Abstract
A 64-channel brain array coil was developed and compared to a 32-channel array constructed with the same coil former geometry in order to precisely isolate the benefit of the two-fold increase in array coil elements. The constructed coils were developed for a standard clinical 3T MRI scanner and used a contoured head-shape curved former around the occipital pole and tapered in at the neck to both improve sensitivity and patient comfort. Additionally, the design is a compact, split-former design intended for robust daily use. Signal-to-noise ratio (SNR) and noise amplification (G-factor) for parallel imaging were quantitatively evaluated in human imaging and compared to a size and shape-matched 32-channel array coil. For unaccelerated imaging, the 64-channel array provided similar SNR in the brain center to the 32-channel array and 1.3-fold more SNR in the brain cortex. Reduced noise amplification during highly parallel imaging of the 64-channel array provided the ability to accelerate at approximately one unit higher at a given noise amplification compared to the sized-matched 32-channel array. For example, with a 4-fold acceleration rate, the central brain and cortical SNR of the 64-channel array was 1.2 and 1.4-fold higher, respectively, compared to the 32-channel array. The characteristics of the coil are demonstrated in accelerated brain imaging.
Introduction
Parallel acquisition has impacted clinical brain imaging to the point where nearly every brain examination is performed with an array comprising multiple smaller surface coil elements. Originally proposed as a method to increase sensitivity by extending the high-sensitivity detection pattern of small surface coil elements to larger areas (1), the term parallel imaging now includes the use of array coils to perform image encoding and thus reduce the image acquisition time (2,3). Because of this dual goal, array performance metrics must include both sensitivity estimates for both non-accelerated and accelerated imaging.
Simulation studies have shown exactly how these metrics change when incrementing the density of array elements. For example, simulations of spherical distributions show that the ultimate possible SNR in the center of the phantom is already closely approached at 3T when as little as 12 array elements are used (4). Larger numbers of elements are required to approach the ultimate SNR in the periphery. When higher image acceleration factors are considered, the image sensitivity of conventional arrays is not as tightly bounded by the ultimate SNR and ultimate G-factor. Simulation studies and ultimate SNR comparisons are limited, however, since they are restricted to a simple geometry. Even to the extent that the head is spherical, a realistic head coil cannot completely surround the brain with array elements. The simulations also make simplifying assumptions about body-noise dominance and element decoupling which are difficult to realize in practice. Therefore experimental comparisons are always needed to guide practical implementations.
Experimental studies contrasting brain arrays with different numbers of array elements have supported the general findings of the simulation studies. For example, a comparison of a 96-channel and 32-channel helmet arrays showed that central SNR is nearly identical and peripheral SNR increased in the higher channel count coil (5). Also, more highly accelerated imaging benefits from both the peripheral SNR gain and a reduced g-factor, thus improving sensitivity in the center for accelerated acquisitions.
In this study, we simulate, design, construct, and evaluate a 64-channel brain array coil and compare it to a 32-channel array, simulated and constructed with the same coil former geometry in order to precisely isolate the benefit of the two-fold increase in array coil elements. Additionally, we sought a split-able coil design so that the patient can lie down into the coil, unlike previous array designs of arrays exceeding 32 elements, which required the helmet to be pulled-down over the head. An additional benefit of the split-former design is that it allows the coil to taper in at the neck, more closely following the anatomy by “capturing” the head and better surrounding it with loop elements in a way precluded by a simple helmet design. Thus, we taper the coil former near the neck and follow the contour of the occipital pole and cerebellum closely. By comparing its performance to a 32-channel array also built with these geometrical features, we assess both the benefit of doubling the number of elements (at a constant geometry) as well as the benefits of these shape changes (at a constant number of elements). A further goal of this design was to assess a compromise coil element size between the previously published 32- and 96-channel designs (5) in order to perform robust clinical and research brain MRI applications. While the 32-channel loops are large enough to be body-noise dominated on nearly every subject, the 96-channel loops were less body-noise dominated, and thus the performance of the coil is more subject specific.
The constructed coils were developed for a state-of-art clinical MRI scanner in a way that addresses patient comfort concerns and can be disseminated for robust daily use. The split-able anatomically shaped 32- and 64-channel arrays were evaluated in phantom and human measurements for SNR, G-factor, and noise correlation. The experimental G-factor data was compared to a full-wave simulation. Finally, we demonstrate the arrays’ capabilities in highly accelerated anatomical images.
Material and Methods
Coil Design and Construction
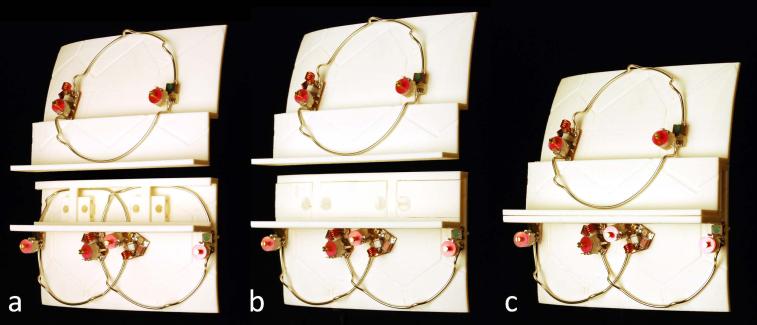
Both coils (32-channel and 64-channel) were designed on an anatomically shaped former consisting of a large posterior head neck part with and an overlapping anterior head portion (Figure 1). The 64-channel array used 42 elements on the posterior part and 22 on the anterior. For the 32-channel array, these numbers were 18 and 14 respectively. The helmet shape was taken by dilating (~3mm) the 95th percentile contour of aligned 3D MRI scans to accommodate additional foam padding. The larger posterior section is designed so the subject can lie down into the coil, rather than a helmet design, which must be pulled down over the head. The anterior head segment closes the helmet via a snap-in mechanism and overlaps with the posterior portion to allow the loops on the two halves to be geometrically decoupled. The mechanical implementation of the housing split is shown in greater detail in Figure 2, while the colored loops in Figure 3 show where the split occurs in relation to the element array. Eye cut-outs facilitate visual stimulation for functional studies. All helmet parts including its covers were printed in acrylonitrile butadiene styrene (ABS) plastic using a rapid prototyping 3D printer (Dimension SST 1200es, Dimension, Inc., Eden Prairie, MN, USA).
