Abstract
This study was conducted to examine the interactions among the innate and adaptive immune components of the liver parenchyma during acute viral hepatitis. Mice were i.v. infected with a recombinant adenovirus, and within the first 24 h of infection, we found a transient, but significant, accumulation of IL-17 and IL-23 in the liver. In vivo neutralization of these interleukins alleviated the liver injury. Further investigations showed that IL-17 neutralization halted the intrahepatic accumulation of CTL and Th1 cells. A majority of the IL-17-producing cells in the liver were γδ T cells. Additionally, intrahepatic IL-17+ γδ T cells, but not the IFN-γ+ ones, preferentially expressed IL-7Rα (CD127) on their surface, which coincided with an elevation of hepatocyte-derived IL-7 at 12 h post-infection. IL-7Rα blockade in vivo severely impeded the expansion of IL-17-producing cells following viral infection. In vitro, IL-7 synergized with IL-23 and directly stimulated IL-17 production from γδ T cells in response to TCRγδ stimulation. Finally, type I interferon (IFN-I) signaling was found to be critical for hepatic IL-7 induction. Collectively, these results showed that the IFN-I/IL-7/IL-17 cascade was important in priming T cell responses in the liver. Moreover, the highly coordinated cross talk among hepatocytes and innate and adaptive immune cells played a critical role in antiviral immunity in hepatitis.
INTRODUCTION
Viral hepatitis is one of the most common health problems in the world. Many viruses (e.g. hepatitis B and C viruses, cytomegalovirus, and adenovirus) can cause liver inflammation, which is often characterized by various degrees of CTL/Th1 responses. Among these viruses, adenovirus (Ad) is a prototypical DNA virus and an important pathogen. It is also one of the preferred vectors for gene therapy, cancer therapy and experimental vaccines (1). When i.v. injected, the virus preferentially targets the liver and encounters the host defense system. Strikingly, a majority of the viruses have been found to be eliminated by innate immune mechanisms within 24 h (2). However, in subsequent periods, viral elimination reportedly slowed, while disease resolution depended on virus-specific CTL and Th1 responses (3, 4). Additionally, it was reported that overzealous T cell responses can result in necroinflammatory hepatitis, treatment failure, and even patient death (5). On the other hand, immunodeficiency in nude mice or administration of immunosuppressants (e.g., cyclosporine A, dexamethasone and FK506) was indicated to significantly prolong the presence of the virus in infected mouse liver (6, 7). Several types of innate cells (NK, NKT, neutrophils, Kupffer cells, and γδ T cells) contribute to antiviral defense and pathogenic processes (8–10). Among them, γδ T cells have been reported to play a critical role in the pathogenesis of Ad-induced hepatitis by their production of IFN-γ, thereby promoting the recruitment of CTLs to the liver (11). However, considering that NK cells are potent and quick IFN-γ producers in the early phase of Ad-induced hepatitis (12), the role of γδ T cells should not be solely restricted to IFN-γ production. Additionally, it remains unclear mechanistically how these non-classical γδ T cells interact with APCs to engage the adaptive arm of the immune system.
The pro-inflammatory cytokine IL-17 is secreted by CD4+ Th17 cells under TCR engagement and can induce the production of numerous other cytokines and chemokines from many cell types. IL-17 is typically associated with destructive tissue damage in autoimmune diseases and bacterial and fungal infections (13). IL-17 could also function as an important regulator orchestrating Th1 and CTL responses in inflammatory bowel disease (14), anti-tumor immunity (15), and intracellular bacterial infections (16). Activated CD4+ T cells were initially deemed the major producers of IL-17 (17, 18). However, γδ T cells can be the main source of IL-17 under certain circumstances (19–26). Differing from the conventional Th17 cells, whose differentiation depends on the presence of TGF-β, IL-6 and IL-23, IL-17-producing γδ T cells seem to differentiate in the thymus independently of IL-6 (27–29). Additionally, in the peripheral, IL-17 production by γδ T cells does not need TCR engagement, but can be merely triggered by IL-23 and other inflammatory factors such as IL-1β (30). In mice, IL-17+ γδ T cells are restricted to the Vγ4 and Vγ6Vδ1 subsets (31, 32). Also, these cells can be phenotypically distinguished from their IFN-γ+ counterparts through cell surface molecules. For example, IFN-γ+ γδ T cells express CD27 (28) and CD122 (33), whereas IL-17+ ones do not. Recently, IL-17 production has been reported in HIV (34), hepatitis B and C infections (35, 36). In mice injected with Con A, which leads to a profound immune activation and fulminant hepatitis, IL-17 has been implicated as a pro-inflammatory regulator, but also more recently as an anti-inflammatory regulator (37, 38). These reports have raised several interesting questions. Do γδ T cells secrete IL-17 in virus-induced hepatitis, especially during the early stage? If so, what role do these cells play in antiviral immune responses?
Another important question in this and other types of viral hepatitis is whether hepatocytes, the most abundant and metabolically active cell type in the liver, participate in these crucial immunological dialogues or not. Our previous reports showed that hepatocytes could regulate the anti-viral reaction in the liver through the expression of some co-stimulatory molecules, including CD40 and CD86 (4, 39). More recently, Sawa et al. reported that hepatocytes could produce IL-7, a potent immune-stimulatory cytokine, in response to IFN-I stimulation, and that hepatocyte-derived IL-7 was pivotal for Th17 cells development in an autoimmune model (40). Also, in vivo blocking IL-7Rα could preferentially suppress Th17 development and IL-17-mediated murine autoimmune disease (41). However, the definite role of IL-7 on IL-17-producing γδ T cells awaits further elucidation.
Given that the role of IL-17 in viral hepatitis is still elusive, and early intrahepatic events are extremely crucial to the development of protective immune responses and disease outcome, we decided to examine the effects and regulation of intrahepatic IL-17-producing cells following an adenovirus infection. Here, we used in vivo antibody neutralization and demonstrated that IL-17 played a critical role in initiating successful anti-viral CTL reactions. In addition, IL-7Rα+ γδ T cells, but not Th17 cells, were the major IL-17 producers. Finally, we demonstrated that IFN-I was pivotal for inducing IL-7 production from hepatocytes following Ad infection, which might be responsible for the IL-17 production from γδ T cells. This study collectively showed that, in addition to the expression of co-stimulatory molecules, hepatocytes could also orchestrate the adaptive anti-viral immune response by secreting IFN-I and IL-7.
MATERIALS AND METHODS
Animals
Female C57BL/6 (B6) mice were purchased from the Jackson Laboratory. Breeding pairs of IFN-α receptor (IFNAR) −/− mice from the 129/Sv background were kindly provided by Dr. Herbert Virgin (Washington University School of Medicine, St. Louis, MO). Wild-type 129/SvEv mice were purchased from Taconic (Germantown, NY). All mice were maintained and bred under specific pathogen-free conditions at the UTMB animal care facility. Mice were used at 7–10 wk of age according to NIH Guidelines and with the approval of the UTMB Institutional Animal Care and Use Committee. Ad carrying the lacZ gene (AdLacZ) was used to induce hepatitis, as described previously (42). The liver histological evaluations were performed and scored as described previously (4, 39).
In vivo neutralization of IL-17 and IL-23 and blocking of IL-7Rα
To block the effects of IL-17 or IL-23, we injected the mice i.p. with 100 µg mAb against IL-17 or IL-23p19 (clones 50104 and 320229, respectively, R&D system) at day -1, 0, 1 and 3 post-infection. To block the effects of IL-7, we injected mice i.p. with 100 µg anti-mIL-7Rα mAb (clone: SB/14, BD Bioscience) at days -1 and 0 and euthanized at day 1 post infection. Normal rat IgG (Sigma) was administered as an isotype control.
In vivo IFN-β and IFN-α treatment
To examine the effect of IFN-I on intrahepatic immune responses, we directly challenged mice i.v. with IFN-β (10,000 IU; PBL InterferonSource) or IFN-α (1000 IU; HyCult, Plymouth Meeting, PA), as described previously (43). Animals were euthanized at 5 h post-challenge. After perfusion with cold PBS to remove the blood, livers were collected for subsequent analyses.
Primary hepatocyte isolation and culture
Primary hepatocytes were isolated from C57BL/6 mice by an adaptation of a two-step collagenase perfusion technique (44). In brief, the mouse liver was perfused with HBSS (pH 7.4, without calcium and magnesium) containing 1 mM EGTA and 10 mM HEPES for 10 min, followed by HBSS with calcium and magnesium plus collagenase D (Roche Applied Science, Indianapolis, IN) for 10 min at 37°C. The digested liver was then excised, rinsed, and disaggregated in a 150-mm polystyrene Petri dish. Subsequently, the disaggregated material was filtered through a 70-µm cell strainer, and the filtrate was gently centrifuged for 3 min at 50 g. The pellet was re-suspended in 45% Percoll in PBS and re-centrifuged at 50 g for 10 min. After enrichment by Percoll isodensity purification, the cells were washed and gently centrifuged, and the pellets were re-suspended in the DEME supplemented with 10 mM HEPES, 2 mM L-glutamine, ITS (Sigma) and 10% fetal bovine serum. The viability of the cells was assessed by the trypan blue dye exclusion method. The cells were placed (2.5 × 105 cells/3.0 ml attachment media) in 6-well plates (Corning, Corning, NY) pre-coated with collagen.
