Abstract
Mutations in the Ceramide kinase like (CERKL) gene are associated with retinitis pigmentosa (RP26) and cone-rod dystrophy. CERKL is homologous to Ceramide kinase (CERK), and its function is still unknown. The purpose of this study was to test the expression and distribution of this gene and its protein in rat and in mouse tissues, in light-stressed rat retinas and in the retinas of NeuroD1 knock-out mice to understand the role of CERKL in the retina. Expression of Cerkl and Cerk mRNA was determined by quantitative RT-PCR. Expression of the protein was determined by Western blotting with anti-CERKL antibody. Localization of the protein was determined by using immunofluorescence microscopy. With qRT-PCR, we revealed that the relative mRNA expression of Cerkl was the highest in the retina among all the rat tissue tested; it was >10-fold higher than in the brain. On the other hand, Cerk has ubiquitous expression and its relative abundance is >2 fold of Cerkl in the retina. Cerkl was expressed minimally in the developing mouse eyes and reached a peak at retinal maturity at 2 months. Western blots of retinal tissues revealed two major CERKL protein bands: 59 kDa (C1) and 37 kDa (C2). However, only C2 CERKL was found in the rat retinal rod outer segment (ROS) at level of that was not changed in light vs. dark adaptation. In the light-stressed retina, expression of Cerkl mRNA increased significantly, which was reflected in only on C2 CERKL protein. The CERKL protein localized prominently to the ganglion cells, inner nuclear layers (INL), retinal pigment epithelial (RPE) cells, and photoreceptor inner segments in the retinal sections. Nuclear localization of CERKL was not affected in RPE, INL and the ganglion cell layers in the light-stressed retina; however, the perinuclear and outer segment locations appear to be altered. In the NeuroD1 knock-out mouse retina, the expression of Cerkl mRNA and protein decreased and that decrease also pertains to C2 CERKL. In conclusion, the retina had the highest level of Cerkl mRNA and protein expression, which reached its maximum in the adult retina; CERKL localized to ROS and RPE cells and the light adaptation did not change the level of CERKL in ROS; light-stress induced Cerkl expression in the retina; and its expression decreased in NeuroD1 knock-out retina. Thus, CERKL may be important for the stress responses and protection of photoreceptor cells.
Keywords: ceramide kinase like (CERKL), ceramide kinase (CERK), NeuroD1, retina, photoreceptor, light-induced retinal degeneration, gene expression
INTRODUCTION
The Ceramide kinase like (CERKL) gene is one of the newest members of the retinitis pigmentosa (RP) family. Mutations of CERKL are associated with recessive, nonsyndromic retinitis pigmentosa (RP26) with significant macular involvement during the early stages of the disease (Ali et al., 2008; Auslender et al., 2007; Bayes et al., 1998; Tuson et al., 2004). Although Bayes et al., (1998) described cases of what they called recessive RP with appreciated heterogeneity in the phenotype, they also reported that younger patients (age 23 and 24 years) had macular alteration and significant central scotoma, which may indicate an early macular phenotype (Bayes et al., 1998). In 2004, Tuson et al. identified this gene and its mutation within members of the same family (Tuson et al., 2004). All affected individuals were homozygous for a nonsense mutation (R257X; CGA→TGA) in exon 5. The gene was named ‘Ceramide kinase like’ based on its homology with Ceramide kinase (CERK) (Tuson et al., 2004). From the discovery of new mutations and from further characterization of the phenotype of the previously identified mutations, CERKL mutations are now considered as the cause of cone-rod dystrophy (CRD), which progresses to an RP-like phenotype in advanced stages (Aleman et al., 2009; Avila-Fernandez et al., 2008; Littink et al., 2010; Tang et al., 2009).
CERKL was initially considered as a retinal ceramide kinase. However, no kinase activity so far has been identified for this protein. CERKL expression is highly complex; more than 20 transcripts, which may generate various protein products, have been found in human and mouse tissues (Garanto et al., 2011). Attempts have been made to generate Cerkl knock-out mice; however, the transcriptional complexity of the gene makes it challenging to develop knock-out mice completely ablated for CERKL function (Garanto et al., 2012). CERKL has been shown to be expressed in various cell types in the retina, and a cone-dominant expression in mouse photoreceptors supports the notion that cone cell death precedes rods in humans with the CERKL mutation (Vekslin and Ben-Yosef, 2010). CERKL is also expressed significantly in ganglion cells and patients with CERKL mutations is known to develop significant pathology in the inner retina (Aleman et al., 2009). Given this transcriptional complexity, the CERKL mutation pathology is also complex.
In this study, we analyzed the expression and tissue distribution of Cerkl in rat tissues, confirmed its expression in mouse tissues and generated novel data on its expression in embryonic and developing mouse eyes to gain a better understanding of the role of this gene in the retina during embryogenesis and development. Because CERKL has previously been speculated as a retinal CERK (ceramide kinase), we performed a side-by-side comparative analysis of the expression of Cerk in every tissue and at developing stages. In a recent study, Nevet et al. showed an interaction between CERKL and neuronal calcium sensor (NCS) proteins, including guanylate cyclase activating protein 1 (GCAP1), GCAP2, and recoverin in the photoreceptor cells (Nevet et al., 2012). We compared expression of these genes with Cerkl and Cerk expression in developing eye tissues. From previous in vitro studies, CERKL was attributed to have a protective effect against oxidative stress (Tuson et al., 2009). We used the light-stressed rat retina model in which photoreceptor cell death occurs by oxidative stress and measured the expression of the Cerkl gene and its protein and determined the localization of CERKL protein to understand whether CERKL is involved in retinal protection against stress.
We further analyzed expression of CERKL in the NeuroD1 knock-out mouse retina. BETA2/NeuroD1 is a neuronal transcription factor; it is highly expressed in the developing retina, its genomic location is immediately adjacent to the 5’ of CERKL, and it is conserved in all known mammalian species (Cho et al., 2007). Interestingly, NeuroD1 knock-out mice develop a characteristic retinal degeneration phenotype; both rod and cone cells begin to degenerate during the very early postnatal days (Pennesi et al., 2003). We speculated that these linked genes may also be functionally related.
MATERIALS AND METHODS
Animal and Tissue Collection
All procedures were performed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the University of Oklahoma Health Sciences Center (OUHSC) Guidelines for Animals in Research. All protocols were reviewed and approved by the Institutional Animal Care and Use Committees of the OUHSC, the Dean A. McGee Eye Institute (DMEI) and the University of Texas M. D. Anderson Cancer Center. All animals were maintained on a 12-h dim cyclic light (5–10 lux at the cage level) /12-h dark cycle, and tissues were collected at the end of a dark cycle at the appropriate age. We collected the fore brain, hind brain, retina, eyecup containing RPE-choroid-sclera (eyecups = RPE+choroids+sclera; no retina), heart, lung, liver, spleen, kidney, skin, testis, and ovary from adult Sprague Dawley (SD) rats. Mouse eye balls (6 to 8) were collected from embryonic day E15 and E18, and postnatal days P1, P3, P7, P15, P30, P60, P90, P210 to study the expression of the genes of interest in the whole eye. To study the distribution of this gene expression in mouse eye tissue, the distalmost 3-mm optic nerve (ON) cut from the scleral surface of the eyeballs, eyecups, retina, lens, iris-ciliary body (I-CB) and cornea were collected after dissection. Retinas from NeuroD1 knock-out mouse were collected at P30 for mRNA and protein expression studies.