Figure 1.
Completed 64-channel (a) and 32-channel (b) array coils without covers. Both sized matched coil helmets are designed with a split-able anatomical shaped former design.
Figure 2.
Mechanical implantation of the housing split to allow the loops on the two halves to be geometrically decoupled. The adjacent anterior loops are bent over the housing's rim structure to achieve critical overlap with their nearest neighbors on the posterior housing segment. Small windows in the posterior rim structure provide access for mechanical decoupling (a). The windows are covered in the final setup (b). A snap-in mechanism overlaps both portions (c).
Figure 3.
Simulation geometries for time-domain finite element computations for the 64-channel (a) and 32-channel (b) coil. Position and loop shape of the elements were transferred from the hexagon/pentagon pattern from the 3D helmet model. The split-housing structure is incorporated into the loop array models (orange: posterior housing segment, blue: anterior housing segment). Loop shapes (e.g. bent loops for critical overlap between housing segments, or “potato-shaped” loops in the neck region) were carefully 3D modeled in order to obtain realistic simulated acceleration capabilities.
The layout of the overlapped circular coil elements was established by a hexagonal and a pentagonal tiling pattern (6), which was printed onto the coil former together with standoffs to mount the preamplifiers. The diameters of the loop coil are derived from the size of the pentagon or hexagon tiles; the loop diameter is slightly larger than the diameter of the circle which inscribes the vertexes of the hexagon/pentagons. For the 64-channel coil, the loops corresponding to the hexagons were 65 mm in diameter, with an inductance of approximately 190 nH. The diameter for the pentagon locations was 55 mm (~170 nH inductance). For the 32-channel array these sizes were 95 mm and 75 mm, respectively, and the corresponding estimated inductances were ~270 nH and ~210 nH. The posterior former of the 64-channel array incorporated 39 hexagons and three pentagons; the anterior section contains 15 hexagons, three pentagons, and two non-circular shaped loops at the edge of the housing, and two somewhat oval shaped eye loops. The 32-channel array has 15 hexagons and three pentagons in the posterior section and 10 hexagons and two pentagons and two oval eye loops in the anterior section. The eye loops of both arrays used a shared capacitance degenerately decoupled design (7). All other loops used critical overlap for decoupling of neighbors (8). Also the 64-channel array's eye loops inscribe a larger diameter compared to the rest of the loop pattern to provide an unobstructed visual field of view. The overlap between the posterior and anterior housing elements is mechanically achieved.
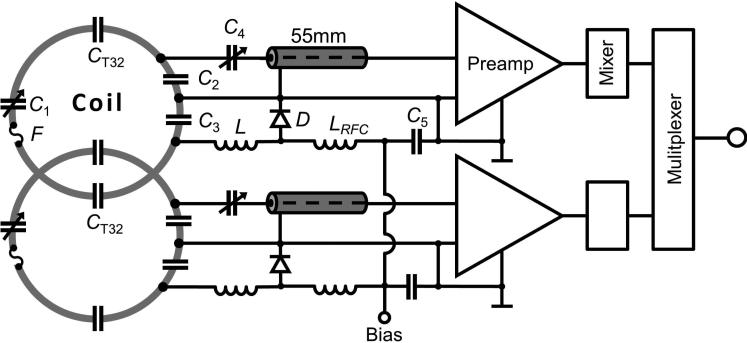
We used 16-awg (1.3 mm) thick tin-plated copper wire to form the loops with bridges bent into the wire to allow the coil conductors to cross-over one another without touching. Wire was chosen over circuit board material to avoid eddy current losses in the surrounding elements (9). We divided each loop symmetrically to place the discrete components (see schematic circuit in Figure 4). The 64-channel array used two divisions and the 32-channel array used four per loop. The discrete components were mounted on small FR4 circuit boards, manufactured with a rapid prototyping circuit router (T-Tech-7000, T-Tech, Inc, Norcross, GA, USA), and then soldered to the loop conductor. These small circuit boards minimize mechanical stress between the loop wires and the ceramic capacitors (Series 11, Voltronics, Danville, NJ, USA). The tuning capacitor circuit board contains a variable capacitor C1 (3-42pF, BFC580811339, Vishay Intertechnology, Inc, Malvern, USA) to fine-tune the loop resonance to 123.25 MHz. The coil matching circuit-board incorporates a capacitive voltage divider (C2, C3) and a variable series capacitor C4 (BFC580811339, Vishay Intertechnology, Inc, Malvern, USA) to impedance match the element's output to the optimized noise match of 50 Ohm desired by the preamplifier. Additionally, the coil matching circuit board contains an active detuning circuit across one of the voltage divider capacitor (C3). The active detuning is achieved using a PIN diode D (Macom, MA4P7446F-1091T, Lowell, MA, USA) in series with a hand wound inductor L (30-48 nH), which together with capacitor C4 resonates at the Larmor frequency. Thus, when the PIN diode is forward biased (transmit mode), the resonant parallel LC3 circuit inserts a high impedance in series with the coil loop, blocking current flow at the Larmor frequency.