Isolation of intrahepatic lymphocytes
Intrahepatic lymphocytes were isolated according to our previous method with slight modifications (4, 39). Briefly, liver tissues were pressed and distributed and then collected in complete RPMI-1640. After washing (300 g, 10 min), cell suspensions were re-suspended in complete RPMI-1640 containing collagenase IV (0.05%, Roche) at 37°C for 30 min. After digestion, cell suspensions were passed through 70-µm nylon cell strainers to yield single-cell suspensions. Intrahepatic mononuclear cells were isolated by centrifugation (400 g) at room temperature for 30 min over a 30/70% discontinuous Percoll gradient (Sigma). The cells were collected from the interphase, thoroughly washed, and re-suspended in complete RPMI-1640 containing 10% FBS (HyClone, Logan, UT).
Splenic γδ T cell purification and stimulation
Splenocytes were processed through immuno-magnetic negative selection to deplete TCRαβ T cells, B220+ cells, NK cells and I-A+ APCs, as described previously (45), and then positively selected through FITC-conjugated anti-TCRγδ (GL-3) and anti-FITC MicroBeads (Miltenyi Biotech, Germany). The purity of resultant cells was determined by flow cytometry and was consistently higher than 85%. Purified γδ T cells were stimulated with plate-coated anti-TCRγδ (GL-4, 5 µg/ml, BD Bioscience), together with a combination of recombinant mouse IL-1β (5 ng/ml), IL-7 (20 ng/ml), IL-23 (20 ng/ml, all from eBioscience), TNF-α (5 ng/ml, Peprotech), or recombinant human IFN-β (3 ng/ml, Peprotech). These concentrations of cytokines were selected according to our preliminary experiments and in vitro Th17 cell differentiation conditions (data not shown). Culture supernatants were collected at 48 h and stored at −20°C for ELISA assays. For detecting proliferation, after a 48-h incubation, cells were pulsed with 1 µCi/well of 3H-thymidine and incubated for another 8 h. Cells were then harvested onto glass fiber filters, and incorporated radioactivity was counted by using a Beta Scintillation Counter (MicroBeta Trilux, MA).
Intracellular staining
Methods for intracellular staining were consistent with previous reports (4, 45). Cells were incubated for 4 h with PMA (50 ng/ml) and ionomycin (750 ng/ml). For the simultaneous detection of surface CD107a/b (LAMP-1/2) and intracellular cytokines, cells were stimulated by plate-coated anti-CD3 mAb (145–2C11, 10 µg/ml, eBioscience) for 4 h, in the presence of GolgiStop (BD Bioscience). At the end of incubation, cells were collected and blocked with FcγR blocker before extracellular staining for corresponding fluorochrome-labeled surface mAb. After surface staining, cells were fixed, permeabilized and stained for cytokines by using a fixation/permeabilization kit (eBioscience).
Flow cytometry
Murine lymphocytes were blocked with anti-mCD16/CD32 (eBioscience) and stained with fluorochrome-labeled antibodies or biotinylated mAbs, followed by fluorochrome-conjugated streptavidin, collected by LSRII FACSFortessa (Becton Dickinson, San Jose, CA), and analyzed by using FlowJo software (TreeStar, Ashland, OR). The following staining panels were used: Respectively staining the intracellular IL-17, IFN-γ or Ki-67: PE-Cy7-anti-mCD3, APC-anti-mTCRγδ, Pacific blue-anti-mCD4, PerCp-Cy5.5-anti-mCD8, FITC-anti-mCD127, and PE-anti-mIL-17 or PE-anti-mIFN-γ or PE-anti-m/rKi-67. We stained IL-17 and IFN-γ simultaneously with PE-Cy7-anti-mCD3, FITC-anti-mIFN-γ, PE-anti-mIL-17, APC-anti-mTCRγδ or other fluorochrome-labeled anti-CD4, CD8, and NK1.1. Intracellular IFN-γ and surface CD107a/b were simultaneously stained with PE-Cy7-anti-mCD3, PerCp-Cy5.5-anti-mCD8, PE-anti-mIFN-γ, Alexa Fluor 488-anti-CD107a and Alexa Fluor 488-anti-CD107b. For staining the subsets of γδ T cells, cells were blocked with FcR blockers, and then stained with Biotin-labeled anti-Vγ1, and washed, which was followed by avdin-PerCp-Cy5.5. After washing, the cells were stained with PE-Cy7-anti-mCD3, APC-anti-mTCRγδ, FITC-anti-mVγ4. For intracellular staining, PE-anti-mIL-17 was applied. For staining the Vγ5 subset of γδ T cells, cells were blocked with FcR blockers and then were stained with anti-Vγ5 and washed, followed by FITC-labeled goat-anti-RatIgG. After washing, the cells were stained with PE-Cy7-anti-mCD3, and APC-anti-mTCRγδ.
All fluorochrome-labeled mAbs and their corresponding isotype controls were purchased from BD Pharmingen (San Diego, CA) and eBioscience. The mAb for Vγ1 (clone: 2.11) was kindly provided by Dr. Rebecca O’Brien at the National Jewish Medical and Research Center, Denver. The mAb for Vγ5 was clone 17D1, a kind gift from Dr. Robert Tigelaar at Yale School of Medicine. If used alone, it stains Vγ5, but in the presence of anti-TCRγδ (clone: GL-3) clone 17D1 stains Vγ6δ1. In all two-step surface staining and intracellular staining, isotype controls were strictly used.
Western blot assay
Proteins were extracted by homogenization with a syringe plunger on ice from frozen liver tissues in a lysis buffer. Subsequently, equal amounts of proteins were loaded onto 10% SDS-polyacrylamide gels and then transferred onto polyvinylidene difluoride membranes (BioRad Laboratories). Membranes were incubated with goat anti-mouse IL-23p19, IL-17 (clones 320229 and 50104, respectively, R&D system), or anti-β-actin (clone AC-15, Sigma), followed by HRP-conjugated secondary Abs for 1 h. Blots were visualized by enhanced chemiluminescence (ECL-Plus, Amersham Biosciences, Piscataway, NJ).
Real-time PCR
Total RNA was extracted from the liver and other tissues (e.g., the lungs, heart, kidneys, spleen, bone marrow, and thymus) with an RNAqueous kit and digested with DNase I (Ambion, Austin, TX). For relative quantitation of the cytokine and chemokine mRNA levels, cDNA was prepared from 1 µg of RNA by using a Taqman Reverse Transcription Kit (Bio-Rad), and 2 µl of the cDNA was amplified in a 25-µl reaction mixture containing 12.5 µl of DNA Master SYBR Green 1 (Roche) and 0.9 µM each of gene-specific forward and reverse primers. The qRT-PCR assays were performed in triplicate with the SYBR Green PCR Master Mix (ABI 4364344), as specified by the manufacturer. The PCR assays were denatured for 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. The PCR was performed with the ABI Prism 7000 Sequence Detection System. Relative quantitation of mRNA expression was calculated as the fold increase in expression by using the ΔΔCt method. PCR products were subjected to melting curve analysis to assure that a single amplification product was produced. To assess IL-7 expression among different tissues, we used the method described by Sawa et al. (40). To detect the viral DNA copy numbers, DNA was isolated from the livers and PCR amplification was done according to our previous description (4). The sequences of the forward and reverse gene-specific primers are listed in Supp. Table I.
ELISA assays
The levels of IL-17A in serum or culture supernatant were assayed by using an ELISA kit (eBioscience) according to the manufacturer’s instructions. Detection limit of IL-17A was 1.6 pg/ml.
Statistical analysis
For statistical analyses, the 2-tailed student t test was used. Analysis of variance was performed by using ANOVA. The p values < 0.05 were considered significant* and < 0.01, as highly significant**.