For the immunofluorescence microscopy, adult albino (BALBc) mice and NeuroD1 knock-out mice (at P60) eyeballs were fixed in 4% paraformaldehyde and embedded in OCT. Cryosections (10 μm) were prepared for immunolocalization studies of the CERKL protein.
Light damage of Albino Rats
Eight- to ten week-old SD rats were exposed to damaging light (white cool light) for 6 h (9 AM to 3 PM) at an intensity of 2,700 lux following previously published protocols (Mandal et al., 2011; Mandal et al., 2009). After light damage, the retinas and eyecups were harvested at select time points (0 h, 3 h, 6 h, 12 h, and 24 h after the completion of light-damage), snap frozen in liquid N2, and prepared for RNA and protein analysis. For studying the localization of the CERKL protein in the light-exposed retina, dark-adapted non-light-damaged (NLD) and light-damaged (LD) eyes at 0 h and 24 h after LD were harvested, fixed in 4% paraformaldehyde, embedded in OCT and sectioned.
RNA Isolation and cDNA Synthesis for Quantitative RT-PCR (qRT-PCR)
RNA was isolated from all of the collected tissues using TRIzol (Invitrogen) reagent following the manufacturer’s protocol. First-strand cDNA synthesis was carried out (SuperScript II First-Strand Synthesis System; Invitrogen) for qRT-PCR. Primers for qRT-PCR were designed in such a way that they spanned at least one intron, which eliminated the possibility of amplification from residual genomic DNA contamination. Degenerative forward and reverse primers for qRT-PCR were designed to amplify both mouse and rat Cerkl sequences. As Cerkl is known to have several transcripts (Garanto et al., 2011; Tuson et al., 2009), the primers were selected from exon 11 (forward) and exon 12 (reverse) of the known protein encoding the Cerkl transcript (ensemble.org: Cerkl-001 ENSMUST00000143974; NCBI CCDS database: Mouse CCDS set: CCDS38157). These two exons are common in almost all of the known transcripts of Cerkl (Garanto et al., 2011), so that the amplification can represent the total Cerkl expression. Primer pairs were forward: 5’- AACAATGGAAGCATGGCTCT-3’; reverse, 5’- CTCCTGTGGGCTGTATCCAT-3’ for Cerkl and forward: 5’-GTCCTTCCTCCCAGCACAG-3’; reverse, 5’- GCACTTCCGGATAAGGATGA-3’ for Cerk. The sequence of primers used for other retinal genes will be obtained from the corresponding author upon request. Quantitative PCR (using iQ SYBR Green Supermix; Bio-Rad, Hercules, CA) and melt-curve analysis (using iCycler; Bio-Rad) were performed. Mean values (± SD) were calculated and are presented as fold over the most consistent housekeeping genes, RPL19, following previously published analysis methods (Mandal and Ayyagari, 2006; Mandal et al., 2009; Mandal et al., 2006a; Mandal et al., 2006b).
Immunofluorescence Microscopy
The localization of the CERKL protein in mouse and rat cryosections was determined by using immunofluorescence microscopy with a rabbit polyclonal anti-CERKL antibody (Abcam Inc. Cambridge, MA) as described previously (Mandal and Ayyagari, 2006; Mandal et al., 2006a; Mandal et al., 2006b; Sherry et al., 2005). Frozen sections were thawed, rehydrated, and treated with 1% NaBH4 to reduce autofluorescence. Nonspecific labeling was blocked by using a ‘blocker’ containing 10% normal horse serum, 5% bovine serum albumin, 1% fish gelatin, and 0.5% Triton X-100 in PBS. Excess blocker was removed, and primary antibody was applied overnight at 4°C. Sections were rinsed and incubate d in anti-rabbit secondary antibody with Alexa Flour 488 (Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature to visualize labeling. Sections were rinsed and cover-slipped with ProLong Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA, USA). Omission of primary antiserum eliminated immunolabeling and served as control.
Confocal microscopy was performed by using an Olympus FluoView FV500 microscope (Olympus Microsystems, Center Valley, PA, USA). To ensure quantitative image quality, laser power, pinhole settings, photomultiplier tube settings, and intensity thresholds were kept constant for each retinal section imaged. Images from NeuroD1 retina captured using epifluorescence microscope.
Preparation of Rat Retinal ROS
Rats were dark adapted for 12 h and their retinas were harvested under red light to prepare dark-adapted (D) rod outer segment (ROS); another group of rats were light adapted for 1 h after 12 h dark adaptation, and their retinas were harvested and used to prepare light-adapted (L) ROS. Four retinas from 2 rats formed one sample and we prepared ROS from 6 independent light-adapted and 6 independent dark-adapted samples. Retinal homogenates were used to isolate the ROS fractions by using methods described by Papermaster and Dreyer (Papermaster and Dreyer, 1974) with slight modification, as published earlier (Brush et al., 2010; Martin et al., 2005). The band containing purified ROS was collected, as well as the pellet from each sample. The collected fragments were diluted with homogenization buffer, and pelleted by centrifugation at 27,000g for 30 min at 4°C. The pellets were then used for protein extraction.
Protein Isolation and Western Blotting
Total protein was extracted from mice and rat retinal tissues, rat ROS and the pellet fraction using the lysis buffer T-Per (Pierce, Rockford, IL) with a protease inhibitor cocktail (Roche, Indianapolis). This was followed by centrifuging at 10,000g for 15 min at 4°C to collect the supernatants. After protein concentrations were determined by using BCA reagent (Pierce), equal aliquots (25–30 μg) of protein samples were applied to 10% sodium dodecyl sulfate polyacrylamide gels and electrophoretically separated. Resolved proteins were electrophoretically transferred to nitrocellulose membranes (Bio-Rad) and blocked with 5% BSA for 1 h at room temperature. The membranes were incubated with anti-CERKL (1:1000) and anti-β-Actin (1:2000) antibodies for 16 h at 4°C, after which they were incubated with the appropriate secondary antibodies for 1 h at room temperature. Proteins were visualized by using chemiluminescence signals generated after the addition of Supersignal Chemiluminescence Substrate (Pierce, Rockford, IL). The intensities of the protein bands were determined by using Image J 1.32j software.