Figure 4.
Circuit schematic for a coil pair of the 64-channel array coil. Each coil element consists of four (C1-C4) capacitors. The variable capacitor C1 fine-tunes the coil element frequency. Where C2 and C3 are equally valued and provide a capacitive voltage divider at the coil output circuit. A detuning trap is formed around C3 using a variable inductor L and a Diode D. A series capacitive matching (C4) transforms the element impedance to 50 Ohm. After the preamplifiers the detected signals are down converted to an intermediate frequency and multiplexed onto a single coaxial output. The signals are later de-multiplexed back into individual channels in the receiver unit. (Values for a 64ch loop (65mm dia.): C1≈14pF, C2=C3=47pF, C4≈8pF, C5=1nF, L≈36nH, LRFC=3.3nH. Values for a 32ch loop (95mm dia.): C1≈32pF, CT32=27pF, C2=C3=33pF, C4≈11pF, C5=1nF, L≈48nH, LRFC=3.3nH).
While nearest neighbor decoupling was addressed with critical overlap, next-nearest neighboring coil elements were decoupled using preamplifier decoupling (1). To transform the preamplifier input impedance to a high series impedance within the loop, we used the impedance transformation of the series matching capacitor (C4) and an additional phase shift from a 55 mm long coaxial cable. Together these transform the preamplifier input impedance to a parallel inductance across C2 of the capacitive voltage divider. This parallel LC circuit is set to resonate at the Larmor frequency and introduces the needed high series impedance in the coil loop (1). In this mode, minimal current flows in the loop and inductive coupling to other coils is minimized despite the presence of residual mutual inductance. The fine adjustment of the impedance transformation of the preamplifier input was done by carefully controlling the coaxial cable (UT-85C-form, Micro-Coax, Pottstown, PA, USA) length (~55 mm) between preamplifier and coil matching circuit board. Pairs of coils were attached to low-noise converters (Siemens AG, Healthcare Sector, Erlangen, Germany), which include low-noise preamplification as well as a down-conversion to an intermittent frequency. These two intermittent frequencies were multiplexed onto a single coaxial output. The signals are later de-multiplexed back into individual channels in the receiver unit. Ultimately, this procedure reduced the number of output cables of the constructed array coil, creating sparser cable routing. Each preamplifier channel also contains a common mode rejection transformer between the first and second stages (10). All preamplifiers were carefully oriented in z-direction to minimize Hall effect issues within the field effect transistors used in the preamplifiers (11–13). Preamp outputs passed through an additional cable trap prior to the coil plug to suppress common mode currents and avoid interaction with the radio frequency (RF) transmit system. The posterior segment is connected to the patient table using a sliding connection mechanism integrated into the coil housing, thus eliminating the use of a conventional coil plug. The anterior segment outputs used a single standard coil plug on a short cable plugging into the table on the patient's left side.
Coil bench tests
Bench testing during construction was carried out with a custom-made coil-plug simulator which provides the 3V power for the preamplifiers and the ability to manually apply bias current (100 mA) to each PIN diode pair on the array elements. Bench measurements verified the element tuning, active detuning, nearest-neighbor coupling and preamplifier decoupling for each coil element. Additionally, the ratio of unloaded-to-loaded quality factors (QU/QL) were measured for each coil element size. The QU/QL-ratios were obtained with a dual-loop probe lightly coupled into the loop under test (14), with no coaxial cable or preamplifier present. The QU/QL-ratios were tested with the loops both external to the array assembly and within the populated but detuned array.
After populating all of the elements, the detuning trap circuits were adjusted so that elements not under test could be actively detuned using the coil-plug simulator. Active detuning was measured with a S21 measure between two decoupled (~80dB) inductive probes lightly coupled to the array element under test. Nearest neighbor coupling was measured using a direct S21 measurement between pairs of elements using coaxial cables which could directly connect to the preamplifier sockets of the two elements under test. The crossing bridges in the loops were bent to empirically optimize the decoupling by watching the S12 between the two loops while bending the wire bridges. When measuring the S21 between an adjacent pair, all other elements of the array were detuned.
We measured the preamplifier decoupling of a given loop with all other loops detuned. Preamplifier decoupling was measured as the change in the double-probe S21, when the coil output coaxial cable was terminated in each of two different match conditions (15). In the first case the coil was terminated with the powered preamplifier (low impedance termination). In the second case the coil was terminated with 50 Ohm. When necessary, we adjusted the cable length of the short coaxial cable between coil element and preamplifier to provide optimal preamplifier decoupling.
Simulation
Finite element computations (XFDTD, Remcom, Stage College, PA, USA) in the time-domain were computed in order to simulate acceleration capabilities of both constructed arrays. First, all overlapped coil elements were modeled in a CAD software (SketchUp, Google, Mountain View, CA, USA) as a 1.3 mm diameter solid conductor. Position and loop shape of the elements were transferred from the hexagon/pentagon pattern from the 3D helmet model. We also incorporated the split-housing structure into our loop array model. Thus, the adjacent anterior loops are bent over the housing's rim structure to achieve critical overlap with their nearest neighbors on the posterior housing segment (Figure 3). For simplification each loop element was approximated in 24 straight segments.
