Abstract
GPR56, a member of the adhesion G-protein coupled receptor (GPCR) family, is integral to the development of the cortex, as mutations in GPR56 cause bilateral frontoparietal polymicrogyria (BFPP). BFPP is a cobblestone-like cortical malformation, characterized by overmigrating neurons and the formation of neuronal ectopias on the surface of the brain. Since its original cloning a decade ago, GPR56 has emerged from an orphaned and uncharacterized protein to an increasingly well-understood receptor, both in terms of its signaling and function. Collagen III is the ligand of GPR56 in the developing brain. Upon binding to collagen III, GPR56 activates RhoA via coupling to Gα12/13. This pathway appears to be particularly critical in the preplate neurons, which are the earliest born neurons in the cortex, as the expression pattern of GPR56 in these neurons mimics the anterior to posterior gradient of malformation associated with loss of GPR56 in both human and mice. Further characterizing the role of GPR56 in the preplate will shed light on the mechanism of cortical development and patterning.
Keywords: adhesion G protein-coupled receptor, GPR56, bilateral frontoparietal polymicrogyria, extracellular matrix, neuronal migration
Introduction
GPCRs are an extremely diverse group of proteins encoded by over 800 genes in the human genome [1]. This receptor “superfamily” is united by commonalities in structure consisting of a seven-transmembrane α-helix, an extracellular N-terminus, an intracellular C-terminus, and three interhelical loops on each side of the plasma membrane [2]. Adhesion GPCRs are one of the five subgroups of GPCRs that facilitate cell-cell and cell-matrix interaction. Structurally, they are differentiated from other subgroups by the presence of an exceptionally long extracellular N-terminal region and a GPCR proteolysis site (GPS), which is an autocleavage site that cleaves the receptor into N- and C- terminal fragments during the maturation process [3,4]. Although there are a total of 33 human adhesion GPCRs, GPR56 is the first and only member to be linked thus far to a human developmental disorder – bilateral frontoparietal polymicrogyria (BFPP) [5–7].
Much has been learned about GPR56 and its role in brain development and cancer metastasis. In this review, we will mainly focus on GPR56’s biochemical properties and signaling pathways as they relate to the development of the cerebral cortex. We will also specifically comment on how it interacts with its ligand, collagen III, to regulate cerebral cortical development.
GPR56-related brain malformation
BFPP
The first clinical description of BFPP dates to 1990 when Harbord and colleagues reported two sisters, ages seven and ten, who presented with developmental delays and nonprogressive cerebellar ataxia [8]. The reported MRI was interpreted as a “neuronal migration abnormality.” However, subsequent high-resolution MRIs revealed the characteristics of BFPP, including (1) bilateral polymicrogyria with an anterior to posterior gradient of severity; (2) bilateral patchy white matter signal changes without a specific pattern; and (3) brainstem and cerebellar hypoplasia [9].
The first linkage study of BFPP was carried out in two unrelated consanguineous Palestinian pedigrees [10]. Given the fact that 1) the cases arose from consanguineous healthy parents and 2) individuals of both sexes were affected, the disorder is suggestive of an autosomal recessive inheritance. Therefore linkage analysis and homozygosity mapping were carried out. Statistical analysis provided a strong evidence linking BFPP to chromosome 16q12.2-21, with all five affected individuals from these two pedigrees sharing identical marker alleles in a 17cM region bordered by microsatellite markers. This discovery indicated that the two families share a founder mutation, even though there was no known relationship between them. When this study was extended to 12 pedigrees, the region of interest was narrowed down to an interval of 2.7cM with a total of 27 characterized genes, of which 17 were identified as candidate genes and subjected to sequencing analysis in the genomic DNA of BFPP patients [6]. Multiple independent mutations were identified in GPR56, all of which were homozygous germline mutations. To date, a total of 22 mutations have been reported in humans (Table 1) [5,7,11–13].
Table 1.
BFPP-associated mutations in human
| Nucleotide change | Predicted aa change | Domain | Mutation type | Ref |
|---|---|---|---|---|
| c.97C>G | p.R33P | Ligand binding | Missense | 13 |
| c.112C>T | p.R38W | Ligand binding | Missense | 6, 7 |
| c.113G>A | p.R38Q | Ligand binding | Missense | 7 |
| c.174_175insC | p.E56RfsX24 | Ligand binding | Frameshift | 11 |
| c.235C>T | p.R79X | Ligand binding | Missense | 13 |
| c.263A>G | p.Y88C | Ligand binding | Missense | 6 |
| c.272G>A | p.C91Y | Ligand binding | Missense | 11 |
| c.272G>C | p.C91S | Ligand binding | Missense | 6 |
| c.367c C>T | p.Q123X | Ligand binding/STP | Nonsense | 11 |
| c.671delA | p.D224WfsX96 | Between STP/GPS | Frameshift | 11 |
| c.739_746delCAGGACC | p.Q246TfxX72 | Between STP/GPS | Frameshift | 6 |
| c.768-1G>C | † | Between STP/GPS | Splicing | 6 |
| c.1036T>A | p.C346S | GPS | Missense | 6 |
| c.1046G>C | p.W349S | GPS | Missense | 7 |
| c.1167+3G>C | ‡ | GPS | Splicing | 6 |
| c.1254C>G | p.C418W | TM1 | Missense | 11 |
| c.1215–1216delC | p.L406S406fsX41(M447X) | TM2 | Frameshift | 11 |
| c.1345delCTG | p.L449del | TM2 | In frame deletion | 11 |
| c.1453C>T | p.S485P | TM3 | Missense | 11 |
| c.1486G>A | p.E496K | TM3 | Missense | 12 |
| c.1693C>T | p.R565W | EC2 | Missense | 6, 7, 13 |
| c.1919T>G | p.L640R | TM7 | Missense | 7 |
TM, transmembrane domain; EC, extracellular loop
Unknown due to the presence of potential cryptic splice acceptor site(s).
Unknown due to the presence of potential cryptic splice donor site(s).
Since the initial report, a total of 48 molecularly confirmed BFPP cases have been reported worldwide [5,7,11–13]. The prevalence of BFPP is suspected to be significantly higher than the number of cases reported, however, due to the fact that it was frequently misdiagnosed prior to the availability of high resolution MRI and genetic testing. Indeed, the confirmed BFPP cases were previously reported under four additional different diagnoses: “autosomal recessive syndrome of pachygyria,” “neuronal migration abnormality,” “cobblestone lissencephaly with normal eyes and muscle”, and “lissencephaly with cerebellar hypoplasia,” making it particularly difficult to discern the true prevalence of BFPP.
The expression of GPR56 in the developing cerebral cortex
GPR56 is a member of the adhesion G protein-coupled receptor family. It was originally cloned in 1999 by two independent groups [14,15]. Its association with a severe human brain malformation, BFPP, suggests an important role of GPR56 in brain development. Therefore, significant efforts were undertaken to characterize the expression pattern of GPR56 in the developing brain. In situ hybridization showed preferential expression of Gpr56 mRNA in neuronal progenitor cells of the cerebral cortical ventricular and subventricular zones during periods of neurogenesis [6]. Immunohistochemistry (IHC) with a mouse monoclonal antibody against mouse GPR56 revealed a broad expression of the protein in multiple cell types in the preplate, marginal zone, subventricular zone and ventricular zone [16].
The histopathology of BFPP
Polymicrogyria is a highly heterogeneous cortical malformation [17]. The normally convoluted gyri are replaced by numerous (poly) and noticeably smaller (micro) gyri. Histologically, the normal six-layered cerebral cortical structure is distorted into presenting with either four layers, the absence of discernible layers altogether, or the presence of leptomeningeal heterotopia [18,19]. The latter form is commonly known as cobblestone lissencephaly, or type II lissencephaly, and results from aberrant neuronal migration through breaches in the basal lamina [20].