Statistical Analyses
Statistical analyses were performed by using Graphpad Prism 5.0 software. The quantitative data are expressed as mean ± SD for each group. An unpaired Student's t test and one-way ANOVA were performed for the means of two unmatched groups.
RESULTS
Expression Distribution of Cerkl in Murine Ocular Tissue
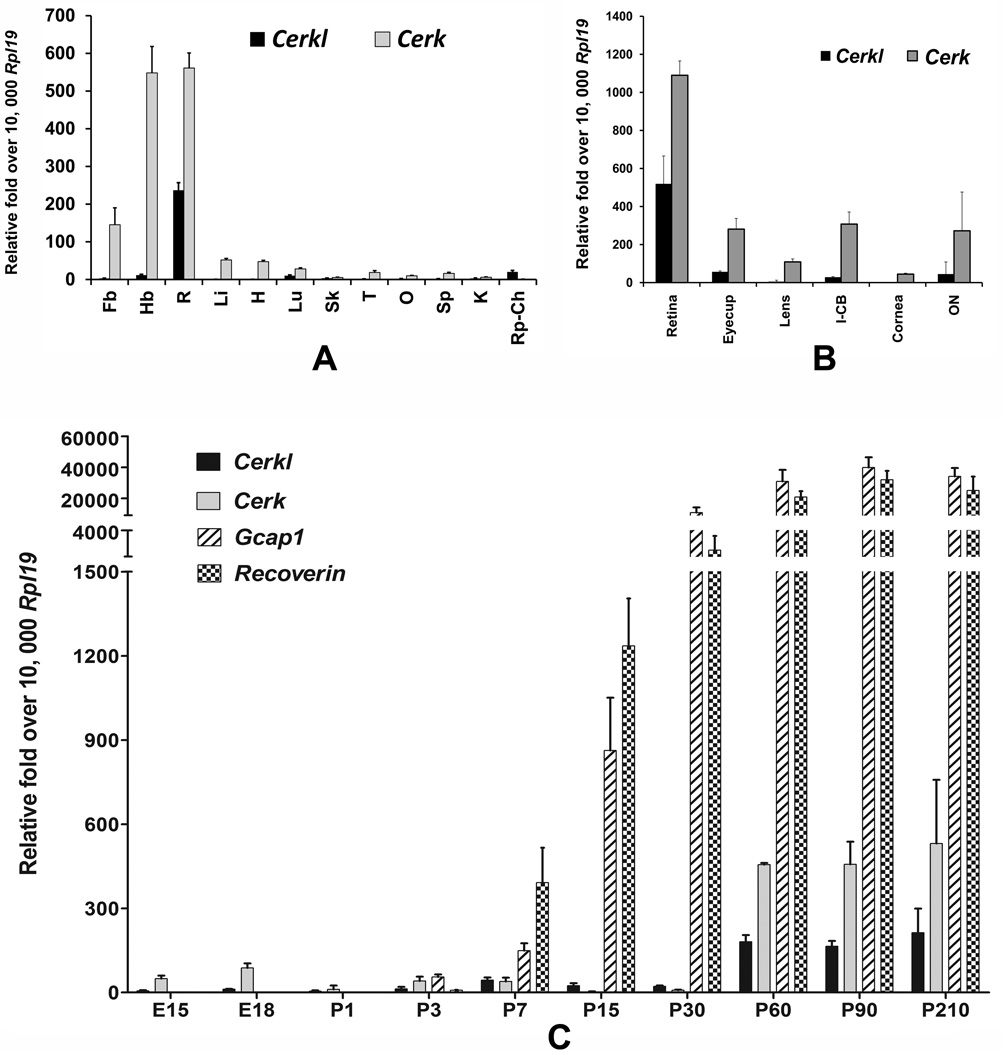
Mutations in the CERKL gene cause nonsyndromic RP or CRD in humans (Aleman et al., 2009; Ali et al., 2008; Auslender et al., 2007; Bayes et al., 1998). To understand the expression distribution of Cerkl in different tissues, we tested mRNA expression in various rat tissues. The distribution of Cerkl in human and mouse tissues has been reported and since its discovery CERKL has been speculated as retinal CERK (Bornancin et al., 2005; Garanto et al., 2011; Vekslin and Ben-Yosef, 2010). We tested expression and performed a side-by-side comparison of Cerkl with Cerk in rat tissues including the retina. The other tissues that were included were the forebrain, hindbrain, liver, lung, heart, skin, testis, ovary, spleen, kidney, and eyecup. Cerkl expression was abundant in the retina (Figure 1A). Other tissues that expressed Cerkl included the hind-brain, lung, kidney and eyecup (Figure 1A); however, the level of expression in those tissues was at least 10 fold lower than the expression in the retina (Figure 1A). We found that Cerk was also highly expressed in the retina and brain and that the relative abundance of Cerk was ~2 fold of Cerkl in the retina. All other tissues had some levels of expression of Cerk (Figure 1A). Therefore, the tissue distribution data indicated Cerkl is predominantly a retina-expressing gene. Cerk, on the other hand, has ubiquitous expression and its level of expression in the retina is higher than Cerkl, suggesting that Cerkl may not be an alternative of Cerk in the retina, as previously indicated (Vekslin and Ben-Yosef, 2010).
FIGURE 1.
A. Expression and distribution of ceramide kinase like (Cerkl) and ceramidekinase (Cerk) genes in rat tissues. Quantitative RT-PCR was performed with the primers that selectively amplified the major reported transcript of the Cerkl gene; Cerk expression was tested for comparison. Three independent reactions were performed per sample per primer pair, and the mean values of 3–4 samples (mean + SD, n = 3–4) were then compared with the expression of housekeeping gene Rpl19. Fb, forebrain; Hb, hindbrain; R, retina; Li, liver; H, heart; Lu, lung; Sk, skin; T, testis; O, ovary; Sp, spleen; K, kidney; and Rp-Ch, RPE-choroids. B. Distribution of Cerkl in mouse eye tissues. Cerk expression was tested for comparison. Eyes from adult mice were dissected in nuclease-free PBS, and a clean tissue pool of the optic nerve (ON; 3-mm distal-most optic nerve cut from the surface of the sclera), the eyecup containing RPE-choroid-sclera, retina, iris-ciliary body (I-CB), lens, and cornea were collected for RNA isolation and cDNA synthesis. Three independent reactions were performed per sample per primer pair, and mean values (+ SD, n = 4)) are presented over the values of mouse Rpl19 gene that is amplified in parallel replica reactions for each sample. C. Expression of Cerkl in developing mouse eyes and eyes with age. Expression of Cerk, Gcap1 and Recoverin was tested for comparison. RNA was isolated from mouse whole eyes from embryonic days 15 and 18 (E15 and E18) and postnatal days 1 to 210 (P1 to P210) and was used to measure the quantitative expression of Cerkl and Cerk genes. Quantitative RT-PCR was performed and data were analyzed as described in Figure 1A (mean+ SD, n = 4).