After importing the loops into the simulation package, we defined the conductor material as perfect electric conductors (PEC). The simulation included a conductive load using a human-meshed model (Duke, Virtual Family, with 1mm isotropic resolution) (16). Excitation was emulated using a sinusoidal unit current at the Larmor frequency of 123.25 MHz. The complex reception profile of the coil was taken as the B1- component of the simulated field in the transverse plan (17). However, simulations did not account for coil tuning or matching conditions and neglected inter-element coupling. To match the acquired B1- maps and its derived G-factor maps the simulated fields were averaged into 5mm slices and computed and visualized in Matlab (MathWorks Inc., Natick, MA, USA). To calculate the G-factor maps for the simulated arrays, the simulated B1- field maps were used together with the mutual noise resistance matrix. The latter was determined as the inner product of the spatially varying electric fields of the coils over the volume of the sample (1).
MRI Data Acquisition and Reconstruction
Data were acquired on a 64-channel 3T clinical MRI Siemens scanner (MAGNETOM, Skyra, Tim 4G, Dual Density Signal Transfer, Siemens AG, Healthcare Sector, Erlangen, Germany) equipped with customized gradient coil (AS302 CONNECTOM† gradient) but using the standard maximum gradient strengths 45mT/m maximum amplitude gradient strength and a maximum slew rate of 200mT/m/ms. Prior to imaging, the coils was subjected to a number of tests on the scanner to validate its performance. We measured the percentage of the body RF power dissipated in the detuned array coil by comparing the RF body coil forwarded/reflected power levels required to achieve a 180° excitation in a phantom with and without the detuned array coil present. We considered an arrays validated, when the ratio of the absorbed power with the array present to the absorbed power without the array, is between 0.9 and 1.1. We also tested the coil for heating. After switching off the SAR monitor and the gradient stimulation monitor, we measured the temperature increase in the coil caused by RF transmit power being absorbed by the receive circuitry or heating by induced currents from the gradient switching. The detuned coil and phantom were scanned for 15 min with a body-coil B1 field of 30 μT applied at a 10% duty cycle and repetition time of 60 ms; an RF power level well above the SAR limit.
The time course stability of the coils were evaluated in a single-shot gradient echo EPI time-series (500 time points, TR/TE/FA: 1000ms/30ms/90°, FOV: 200×200mm2, M: 64×64, BW: 2298 Hz/Pixel, 16 slices of 5 mm each) using a spherical phantom filled with agar. The mean intensity of a 15-pixel square region of interest (ROI) in the center of the phantom was plotted after detrending linear and quadratic temporal trends. The stability was measured as the peak-to-peak variation in the signal intensity expressed as a percentage of the mean signal intensity.
SNR and G-factor measurements were obtained from a healthy volunteer measurement following informed consent and adhering to a protocol approved by our institution's Human Research Committee. To assess SNR, proton density weighted gradient echo images (Repetition Time (TR) / Echo Time (TE) / Flip Angle (FA) = 30ms/6ms/30°, Matrix (M): 192×192, Field-of-View (FOV): 170×170 mm2, slice thickness (SL) : 5 mm, bandwidth (BW)= 200 Hz/Pixel) were acquired. Noise covariance information was acquired using the same pulse sequence but with no RF excitation. The SNR maps were calculated for images formed from noise-covariance weighted root sum-of-squares (cov-RSS) of the individual channel images (1,18). In addition we investigated potential SNR degradation, in the case when small head sizes (e.g. female subjects) are imaged with large-count element coils. We designed and 3D-printed two head sized phantoms, which correspond to the 50th percentile of the male and female population, with a head circumference of 58cm and 56cm, respectively. The phantoms were filled with 1.955 g CuSO4·5H2O and 3.6 g NaCl per 1000 g H2O. The G-factor maps were calculated in order to assess noise amplification in SENSE reconstructions caused by the ill-conditioned unaliasing of the under-sampled images. SENSE G-factor maps were estimated from the measured complex coil sensitivities and noise correlation matrix (2). The maximum G-factor was determined after applying a 5×5 pixel smoothing filter to avoid biasing by noise singularities. For a fair assessment of the G-factor characterizations, the FOV tightly enclosed the object to maximize the aliasing induced by the acceleration.
Furthermore, parallel imaging capabilities of both coils were compared using simultaneous multi-slice Echo Planar Imaging (EPI) acquisition techniques (19) with combined blipped-Controlled Aliasing in Parallel Imaging (blipped-CAIPI) (20). The blipped-CAIPI acquisition introduces an inter-slice image shift in the phase encoding direction between the simultaneously acquired slices to mitigate the G-factor penalty associated with such acquisitions. We evaluated the potential of G-factor penalty mitigation, when moving from 32 to 64 array coil elements using an in-plane acceleration factor of R=2 and slice acceleration of Rsl=5 for a whole-brain acquisition. The inverse G-factor maps of blipped-CAIPI acquisitions were calculated using the pseudo-multiple replica method (21) with 1000 image pseudo time series of the coil-combined images. A 5×5 kernel for slice-GRAPPA and a 3×3 for in-plane-GRAPPA were used for these calculations. The resulting 1/G-factor maps were evaluated over a brain-sized ROI of a representative slice group. For this comparison, data from the same subjects acquired using an accelerated spin-echo EPI acquisition (TR/TE/FA: 8.9s/94ms/90° FOV: 220×220mm2, 2mm isotropic, M: 110×110, BW: 1976 Hz/Pixel, 63 slices, Rsl × R=5×2)
Comparison of in-vivo accelerated imaging capabilities is demonstrated with a T1-weighted multi-echo MPRAGE sequence (22), with TR/TI/FA = 2.53s/1.1s/7°, matrix=256×256×176, 1mm isotropic voxel size, BW=651 Hz/pixel, acceleration of R=4, one average, and an acquisition time of 3:38 min.