BFPP is a radiological diagnosis. Some of the MRI findings, namely abnormal signal in the cerebral white matter and hypoplasia of the pons and cerebellar vermis, have many similarities to those seen in conditions with cobblestone lissencephaly, such as muscle-eye-brain disease (MEB), Fukuyama congenital muscular dystrophy (FCMD), and Walker-Warburg syndrome (WWS). Indeed, some of the molecularly confirmed BFPP cases were originally diagnosed as cobblestone lissencephaly with normal eyes and muscle [9,21]. Therefore, it has been suggested that BFPP should be renamed as cobblestone-like cortical malformation (Dobyns, personal communication).
Histological studies in a Gpr56 knockout mouse model and a human postmortum BFPP brain further supported the connection of BFPP to cobblestone lissencephaly. Homozygous deletion of mouse Gpr56 results in cortical lamination defects with neurons overmigrating through a breached pial basement membrane (BM). The cortical malformation strongly resembles human cobblestone lissencephaly, thereby suggesting that the histopathology of BFPP is a cobblestone-like cortical malformation [22]. One reported foetopathological BFPP case confirmed the presence of ectopic neuronal overmigration, a key feature of cobblestone-like cerebral dysgenesis, further supporting this suggested pathology [11].
The MRIs of BFPP brains also shows rostral cerebellar cortical hypoplasia as well as mild hypoplasia of the cerebellar vermis. Histological analysis of adult Gpr56 knockout mice showed a malformed rostral part of the cerebellum, encompassing lobules I-V in the form of a fragmented pial BM and disruptions in folding of the cerebellar lobes [23].
Cobblestone lissencephaly
Cobblestone lissencephaly is typically seen in three distinct human disorders: MEB, FCMD, and WWS [20]. These three disorders are autosomal recessive diseases that encompass congenital muscular dystrophy, ocular malformations, and cobblestone lissencephaly. MEB, FCMD, and some WWS cases are caused by aberrant glycosylation of α-dystroglycan, another matrix receptor [24–27]. The leading pathology of cobblestone lissencephaly is the presence of neuronal ectopias on the brain surface [20]. Mutant mice with deletions in some members of the integrin pathway and constituents of the ECM also exhibit cortical migration defects with deficiencies in basal lamina integrity as well as cortical ectopias, which are features of the human cobblestone malformation [28–33]. Traditionally, the suggested pathology leading to cobblestone lissencephaly has been a defective pial BM [20]. However, recent literature demonstrates that abnormal neuronal migration may partially account for the improper formation of the cobblestone-like cortex [34–37].
The biochemical properties of GPR56
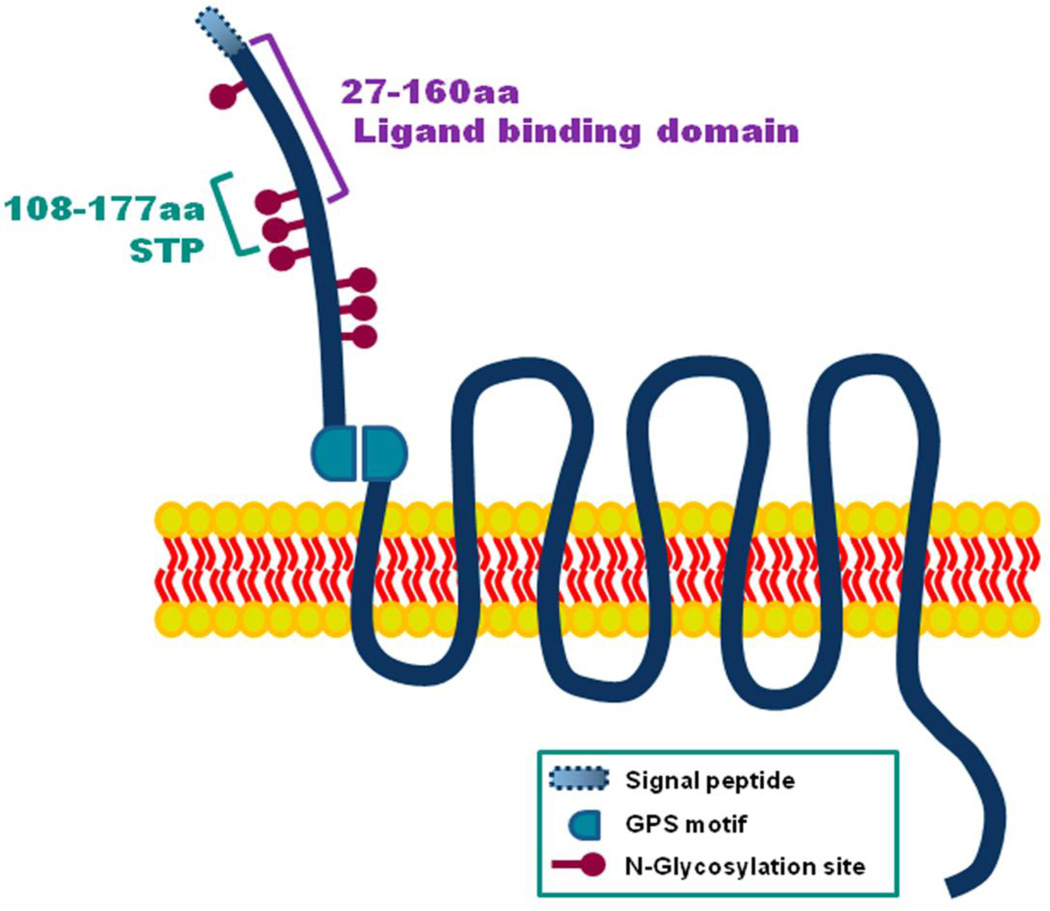
As a member of the adhesion GPCRs, GPR56 has an exceptionally long extracellular N-terminus, which can be subdivided into several regions, including the serine threonine proline (STP) segment (aa 108–177) and ligand binding domain (aa 27–160) [38,39] (Fig. 1). GPR56 also contains a GPS motif, responsible for cleaving the protein into N- and C-terminal fragments [40–42]. Finally, GPR56 is significantly glycosylated, with seven different sites along its N-terminal fragment [42]. The details of these various biochemical properties are described in this section.
Fig. 1.
Structure of GPR56 protein. GPR56 consists of an exceptionally long extracellular N-terminal region, GPCR protolysis site (GPS), and a seven transmembrane α-helix (7TM) C-terminal region. The GPS domain is an autocleavage site. There is a signal peptide (aa 1–26) in the N-terminus of GPR56. The ligand binding domain spans the region of aa 27–160 that is essential for binding to collagen III. The STP segment (aa 108–177) is the binding site for TG2. There are a total of seven N-glycosylation sites
GPS-mediated autocatalytic process
The GPS motif was first demonstrated to be an internal cleavage site in latrophilin and was later found to be present in all 33 members of the adhesion GPCRs, as well as in several other proteins outside the adhesion GPCR family, including all five human polycystic kidney disease proteins [43–48]. The GPS-motif is comprised of four cysteine and two tryptophan residues in the conserved sequence, C-x2-Wx6-16-W-x4-C-x11-C-x-C, which is essential for its catalytic properties. It has recently been showed that the GPS motif is a part of a much larger evolutionarily conserved domain referred to as the GPCR-Autoproteolysis Inducing (GAIN) domain [49].
Post-transcriptionally, GPR56 is autocatalytically cleaved via the GAIN domain between amino acids Histidine-381 and Leucine-382 into N- and C- terminal fragments, GPR56N and GPR56C, respectively [40–42]. GPR56N can either remain associated with GPR56C to form non-covalent heterodimers or be secreted into the conditioned media [42]. Although the biological significance of its secretion is not clear, the fact that disease-associated mutations in GPR56N were not detected in the conditioned media suggest that this is a key step for the receptor function [42].
Two BFPP-associated mutations, C346S and W349S, are found within the GPS motif and have been found to abolish proteolytic cleavage of GPR56 [42,50]. Consequently, C346S and W349S mutant proteins become trapped in the endoplasmic reticulum (ER), thereby preventing GPR56 expression on the cell surface. Interestingly, mutations in GPR56 found in other regions of the protein do not meet the same fate, although there is still a decrease in their expression on the cell membrane [42]. A significant amount of C346S and W349S mutant proteins can be rescued by providing a pharmacological chaperone to force the ER to release its retained proteins, resulting in a cell surface expression in these mutants. The results of these experiments also differentiate the C346S from W349S mutations, as W349S is much more receptive to treatment with pharmacological chaperones, perhaps indicating that the C346S mutation results in a significantly larger distortion of the protein [42]. Moreover, individuals harboring the C346S mutation present with both BFPP and microcephaly, a more severe phenotype than individuals carrying other GPR56 mutations, including deletion mutations [6,7].