RESULTS
IL-17 and IL-23 production increased at an early stage in Ad-induced hepatitis
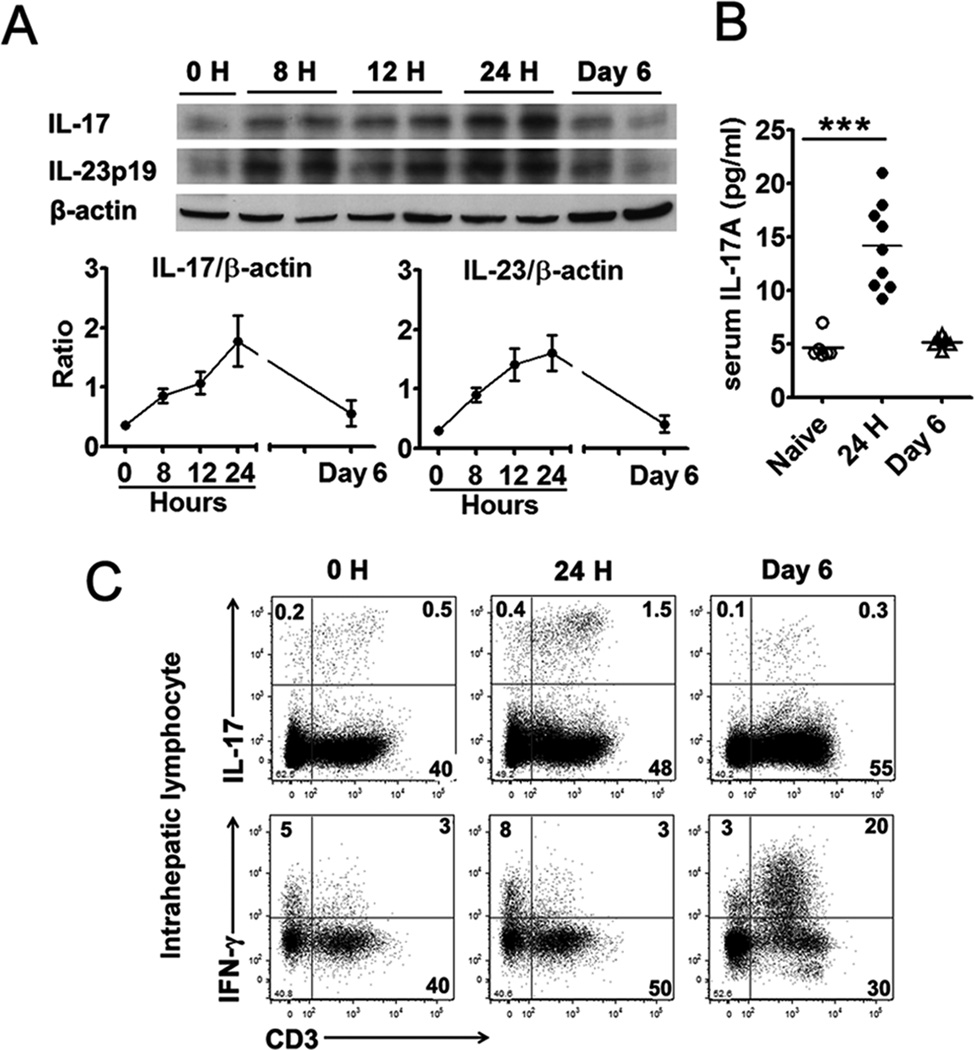
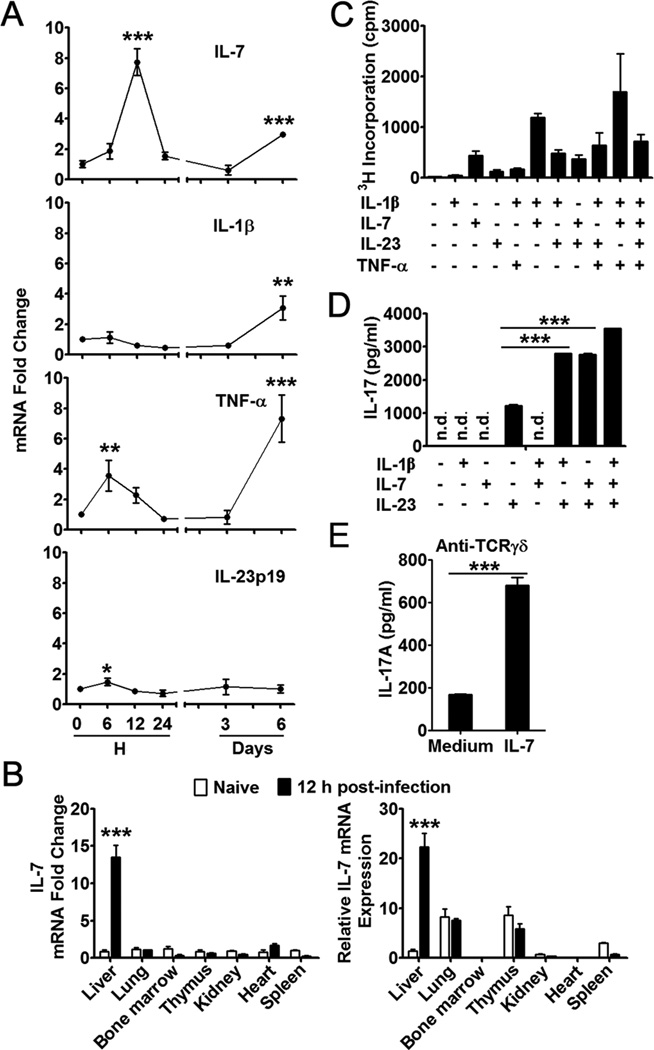
Intravenous injection of Ad can induce acute hepatitis, which reaches its peak in B6 mice at around days 5–6; the clinical and pathological manifestations of liver inflammation resolve spontaneously about 3 to 4 weeks thereafter (3). To determine the role of IL-17 and IL-23 in this model, we injected i.v. B6 mice with 2 × 109 pfu of Ad carrying the lacZ gene (AdLacZ), as described previously (4). The animals were sacrificed at 0, 8, 12 and 24 h, and 6 days post-infection. Western blot analysis revealed a significant accumulation of IL-17 in the liver during the first 24 h (Fig. 1A). A parallel elevation of IL-23p19, an important cytokine to induce IL-17 production, was also observed. An ELISA assay confirmed that the intrahepatic surge of IL-17 was accompanied by its increase (3 fold) in the serum at the same time (Fig. 1B).
Figure 1. Ad infection induced IL-17 and IL-23 production in the liver during the initial 24 hours.
C57BL/6 mice were injected i.v. with 2 × 109 pfu of AdLacZ and sacrificed at the indicated time points. The serum was prepared and liver tissues were isolated after perfusion. A) Western blot analysis of protein levels of IL-17A and IL-23p19 in the liver. Top panel, representative western blot; Bottom panel, graphical representation of the individual band densities normalized to that of the respective β-actin band. One blot was the mix of two livers, and the experiment was repeated twice with a similar pattern. B) Serum IL-17 levels detected by an ELISA. N = 6 to 9 mice per group. C) IHLs were isolated and examined for IL-17 and IFN-γ by flow cytometry. Results were pooled from 3 independent experiments with similar results. N = 3 in each time point.
To examine the source of IL-17 in the liver, we isolated the IHLs and analyzed these cells by flow cytometry. As shown in Fig. 1C, most IL-17-producing cells in the liver were CD3+ T lymphocytes, and these cells expanded from 0.5% at 0 h to 1.5% at 24 h post-infection. This surge of IL-17+ T cells was liver-specific, since their frequencies remained low (0.1%) in the spleen, either on day 1 or day 6 (Supp. Fig. 1A). While IFN-γ+ T cells did not expand during the first 24 h post-infection, either in the liver or the spleen (Fig. 1C and Supp. Fig. 1A), they accumulated considerably in the liver at 6 days post-infection (comparing 3% vs. 20% in Fig. 1C). Therefore, Ad infection triggers a liver-specific production of IL-17 and IL-23 at day 1, followed with IFN-γ production at day 6.
Neutralization of IL-17 or IL-23 ameliorated Ad-induced hepatitis
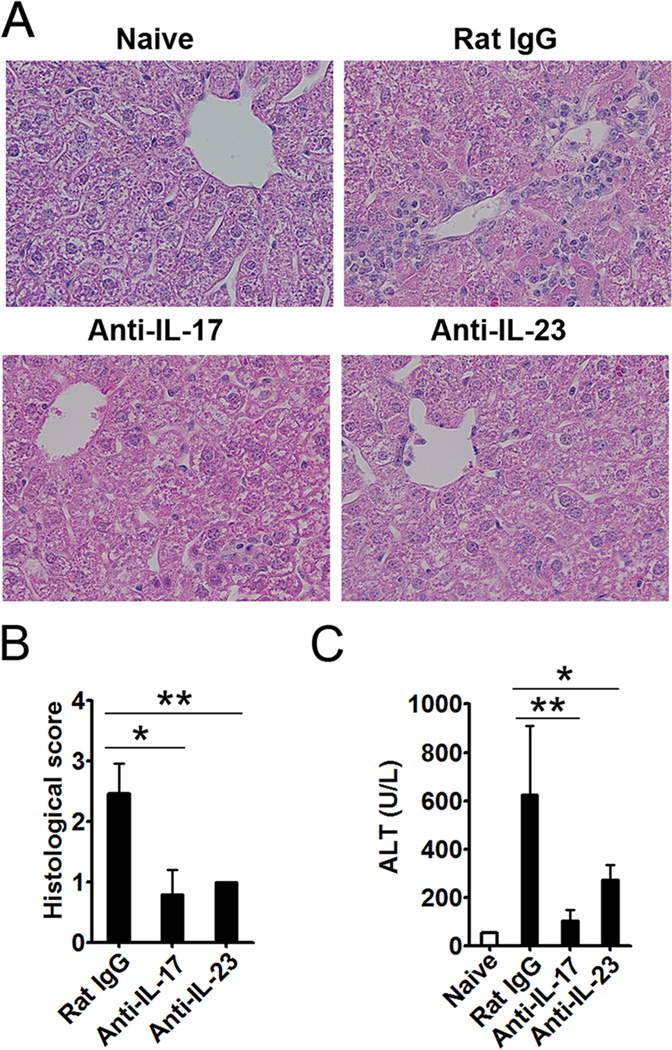
To determine whether this early elevation of IL-17 and IL-23 affects liver injury, we injected the mice i.p. with anti-IL-17 or anti-IL-23 mAb on days -1, 0, 1 and 3 following Ad infection. The mice treated with isotype control Ab developed hepatitis, characterized by inflammatory infiltration, hepatocytes with megaloblastic changes, and single-cell necrosis, at 6 days post-infection (Fig. 2A). The animals treated with anti-IL-17 or -IL-23 mAb, on the other hand, displayed milder liver inflammation and lower pathological scores (Fig. 2B). IL-17 or IL-23 neutralization also greatly reduced the serum ALT levels (Fig. 2C). These data demonstrated that IL-17 or IL-23 neutralization could alleviate liver injury.