Using mouse and human tissues, Cerkl mRNA expression was found in the retina (Garanto et al., 2011; Vekslin and Ben-Yosef, 2010). We asked whether Cerkl was expressed in other eye tissues besides the retina, especially in the eyecup that includes RPE-choroids and sclera, which may indicate expression in the RPE cells. We detected Cerkl expression in mouse eyecup, I-CB and ON (Figure 1B). The highest level of expression was detected in the retina, which was similar to its expression in rat tissues (Figure 1A). However, Cerk had ubiquitous and higher levels of expression in all tested ocular tissues (Figure 1B).
Cerkl expression has been noted in mouse embryonic eyes (E14) and newborn eyes (P0), and a higher level of expression is found in 5-month-old eyes (Vekslin and Ben-Yosef, 2010). We studied the expression of Cerkl with other genes at various timepoints starting at E15 to P210. We included in this study the Cerk and two other genes, Gcap1 and Recoverin, protein products of which have recently been shown to interact with CERKL in the photoreceptor cells (Nevet et al., 2012). We detected only a minimal level of expression of Cerkl in prenatal and postnatal developing eyes (Figure 1C). We found that a peak in the expression Cerkl at P7, the stage at which the photoreceptor cells start developing their outer segments (Figure 1C). However, its expression reached its maximum at the maturity of the retina (P60) and was maintained for 7 months (in mice) (Figure 1C) and 14–18 months (in rats, data not shown), the highest time-points examined. Cerk expression also had a similar pattern; however, embryonic tissues had significantly higher levels of Cerk expression than Cerkl (Figure 1C). As expected, the photoreceptor-specific Gcap1 and Recoverin expression jump started at P7 and reached their maximum with the maturity of the eye (P60) and then maintained their levels in the mature retina (Figure 1C). The development and differentiation of mouse photoreceptor layer takes place between P7 and P30 (Smith et al., 2002). Increase in Cerkl expression at P7 may indicate its presence in the photoreceptor cells at a stage when photoreceptor outer segment starts developing and a role for CERKL in photoreceptor outer segment development or function or both. However, maintenance of an increased level of Cerkl in the adult retina may also indicate its presence in the photoreceptor outer segment, which is renewed and replaced continuously. This is true for other photoreceptor proteins such as Rhodopsin (Mandal et al., 2004), GCAP1 and Recoverin (Figure 1C). CERKL’s interaction with GCAPs and Recoverin indicates its presence in the outer segment (Nevet et al., 2012).
Effect of Damaging Light on Cerkl mRNA Expression
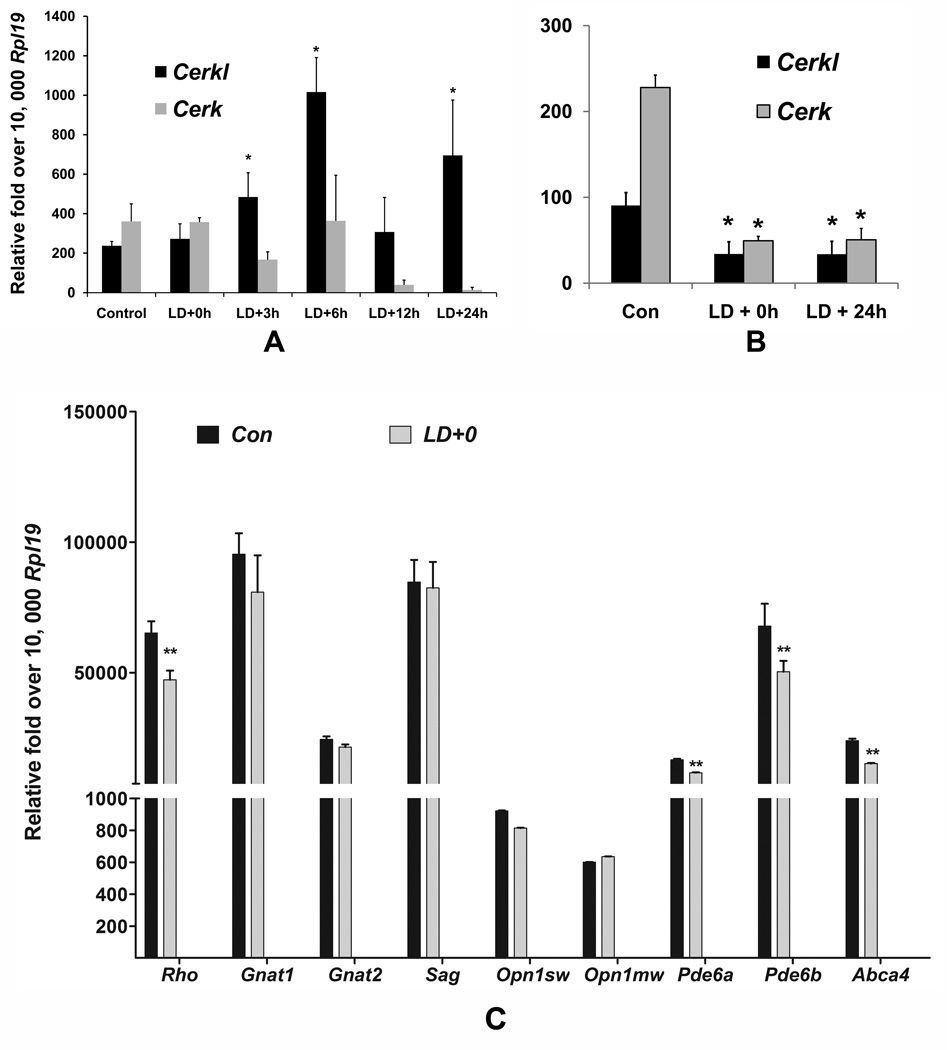
In vitro studies suggested that CERKL is a stress response protein (Tuson et al., 2009). To examine its expression under stress, we used our light-damaged retinal model in which photoreceptor cell death occurs by apoptosis mainly due to oxidative stress. Apoptosis starts after 8–12 h and by 24 h almost all of the photoreceptor cells in the central retina enter into apoptosis (Gordon et al., 2002; Hafezi et al., 1997; Mandal et al., 2011; Mandal et al., 2009). We observed that Cerkl mRNA expression increased by 2 fold by 3 h and 4 fold by 6 h after light-damage (Figure 2A); and the expression remained at normal levels or higher until 24 h. However, the expression of Cerk was not affected until 6 h after light-damage and was significantly reduced at 12 h and 24 h after light-damage (Figure 2A), the time when the cells actively enter into apoptosis.
FIGURE 2.