N-glycosylation
Glycosylation occurs when sugars are added to a protein at either the amide nitrogen in asparagines (N-linked) or the oxygen in serine and threonine side chains (O-linked) [51]. In addition to the presence of multiple potential glycosylation sites, GPR56N was found to be much larger than its predicted molecular weight, further indicating the presence of protein modification. To analyze the extent and nature of the potential glycosylation of GPR56, the protein was treated with Peptide: N-Glycosidase F and neurominidase to remove N-linked and O-linked oligosaccharides respectively. Only Peptide: N-Glycosidase F treatment, not neurominidase, resulted in a substantial shift of the protein band from 60–80 kDa to 40 kDa, which correlates to the size of unmodified GPR56N [42]. These results suggest that GPR56 is modified by N-glycosylation alone. This observation was further confirmed by site-directed mutagenesis analysis, which demonstrated that when all seven potential N-glycosylation sites (Asn-39, -148, -171, -234, -303, -324, and -341) were altered, only a single 40kDa protein band was detected [42].
Interestingly, disease-associated mutations, including the two mutations in the GPS motif, C346S and W349S, did not affect the glycosylation of GPR56. However, glycosylation proved to be essential for GPR56 protein trafficking to the cell surface and its secretion; when all of the seven N-glycosylation sites were removed by site-directed mutagenesis, the mutant protein failed to express on the cell surface and secret into the conditioned media [42].
STP segment
A seventy amino acid-long STP segment is found between aa 108–177 in GPR56N. As indicated by its name, the STP domain consists mainly of serine, threonine, and proline amino acids. Functionally, this region is best known for the potential role of GPR56 in cancer metastasis. The STP segment is the binding site of the tissue transglutaminase, TG2, which is a crosslinking enzyme that is mainly present in the extracellular matrix (ECM) [38]. In addition to crosslinking, TG2 can modify proteins by amine incorporation, deamidation, and by acting as an isopeptidase in a Ca2+-dependent manner [52]. Furthermore, TG2 mediates the interaction of integrins with fibronectin and crosslinks proteins of the ECM in order to strengthen its integrity [53,54]. Therefore, it is not surprising that down-regulation of TG2 has been associated with aggressive tumors and metastasis.
More specifically, the binding of TG2 to GPR56 likely prevents metastasis through suppressing angiogenesis and tumor progression. This claim is further supported through data indicating that when the STP segment is deleted in GPR56, thereby preventing TG2 binding, vascular endothelial growth factor (VEGF) production is increased and angiogenesis is upregulated [38]. However, the mechanism by which TG2 inhibits VEGF production remains unknown. Although deleting the STP segment is associated with an increase in angiogenesis, knocking down TG2 mRNA fails to show an increase in VEGF production [38].
Ligand binding domain
There is a separate ligand binding domain specific to collagen III, which is the ligand of GPR56 in the developing brain (further discussion in the upcoming section). In our quest to discover the location of the putative ligand binding region, we found that deleting aa 93–143 completely abolished the putative ligand binding ability of GPR56. This discovery suggests that the ligand binding domain lies in the most N-terminal region of GPR56 [22]. Indeed, using truncated GPR56N fragments of various lengths, we confirmed that the ligand binding domain of GPR56 lies within aa 27–160. Within this domain, there are seven reported disease-associated missense mutations, of which four were tested and shown to abolish the ability of GPR56 to bind to its ligand, collagen III [39]. Although two of the seven glycosylation sites are found in this ligand binding domain, glycosylation appears to be unnecessary for the receptor-ligand interaction [39].
GPR56C
Unlike the GPR56N, which has several defined domains and motifs, the 7TM region of GPR56 remains uncharacterized. Interestingly, mutations in GPR56C do not affect the non-covalent interaction between GPR56N and GPR56C [42]. In fact, the only abnormality in these mutations was either a lack of cell surface expression of GPR56C (R565W mutant) or a higher level of surface GPR56C in comparison to surface expression of GPR56N (L640R mutant). These findings suggest that GPR56N and GPR56C are independently trafficked and that disease-associated mutations affect the protein trafficking of GPR56C.
The signaling of GPR56
TG2, an endogenous binding partner of GPR56
Two different research groups ventured to identify the ligand of GPR56, both of which took a receptor affinity probe in situ approach to first determine the putative ligand expression pattern in a variety of tissues. The Hynes’ group found that the putative ligand of GPR56 is expressed in the ECM of a diverse set of tissues. Through using overlay assays and mass spectrometry analyses, TG2 was identified as an endogenous binding partner of GPR56 [41].
Although TG2 binds to GPR56 at the STP segment, it remains unclear whether TG2 functions as a ligand in a traditional fashion, i.e. activating a downstream signaling pathway upon binding to its receptor. The downstream signaling as well as biological consequence of this binding remains largely unknown. TG2 seems to have multifaceted functions in in vitro cell culture systems [52]. However, its function in vivo during development remains unclear, as TG2 knockout mice do not show any developmental abnormalities [55]. Our unpublished studies further confirm these results, as homozygous TG2 mutant mice did not display any identifiable cortical malformation.
There is no published data on the characterization of the temporal and spatial expression of TG2 in embryonic brains. In adult rat brain, TG2 is widely expressed in neurons along pyramidal and extrapyramidal pathways with less expression in the somatosensory system [56]. Developmental profiling of TG2 mRNA by real time RT-PCR in the mouse forebrain indicates that its levels increase after birth [57].
Collagen III, the major ligand of GPR56 in the developing brain
To identify the ligand of GPR56 in the developing brain, we engineered a GPR56N-mFc fusion construct as well as a mutant fusion protein, GPR56Ndel-mFc, which lost the putative ligand binding through deleting aa 93–143. Receptor affinity probe in situ revealed that the GPR56 putative ligand was found to localize in the meninges and pial BM. Therefore, primarily cultured meningeal fibroblasts were used as the ligand source for the subsequent ligand search. Through a combined approach of in vitro biotinylation/proteomics and genetics, we discovered that collagen III is the ligand of GPR56 in the developing brain [58,59].
Fibrillar collagen type III (gene symbol COL3A1) is a major structural component of the ECM of skin, cardiac, and vascular tissues with integrins α1β1 and α2β1 serving as its typical receptor [60,61]. Collagen III mutations are associated with human type IV Ehlers–Danlos syndrome (EDS) [62–67]. The vast majority of reported type IV EDS cases are the result of autosomal dominant inheritance, which causes excessive bleeding, bruising, and vascular problems, including aortic dissection, in afflicted individuals. There is one reported case of recessive type IV EDS with a homozygous mutation in the COL3A1 gene, resulting in a diffuse cortical dysplasia, suggesting a possible role of collagen III in the developing brain [68]. Interestingly, the cortical dysplasia did not occur uniformly throughout the cortex. Rather, the malformation was most prominent in the frontal cortex.
Little was known about collagen III expression in the developing brain prior to the discovery of its role as the ligand of GPR56, with the exception of one scant report of its expression in the brain via array analysis [69]. Subsequent IHC showed that collagen III is mainly expressed in the meninges, pial BM, and blood vessels in the developing brain [58]. Double IHC of GPR56 and collagen III revealed the direct interaction of collagen III and GPR56-expressing cells at the pia surface.
Collagen III mediated GPR56 signaling
RhoA activation has been shown to regulate cell migration [70]. Given the fact that loss of GPR56 results in neuronal overmigration, it is possible that the interaction of GPR56 and collagen III activates the RhoA pathway. Indeed, a GTP-RhoA pull-down assay, using NIH 3T3 as host cells that express an abundance of endogenous GPR56, revealed that the addition of collagen III to the cells resulted in an increased level of GTP-RhoA in comparison to the control [58]. Furthermore, knockdown of GPR56 via Gpr56 shRNA or the absence of GPR56 altogether attenuated RhoA activation upon the addition of collagen III, suggesting that collagen III activates RhoA through GPR56. These data are consistent with the finding that RhoA pathway is activated by a rabbit polyclonal antibody against GPR56N, presumably by functioning as an agonist of GPR56 [71].