Results
Coil bench tests
Coil elements from the 64-channel array, showed an unloaded-to-loaded Q-ratio of 264/45=5.9 and 256/62=4.1 for the hexagons and pentagons respectively for the case of the single isolated loop (no neighbors present). We also observed a resonance frequency drop of 0.4 MHz upon loading. With six non-resonant neighboring elements present we measured a QU/QL-ratio of 235/46=5.1 and 224/62=3.6 for the hexagons and pentagons, respectively. Furthermore, with the 6 neighbors present there was no shift in resonant frequency observable upon loading. The QU/QL-ratio for the 32-channel head array was 250/23 = 10.9 and 273/36 = 7.6 for the hexagons and pentagons respectively without neighbors present. In an array arrangement, where six neighbors were present, the Q-ratio was slightly decreased (hexagon: 242/24 = 10.1, pentagon: 257/36 = 7.1).
Active PIN diode detuning provided >42 dB isolation between the tuned and detuned states for both constructed array coils. Bench tests obtained from both coils showed a range of decoupling between nearest neighbor elements of -13 dB to -25 dB with an average of -17 dB and -18 dB for 64-channel and 32-channel coil, respectively. The coupling between next-nearest neighbors (non-adjacent pairs) ranged from -6 dB to -25 dB. In addition to this geometric decoupling, the elements received an additional isolation of 18 dB from preamplifier decoupling.
Imaging Performance
Figure 5 shows representative noise correlation matrices for both constructed arrays. The 64-channel noise correlation ranged from 0.1% to 38% with an average of 8% for the off-diagonal elements. For the 32-channel these numbers ranged from 0.1% to 35% with a mean of 11%. Both constructed array coils passed the in-scanner safety tests without additional adjustment. We observed a slight increase (2% for the 64-channel and 1.5% for the 32-channel array) in the RF power required to achieve a given flip angle when the de-tuned array was placed inside the scanner. Temperature changes induced by the heating tests were below 2°C on critical components (e.g. cable traps) or on the inner side of the array helmets. Stability tests indicated a peak-to-peak variation of 0.5% and 0.3% over a 15×15 pixel ROI for 500 time-points for 3×3×5 mm3 resolution EPI for the 64-channel and 32-channel, respectively.
Figure 5.
Noise correlation coefficient matrices generated from an acquisition without RF excitation. The averaged off-diagonal of the matrix was 9% (min: 0.1%, max: 38%) for the 64-channel array coil and 11% (min: 0.1%, max: 38%) for 32-channel coil.
Figures 6 and 7 show SNR map comparisons between the constructed 64-channel and 32-channel array coils for unaccelerated and accelerated images combined with the cov-RSS. The SNR in the sagittal slice acquired through the center of each coil is shown in Figure 6. At the location of the lowest brain-SNR, which corresponds to the upper brainstem/pons, the measured SNR was similar in both constructed coils. Figure 7 shows the SNR map of a transversal slice through the center of the brain (~3cm superior to the brain-stem/pons location reported above) for both unaccelerated and accelerated imaging. Figure 8 shows the computed relative SNR increase of the 64-channel coil over the 32-channel coil in three region-of-interests (ROI): brain center, cortex, and skull for these three ROIs for R=1 through R=8. For unaccelerated (R=1) imaging, both figures 7 and 8 show nearly identical SNR at the central ROI (~4% higher SNR for the 64-channel coil) and a 1.3-fold SNR improvement over the 32-channel coil at the outer cortex ROI. The largest SNR increase of up to 1.5-fold compared to the 32-channel coil, was measured in the close proximity to the 64-channel coil elements, which corresponds to the head's skin and skull, an unimportant area in brain MRI. In the case of accelerated image SNR, the 64-channel coil increasingly outperforms the 32-channel array with higher acceleration factors. For the R=4 fold accelerated image, the central brain and cortical SNR were 1.2- and 1.4-fold higher for the 64-channel array compared to the 32-channel array.
Figure 6.
In vivo SNR comparisons in a sagittal plane between the sized-matched 64-channel (a) and 32-channel (b) array coils. The SNR measurements show a 1.3-fold peripheral SNR increase in the brain and nearly the same SNR is achieved in the coil's center.
Figure 7.
Measured SNR in non-accelerated (R=1) and 2D accelerated (R=3 to R=5) cases for the 64-channel coil (top row) 32-channel coil (bottom row) in a transversal mid-brain slice. The 64-channel coil increasingly outperforms the 32-channel coil with higher acceleration factors in both central and peripheral SNR. The scans were accelerated in anterior–posterior direction.
Figure 8.
Relative SNR gain of the 64-channel coil over the 32-channel coil as a function of acceleration. The higher peripheral SNR of the 64-channel coil and the reduced G-factors, mainly obtained in the center of the 64-channel coil, show substantial better SNR performance in all brain areas with higher accelerated acquisitions compared to the 32-channel array coil.
SNR measurements with the head phantoms, corresponding to average female and male head circumferences, showed that the 64-channel array with a female head phantom lost 8% of its mean SNR obtained from the bigger male-sized phantom. For the 32-channel array this loss was only 3.
Figure 9 shows the inverse G-factor maps in transversal brain planes for one-dimensional (1D) and two-dimensional (2D) accelerations for the constructed and simulated array coils. The simulated G-map calculations show 5% overall lower noise amplifications compared to the measured G-factors. Both, simulated and measured G-factors estimations show that the 64-channel coil provides one additional unit of acceleration for a given noise amplification. For example, at acceleration factor of R=4, the peak G-factor for the 64-channel coil was only 24% of that of the 32-channel coil.