Figure 2. IL-17 and IL-23 neutralizations alleviated the liver injury induced by Ad infection.
C57BL/6 mice were treated i.p. with anti-IL-17, anti-IL-23, or isotype control (Rat IgG2a) mAb at days -1, 0, 1, and 3 following Ad infection. The serum was prepared, and liver tissues were isolated after the perfusion. A) Paraffin-embedded sections were stained with H&E for histological evaluation, shown are representative pictures. B) Cumulative graphical representation of the histological scores. C) Serum ALT levels. N = 6 to 8 mice per group. *P < 0.05; **P < 0.01.
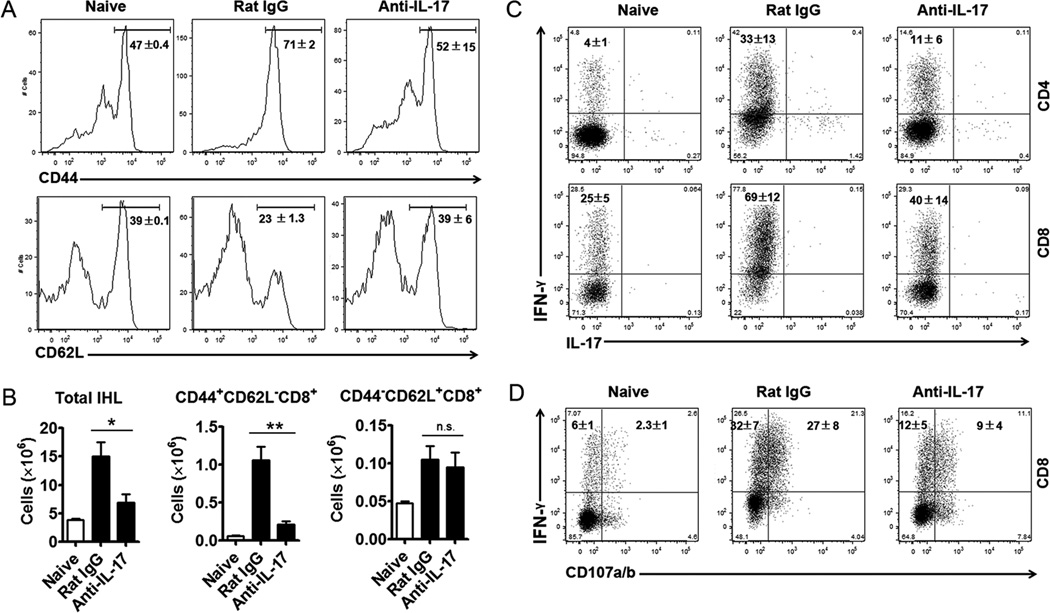
Since Ad infection induces strong CTL and Th1 responses in the liver, resulting in tissue destruction and serum ALT elevation (3), we then examined the activation and accumulation of CTL and Th cells in the IL-17-neutralized group. Ad infection resulted in the infiltration of activated CD8+ T cells, with significantly increased CD44 expression and decreased CD62L expression on their surface. In contrast to isotype control mice, IL-17 neutralization significantly suppressed the infiltration of activated CD44hiCD8+ T cells (Fig. 3A and B). Functionally, anti-IL-17 mAb administration significantly suppressed the IFN-γ-producing ability of both CD4+ and CD8+ T cells in the liver (11 ± 6% vs. 33 ± 13% and 40 ± 14% vs. 69 ± 12%, respectively; Fig. 3C). IL-17 neutralization not only reduced IFN-γ+CD8+ frequencies, but also interfered with their effector function in the liver (Fig. 3D). In the control group, considerable IFN-γ+ cells (27 ± 8%) expressed LAMP-1/2 (CD107a/b), indicative of their ability to de-granulate cytolytic vesicles. In the anti-IL-17-treated animals, however, a lower percentage of cells (9 ± 4%) expressed surface CD107a/b.
Figure 3. IL-17 neutralization suppressed the intrahepatic Th1 and CTL responses induced by Ad infection.
C57BL/6 mice were treated i.p. with anti-IL-17 or isotype control (Rat IgG2a) mAb at days -1, 0, 1, and 3 following Ad infection. Mice were sacrificed at day 6 post-infection. The liver tissues were isolated after the perfusion. A) Representative flow cytometric examination of CD44 and CD62L expression on CD8+ T cells. B) Calculated absolute cell numbers according to total IHLs and individual percentages. *P < 0.05; **P < 0.01; n.s. = no significance. C) IHLs were isolated and stimulated with PMA and ionomycin for 4 h in the presence of GolgiStop. D) IHLs were isolated and stimulated with anti-CD3 for 4 h in the presence of GolgiStop. The cells were then stained for surface markers and intracellular cytokines and examined by flow cytometry. Shown are representative flow cytometric results, n = 6 to 8 mice per group, cumulative mean ± SD was shown in quadrant.
Although neutralization of IL-17 alleviated liver inflammation, hepatic Ad viral copy numbers in IL-17 neutralization mice did not significantly differ from that in control mice at day 6 after AdLacZ infection (p > 0.05). For the control mice and IL-17 neutralization mice, viral copy numbers in liver tissue (means ± SEM; n = 4 to 6 mice per group) were 4,683 ± 698 and 3,923 ± 733 per gram, respectively.