RhoA activation by GPCRs principally occurs through coupling with the Gα12 and Gα13 proteins [72]. The conditional deletion of the genes encoding both Gα12 and Gα13 in the nervous system results in a cobblestone-like cortical malformation [34]. Embryonic cortical neurons from G12/G13 knockout mice also fail to retract their neurites in response to lysophosphatidic acid and sphingosine-1-phosphate, implying that they have lost the ability to respond to repulsive mediators acting via GPCRs [34]. Taken together, GPR56 likely couples to Gα12/13 to activate RhoA. Indeed, the expression of the dominant negative mutant of Gα13 blunted the activation of RhoA by recombinant collagen III [58].
Constitutively activated GPR56C
It has been recently demonstrated that truncating GPR56 through removing GPR56N up to the GPS domain enhances GPR56-mediated RhoA activation [73]. These results indicate that GPR56C is constitutively activated and its association with GPR56N inhibits its signaling. However, the consequences of GPR56 activation on cell migration appear to vary depending on which GPR56 pathway is involved. Data from Hall, Itoh, and our own laboratory demonstrates that the activated GPR56 leads to an inhibition of cell migration through the RhoA pathway [58,71,73]. Research from Xu’s lab, however, indicates that activated GPR56 causes an increase in cell migration and angiogenesis via the PKCα pathway [38]. Moreover, the expression of GPR56C alone in MC-1, a melanoma cell line, showed dramatically induced melanoma growth and angiogenesis in vivo [38]. Therefore, it appears that the result of GPR56 activation is specific to the cell population in question.
The possible mechanism for GPR56 related cobblestone cortex
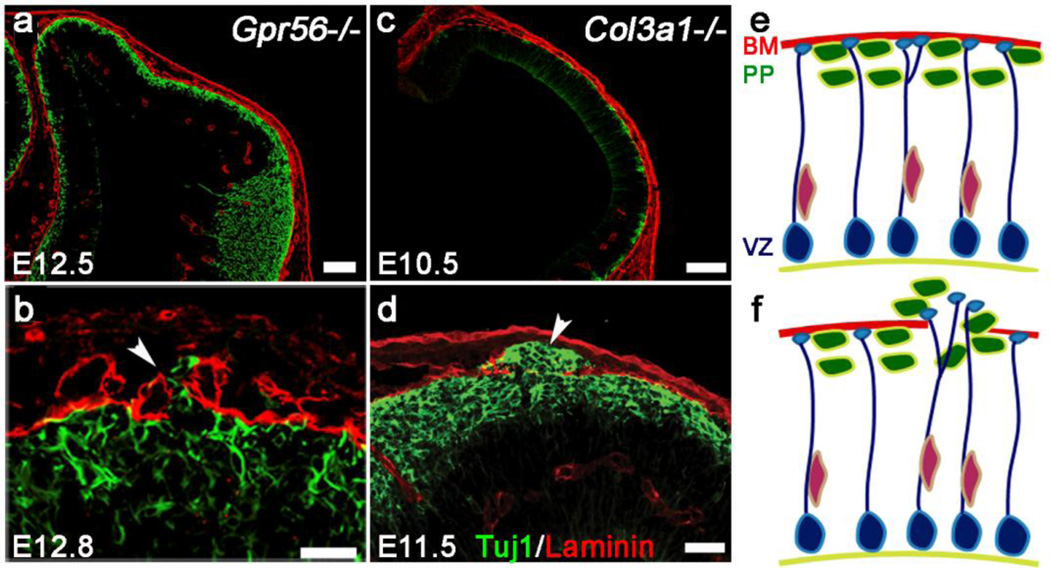
In both Gpr56 and Col3a1 mutant mice, the pial BM was initially properly formed but subsequently breached with concurrent neuronal overmigration in later developmental stages (Fig. 3a and c) [22,59]. The onset of overmigration was highly dependent on the mutation. In Gpr56−/−, the pial BM was breached on E12.8 (6 pm on the 12th day of vaginal plugging), as seen in Figure 3b; overmigration was observed in E11.5 (10am on the 11th day of vaginal plugging) Col3a1−/− brains (Fig. 3d). In mice, the preplate is formed in the dorsal forebrain between E10.5 and E12.5, and layer 6 neurons start to migrate into the preplate at E13.5 [74–77]. These observations are extremely interesting for two reasons. First, the timing of the malformation suggests that the overmigrating neurons are preplate neurons in both Col3a1 and Gpr56 knockout brains. Second, the fact that the pial BM is intact until these preplate neurons actively penetrate through in the absence of GPR56 signaling indicates that the pial BM cannot be the sole culprit responsible for the formation of neuronal ectopias; unsuppressed neuronal migration through the pial BM could also be responsible. This hypothesis is further supported through data obtained by in vitro neurosphere migration assays, which emonstrated that the interaction of GPR56 and collagen III inhibits neuronal migration [58].
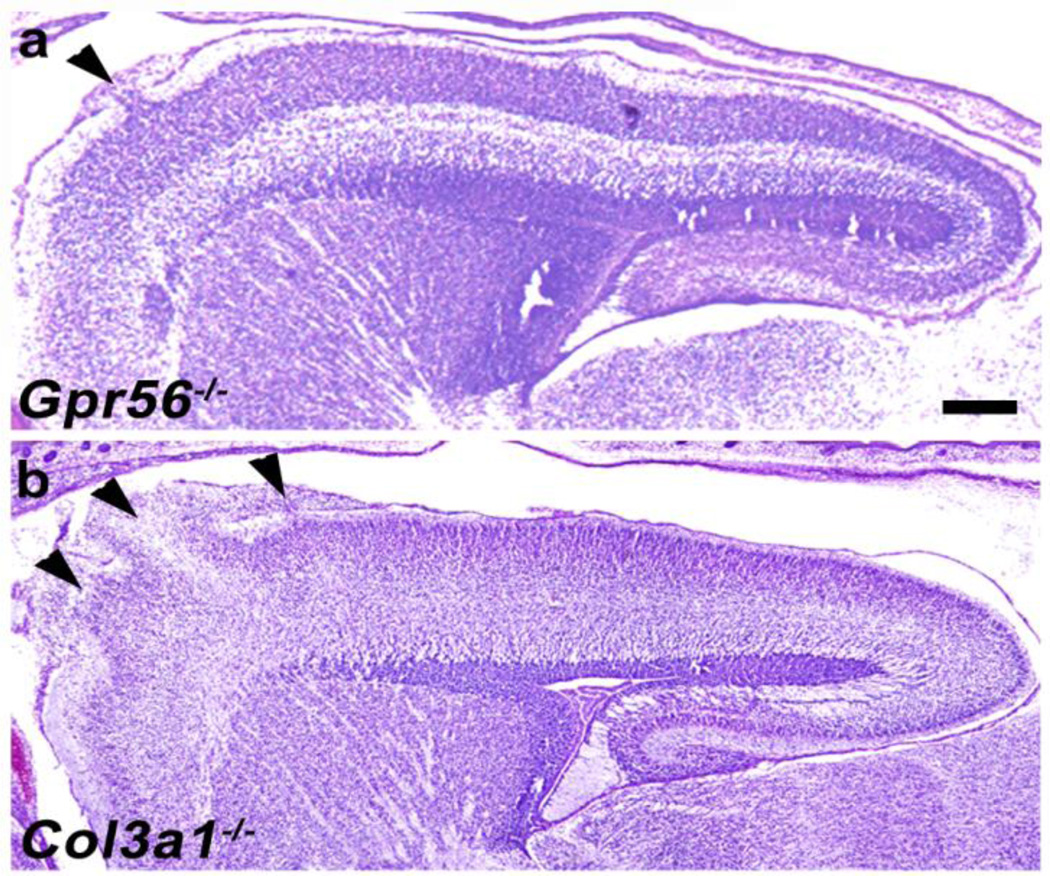
Fig. 3.