Figure 9.
Measured and simulated inverse G-factor maps of transverse in-vivo images and digital anatomical head model slices, respectively. The maps from the measured data were calculated using images from a PD-weighted FLASH sequence and additionally noise correlation information. The maps of simulated data were computed from the complex reception profile of the coil (B1-). The simulated data show slightly lower mean G-factors . Both methods for G-factors calculation show most favorable G-factors for the 64-channel array coil, roughly providing one additional unit of acceleration for a given noise amplification factor.
Figure 10 shows the fully encoded brain images obtained from the accelerated simultaneous multi-slice acquisition and its corresponding inverse G-factor maps. For the 64-channel coil the average G-factor was 1.09 across the five slices, with a maximum 1.45. The 32-channel coil showed overall higher noise amplifications with an average G-factor of 1.22 and a maximum of 1.84.
Figure 10.
Inverse G-factor map comparison between the 64-channel and 32-channel coil, obtained from the accelerated simultaneous multi-slice acquisition with blipped-CAIPI. In-plane acceleration factor of R=2 and slice acceleration of Rsl=5 are used. The 64-channel array coil provides an average G-factor reduction of 10.7% across all five slices and a 21.2% noise amplification decrease of the peak G-factors, when compared to the 32-channel array.
Figure 11 shows highly accelerated (R=4) MPRAGE images between both constructed array coils, obtained from the same subject. This acquisition captures the whole head brain morphology with 1mm isotropic resolution in 3:38 minutes. The combination of reduced G-factors and cortical brain SNR, translates to improved highly accelerated image quality in the 64-channel array coil compared to the 32-channel coil.
Figure 11.
Fully encoded sagittal 1-mm isotropic MPRAGE images with GRAPPA acceleration factor of R = 4 and an acquisition time of 3:38 minutes for whole brain coverage. The combination of reduced G-factors and improved cortical SNR of the 64-channel coil (a), translates to improved image quality when compared to the 32-channel coil (b). Images from both coils are obtained from the same subject.
Discussion
In this study we construct and characterize size- and shape-matched 64-channel and 32-channel brain array coils, designed for highly accelerated imaging on a clinical 3T scanner for robust daily use. The coils’ performance were evaluated in bench and phantom imaging experiments, including bench-level measures of element performance and decoupling and system-level tests of heating and transmit field interactions. The benefit of the increased number of array elements was assessed in human measurements through SNR and G-factor maps, as well as with accelerated in-vivo imaging.
A number of technical issues arise in the implementation of large channel-count arrays employing relatively small element sizes (such as the 65 mm diameter elements in the 64-channel array). In particular, the inter-element decoupling becomes more difficult and time-consuming as the element density increases. Additionally, maintaining a high QU/QL-ratio becomes problematic. For example, while a 32-channel adult coil with a loop diameter of ~90 mm can be constructed out of flexible circuit material, array coils with smaller elements size show significant eddy current losses in the conductors of the neighboring elements leading to a lower QU/QL-ratio and diminished SNR (6,9). Spatially sparse wire conductors, and relocation of the preamplifier and its motherboard at least 30 mm from the loop elements reduces the losses in these copper components of the dense arrays. Also, the ability to mechanically optimize the overlap between two loops by bending the wire facilitated the element decoupling procedure. Despite these precautions, the unloaded Q of a given loop was measurably diminished when the loop under test was placed in an array configuration suggesting that losses within the conductors of neighboring elements were still present. Nonetheless, the QU/QL-ratio for constructed 64-channel array shows sample noise dominance, but this metric was less favorable for the smaller loop sizes comprised in the array (e.g. loop sizes corresponding to the pentagons). In the larger elements of the 32-channel coil these issue were much less critical. A frequency drop of 0.4 MHz upon sample loading, measured with an isolated coil element of the 64-channel coil, indicates imbalance in how the sample and coil interact through electric and magnetic fields. This source of loss could be compensated with more equally distributed series tuning capacitors to further balance the electrical field around the loop. However, when loop sizes are small, additional series capacitors increase the effective series loop resistance (5,9) and also the practical implementation of higher capacitor counts in high-density array coils is challenging.
The need to align the preamplifier to z-direction (11–13) reduced the choices for preamplifier positioning. The layout was further constrained by the need to avoid passing an output coax of a preamplifier near (<2cm) the input of other preamps to avoid positive feedback and possible oscillation. These preamplifier placement issues become more challenging as coil element density increases. Finally, it becomes more difficult to enclose very high-density coil arrays in compact housings.
Measurements of the transmitted RF field with and without the constructed detuned array coils inside the scanner showed up to 4% difference in transmit power. This indicates sufficient coil element detuning and suppression of common mode currents on the cables. The modest increase in power requirement when arrays are present might be due to power dissipation in the copper-wire used in the element construction, cables, and preamplifiers (including circuitry and motherboard.) Similar observations can be found in literature about experimental high-density array coils (5,23–25).
The noise correlation between the coil elements revealed a mean of 13%. However, some pairs in each coils showed correlations over 38%. These “worst case” correlations were found predominantly in adjacent loops. Some of the correlation is likely due to inductive coupling, but some is due to shared resistance through the sample (1). In principle it might be possible to reduce the effect of inductive coupling with a bridging capacitor. The noise-covariance weighted root sum-of-squares reconstruction method is able to reduce the impact of these noise correlations within the array by utilizing coil sensitivity and noise correlation information.
