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis and Rheumatism. 2007;56:1204–1211. doi: 10.1002/art.22515. [DOI] [PubMed] [Google Scholar]
- 17.Lane NE, Lin P, Christiansen L, Gore LR, Williams EN, Hochberg MC, et al. Association of mild acetabular dysplasia with an increased risk of incident hip osteoarthritis in elderly white women: the study of osteoporotic fractures. Arthritis and Rheumatism. 2000;43:400–404. doi: 10.1002/1529-0131(200002)43:2<400::AID-ANR21>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 18.Reichenbach S, Juni P, Werlen S, Nuesch E, Pfirrmann CW, Trelle S, et al. Prevalence of cam-type deformity on hip magnetic resonance imaging in young males: a cross-sectional study. Arthritis Care Res (Hoboken) 2010;62:1319–1327. doi: 10.1002/acr.20198. [DOI] [PubMed] [Google Scholar]
- 19.Agricola R, Heijboer M, Bierma-Zeinstra S, Verhaar J, Weinans H, Waarsing J. Cam-type deformities strongly predict total hip replacement within 5 years in those with early symptomatic OA: A prospective cohort study (check) (Abstract) Osteoarthritis and Cartilage. 2012;20:S203-. [Google Scholar]
- 20.Thomas GE, Batra RN, Kivan A, Pennant S, Hart D, Spector TD, et al. The association between hip morphology and 19-year risk of osteoarthritis in the hip (Abstract) Osteoarthritis and Cartilage. 2012;20:S23–S24. [Google Scholar]
- 21.Thomas GE, Kiran A, Batra RN, Hart D, Spector T, Taylor A, et al. The association between hip morphology and end-stage osteoarthritis at 12-year followup (Abstract) Osteoarthritis and Cartilage. 2012;20:S204-. [Google Scholar]
- 22.Nevitt MC, Xu L, Zhang Y, Lui LY, Yu W, Lane NE, et al. Very low prevalence of hip osteoarthritis among Chinese elderly in Beijing, China, compared with whites in the United States: the Beijing osteoarthritis study. Arthritis and Rheumatism. 2002;46:1773–1779. doi: 10.1002/art.10332. [DOI] [PubMed] [Google Scholar]
- 23.Dudda M, Kim YJ, Zhang Y, Nevitt MC, Xu L, Niu J, et al. Morphological differences between Chinese and Caucasian female hips: Could they account for the ethnic difference in hip osteoarthritis? Arthritis Rheum. 2011 doi: 10.1002/art.30472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800–1806. doi: 10.1007/s11999-008-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felson DT. Obesity and vocational and avocational overload of the joint as risk factors for osteoarthritis. J Rheumatol Suppl. 2004;70:2–5. [PubMed] [Google Scholar]
- 26.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Vlad SC, Neogi T, Aliabadi P, Fontes JD, Felson DT. No association between markers of inflammation and osteoarthritis of the hands and knees. J Rheumatol. 2011;38:1665–1670. doi: 10.3899/jrheum.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott AL, Kraus VB, Luta G, Stabler T, Renner JB, Woodard J, et al. Serum hyaluronan levels and radiographic knee and hip osteoarthritis in African Americans and Caucasians in the Johnston County Osteoarthritis Project. Arthritis and Rheumatism. 2005;52:105–111. doi: 10.1002/art.20724. [DOI] [PubMed] [Google Scholar]
- 29.MacGregor A, Li Q, Spector TD, Williams FMK. The genetic influence on radiographic osteoarthritis is site specific at the hand, hip and knee. Rheumatology. 2009:277–280. doi: 10.1093/rheumatology/ken475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter DJ, Niu JB, Felson DT, Harvey WF, Gross KD, McCree P, et al. Knee alignment does not predict incident osteoarthritis: the Framingham osteoarthritis study. Arthritis Rheum. 2009;56:1212–1218. doi: 10.1002/art.22508. [DOI] [PubMed] [Google Scholar]
- 31.Felson DT, McLaughlin S, Goggins J, Lavalley MP, Gale ME, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Annals of Internal Medicine. 2003;139:330–336. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 32.Taljanovic MS, Graham AR, Benjamin JB, Gmitro AF, Krupinski EA, Schwartz SA, et al. Bone marrow edema pattern in advanced hip osteoarthritis: quantitative assessment with magnetic resonance imaging and correlation with clinical examination, radiographic findings, and histopathology. Skeletal Radiology. 2008;37:423–431. doi: 10.1007/s00256-008-0446-3. [DOI] [PubMed] [Google Scholar]
- 33.Niu J, Zhang YQ, Torner J, Nevitt M, Lewis CE, Aliabadi P, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329–335. doi: 10.1002/art.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Annals of the Rheumatic Diseases. 2002;61:617–622. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prodromos CC, Andriacchi TP, Galante JO. A relationship between gait and clinical changes following high tibial osteotomy. J Bone Joint Surg Am. 1985;67:1188–1194. [PubMed] [Google Scholar]
- 37.Myers SL, Brandt KD, O'Connor BL. Low dose prednisone treatment does not reduce the severity of osteoarthritis in dogs after anterior cruciate ligament transection. Journal of Rheumatology. 1991;18:1856–1862. [PubMed] [Google Scholar]
- 38.Insall JN, Ranawat CS, Aglietti P, Shine J. A comparison of four models of total knee-replacement prostheses. Journal of Bone and Joint Surgery. 1976;58:754–765. [PubMed] [Google Scholar]
- 39.Bellamy N, Campbell J, Welch V, Gee T, Bourne R, Wells GA. Intraarticular corticosteroid for treatment of osteoarthritis of the knee: The Cochrane Collaboration. 2009:1–220. [Google Scholar]
- 40.Roemer F, Guermazi A, Hunter DJ, Niu J, Zhang Y, Englund M, et al. The association of meniscal damage with joint effusion in persons without radiographic osteoarthritis: the Framingham and MOST osteoarthritis studies. Osteoarthritis and Cartilage. 2009;17:748–753. doi: 10.1016/j.joca.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higuchi H, Shirakura K, Kimura M, Terauchi M, Shinozaki T, Watanabe H, et al. Changes in biochemical parameters after anterior cruciate ligament injury. International Orthopaedics. 2006;30:43–47. doi: 10.1007/s00264-005-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis and Rheumatism. 2008;58:744–753. doi: 10.1002/art.23288. [DOI] [PubMed] [Google Scholar]