Decreasing anterior-to-posterior gradient of severity of brain malformation. Cresyl violet staining of sagittal section of Gpr56−/− (a) and Col3a1−/− (b) E16.5 mouse brains. Both Gpr56−/− and Col3a1−/− brains show ectopias in the rostral cortex. Scale bar=200µm
The role of GPR56 in the developing cerebral cortex
Mutations in both GPR56 and Col3a1 cause a cortical malformation that most severely affects the formation of the rostral cortex. MRIs of BFPP brains show the cortical abnormality extends diffusely across the cerebral cortex with a decreasing anterior-to-posterior gradient of severity [6,7,9,10]. In mice, however, the cortical malformation associated with the deletion of Gpr56 or Col3a1 occurs exclusively in the frontal regions of the brain (Fig. 4) [22,58,59]. These findings support the hypothesis that GPR56 signaling, particularly in the context of GPR56 and collagen III, is important for the formation of the rostral cortex.
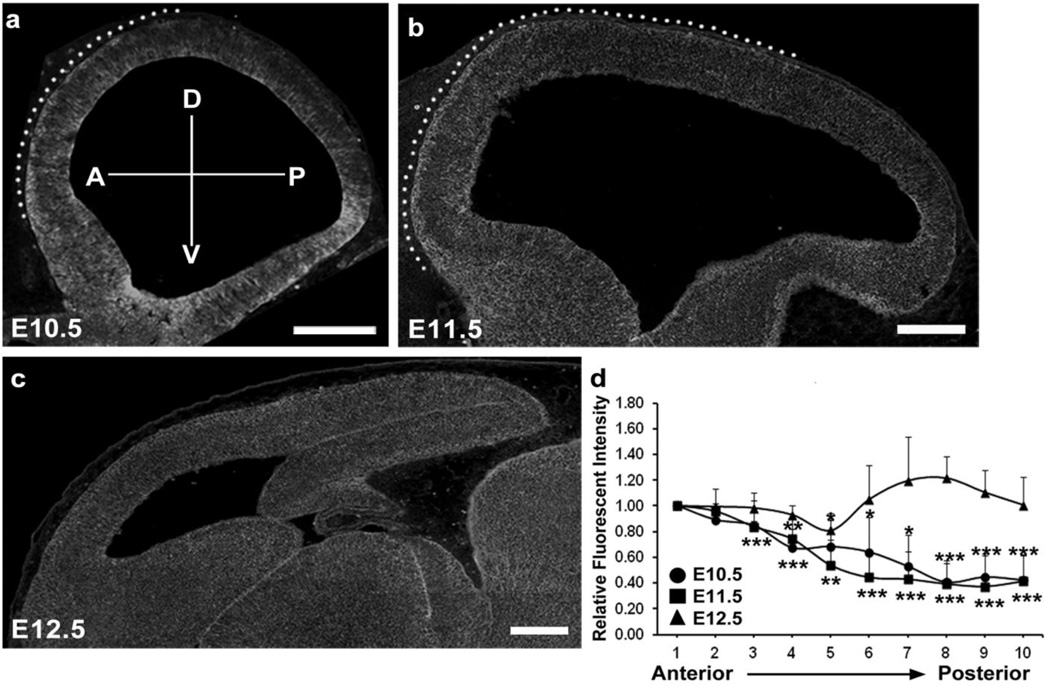
Fig. 4.
Gradient expression pattern of GPR56 in preplate neurons during early cortical development. IHC of GPR56 on the brain sections of E10.5 (a), E11.5 (b), and E12.5 (c) mice. Gradient profile was observed at E10.5 and E11.5 but it was disappeared at E12.5. The dotted area in (a) and (b) indicates the region of GPR56 expression in the preplate neurons. The relative fluorescent intensity of GPR56 expression was quantified from anterior to posterior and presented as mean ± SD. P<0.05*, <0.01**, <0.005*** (Two-tailed Student’s t-test). Scale bars=200 µm
The regulation of regional cortical development by GPR56 signaling can be accomplished by regional expression of either GPR56 or its ligand, collagen III. IHC of collagen III on sagittal sections of mouse embryonic brains ranging in age from E10.5 to E11.5 did not reveal a visible expression gradient of collagen III during these developmental stages [16]. On the contrary, an anterior-to-posterior gradient of GPR56 protein expression was found on the basal surface of the neocortex in both E10.5 and E11.5 brains (Fig. 5a and b) [16]. The gradient expression pattern, however, dissipated by E12.5 (Fig. 5c). This finding is particularly interesting, as the change in the expression pattern occurs in the region where preplate neurons reside.
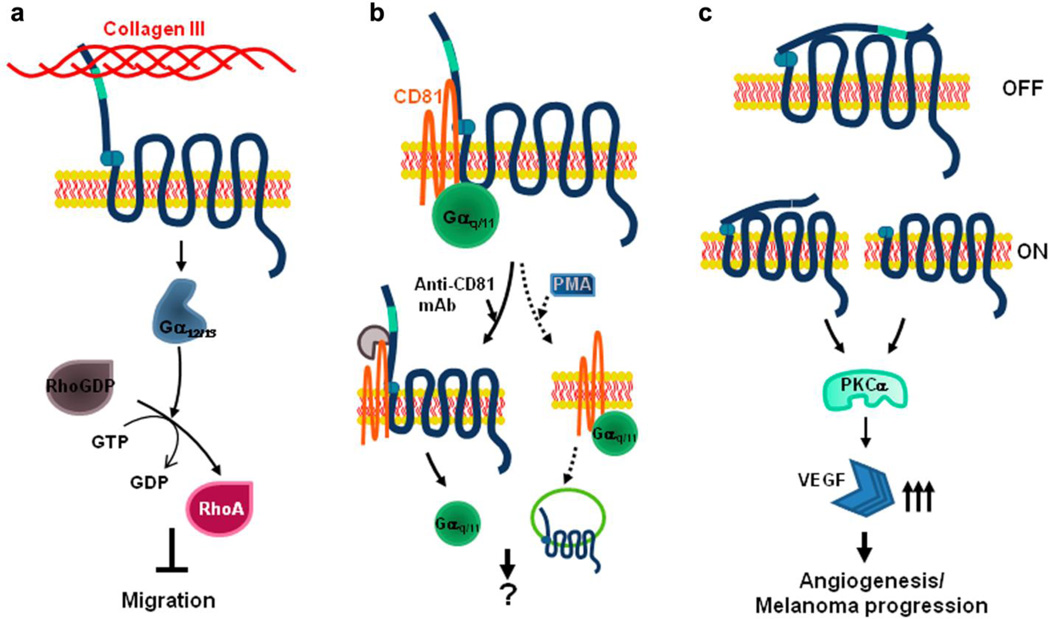
Fig. 5.
Schematic diagram of GPR56 signal pathways. a Collagen III-mediated RhoA activation. The binding of collagen III and GPR56 couples to Gα12/13 and leads to the activation of RhoA. This signaling cascade contributes to the inhibition of neuronal migration. b GPR56/CD81/Gαq/11 signaling complex. Tetraspanins CD9/CD81play a role as scaffolding proteins and stabilize GPR56/CD81/Gαq/11 complex. This complex is disrupted when CD81 protein is engaged with its antibody resulting in the separation of Gαq/11 from the complex. The complex can also be dismantled upon phorbol ester (PMA) stimulation, which results in the internalization of GPR56 (adapted from [81]). c Role of GPR56 in angiogenesis and metastasis. After cleavage, GPR56N and GPR56C form heterodimers to maintain the signaling “off” state. However, the state is switched to “on” state when “free” GPR56C is increased by GPR56C overexpression or deletion of STP segment. This “free” GPR56C activates protein kinase Cα (PKCα), resulting in increased production of VEGF, thus promoting angiogenesis and tumor progression (adapted from [38])
Preplate neurons are important in establishing the framework for the subsequent development of the cerebral cortex. Cajal-Retzius (C-R) cells, which secrete reelin, are one of the major cell types in the preplate. In the absence of reelin (reeler mice), layer 6 neurons fail to split the preplate, resulting in what is essentially a “pile-up” of later-born neurons and the formation of an upside-down cortex [20,78–80]. However, in the absence of GPR56 or collagen III, neurons overmigrate through a breached pial BM into the arachnoid space, a antithetical phenotype of what is observed in reeler mice [11,22,58,59]. The fact that a gradient expression of GPR56 in preplate neurons matches the regional cortical defects associated with loss of GPR56 suggests a novel receptor-ligand pair is responsible for mediating the interaction between preplate neurons and the pial BM, thus defining the boundary between the neocortex and the meninges while providing a framework for the developing cortex. Further testing of this hypothesis will undoubtedly advance our understanding of the molecular mechanisms underlying how preplate neurons regulate cortical development.