IL-17 predominantly produced by γδ T cells in the liver
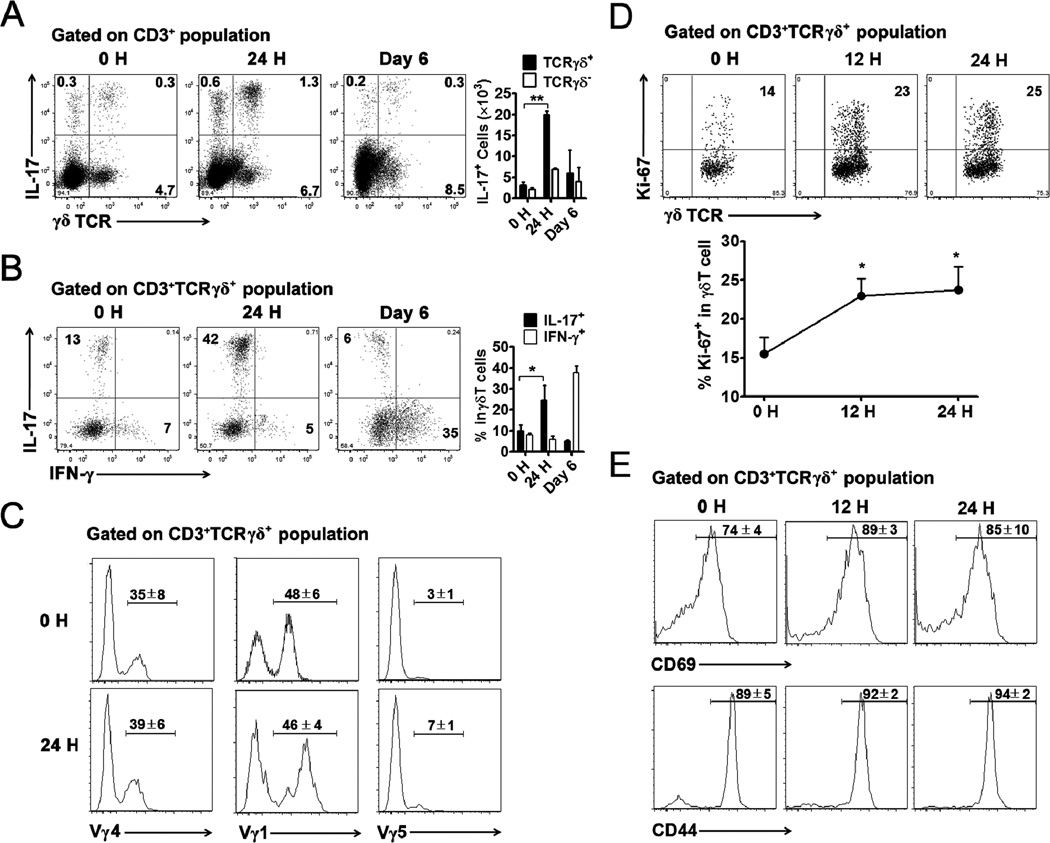
Both innate and acquired T cells were reported to produce IL-17. We found that in the liver more than two-thirds of IL-17-producing cells expressed TCRγδ on their surface, as judged by their percentages and absolute numbers (Fig. 4A). Small populations of IL-17+ γδ− intrahepatic T cells were heterogeneous and include CD4+CD8+ and NKT cells (Fig. 4A and Supp. Fig. 1B). However, compared with the high percentage of IL-17+ γδ T cells (42%, Fig. 4B), the IL-17+ γδ− T cell subsets were all below 0.9% within their respective populations (Supp. Fig. 1B). We found that the IL-17+ γδ T cells in the liver expanded more than 2-fold on day 1 and waned during the next several days, whereas the IFN-γ+ γδ T cells did not expand until after day 1. On day 6, most (about 35%) of the intrahepatic γδ T cells expressed IFN-γ, but not IL-17 (Fig. 4B). It has been reported that most γδ T cells in the liver of naïve mice belong to the Vγ1 and Vγ4 subsets, and the latter are the major IL-17 producers in peripheral lymphoid organs (46). In this study, we found that the proportions of intrahepatic γδ T cell subsets (Vγ1, Vγ4, and Vγ5) did not change significantly at day 1 (Fig. 4C). During the initial 24 h of infection, intrahepatic γδ T cells were activated and progressed into the cell cycle, as reflected by their elevated intracellular Ki-67 as well as surface CD69 and CD44 expression (Figs. 4D and E). Consistent with an earlier report (22), we found that the Vγ4 subset was the major IL-17 producer in the liver following Ad infection (Supp. Fig. 2A).
Figure 4. γδ T cells were the major cellular source of IL-17 in the liver.
C57BL/6 mice were injected i.v. with 2 × 109 pfu of Ad and sacrificed at 0, 1 and 6 days post-infection. Following perfusion, IHLs were isolated and stimulated with PMA and ionomycin for 4 h in the presence of GolgiStop. The cells were then collected and stained for surface markers and intracellular cytokines and examined by flow cytometry. A) Left: flow cytometric examination of IL-17 expression in TCRγδ+ and TCRγδ− subsets of intrahepatic CD3+ T cells; Right: cumulative statistical results of absolute cell number. B) Left: flow cytometric examination of IL-17 and IFN-γ expression in intrahepatic γδ T cells; Right: cumulative statistical results of percentage. C) Representative flow cytometric examination of surface CD3, TCRγδ, Vγ1, Vγ4, and Vγ5 expression. Cumulative mean ± SD was shown in quadrant. n = 3 mice per time point. Experiments were repeated at least three times with similar pattern. *P < 0.05; **P < 0.01. D and E) Mice were injected i.v. with 2 × 109 pfu of Ad and sacrificed at 0, 12 and 24 h post-infection (4 mice per time point), and liver cells were collected for FACS staining. Cells were gated on CD3+ TCRγδ+ populations. D) Intracellular staining of Ki-67. The up panel shows representative dot plots, the bottom panel shows statistical results. *, p < 0.05. E) Surface staining of CD69 and CD44. Cumulative mean ± SD was shown in quadrant.
IL-17-producing γδ T cells regulated by hepatic IL-7
Since γδ T cells can proliferate and secrete IL-17 merely in the presence of IL-7, IL-1β and/or IL-23 in sepsis and autoimmune disease (23, 47), we investigated the levels of these cytokines in livers infected with Ad virus. We injected i.v. B6 mice with 2 × 109 pfu AdLacZ, and sacrificed the animals at 0, 6, 12 and 24 h, as well as on days 3 and 6 post-infection. We detected a significant increase in TNF-α and IL-23p19 expression at 6 h, followed by a robust elevation of IL-7 mRNA levels at 12 h (Fig. 5A). This increased level of IL-7 expression was only detected in the liver, but not in the lungs, heart, kidneys, bone marrow, spleen, and thymus (Fig. 5B). Along with an accumulation of total IHLs and CD44hiCD62lo CTL infiltrates during the peak of liver injury at day 6 (Fig. 3B), there was another increase in IL-7, IL-1β and TNF-α messengers in the liver (Fig. 5A). To examine the proliferative potential of γδ T cells in response to these cytokines in vitro, we used purified splenic γδ T cells [because of comparable percentages of γδ T cell subsets in naïve mouse liver and spleen (Supp. Fig. 2B) and technical issues of obtaining sufficient numbers of hepatic γδ T cells]. Similar to a previous report (47), our results showed that IL-7 alone was sufficient to induce γδ T cell proliferation but not IL-17 secretion (Figs. 5C and D). IL-7 could synergize with IL-1β and TNF-α for γδ T cell proliferation, as well as acting synergistically with IL-23 and IL-1β for IL-17 secretion. In the presence of an anti-TCRγδ mAb, IL-7 dramatically augmented IL-17 production in γδ T cells (Fig. 5E), suggesting that IL-7 could affect the γδ T cells to produce IL-17 with or without TCR engagement. Finally, IL-7 seemed to prolong the survival of γδ T cells ex vivo (Supp. Fig. 2C).
Figure 5. IL-7 induced proliferation and IL-17 production of γδ T cells.
A) C57BL/6 mice were injected i.v. with 2 × 109 pfu of Ad and sacrificed at the indicated time points. Following perfusion, liver tissues were isolated, and total RNA was extracted for qRT-PCR analysis of cytokines. Results were expressed as fold change to corresponding mRNA levels in naive mice. N = 6 mice in each time point. B) Mice were injected i.v. with 2 × 109 pfu of Ad and sacrificed at 12 h post-infection. The liver, lungs, kidneys, heart, spleen, bone marrow, and thymus were isolated and weighted, and total RNA was extracted for qRT-PCR analysis of IL-7. Left: IL-7 mRNA expression in various tissues after virus infection. Data are shown as folds of change for IL-7 expression in the respective tissues in naive mice. Right: relative amounts of IL-7 mRNA in various tissues. IL-7 mRNA expression in the naïve liver was referred to as the control. Data are shown as folds of change for IL-7 mRNA expression in various tissues as compared to naïve liver and respective average tissue weights (n = 3 in both naïve and infected groups). *** P < 0.001. C) Proliferation of splenic γδ T cells. (D and E) IL-17 production from splenic γδ T cells stimulated by IL-1β, IL-7 and/or IL-23 (D) or by anti-TCRγδ in the presence or absence of IL-7 (E). For C, D, and E, splenic γδ T cells were purified from naïve mice through magnetic bead selection; shown are the mean ± SD of triple wells in one experiment. Experiments were repeated at least 3 times with a similar pattern. n.d. means the value was under the detection limit. *P < 0.05; **P < 0.01; *** P < 0.001 vs 0 hour (A) or as indicated (C, D and E).