The G-factor maps show the highest noise amplification in the central brain regions. The comparison between the 64- and 32-channel coil shows that the central brain regions also has the highest relative improvement in G-factor when the element count is doubled. We attribute the G-factor improvement to the smaller elements, which offers a stronger spatial modulation of signal intensity and thus improved ability to unalias folded images (SENSE method) or synthesize spatial harmonics (SMASH or GRAPPA methods). Finally, we note that the improvement in G-factors at the brain center spatially compliments the SNR gains in that higher channel count coils show in unaccelerated imaging, which are only at the brain periphery. The accelerated images obtained from the constructed 64-channel array provided the ability to accelerate at approximately one unit higher at a given noise amplification compared to the sized-matched 32-channel array. Our simulated G-factors are quantitatively in line with the measured ones, which show only slightly lower noise amplifications. It also matches the results from previously published simulation studies (4) with simple geometries (spherical phantom and spherical array coils). This study predicted a 21% decrease of maximum G-factors when the element count is doubled from 32 channels to 64 channels. Our data show a 24% reduction on peak G-factors moving from 32 to 64 channels. Additional improvements in parallel imaging encoding with 64-channel head coils can be accomplished using simultaneous multi-slice EPI acquisitions. For example, in a 5x2-fold accelerated multi-slice acquisition, the 64 channel array coil provides an average G-factor reduction of 10.7% across all five slices and a 21.2% noise amplification decrease of the peak G-factors, when compared to the 32-channel array. Similar to the regular parallel imaging techniques, the smaller elements sizes and its increased loop count of the 64-channel coil provide a stronger spatial distinction of the sensitivity profiles, which permit a better separation of the simultaneously exited slices (19,26,20). The combination of the 64-channel brain array coil with simultaneous multi-slice EPI acquisition techniques and regular parallel imaging, substantially reduces the acquisition time and allows full-brain high-resolution fMRI acquisitions with reasonable TR and very low noise amplifications. Furthermore, in some regions of the brain we observed 1/G-factors greater than unity; indicating some noise cancellation in the reconstruction process as previously demonstrated in low acceleration in-plane GRAPPA acquisitions (27).
The narrow fit around the neck prohibits the subject from exiting the coil without opening the halves. This design choice initially raised the concern that subjects would have an adverse psychological reaction to having one's head “captured.” However, our experience has showed that the design does not induce additional anxiety. A secondary benefit of the contoured design was that the helmet was comfortable to lie on with minimal foam padding. This stems from the bowl shaped support for the occipital pole, the curving neck support.
Further housing design consideration regarding small looped arrays, arise when we compare the coil performance between big and small head sized subject (e.g. as expected between male and female subjects who have a 13.3 mm smaller head circumference on average compared to men (28). Assuming a spherical head shape, this would suggest the elements would be 3mm further from the head for the average female subject. The concern is that the female group is potentially even further underserved by the higher element count coil. SNR measurements with the head phantoms corresponding to average female and male head circumferences showed that this was partially true; the 64 channel array lost 8% of its mean head SNR compared to 3% for the 32-channeal array. But even for the smaller phantom, there were SNR benefits from the higher element count coil. This result qualitatively agrees with the data of Hayes et al. and Lawry et al., who studied SNR drop-off as a surface coil is backed away from the loaded object. These studies suggested that significant SNR is not lost until the coil is more than one quarter of the coil diameter from the load (29,30). For our constructed array coil with 65 mm diameter coils, this suggests, coil elements can be up to 16 mm from the head load. However, when the loop count increases beyond 64 channels (with correspondingly smaller loop diameters), array designs will likely need at least a few spatial degrees of freedom to allow the array to better fit individual subjects.
Conclusion
A 64-channel array coil for accelerated brain imaging was constructed and tested with phantoms and in vivo MRI scans. We compared the parallel imaging and SNR performance of the constructed array to a sized-matched 32-channel head coil, which we specially constructed for this purpose. For unaccelerated imaging the 64-channel coil produced substantial SNR improvement only in the brain periphery. However, for image accelerations of R=3 or above, the G-factor improvements in the brain center translated to improved imaging at all locations in the head.
Acknowledgements
We would like to thank Siemens AG, Healthcare Sector, Erlangen, Germany, for providing scanner specific components. This project was supported by National Institutes of Health Grants U01MH093765, K99EB012107, and P41RR14075.
Footnotes
Product under development and not commercially available in the U.S. and its future availability cannot be assured.