Closing remarks
Much has been learned since the original cloning of GPR56 a decade ago. In many ways, the progress made on GPR56 characterization is groundbreaking for the whole subgroup adhesion GPCRs. Through discovering that mutations in GPR56 are associated with BFPP, for the first time, an adhesion GPCR was implicated in a human disease. Then, by demonstrating that collagen III is its ligand, GPR56 was further set apart from other adhesion GPCRs, as the vast majority remains orphaned. Additional advancements have been made through characterizing GPR56’s biochemical properties and signaling (Fig. 1 and 6).
Although the recent progress on GPR56 is exciting, the next stage of characterizing GPR56 will be even more so, as the research shifts from understanding the structure of the protein to learning about its function. On the most basic level, the downstream signaling pathway is still being elucidated. Although GPR56 has been shown to associate with tetraspanins on the cell membrane, the nature of its association and the effects of GPR56 mutations on its co-signaling molecules remain unknown.
Currently, the functionality of GPR56 is being investigated principally from two different global perspectives: cortical development and cancer metastasis. It has already been shown that GPR56-collagen III interaction is responsible for neuronal migration regulation in the developing cerebral cortex. When this interaction fails due to mutation, preplate neurons overmigrate and pierce the pial BM. This mechanism strongly resembles cancer metastasis, in which cells breakthrough the ECM surrounding the initial tumor in an attempt to metastasize. However, the relationship between GPR56 and cancer is more multifaceted than just its involvement in cell migration; GPR56-TG2 interaction is thought to play a separate role in cancer progression by regulating angiogenesis. As applicable as the mechanism of cortical formation is to cancer research, the mechanisms of angiogenesis are equally relevant to brain research, as the nervous system and the vascular system develop in tandem within the emerging cortex. As the cerebral cortex develops and neurons migrate to their proper laminar location, angiogenesis must occur as well in order to provide the ever-growing brain with much needed oxygen and nutrients. Finally, as mentioned previously, GPR56 is simply one of 33 adhesion GPCRs found in humans, therefore, further characterizing GPR56’s structure and function may translate to the other still elusive proteins.
Fig. 2.
The pial BM is initially well formed but subsequently disrupted by deletion of Gpr56 or Col3a1. a–d Double IHC of Tuj1 (green) and laminin (red) in Gpr56−/− (a, b) and Col3a1−/− (c, d) neocortex. Tuj1+ postmitotic neurons are very well organized beneath the pial BM in both Gpr56−/− at E12.5 (a) and Col3a1−/− at E10.5 (c), whereas Tuj1+ postmitotic neurons migrate past the pial BM into the arachnoid space (arrowheads) in E12.8 Gpr56−/− (b) and E11.5 Col3a1−/− (d) brains. e, f Preplate neurons (green) are well organized beneath the pial BM (red line). Radial glial cells are located at ventricle zone (VZ) and extend their endfeet toward the pial BM (blue). Preplate neurons and radial glial endfeet are in direct contact with the pial BM, which is intact in normal cortex at early embryonic stage. In contrast, Gpr56 or Col3a1 mutant brain shows three major features: overmigration of preplate neurons including Cajal-Retzius (C–R) cells, misplacement of radial glial endfeet in defective region, and a breached pial BM. Scale bars: a and c=100 µm; b=20 µm; d=50 µm
Acknowledgments
This research was supported in part by NINDS grant RO1 NS057536 (X.P.), the William Randolph Hearst Fund Award (S.J. and R.L.), the Leonard and Isabelle Goldenson Research Fellowship (R.L.), and the NIH Training Grant 5T32HD7466-15 (S.J.).
References
- 1.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 2.Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 3.Yona S, Lin HH, Siu WO, Gordon S, Stacey M. Adhesion-GPCRs: emerging roles for novel receptors. Trends Biochem Sci. 2008;33:491–500. doi: 10.1016/j.tibs.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Stacey M, Lin HH, Gordon S, McKnight AJ. LNB-TM7, a group of seven-transmembrane proteins related to family-B G-protein-coupled receptors. Trends Biochem Sci. 2000;25:284–289. doi: 10.1016/s0968-0004(00)01583-8. [DOI] [PubMed] [Google Scholar]
- 5.Strokes N, Piao X. Adhesion-GPCRs in the CNS. Adv Exp Med Biol. 2011;706:87–97. doi: 10.1007/978-1-4419-7913-1_7. [DOI] [PubMed] [Google Scholar]
- 6.Piao X, Hill RS, Bodell A, Chang BS, Basel-Vanagaite L, Straussberg R, et al. G proteincoupled receptor-dependent development of human frontal cortex. Science. 2004;303:2033–2036. doi: 10.1126/science.1092780. [DOI] [PubMed] [Google Scholar]
- 7.Piao X, Chang BS, Bodell A, Woods K, Benzeev B, Topcu M, et al. Genotype-phenotype analysis of human frontoparietal polymicrogyria syndromes. Ann Neurol. 2005;58:680–687. doi: 10.1002/ana.20616. [DOI] [PubMed] [Google Scholar]
- 8.Harbord MG, Boyd S, Hall-Craggs MA, Kendall B, McShane MA, Baraitser M. Ataxia, developmental delay and an extensive neuronal migration abnormality in 2 siblings. Neuropediatrics. 1990;21:218–221. doi: 10.1055/s-2008-1071501. [DOI] [PubMed] [Google Scholar]
- 9.Chang BS, Piao X, Bodell A, Basel-Vanagaite L, Straussberg R, Dobyns WB, et al. Bilateral frontoparietal polymicrogyria: clinical and radiological features in 10 families with linkage to chromosome 16. Ann Neurol. 2003;53:596–606. doi: 10.1002/ana.10520. [DOI] [PubMed] [Google Scholar]
- 10.Piao X, Basel-Vanagaite L, Straussberg R, Grant PE, Pugh EW, Doheny K, et al. An autosomal recessive form of bilateral frontoparietal polymicrogyria maps to chromosome 16q12.2-21. Am J Hum Genet. 2002;70:1028–1033. doi: 10.1086/339552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahi-Buisson N, Poirier K, Boddaert N, Fallet-Bianco C, Specchio N, Bertini E, et al. GPR56- related bilateral frontoparietal polymicrogyria: further evidence for an overlap with the cobblestone complex. Brain. 2010;133:3194–3209. doi: 10.1093/brain/awq259. [DOI] [PubMed] [Google Scholar]
- 12.Luo R, Yang HM, Jin Z, Halley DJ, Chang BS, MacPherson L, et al. A novel GPR56 mutation causes bilateral frontoparietal polymicrogyria. Pediatr Neurol. 2011;45:49–53. doi: 10.1016/j.pediatrneurol.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parrini E, Ferrari AR, Dorn T, Walsh CA, Guerrini R. Bilateral frontoparietal polymicrogyria, Lennox-Gastaut syndrome, and GPR56 gene mutations. Epilepsia. 2008 doi: 10.1111/j.1528-1167.2008.01787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zendman AJ, Cornelissen IM, Weidle UH, Ruiter DJ, van Muijen GN. TM7XN1, a novel human EGF-TM7-like cDNA, detected with mRNA differential display using human melanoma cell lines with different metastatic potential. FEBS Lett. 1999;446:292–298. doi: 10.1016/s0014-5793(99)00230-6. [DOI] [PubMed] [Google Scholar]
- 15.Liu M, Parker RM, Darby K, Eyre HJ, Copeland NG, Crawford J, et al. GPR56, a novel secretin-like human G-protein-coupled receptor gene. Genomics. 1999;55:296–305. doi: 10.1006/geno.1998.5644. [DOI] [PubMed] [Google Scholar]