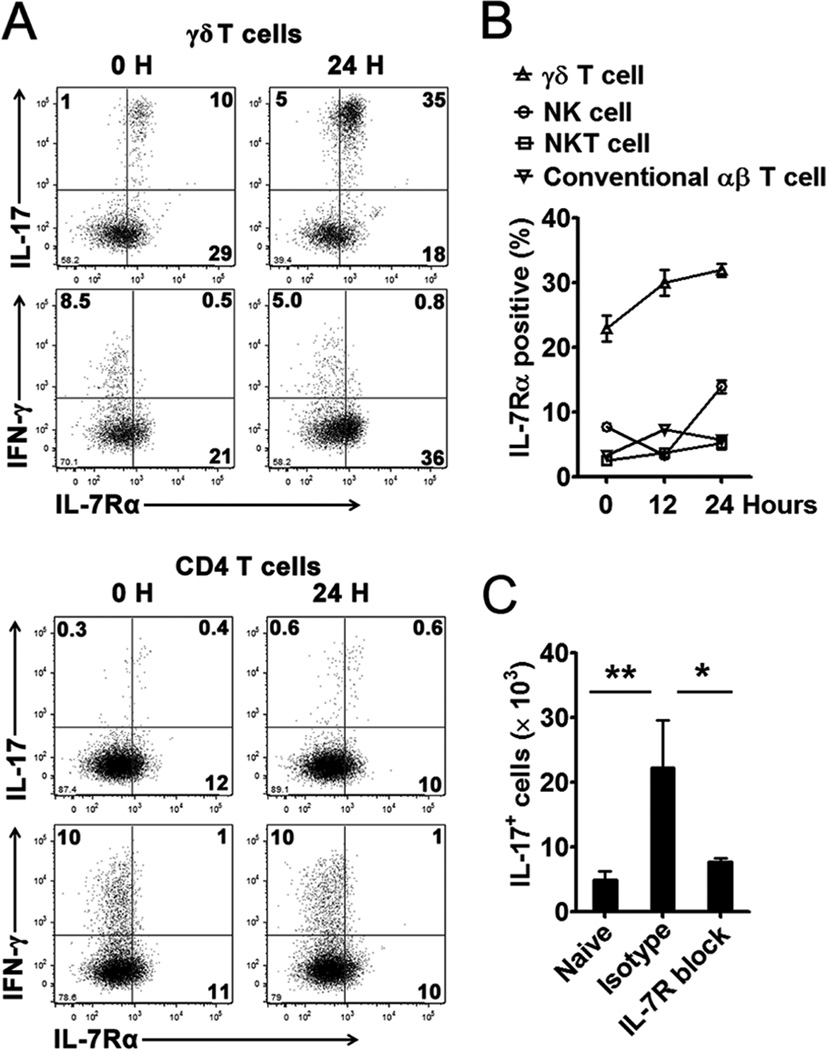
Flow cytometric analysis demonstrated that the intrahepatic IL-17+ γδ T cells preferentially expressed high levels of IL-7Rα (Fig. 6A). In contrast, IFN-γ+ γδ T or CD4+ T cells did not express IL-7Rα (Fig. 6A). Compared with NK, NKT, and αβ+ T cells, γδ T cells in the liver expressed the highest level of IL-7Rα (Fig. 6B). When we treated the mice with an antagonistic mAb against IL-7Rα (48), the blockade of IL-7R signaling halted the expansion of IL-17+ cells in the liver (Fig. 6C). These data may mean that hepatocytes regulate the γδ T cell population and function through the IL-7/IL-7R interaction following Ad infection. The early IL-7 and IL-17 production creates a favorable cytokine environment in the liver for effective antigen presentation and T cell development.
Figure 6. IL-7 controlled expansion of IL-17+ γδ T cells in the liver following Ad infection.
C57BL/6 mice were injected i.v. with 2 × 109 pfu of Ad and sacrificed at the indicated time points. (A and B) IHLs were isolated and stimulated with PMA and ionomycin for 4 h in the presence of GolgiStop. The cells were then examined for surface IL-7Rα (CD127) and intracellular IL-17 and IFN-γ in γδ T cells (Up panel) and CD4+ cells (Bottom panel) through flow cytometry. B) Percentage of IL-7Rα-positive population in γδ T cells, NK cells (NK1.1+ CD3−), NKT cells (NK1.1+ CD3+) and conventional αβ T cells (CD3+ NK1.1− TCRγδ− TCRαβ +) at the indicated time point post-infection. C) C57BL/6 mice were injected i.p. with anti-IL-7Rα mAb at days -1 and 0 and subsequently infected with 2 × 109 pfu of Ad. One day later, mice were sacrificed, and IHLs were stained for surface markers and intracellular IL-17. N = 3 to 8 mice per group. In A and B, shown were results from 3 independent experiments with similar patterns (n = 3 per time point).
Hepatocyte-derived IL-7 induced by IFN-I signaling
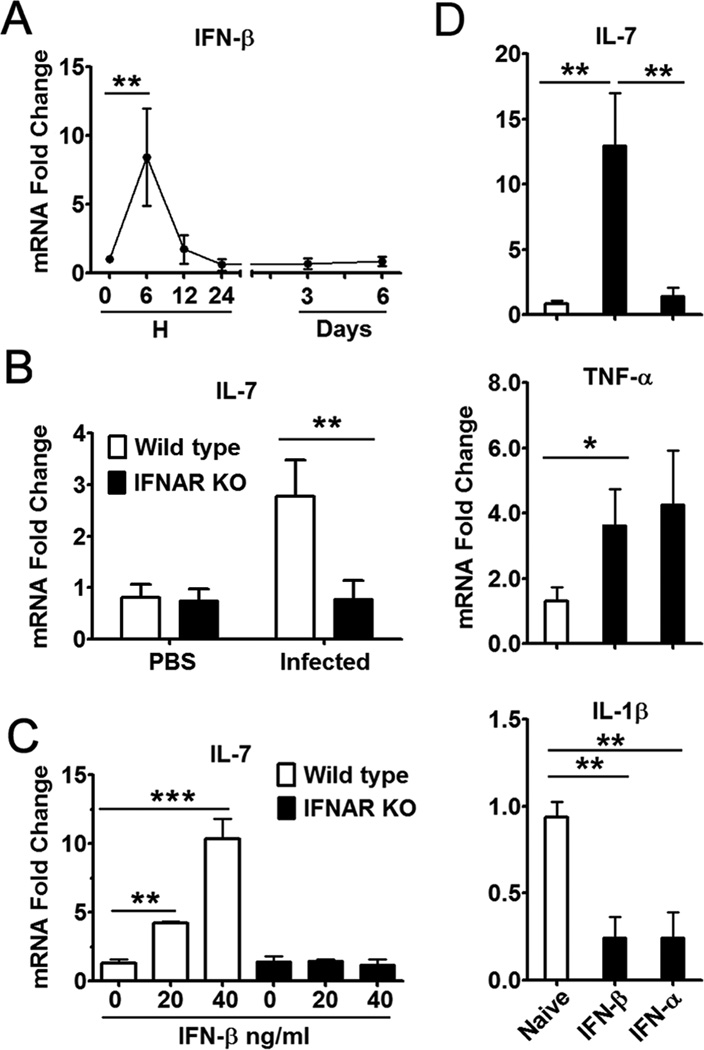
IL-7 was previously thought to be produced constitutively in the thymus and bone marrow (49, 50). A more recent study reported that liver can produce IL-7 in response to TLR4 signaling in vivo (40). Here, we found a transient, but robust, IFN-β expression at 6 h post-infection (Fig. 7A), followed by a similar rise in IL-7 mRNA levels at 12 h (Fig. 5A). We hypothesized that IFN-I signaling was partially responsible for hepatic IL-7 production following Ad infection. By using Ad-infected wild-type and IFNAR−/− mice, we found that in the absence of IFN-I signaling, the animals failed to express IL-7 in the liver despite viral challenge (Fig. 7B). In vitro studies indicated that IFN-β could significantly induce the IL-7 expression only in primary hepatocytes isolated from wild-type mice, but not from IFNAR−/− mice (Fig. 7C). To further confirm the inducible effect of IFN-I signaling on hepatic IL-7 production, we injected naïve mice i.v. with murine IFN-α or IFN-β and examined the expression levels of IL-7, IL-1β, TNF-α, IL-12 and IL-23 in the perfused liver at 5 h post-treatment. Results showed that IFN-β injection resulted in a significant increase in the expression levels of IL-7 and TNF-α (Fig. 7D), but not in those of IL-23 and IL-12 (data not shown). In contrast, both IFN-β and -α significantly suppressed IL-1β mRNA levels in the liver. These results may indicate that the IFN-I signaling pathway is pivotal in inducing IL-7 expression by hepatocytes, which might exert immunomodulatory functions during Ad infection.
Figure 7. IFN-I controlled hepatic IL-7 expression following viral infection.
A) C57BL/6 mice were injected i.v. with 2 × 109 pfu of Ad and sacrificed at the indicated time points. Liver mRNA was isolated for qRT-PCR analysis. B) IFNAR−/− mice and the control 129/Sv mice were injected i.v. with 2 × 109 pfu of Ad and sacrificed at 12 h post-infection. Liver mRNA was isolated for qRT-PCR analysis. C) qRT-PCR analysis of IL-7 expression in primary hepatocytes from IFNAR−/− mice and wild-type control mice under the stimulation of IFN-β. D) C57BL/6 mice were injected i.v. with murine IFN-α or IFN-β. Five hours later, liver mRNA was isolated for qRT-PCR analysis. For A, B, and D, n = 6 to 7 in each group. For C, shown were mean ± SD of triple wells in one experiment. Experiments were repeated at least 3 times, resulting in similar patterns. *P < 0.05; **P < 0.01; *** P < 0.001.