A. Light-stress modulates Cerkl expression in the rat retina. SD rats were exposed to damaging light at an intensity of 2,700 lux for 6 h. The retinas and eyecups were harvested at different time points after light damage starting at 0 h (LD+0h), 3 h (LD+3h), 6 h (LD+6h), 12 h (LD+12h), and 24 h (LD+24h) and prepared for RNA extraction and subsequent qRT-PCR. Expression data were calculated by dCt methods after normalizing with three housekeeping genes (Gapdh, Hgprt and Rpl19) and presented against the most consistent housekeeping gene, Rpl19 (* = p < 0.01; n=4–6; Student’s t test). B. Expression Cerkl and Cerk in the eyecups after light damage. Expression in the eyecups was tested at two time points after light damage, Ld+0 and LD+24 (* = p < 0.01; n=4–6; Student’s t test). C. Expression of some photoreceptor markers after light damage. Expression of Rho, guanine nucleotide binding protein, alpha transducing 1 (Gnat1), Gnat2, Rod arrestin (Sag), cone opsin short wave-length (Opn1 sw), cone opsin medium wave-length (Opn1 mw), Pde6a, Pde6b, and Abca4 was tested in the retinas harvested immediately after light damage (** = p < 0.005; n=6; one-way ANOVA). The retinas from non-light exposed rats served as controls (Con)..
Expression of both Cerkl and Cerk in the eyecup tissue was reduced significantly immediately after (LD+0h) and 24 h after light damage (Figure 2B). We then tested some known photoreceptor genes in the rat retinas immediately after light-damage (LD+0). We found expression of the outer segment protein genes, namely, rhodopsin (Rho), phosphodiesterase 6A, cGMP-specific, rod, alpha (Pde6a), phosphodiesterase 6B, cGMP, rod receptor, beta (Pde6b), ATP-binding cassette, and sub-family A (ABC1), member 4 (Abca4) were significantly reduced (Figure 2C). The reduction was much higher in samples harvested at 24 h after light damage (data not shown).
Effect of Damaging Light on CERKL Localization in the Retina
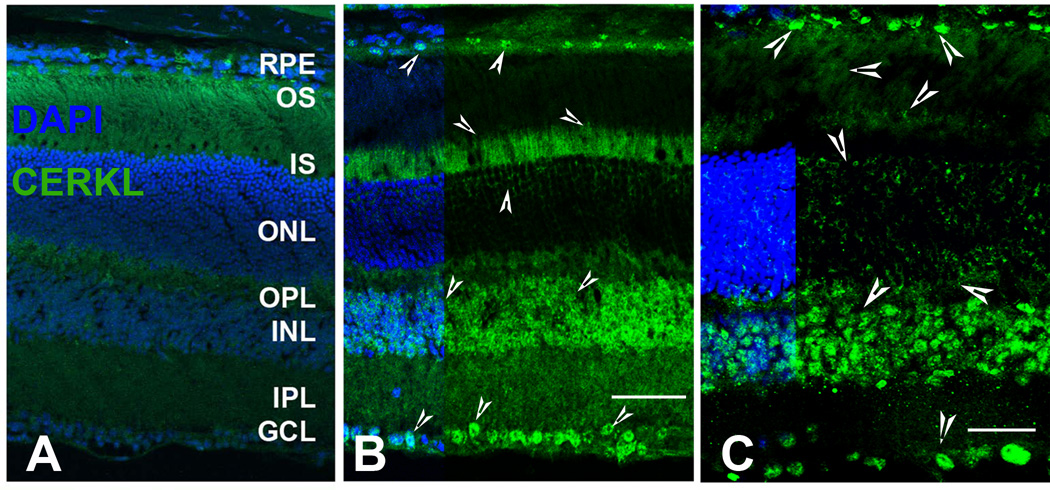
Similar to the data previously shown for the mouse retina (Garanto et al., 2011; Vekslin and Ben-Yosef, 2010), we also detected CERKL localization in various regions and cell types (arrow-heads) of the retinas from albino mouse (Figure 3B) and rat (Figure 3C). Figure 3A is a serial mouse retinal section in which the primary antibody was omitted and served as negative control. Very prominent CERKL expression was found on ganglion cells, which appeared to be mostly nuclear (Figure 3B and 3C). The inner nuclear layer (INL) also had very prominent expression of CERKL (Figure 3B and 3C). CERKL was found to localize in the inner and outer segments of the photoreceptor cells, and lightly on the intranuclear spaces of the ONL (Figure 3B and 3C). In addition to the previous observations we found distinct CERKL labeling on the RPE cells, especially on the RPE nucleus in both mouse and rat retina (Figure 3B and 3C).
FIGURE 3. Localization of the CERKL protein in the albino mouse (BALBc) and rat retinas.
CERKL protein localization was detected by rabbit polyclonal anti-CERKL antibodies (Abcam Inc.). A. Retinal cryosections incubated with normal rabbit serum, served as negative control. B. BALBc retinal sections incubated with anti-CERKL antibodies. C. SD rat retinal sections incubated with anti-CERKL antibodies. CERKL localization is shown by arrow-heads. CERKL labeling was observed in various layers of the retina including ganglion cells, inner nuclear layers, photoreceptor cells, and in the RPE cells. RPE, retinal pigment epithelium; OS, photoreceptor outer segments; IS, photoreceptor inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
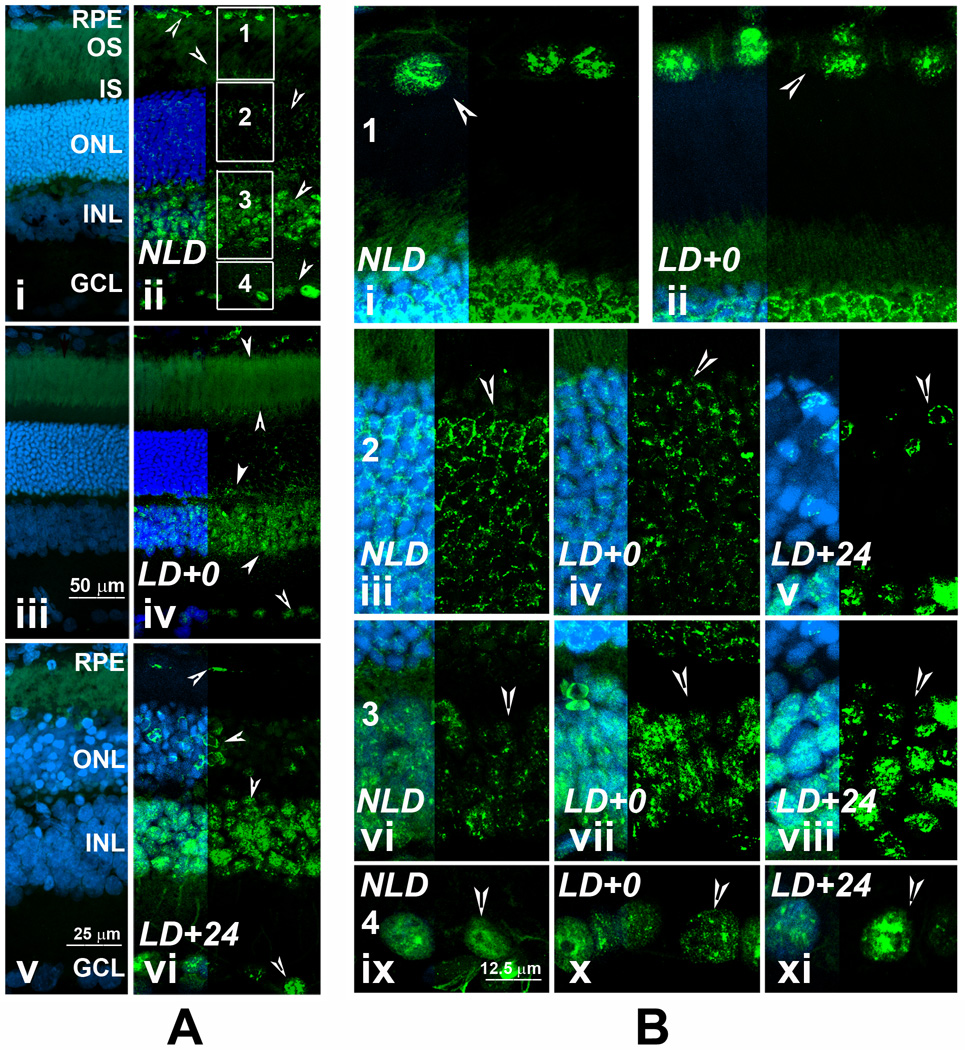