- 16.Jeong SJ, Luo R, Li S, Strokes N, Piao X. Characterization of G protein-coupled receptor 56 protein expression in the mouse developing neocortex. J Comp Neurol. 2012;520:2930–2940. doi: 10.1002/cne.23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen A, Andermann E. Genetics of the polymicrogyria syndromes. J Med Genet. 2005;42:369–378. doi: 10.1136/jmg.2004.023952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrer I, Catala I. Unlayered polymicrogyria: structural and developmental aspects. Anat Embryol (Berl) 1991;184:517–528. doi: 10.1007/BF01236058. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson SH, Thom M, Heffernan J, Lin WR, Harding BN, Squier MV, et al. Persistent reelin-expressing Cajal-Retzius cells in polymicrogyria. Brain. 2001;124:1350–1361. doi: 10.1093/brain/124.7.1350. [DOI] [PubMed] [Google Scholar]
- 20.Olson EC, Walsh CA. Smooth, rough and upside-down neocortical development. Curr Opin Genet Dev. 2002;12:320–327. doi: 10.1016/s0959-437x(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 21.Dobyns WB, Patton MA, Stratton RF, Mastrobattista JM, Blanton SH, Northrup H. Cobblestone lissencephaly with normal eyes and muscle. Neuropediatrics. 1996;27:70–75. doi: 10.1055/s-2007-973752. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Jin Z, Koirala S, Bu L, Xu L, Hynes RO, et al. GPR56 regulates pial basement membrane integrity and cortical lamination. J Neurosci. 1996;28:5817–5826. doi: 10.1523/JNEUROSCI.0853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koirala S, Jin Z, Piao X, Corfas G. GPR56-regulated granule cell adhesion is essential for rostral cerebellar development. J Neurosci. 2009;29:7439–7449. doi: 10.1523/JNEUROSCI.1182-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi K, Nakahori Y, Miyake M, Matsumura K, Kondo-Iida E, Nomura Y, et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 1998;394:388–392. doi: 10.1038/28653. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, et al. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell. 2001;1:717–724. doi: 10.1016/s1534-5807(01)00070-3. [DOI] [PubMed] [Google Scholar]
- 26.Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, Kato Y, Kawaguchi M, Shibata N, Kobayashi M. Expression and localization of fukutin, POMGnT1, and POMT1 in the central nervous system: consideration for functions of fukutin. Med Electron Microsc. 2004;37:200–207. doi: 10.1007/s00795-004-0260-5. [DOI] [PubMed] [Google Scholar]
- 28.Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 29.De Arcangelis A, Mark M, Kreidberg J, Sorokin L, Georges-Labouesse E. Synergistic activities of alpha3 and alpha6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development. 1998;126:3957–3968. doi: 10.1242/dev.126.17.3957. [DOI] [PubMed] [Google Scholar]
- 30.Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, et al. Beta1- class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 31.Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, et al. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niewmierzycka A, Mills J, St-Arnaud R, Dedhar S, Reichardt LF. Integrin-linked kinase deletion from mouse cortex results in cortical lamination defects resembling cobblestone lissencephaly. J Neurosci. 2005;25:7022–7031. doi: 10.1523/JNEUROSCI.1695-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costell M, Gustafsson E, Aszodi A, Morgelin M, Bloch W, Hunziker E, et al. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moers A, Nurnberg A, Goebbels S, Wettschureck N, Offermanns S. Galpha12/Galpha13 deficiency causes localized overmigration of neurons in the developing cerebral and cerebellar cortices. Mol Cell Biol. 1999;28:1480–1488. doi: 10.1128/MCB.00651-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwiatkowski AV, Rubinson DA, Dent EW, Edward van Veen J, Leslie JD, Zhang J, et al. Ena/VASP Is Required for neuritogenesis in the developing cortex. Neuron. 2007;56:441–455. doi: 10.1016/j.neuron.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Sarkisian MR, Bartley CM, Chi H, Nakamura F, Hashimoto-Torii K, Torii M, et al. MEKK4 signaling regulates filamin expression and neuronal migration. Neuron. 2006;52:789–801. doi: 10.1016/j.neuron.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaglin XH, Poirier K, Saillour Y, Buhler E, Tian G, Bahi-Buisson N, et al. Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat Genet. 2009;41:746–752. doi: 10.1038/ng.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang L, Chen G, Mohanty S, Scott G, Fazal F, Rahman A, et al. GPR56 Regulates VEGF Production and Angiogenesis during Melanoma Progression. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo R, Jin Z, Deng Y, Strokes N, Piao X. Disease-associated mutations prevent GPR56-collagen III interaction. PLoS One. 2012;7:e29818. doi: 10.1371/journal.pone.0029818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shashidhar S, Lorente G, Nagavarapu U, Nelson A, Kuo J, Cummins J, et al. GPR56 is a GPCR that is overexpressed in gliomas and functions in tumor cell adhesion. Oncogene. 2005;24:1673–1682. doi: 10.1038/sj.onc.1208395. [DOI] [PubMed] [Google Scholar]
- 41.Xu L, Begum S, Hearn JD, Hynes RO. GPR56, an atypical G protein-coupled receptor, binds tissue transglutaminase, TG2, and inhibits melanoma tumor growth and metastasis. Proc Natl Acad Sci U S A. 2005;103:9023–9028. doi: 10.1073/pnas.0602681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin Z, Tietjen I, Bu L, Liu-Yesucevitz L, Gaur SK, Walsh CA, et al. Disease-associated mutations affect GPR56 protein trafficking and cell surface expression. Hum Mol Genet. 2007;16:1972–1985. doi: 10.1093/hmg/ddm144. [DOI] [PubMed] [Google Scholar]
- 43.Lin HH, Stacey M, Yona S, Chang GW. GPS proteolytic cleavage of adhesion-GPCRs. Adv Exp Med Biol. 2010;706:49–58. doi: 10.1007/978-1-4419-7913-1_4. [DOI] [PubMed] [Google Scholar]
- 44.Volynski KE, Silva JP, Lelianova VG, Atiqur Rahman M, Hopkins C, Ushkaryov YA. Latrophilin fragments behave as independent proteins that associate and signal on binding of LTX(N4C) EMBO J. 2004;23:4423–4433. doi: 10.1038/sj.emboj.7600443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fredriksson R, Lagerstrom MC, Hoglund PJ, Schioth HB. Novel human G protein-coupled receptors with long N-terminals containing GPS domains and Ser/Thr-rich regions. FEBS Lett. 2002;531:407–414. doi: 10.1016/s0014-5793(02)03574-3. [DOI] [PubMed] [Google Scholar]
- 46.Bjarnadottir TK, Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics. 2004;84:23–33. doi: 10.1016/j.ygeno.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Qian F, Boletta A, Bhunia AK, Xu H, Liu L, Ahrabi AK, et al. Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1-associated mutations. Proc Natl Acad Sci U S A. 2002;99:16981–16986. doi: 10.1073/pnas.252484899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mengerink KJ, Moy GW, Vacquier VD. suREJ3, a polycystin-1 protein, is cleaved at the GPS domain and localizes to the acrosomal region of sea urchin sperm. J Biol Chem. 2002;277:943–948. doi: 10.1074/jbc.M109673200. [DOI] [PubMed] [Google Scholar]
- 49.Arac D, Boucard AA, Bolliger MF, Nguyen J, Soltis SM, Sudhof TC, et al. A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis. EMBO J. 2012;31:1364–1378. doi: 10.1038/emboj.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiang NY, Hsiao CC, Huang YS, Chen HY, Hsieh IJ, Chang GW, et al. Diseaseassociated GPR56 mutations cause bilateral frontoparietal polymicrogyria via multiple mechanisms. J Biol Chem. 2011;286:14215–14225. doi: 10.1074/jbc.M110.183830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boscher C, Dennis JW, Nabi IR. Glycosylation, galectins and cellular signaling. Curr Opin Cell Biol. 2011;23:383–392. doi: 10.1016/j.ceb.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Fesus L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci. 2002;27:534–539. doi: 10.1016/s0968-0004(02)02182-5. [DOI] [PubMed] [Google Scholar]