Discussion
The primary goal of this study is to understand the complex interactions of the innate and adaptive immune components in the liver parenchyma in Ad-induced hepatitis. We and others have shown previously that serum ALT levels peak around day 6 to 7 post-adenoviral infection in C57BL/6 mice (42). We further demonstrated in this study that hepatic IL-17-producing cells, as well as liver and serum IL-17 levels, peaked at day 1 and subsided at day 6 post-infection (Figs. 1 and 4, respectively), which was critical for regulating the subsequent CTL and Th1 responses (Figs. 1–3). These results are consistent with previous observations that IL-17 stimulates dendritic cells to promote Th1 responses (14, 16, 35). Importantly, we found that it is γδ T cells that predominantly expanded and produced a bolus of IL-17 early in the infection (Fig. 4). Other minor populations of intrahepatic cells also produced IL-17 at this stage of infection, though at much lower levels (Fig. 4B and Supp. Fig. 1B). These cells were TCRγδ-negative and contained CD4+, CD8+ and NKT cells. Compared with the high percentage of IL-17+ γδ T cells (42%, Fig. 4B), the IL-17+ γδ− T cell subsets were all below 0.9% within their respective populations (Supp. Fig. 1B). Unlike the conventional Th17 effectors (13), the IL-17-producing T cells in the liver do not require a lengthy Ag presentation process, but rather behaved more as the sentinels of the immune system (32). Based on these data, we conclude that early IL-17 production from γδ T cells is critical for the development of virus-specific adaptive immune responses in the liver.
As with CD4+ helper T cells, subsets of γδ T cells could also be defined based on distinct cytokine profiles, including IFN-γ-producing and IL-17-producing γδ T cells. Recent reports showed that IL-17-producing γδ T cells express CCR6, IL-23R and CD25, whereas IFN-γ producing γδ T cells preferentially express CD27 and NK1.1 (reviewed in (51)). Given that the ligand of CD27 is CD70, which is mostly expressed on the peripheral APCs, it’s not surprising that there is an expansion of IFN-γ-producing γδ T cells at 6 days after Ad infection (Fig. 1). Furthermore, in this study, we found that IL-17-producing γδ T cells expressed high levels of IL-7Rα, meaning possibly that IL-7 plays a critical role in the activation of IL-17-producing γδ T cells, as it is known to do in the expansion of IL-17+γδ T cells in autoimmune diseases and bacterial sepsis in animals (40, 41, 52).
The liver is a metabolically active, but immunologically quiescent, organ. Previously, we and others have shown that hepatocytes are capable of activating and altering homeostasis and effector function of Th and CTLs in the liver (4, 39, 53). Moreover, there is recent evidence that liver tissues can produce large amounts of IL-7 in response to TLR4 activation (40). Also, in mice infected with lymphocytic choriomeningitis virus (LCMV), IL-7 injections controlled viral infection by augmenting T cell responses, leading to an increase in serum IL-17 levels (54). However, it is unclear in that infection model whether hepatocytes are capable of producing IL-7 directly. Here, by using Ad-induced hepatitis and primary hepatocyte cultures (Figs 5 and 7), we provide the first evidence that hepatocytes are capable of producing IL-7 during viral hepatitis infection. Thus, hepatocytes can regulate neighboring cells through cytokine production and promote antigen presentation and T cell activation. We propose that the liver parenchymal cells and intrahepatic lymphocytes mounted a highly coordinated immune response soon after viral infection. As hepatocytes produced IL-7, a group of γδ T cells began to upregulate their surface IL-7Rα and secreted IL-17 in situ.
Ad infection has been reported to induce quick and successive IFN-β and -α responses in the liver (55). The binding of IFN-β and -α to a shared IFNAR can induce IFN-stimulated genes and chemokine/cytokine production, resulting in the establishment of an antiviral state in the liver. IFN-I also could induce the development of anti-viral CTL responses through the activation of APC (Supp. Fig. 3A). In this study, we found that Ad infection resulted in a sharp IL-7 response only in the livers of wild-type mice, but not in those of the IFNAR−/− animals (Fig. 7B). Using in vitro primary hepatocyte cultures, we have, for the first time, demonstrated that hepatocytes are capable of producing IL-7 in response to IFN-β stimulation. Since IFN-β itself did not directly change γδ T cell functions, as judged by the levels of IL-17 production or cell surface expression of IL-7Rα (Supp. Fig. 3B and C), we believe that hepatic IL-7 serves as a crucial player in orchestrating cross-talk between type I IFN signaling and IL-17+ γδ T cells, albeit type I IFN signaling was reported to suppress Th17 development in some autoimmune diseases (56).
Ad is a prototypical DNA virus and an important pathogen. It is also one of the preferred vectors for gene therapy, cancer therapy and experimental vaccines (1). When i.v. injected in mice, a majority of the viruses was eliminated quickly by the innate immune mechanisms within 24 h (2). However, subsequently, they were slowly cleared by the virus-specific CTL and Th responses (3, 4, 6, 7). Surprisingly, γδ T cell-deficient mice developed significantly less severe liver inflammation, but showed no significant difference in viral clearance (11). On the other hand, overzealous T cell responses may result in increased necroinflammatory hepatitis without accelerating viral elimination in vivo (4, 39). Thus, further investigations using chronic infection models are needed to define the role of IL-17 in virus clearance.
In summary, our in vivo and in vitro studies indicated that shortly after adenoviral infection, hepatocytes secreted IL-7, and that IFN-I could promote hepatocyte activation and IL-7 production. Hepatocyte-derived IL-7 selectively regulated the expansion of IL-17-producing T cells in the liver, especially those IL-7Rα-expressing T cells. Moreover, at the early stages of infection in the liver, the majority of IL-17+ cells are γδ T lymphocytes, and their IL-17 secretion is critical for the subsequent anti-viral Th and CTL responses. Thus, the highly coordinated events taking place among hepatocytes, as well as innate and adaptive immune cells, eventually lead to viral clearance and disease resolution in the liver. While this study clearly revealed an essential role for the IFN-β/IL-7/IL-17 cascade in regulating innate and adenovirus-specific immune responses in the liver, it will be important to investigate the potential cross-talk of these cytokines in other types of viral hepatitis.
Supplementary Material
Acknowledgments
We thank Drs. Rebecca O’Brien at the National Jewish Medical and Research Center and Robert Tigelaar at Yale School of Medicine for 2.11 and 17D1 mAbs, respectively; Drs. Gregg Milligan and Slobodan Paessler for assistance with knockout animals; Dr. Yingzi Cong for critical review of the manuscript; and Ms. Mardelle Susman for assistance with manuscript preparation.
Financial Support: This work was supported by a grant from the National Institutes of Health AI69142 (to J. S.) and by the James W. McLaughlin Fellowship Fund (to Z. J.).
Abbreviations
- ALT
alanine aminotransferase
- IHL
intrahepatic lymphocyte
- LAMP
lysosomal-associated membrane protein
- MFI
mean fluorescence intensity
- Th17
T helper 17
References
- 1.Liu MA. Immunologic Basis of Vaccine Vectors. Immunity. 2010;33:504–515. doi: 10.1016/j.immuni.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Worgall S, Wolff G, Falck-Pedersen E, Crystal RG. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum. Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Ertl HC, Wilson JM. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 4.Yan J, Jie Z, Hou L, Wanderley JL, Soong L, Gupta S, Qiu S, Chan T, Sun J. Parenchymal expression of CD40 exacerbates adenovirus-induced hepatitis in mice. Hepatology. 2011;53:1455–1467. doi: 10.1002/hep.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raper SE. Gene therapy: The good, the bad, and the ugly. Surgery. 2005;137:487–492. doi: 10.1016/j.surg.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Zsengeller ZK, Wert SE, Hull WM, Hu X, Yei S, Trapnell BC, Whitsett JA. Persistence of replication-deficient adenovirus-mediated gene transfer in lungs of immune-deficient (nu/nu) mice. Hum. Gene Ther. 1995;6:457–467. doi: 10.1089/hum.1995.6.4-457. [DOI] [PubMed] [Google Scholar]
- 7.Vilquin JT, Guerette B, Kinoshita I, Roy B, Goulet M, Gravel C, Roy R, Tremblay JP. FK506 immunosuppression to control the immune reactions triggered by first-generation adenovirus-mediated gene transfer. Hum. Gene Ther. 1995;6:1391–1401. doi: 10.1089/hum.1995.6.11-1391. [DOI] [PubMed] [Google Scholar]
- 8.Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 9.Liu ZX, Govindarajan S, Okamoto S, Dennert G. NK cells cause liver injury and facilitate the induction of T cell-mediated immunity to a viral liver infection. J. Immunol. 2000;164:6480–6486. doi: 10.4049/jimmunol.164.12.6480. [DOI] [PubMed] [Google Scholar]