CERKL can localize in the nucleus and CERKL protein contains a nuclear localization signal and nuclear export signals (Inagaki et al., 2006; Tuson et al., 2009), suggesting that CERKL might be a signal transporter or carrier protein that communicates between the nucleus and cytoplasm. To determine whether CERKL localization in the retina changes upon stress (light-damage), we studied CERKL localization in detail in the light-damaged rat retina at 0 h and 24 h after LD; the dark-adapted, non-light-damaged (NLD) retina served as control (Figure 4). In Figure 4A we present low magnification images of the entire width of retina in which Figure 4Ai, 4Aiii, and 4Av present the no primary antibody control for NLD (Figure 4Aii), LD+0 (Figure 4Aiv), and LD+24 (Figure 4Avi) samples. In Figure 4B, we present the magnified portions of the retina, regions 1–4 (boxed, Figure 4Aii) from all three samples. The RPE cells are almost disappeared or changed in morphology in the central retina at 24 h after light damage and therefore not included in the comparison in Figure 4B region 1.
FIGURE 4. Localization of CERKL protein in rat retina after light stress.
A. Retinal cryosections incubated with normal rabbit serum, served as the negative control (4Ai, 4Aiii, 4Av). SD rat retinal sections incubated with anti-CERKL antibodies: 4Aii, no-light-damaged retina (NLD); 4Aiv, LD+0 h retina; 4Avii, LD+24 h retina. B. Four regions of the retinal sections shown in 4Aii were captured at higher magnification to better visualize the localization of CERKL after light–stress. CERKL expression is shown by arrow-heads. Labeling intensity of CERKL increases in the outer segments at LD+0 (Compare 4Aiv with 4Aii). CERKL labeling pattern changed from the nuclear to the base of apical membrane in the disappearing RPE cells (4Avi). The CERKL labeling in the ONL in LD+0 retina changed from continuous perinuclear to diffused and punctuated (4Biv, compare with 4Biii) and in LD+24 the labeling intensified in the perinuclear region of some of the nuclei and disappearing from the dying photoreceptor nuclei (4Bv, compare with 4Biii). No change in the nuclear localization was observed in the RPE cells immediately after light-damage (4Bii, compare with 4Bi); in the ganglion cells immediately and 24 h after light damage (4Bx, 4Bxi; compare with 4Bix). The CERKL labeling intensity appeared to be increased in the nucleus of the inner retinal neurons after light damage (4Bvii, 4Bviii; compare with 4Bvi). Abbreviations of the retinal layers are as in figure 3.
Immediately after light-damage, CERKL labeling appeared greater in the outer segments (Figure 4Aiv). At 24 h CERKL labeling pattern changed in the disappearing RPE cells, localization moved from the nuclear to the base of apical membrane (Figure 4Avi). The CERKL labeling in the ONL in LD+0 retina changed from continuous perinuclear to diffused and punctuated (Figure 4Biv vs. 4Biii). With photoreceptor cells entering into apoptosis, the CERKL labeling in the ONL appeared to be intensified in the perinuclear region of some of the nuclei and disappearing from the dying photoreceptor nuclei (Figure 4Avi, 4Bv). CERKL protein localizes to the nucleus in the RPE cells, inner retinal neurons and in the ganglion cells (Figure 4Aii, 4Bi, 4Bvi, and 4Bix). No change in the localization was observed in the RPE cells immediately after light-damage (Figure 4Bii, compare with Figure 4Bi); in the ganglion cells immediately and 24 h after light damage (Figure 4Bx, 4Bxi; compare with Figure 4Bix). However, labeling intensity appeared to be increased in the nucleus of the inner retinal neurons after light damage (Figure 4Bvii, 4Bviii; compare with 4Bvi).
Effect of Light-stress and Light-adaptation on CERKL Protein Expression
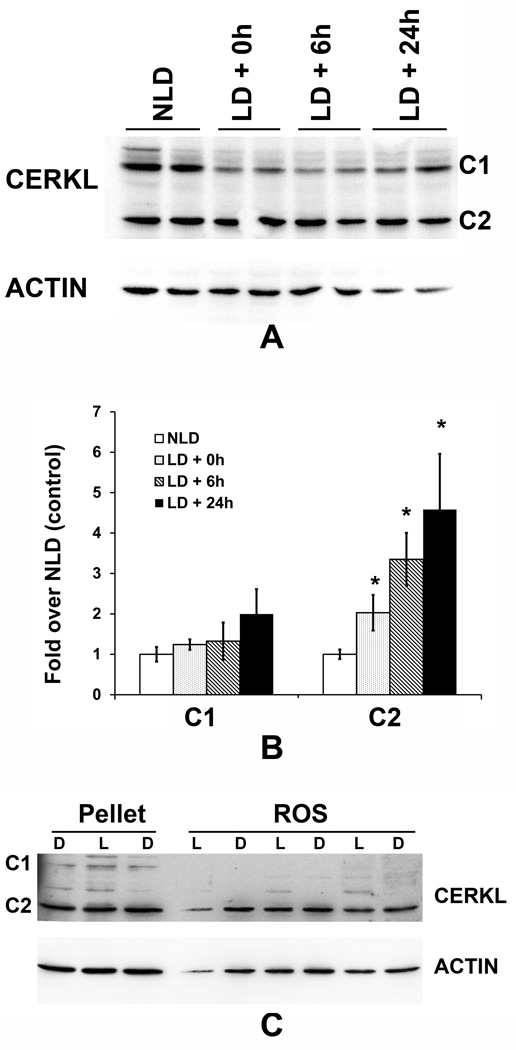
Using the anti-CERKL antibody, we detected two major protein bands of 59 kDa and 37 kDa in rat retinal tissues and designated those as C1 and C2, respectively (Figure 5A-NLD, and Supplement Figure 1). When we examined the expression of the CERKL protein in the light-damaged retina we found no change in the levels of the C1 protein; however, the levels of C2 (37 kDa) protein increased significantly (3–5 fold) after light damage (Figure 5A, 5B), which was consistent with the increase of the Cerkl mRNA (Figure 2A).