- 53.Gaudry CA, Verderio E, Aeschlimann D, Cox A, Smith C, Griffin M. Cell surface localization of tissue transglutaminase is dependent on a fibronectin-binding site in its N-terminal beta-sandwich domain. J Biol Chem. 1999;274:30707–30714. doi: 10.1074/jbc.274.43.30707. [DOI] [PubMed] [Google Scholar]
- 54.Telci D, Wang Z, Li X, Verderio EA, Humphries MJ, Baccarini M, et al. Fibronectin-tissue transglutaminase matrix rescues RGD-impaired cell adhesion through syndecan-4 and beta1 integrin cosignaling. J Biol Chem. 2008;283:20937–20947. doi: 10.1074/jbc.M801763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Laurenzi V, Melino G. Gene disruption of tissue transglutaminase. Mol Cell Biol. 2001;21:148–155. doi: 10.1128/MCB.21.1.148-155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maggio N, Sellitti S, Capano CP, Papa M. Tissue-transglutaminase in rat and human brain: light and electron immunocytochemical analysis and in situ hybridization study. Brain Res Bull. 2001;56:173–182. doi: 10.1016/s0361-9230(01)00649-9. [DOI] [PubMed] [Google Scholar]
- 57.Bailey CD, Johnson GV. Developmental regulation of tissue transglutaminase in the mouse forebrain. J Neurochem. 2004;91:1369–1379. doi: 10.1111/j.1471-4159.2004.02825.x. [DOI] [PubMed] [Google Scholar]
- 58.Luo R, Jeong SJ, Jin Z, Strokes N, Li S, Piao X. G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1104821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeong SJ, Li S, Luo R, Strokes N, Piao X. Loss of Col3a1, the gene for Ehlers-Danlos syndrome type IV, results in neocortical dyslamination. PLoS One. 2012;7:e29767. doi: 10.1371/journal.pone.0029767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim JK, Xu Y, Xu X, Keene DR, Gurusiddappa S, Liang X, et al. A novel binding site in collagen type III for integrins alpha1beta1 and alpha2beta1. J Biol Chem. 2005;280:32512–32520. doi: 10.1074/jbc.M502431200. [DOI] [PubMed] [Google Scholar]
- 61.Nykvist P, Tu H, Ivaska J, Kapyla J, Pihlajaniemi T, Heino J. Distinct recognition of collagen subtypes by alpha(1)beta(1) and alpha(2)beta(1) integrins. Alpha(1)beta(1) mediates cell adhesion to type XIII collagen. J Biol Chem. 2000;275:8255–8261. doi: 10.1074/jbc.275.11.8255. [DOI] [PubMed] [Google Scholar]
- 62.Germain DP. Ehlers-Danlos syndrome type IV. Orphanet J Rare Dis. 2007;2:32. doi: 10.1186/1750-1172-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kontusaari S, Tromp G, Kuivaniemi H, Romanic AM, Prockop DJ. A mutation in the gene for type III procollagen (COL3A1) in a family with aortic aneurysms. J Clin Invest. 1990;86:1465–1473. doi: 10.1172/JCI114863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuivaniemi H, Tromp G, Bergfeld WF, Kay M, Helm TN. Ehlers-Danlos syndrome type IV: a single base substitution of the last nucleotide of exon 34 in COL3A1 leads to exon skipping. J Invest Dermatol. 1995;105:352–356. doi: 10.1111/1523-1747.ep12320704. [DOI] [PubMed] [Google Scholar]
- 65.Pope FM, Martin GR, Lichtenstein JR, Penttinen R, Gerson B, Rowe DW, et al. Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc Natl Acad Sci U S A. 1975;72:1314–1316. doi: 10.1073/pnas.72.4.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prockop DJ, Kivirikko KI. Heritable diseases of collagen. N Engl J Med. 1984;311:376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- 67.Schwarze U, Schievink WI, Petty E, Jaff MR, Babovic-Vuksanovic D, Cherry KJ, et al. Haploinsufficiency for one COL3A1 allele of type III procollagen results in a phenotype similar to the vascular form of Ehlers-Danlos syndrome, Ehlers-Danlos syndrome type IV. Am J Hum Genet. 2001;69:989–1001. doi: 10.1086/324123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Plancke A, Holder-Espinasse M, Rigau V, Manouvrier S, Claustres M, Khau Van Kien P. Homozygosity for a null allele of COL3A1 results in recessive Ehlers-Danlos syndrome. Eur J Hum Genet. 2009;17:1411–1416. doi: 10.1038/ejhg.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Semeralul MO, Boutros PC, Likhodi O, Okey AB, Van Tol HH, Wong AH. Microarray analysis of the developing cortex. J Neurobiol. 2006;66:1646–1658. doi: 10.1002/neu.20302. [DOI] [PubMed] [Google Scholar]
- 70.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 71.Iguchi T, Sakata K, Yoshizaki K, Tago K, Mizuno N, Itoh H. Orphan G protein-coupled receptor GPR56 regulates neural progenitor cell migration via a G alpha 12/13 and Rho pathway. J Biol Chem. 2008;283:14469–14478. doi: 10.1074/jbc.M708919200. [DOI] [PubMed] [Google Scholar]
- 72.Worzfeld T, Wettschureck N, Offermanns S. G(12)/G(13)-mediated signalling in mammalian physiology and disease. Trends Pharmacol Sci. 2008;29:582–589. doi: 10.1016/j.tips.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 73.Paavola KJ, Stephenson JR, Ritter SL, Alter SP, Hall RA. The N terminus of the adhesion G protein-coupled receptor GPR56 controls receptor signaling activity. J Biol Chem. 2011;286:28914–28921. doi: 10.1074/jbc.M111.247973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caviness VS., Jr Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 1982;256:293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- 75.Del Rio JA, Martinez A, Auladell C, Soriano E. Developmental history of the subplate and developing white matter in the murine neocortex. Neuronal organization and relationship with the main afferent systems at embryonic and perinatal stages. Cereb Cortex. 2000;10:784–801. doi: 10.1093/cercor/10.8.784. [DOI] [PubMed] [Google Scholar]
- 76.Hevner RF, Daza RA, Rubenstein JL, Stunnenberg H, Olavarria JF, Englund C. Beyond laminar fate: toward a molecular classification of cortical projection/pyramidal neurons. Dev Neurosci. 2003;25:139–151. doi: 10.1159/000072263. [DOI] [PubMed] [Google Scholar]
- 77.Wood JG, Martin S, Price DJ. Evidence that the earliest generated cells of the murine cerebral cortex form a transient population in the subplate and marginal zone. Brain Res Dev Brain Res. 1992;66:137–140. doi: 10.1016/0165-3806(92)90150-u. [DOI] [PubMed] [Google Scholar]
- 78.Sheppard AM, Pearlman AL. Abnormal reorganization of preplate neurons and their associated extracellular matrix: an early manifestation of altered neocortical development in the reeler mutant mouse. J Comp Neurol. 1997;378:173–179. doi: 10.1002/(sici)1096-9861(19970210)378:2<173::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 79.Nichols AJ, Olson EC. Reelin promotes neuronal orientation and dendritogenesis during preplate splitting. Cereb Cortex. 2010;20:2213–2223. doi: 10.1093/cercor/bhp303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schneider S, Gulacsi A, Hatten ME. Lrp12/Mig13a reveals changing patterns of preplate neuronal polarity during corticogenesis that are absent in reeler mutant mice. Cereb Cortex. 2011;21:134–144. doi: 10.1093/cercor/bhq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Little KD, Hemler ME, Stipp CS. Dynamic regulation of a GPCR-tetraspanin-G protein complex on intact cells: central role of CD81 in facilitating GPR56-Galpha q/11 association. Mol Biol Cell. 2004;15:2375–2387. doi: 10.1091/mbc.E03-12-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]