- 10.Ajuebor MN, Chen Q, Strieter RM, Adegboyega PA, Aw TY. Vα14iNKT cells promote liver pathology during adenovirus infection by inducing CCL5 production: implications for gene therapy. J. Virol. 2010;84:8520–8529. doi: 10.1128/JVI.00605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajuebor MN, Jin Y, Gremillion GL, Strieter RM, Chen Q, Adegboyega PA. γδ T Cells Initiate Acute Inflammation and Injury in Adenovirus-Infected Liver via Cytokine-Chemokine Cross Talk. J. Virol. 2008;82:9564–9576. doi: 10.1128/JVI.00927-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez J, Huang X, Yang Y. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. J. Immunol. 2008;180:1592–1597. doi: 10.4049/jimmunol.180.3.1592. [DOI] [PubMed] [Google Scholar]
- 13.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 14.Feng T, Qin H, Wang L, Benveniste EN, Elson CO, Cong Y. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J. Immunol. 2011;186:6313–6318. doi: 10.4049/jimmunol.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N, Lasarte JJ, Matsuzaki G, Ikuta K, Ryffel B, Benlagha K, Tesniere A, Ibrahim N, Dechanet-Merville J, Chaput N, Smyth MJ, Kroemer G, Zitvogel L. Contribution of IL-17-producing γδ T cells to the efficacy of anticancer chemotherapy. J. Exp. Med. 2011;208:491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, Onishi R, Nyugen N, Walter MJ, Pociask D, Randall TD, Gaffen SL, Iwakura Y, Kolls JK, Khader SA. Interleukin-17 Is Required for T Helper 1 Cell Immunity and Host Resistance to the Intracellular Pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 19.Cui Y, Shao H, Lan C, Nian H, O'Brien RL, Born WK, Kaplan HJ, Sun D. Major role of γδ T cells in the generation of IL-17+ uveitogenic T cells. J. Immunol. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. J. Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blink SE, Miller SD. The contribution of γδ T cells to the pathogenesis of EAE and MS. Curr. Mol. Med. 2009;9:15–22. doi: 10.2174/156652409787314516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, Roark C, Born WK, O'Brien R, Ikuta K, Ishikawa H, Nakae S, Iwakura Y, Ohta T, Matsuzaki G. IL-17A Produced by γδ T Cells Plays a Critical Role in Innate Immunity against Listeria monocytogenes Infection in the Liver. J. Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 24.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 25.Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Venkatakrishna R Jala, Zhang H-g, Wang T, Zheng J, Yan J. Pivotal Role of Dermal IL-17-Producing gd T Cells in Skin Inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing γδ T cells in the delayed phase of ischemic brain injury. Nat. Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 27.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien YH. Thymic selection determines γδ T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing γδ T cell subsets. Nat. Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Do JS, Fink PJ, Li L, Spolski R, Robinson J, Leonard WJ, Letterio JJ, Min B. Cutting edge: spontaneous development of IL-17-producing γδ T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J. Immunol. 2010;184:1675–1679. doi: 10.4049/jimmunol.0903539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Bonneville M, O'Brien RL, Born WK. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 32.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 33.Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y. Identification of CD25+ γδ T cells as fetal thymus-derived naturally occurring IL-17 producers. J. Immunol. 2008;181:5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 34.Yue FY, Merchant A, Kovacs CM, Loutfy M, Persad D, Ostrowski MA. Virus-specific interleukin-17-producing CD4+ T cells are detectable in early human immunodeficiency virus type 1 infection. J. Virol. 2008;82:6767–6771. doi: 10.1128/JVI.02550-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J-Y, Zhang Z, Lin F, Zou Z-S, Xu R-N, Jin L, Fu J-L, Shi F, Shi M, Wang H-F, Wang F-S. Interleukin-17–producing CD4+ T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 2010;51:81–91. doi: 10.1002/hep.23273. [DOI] [PubMed] [Google Scholar]
- 36.Basha HI, Subramanian V, Seetharam A, Nath DS, Ramachandran S, Anderson CD, Shenoy S, Chapman WC, Crippin JS, Mohanakumar T. Characterization of HCV-specific CD4+Th17 immunity in recurrent hepatitis C-induced liver allograft fibrosis. Am. J. Transplant. 2011;11:775–785. doi: 10.1111/j.1600-6143.2011.03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagata T, McKinley L, Peschon JJ, Alcorn JF, Aujla SJ, Kolls JK. Requirement of IL-17RA in Con A induced hepatitis and negative regulation of IL-17 production in mouse T cells. J. Immunol. 2008;181:7473–7479. doi: 10.4049/jimmunol.181.11.7473. [DOI] [PubMed] [Google Scholar]
- 38.Zhao N, Hao J, Ni Y, Luo W, Liang R, Cao G, Zhao Y, Wang P, Zhao L, Tian Z, Flavell R, Hong Z, Han J, Yao Z, Wu Z, Yin Z. Vγ 4 γδ T Cell-Derived IL-17A Negatively Regulates NKT Cell Function in Con A-Induced Fulminant Hepatitis. J. Immunol. 2011;187:5007–5014. doi: 10.4049/jimmunol.1101315. [DOI] [PubMed] [Google Scholar]
- 39.Sun J, Tumurbaatar B, Jia J, Diao H, Bodola F, Lemon SM, Tang W, Bowen DG, McCaughan GW, Bertolino P, Chan T-S. Parenchymal Expression of CD86/B7.2 Contributes to Hepatitis C Virus-Related Liver Injury. J. Virol. 2005;79:10730–10739. doi: 10.1128/JVI.79.16.10730-10739.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawa Y, Arima Y, Ogura H, Kitabayashi C, Jiang JJ, Fukushima T, Kamimura D, Hirano T, Murakami M. Hepatic interleukin-7 expression regulates T cell responses. Immunity. 2009;30:447–457. doi: 10.1016/j.immuni.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Leung S, Wang C, Tan Z, Wang J, Guo TB, Fang L, Zhao Y, Wan B, Qin X, Lu L, Li R, Pan H, Song M, Liu A, Hong J, Lu H, Zhang JZ. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat. Med. 2010;16:191–197. doi: 10.1038/nm.2077. [DOI] [PubMed] [Google Scholar]
- 42.Sun JR, Bodola F, Fan XG, Irshad H, Soong L, Lemon SM, Chan TS. Hepatitis C virus core and envelope proteins do not suppress the host's ability to clear a hepatic viral infection. J. Virol. 2001;75:11992–11998. doi: 10.1128/JVI.75.24.11992-11998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai MM, Gong B, Chan T, Davey RA, Soong L, Kolokoltsov AA, Sun J. Differential, Type I Interferon-Mediated Autophagic Trafficking of Hepatitis C Virus Proteins in Mouse Liver. Gastroenterology. 2011;141:674–685. doi: 10.1053/j.gastro.2011.04.060. 685.e671-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crispe IN. Isolation of Mouse Intrahepatic Lymphocytes. John Wiley & Sons, Inc; 2001. [Google Scholar]
- 45.Hou LF, He SJ, Li X, Yang Y, He PL, Zhou Y, Zhu FH, Yang YF, Li Y, Tang W, Zuo JP. Oral administration of artemisinin analogue SM934 ameliorated the lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum. 2011;63:2445–2455. doi: 10.1002/art.30392. [DOI] [PubMed] [Google Scholar]
- 46.Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL. γδ T cells: an important source of IL-17. Curr. Opin. Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng L, Cui Y, Shao H, Han G, Zhu L, Huang Y, O'Brien RL, Born WK, Kaplan HJ, Sun D. Mouse γδ T cells are capable of expressing MHC class II molecules, and of functioning as antigen-presenting cells. J. Neuroimmunol. 2008;203:3–11. doi: 10.1016/j.jneuroim.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee LF, Axtell R, Tu GH, Logronio K, Dilley J, Yu J, Rickert M, Han B, Evering W, Walker MG, Shi J, de Jong BA, Killestein J, Polman CH, Steinman L, Lin JC. IL-7 promotes T(H)1 development and serum IL-7 predicts clinical response to interferon-beta in multiple sclerosis. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3002400. 93ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine Grow. Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat. Rev. Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 51.Korn T, Petermann F. Development and function of interleukin 17-producing γδ T cells. Ann. N. Y. Acad. Sci. 2012;1247:34–45. doi: 10.1111/j.1749-6632.2011.06355.x. [DOI] [PubMed] [Google Scholar]
- 52.Kasten KR, Prakash PS, Unsinger J, Goetzman HS, England LG, Cave CM, Seitz AP, Mazuski CN, Zhou TT, Morre M, Hotchkiss RS, Hildeman DA, Caldwell CC. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through γδ T-cell IL-17 production in a murine model of sepsis. Inf. Imm. 2010;78:4714–4722. doi: 10.1128/IAI.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J. Clin. Invest. 2004;114:701–712. doi: 10.1172/JCI21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, Assouline B, Lahl K, Sparwasser T, Tedder TF, Paik JH, DePinho RA, Basta S, Ohashi PS, Mak TW. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 55.Huarte E, Larrea E, Hernandez-Alcoceba R, Alfaro C, Murillo O, Arina A, Tirapu I, Azpilicueta A, Hervas-Stubbs S, Bortolanza S, Perez-Gracia JL, Civeira MP, Prieto J, Riezu-Boj JI, Melero I. Recombinant Adenoviral Vectors Turn on the Type I Interferon System without Inhibition of Transgene Expression and Viral Replication. Mol. Ther. 2006;14:129–138. doi: 10.1016/j.ymthe.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 56.Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J. Clin. Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.