References
- 1.Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn Reson Med. 1990;16:192–225. doi: 10.1002/mrm.1910160203. [DOI] [PubMed] [Google Scholar]
- 2.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- 3.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002;47:1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 4.Wiesinger FF, De Zanche N, Pruessmann KP. Approaching ultimate SNR with finite coil arrays.. Proceedings of the 13th Annual Meeting of ISMRM; Miami. 2005.p. 672. [Google Scholar]
- 5.Wiggins GC, Polimeni JR, Potthast A, Schmitt M, Alagappan V, Wald LL. 96-Channel receive-only head coil for 3 Tesla: design optimization and evaluation. Magn Reson Med. 2009;62:754–762. doi: 10.1002/mrm.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med. 2006;56:216–223. doi: 10.1002/mrm.20925. [DOI] [PubMed] [Google Scholar]
- 7.Wang J. A novel method to reduce the signal coupling of surface coils for MRI.. Proceedings of the 4th Annual Meeting of ISMRM; New York. 1996.p. 1434. [Google Scholar]
- 8.Hyde JS, Jesmanowicz A, Froncisz W, Kneeland JB, Grist TM, Campagna NF. Parallel image acquisition from noninteracting local coils. J Magn Reson. 1986;70:512–517. [Google Scholar]
- 9.Kumar A, Edelstein WA, Bottomley PA. Noise figure limits for circular loop MR coils. Magn Reson Med. 2009;61:1201–1209. doi: 10.1002/mrm.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hergt M, Oppelt R, Vester MD, Reykowski A, Huber KM, Jahns K, Fischer HJ. Low noise preamplifier with integrated cable trap.. Proceedings of the 15th Annual Meeting of ISMRM; Berlin. 2007.p. 1037. [Google Scholar]
- 11.Possanzini C, Bouteljie M. Influence of magnetic field on preamplifiers using GaAs FET technology.. Proceedings of the 16th Annual Meeting of ISMRM; Toronto. 2008.p. 1123. [Google Scholar]
- 12.Hoult DI, Kolansky G. A Magnetic-Field-Tolerant Low-Noise SiGe Pre-amplifier and T/R Switch.. Proceedings of the 18th Annual Meeting of ISMRM; Stockholm. 2010.p. 649. [Google Scholar]
- 13.Lagore R, Roberts B, Fallone BG, De Zanche N. Comparison of Three Preamplifier Technologies: Variation of Input Impedance and Noise Figure With B0 Field Strength.. Proceedings of the 19th Annual Meeting of ISMRM; Montreal. 2011.p. 1864. [Google Scholar]
- 14.Darrasse L, Kassab G. Quick measurement of NMR-coil sensitivity with a dual-loop probe. Rev Sci Instrum. 1993;64:1841–1844. [Google Scholar]
- 15.Reykowski A, Wright SM, Porter JR. Design of matching networks for low noise preamplifiers. Magn Reson Med. 1995;33:848–852. doi: 10.1002/mrm.1910330617. [DOI] [PubMed] [Google Scholar]
- 16.Christ A, Kainz W, Hahn EG, Honegger K, Zefferer M, Neufeld E, Rascher W, Janka R, Bautz W, Chen J, Kiefer B, Schmitt P, Hollenbach H-P, Shen J, Oberle M, Szczerba D, Kam A, Guag JW, Kuster N. The Virtual Family--development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol. 2010;55:1041–1055. doi: 10.1088/0031-9155/55/2/N01. [DOI] [PubMed] [Google Scholar]
- 17.Hoult DI. The principle of reciprocity in signal strength calculations - A mathematical guide. Concepts Magn Reson. 2000;12:173–187. [Google Scholar]
- 18.Kellman P, McVeigh ER. Image reconstruction in SNR units: a general method for SNR measurement. Magn Reson Med. 2005;54:1439–1447. doi: 10.1002/mrm.20713. Erratum in Magn Reson Med 2007;58:211–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkman DJ, Hajnal JV, Herlihy AH, Coutts GA, Young IR, Ehnholm G. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J Magn Reson Imaging. 2001;13:313–317. doi: 10.1002/1522-2586(200102)13:2<313::aid-jmri1045>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 20.Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67:1210–1224. doi: 10.1002/mrm.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robson PM, Grant AK, Madhuranthakam AJ, Lattanzi R, Sodickson DK, McKenzie CA. Comprehensive quantification of signal-to-noise ratio and g-factor for image-based and k-space-based parallel imaging reconstructions. Magn Reson Med. 2008;60:895–907. doi: 10.1002/mrm.21728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Kouwe AJW, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008;40:559–569. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt M, Potthast A, Sosnovik DE, Polimeni JR, Wiggins GC, Triantafyllou C, Wald LL. A 128-channel receive-only cardiac coil for highly accelerated cardiac MRI at 3 Tesla. Magn Reson Med. 2008;59:1431–1439. doi: 10.1002/mrm.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keil B, Wiggins GC, Triantafyllou C, Wald LL, Meise FM, Schreiber LM, Klose KJ, Heverhagen JT. A 20-channel receive-only mouse array coil for a 3 T clinical MRI system. Magn Reson Med. 2011;66:582–593. doi: 10.1002/mrm.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keil B, Alagappan V, Mareyam A, McNab JA, Fujimoto K, Tountcheva V, Triantafyllou C, Dilks DD, Kanwisher N, Lin W, Grant PE, Wald LL. Size-optimized 32-channel brain arrays for 3 T pediatric imaging. Magn Reson Med. 2011;66:1777–1787. doi: 10.1002/mrm.22961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Gunther M, Glasser MF, Miller KL, Ugurbil K, Yacoub E. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS ONE. 2010;5:e15710. doi: 10.1371/journal.pone.0015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polimeni JR, Wiggins GC, Wald LL. Characterization of artifacts and noise enhancement introduced by GRAPPA reconstructions.. Proceedings of the 16th Annual Meeting of ISMRM; Toronto. 2008.p. 1286. [Google Scholar]
- 28.Bushby KM, Cole T, Matthews JN, Goodship JA. Centiles for adult head circumference. Arch Dis Child. 1992;67:1286–1287. doi: 10.1136/adc.67.10.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes CE, Axel L. Noise performance of surface coils for magnetic resonance imaging at 1.5 T. Med Phys. 1985;12:604–607. doi: 10.1118/1.595682. [DOI] [PubMed] [Google Scholar]
- 30.Lawry TJ, Weiner MW, Matson GB. Computer modeling of surface coil sensitivity. Magn Reson Med. 1990;16:294–302. doi: 10.1002/mrm.1910160210. [DOI] [PubMed] [Google Scholar]