FIGURE 5. Light-stress increased expression of the CERKL protein in the retina.
A. Anti- CERKL antibody recognized two principal CERKL proteins in the retina: approximately 59 kDa (C1) band and 37 kDa (C2) band. B. Densitometric analysis of the C1 and C2 polypeptides obtained from the light-stressed rat retinas, which are presented as fold over nolight-damaged control (NLD). (LD+0h, LD+6h, LD+24h represent samples harvested at 0 h, 6 h, and 24 h after light damage, respectively). (* = p < 0.01; n=4; Student’s t test). C. CERKL expression in retinal rod outer segments (ROS). ROS was prepared from dark- (D) and light-(L) adapted retinas and subjected to Western blotting with anti-CERKL antibody. Pellet from the ROS prep were also analyzed.
CERKL protein has been shown to be present in photoreceptor outer segments (Garanto et al., 2011; Vekslin and Ben-Yosef, 2010). Its presence and interaction with outer segment proteins has recently been demonstrated using bovine photoreceptor outer segments (Nevet et al., 2012). We asked whether the level of CERKL changes with light and dark adaptation in rat retinal ROS. Proteins from purified ROS from light- and dark-adapted rats were analyzed by Western blotting. We detected only the C2 band (~ 37 kDa) in the ROS samples, and no difference was found in the level of this protein between the dark-adapted and light-adapted samples (Figure 5C). In this analysis, we included a few pellet samples from the same ROS prep and detected both C1 (though lighter) and C2 bands (Figure 5C). Also we did not detect any difference in the intensity of the CERKL pellet bands between D and L samples (Figure 5C).
CERKL Expression in NeuroD1 Knock-out Mice
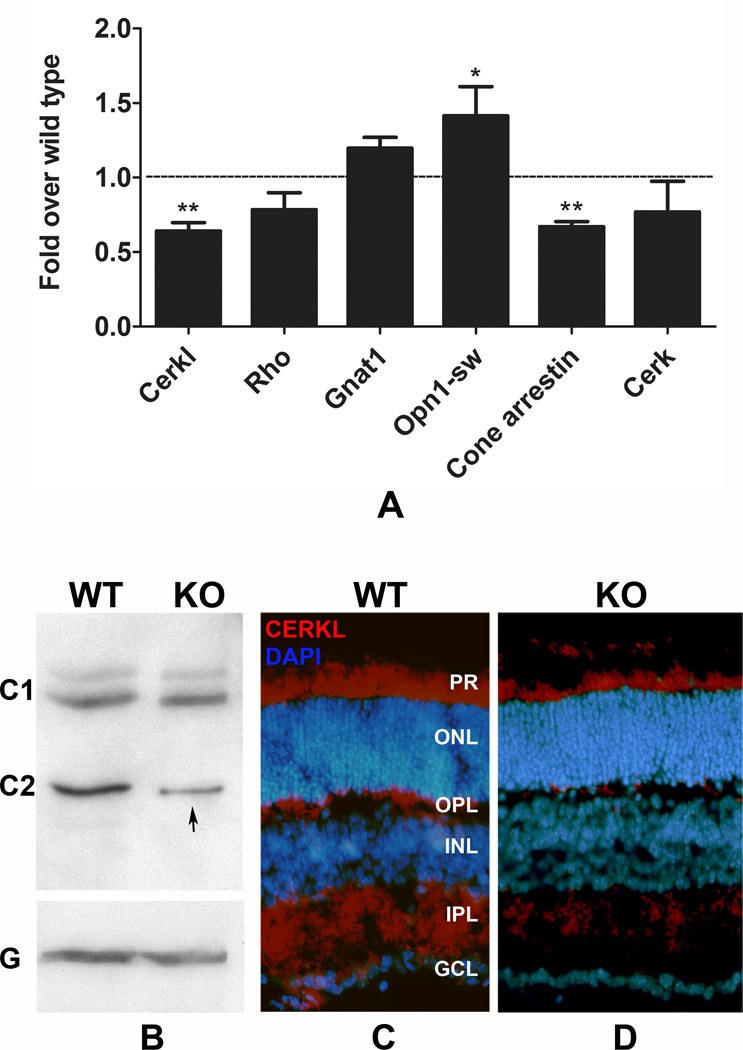
In the human chromosome 2q31.3, CERKL is linked to the NEUROD1 gene, which is located immediate upstream of CERKL in cis configuration. This region is conserved in most of the mammalian genome including the mouse and rat genomes. BETA2/NeuroD1 is a helix-loophelix transcription factor that is expressed widely in the developing nervous system including the retina (Cho et al., 2007; Cho and Tsai, 2006). Interestingly, NeuroD1 knock-out mice develop a retinal degeneration phenotype, in which both rod and cone photoreceptor cells degenerate with age (Pennesi et al., 2003). Although, NeuroD1 is expressed in most of the retinal neurons, it is the photoreceptors at the ONL that are affected by the loss of the protein (Pennesi et al., 2003). We hypothesized that these two linked genes (Cerkl and NeuroD1) are also related functionally, and that NeuroD1 may affect the transcription of Cerkl since NeuroD1 is a transcription factor. Recently, Garanto et al. (2011) showed that one of the transcription initiation sites for Cerkl belongs to the NeuroD1 sequence (Garanto et al., 2011). It would therefore be interesting to see Cerkl expression in the NeuroD1 retina. In a preliminary experiment, we observed that Cerkl mRNA expression was significantly reduced (38%) in the retina of one-month old NeuroD1 knock-out mice (Figure 6A). When we tested other rods and cone cell markers in the same sample, we detected variable expression: the short wave-length cone opsin increased and cone arrestin decreased significantly (6A). In the protein analysis, we found only a reduction in the 37 kDa protein band (C2) and not in the 59 kDa band (C1) (Figure 6B, arrow). This decrease in protein levels was also reflected by a decrease in the intensity of the CERKL labeling in 2-month-old NeuroD1 knock-out retinal sections (Figure 6C).
FIGURE 6. Expression of CERKL in NeuroD1 knock-out retina.
A. Quantitative RT-PCR was used to measure Cerkl mRNA in the retina obtained from 1-month-old NeuroD1 knock-out (KO) mice and from their wild-type littermate (WT). Cerkl expression was significantly lower in the KO retina. Other rod and cone photoreceptor markers tested for comparison. (** = p < 0.001; n=4; Student’s t test). B. Western blot analysis was performed. Similar to the data from the rats, the anti-CERKL antibody recognized two principal polypeptides in the mouse retina, C1 and C2 with the approximate molecular weights of 59 and 37 kDa, respectively. Compared to the WT C2 band, the intensity of C2 band in the KO retina was significantly lower (arrow). G, GAPDH, was used as a loading control. C and D. Immunohistochemical labeling was performed on 2-month-old WT (C) and KO retinas (D). The intensity of CERKL labeling was less in the KO retinas than in the WT retinas.
DISCUSSION
CERKL mutations are involved in both macular and peripheral pathologies. Full-field ERG findings indicate degeneration of both rod and cone photoreceptors and early maculopathy. All of the cases reported thus far from different groups of patients with different mutations by independent investigators point towards a common pathology whereby younger patients (20–30 year old) present with early macular alterations, which ultimately progress towards an RP-like phenotype with peripheral retinal degeneration (Aleman et al., 2009; Ali et al., 2008; Auslender et al., 2007; Avila-Fernandez et al., 2008; Bayes et al., 1998; Littink et al., 2010). Also all of these different mutations identified cause similar phenotypic consequences, which may otherwise rule out the possibility of differential penetrance of different CERKL mutations.
Although CERKL has homology with CERK, the function of this protein is not known. A knock-out mouse was developed by the Bornancin group (Cerkl -/- mice), but its role as a kinase has yet to be established (Graf et al., 2008). The retinas of Cerkl -/- mice were not characterized well by using histological and electrophysiological parameters and therefore it did not result in the generation of much information on CERKL mutation-mediated pathologies. Recently, Garanto et al. (2012) reported another mouse model where they substantially knocked-down the Cerkl expression in the retina but those mouse failed to develop any photoreceptor phenotype (Garanto et al., 2012). The CERKL gene in humans and mice has unusually higher varieties of transcripts that result from alternative exon splicing, alternative promoter uses, and alternative translation initiation sites (Garanto et al., 2011). Therefore, developing a clean knock-out mouse is challenging (Garanto et al., 2012). While we performing our studies on Cerkl expression and distribution in rat and mouse tissues, two papers were published showing the distribution of Cerkl gene expression in mouse tissues and the localization of the protein in the retina (Garanto et al., 2011; Vekslin and Ben-Yosef, 2010). In our studies of rat tissues, we found that the retina had the highest levels of expression of the Cerkl gene and proteins (Figures 1 and S1). This may explain why so far known CERKL mutations in humans cause mostly nonsyndromic retinal pathology; CERKL has more of a retinal-specific expression.Garanto et al. (2011) suggested that higher levels of Cerkl were expressed in the mouse liver; however, we could not detect any measurable levels of Cerkl in the rat liver and no expression was found in the human liver as well (Bornancin et al., 2005; Garanto et al., 2011). Since its discovery, CERKL has been predicted to be a retinal CERK. However, no ceramide kinase activity has been demonstrated for CERKL in vitro and in vivo. Vekslin and Ben-Yosef (2010) reported that the mouse retina had significantly higher Cerkl expression than Cerk expression (Vekslin and Ben-Yosef, 2010). Our quantitative RT-PCR data indicates that Cerk expression was >2 fold higher than Cerkl expression in both mouse and rat retinas (Figure 1), that Cerk transcripts were present in all of the tissues that we tested, and that the expression of Cerk was always higher than the Cerkl in the tissues that also expressed Cerkl (Figure 1). This is similar to the data on expression in human tissues in which both CERKL and CERK expression was measured quantitatively (Bornancin et al., 2005). A recent study, however, provided a possible functional role of CERKL away from CERK in the retina.Nevet et al. (2012) showed the interaction of CERKL with neuronal calcium sensor proteins, including GCAP1, GCAP2, and recoverin in photoreceptor cells (Nevet et al., 2012). These proteins are very important for photoreceptor functioning and therefore may provide clues to how CERKL mutation leads to photoreceptor degeneration pathology. Thus CERKL could have an independent role in the retina and may not be an alternative for CERK in the retina.
For our developmental studies, we used whole mouse eyes. In the whole eye, the major contributing tissue was the retina (Figure 1B). Expression of Cerkl was significantly higher in the adult eyes than in the developing eyes and the higher levels of expression were maintained until an older age. This may indicate that the role of CERKL in the retina is more for maintenance of the retina for its day-to-day activity than its involvement in retinal development. Like previous studies (Garanto et al., 2011; Tuson et al., 2004; Vekslin and Ben-Yosef, 2010), our study found that CERKL localization in various layers of the retina including photoreceptors (Figure 3). We also found CERKL localization in the RPE cells, specifically to the RPE nucleus. CERKL localization in various sub-cellular organelles including the nucleus has been reported (Inagaki et al., 2006; Tuson et al., 2009). We performed a detailed study of CERKL localization in the light-stressed retina (Figure 4). We detected changes in the localization of CERKL in and around the photoreceptor nuclei and increased labeling to the photoreceptor outer segments under light stress. However, nuclear localization of CERKL in the RPE, inner nuclear layer and the ganglion cells were not altered (Figure 4). This is not surprising though, because, photoreceptor cells are the primary target for light damage and the damaging effect of light starts with rhodopsin activation (Humphries et al., 1997; Noell and Albrecht, 1971; Noell et al., 1971; Noell et al., 1966). Alteration in CERKL expression and localization in photoreceptor cells under light stress may indicate a role of CERKL in the stress response of these cells, or it may be a consequence of the induction of apoptosis in photoreceptor cells.
We detected two major protein products, C1 at 59 KDa and C2 at 37 kDa, respectively, from the Cerkl gene in the retinas of mice and rats. The expression levels of both the mature proteins were very high in the retina (Figures 5A, S1), which correlated with the mRNA expression profile of the gene, suggesting that both might have resulted from the Cerkl gene transcription. However, we found only C2 protein in the ROS preparation (Figure 5C) and we found both C1 and C2 in pellet (Figure 5C), but the intensity of the C1 band is much weaker in the pellet than that of the C1 band obtained from the soluble protein from the whole retina as in Figure 5A (NLD). This may indicate C2 is a membrane-associated protein and thus can separate with ROS and can associate with other membranes and get pelleted during the ROS preparation. On the other hand, C1 is the soluble protein. The membrane-associating PH domain has been identified and characterized in CERKL (Rovina et al., 2009). Under light stress, we observed that Cerkl mRNA expression was induced in the rat retina (Figure 2A) and found that this induction was specific to the C2 protein (37 kDa) (Figure 5A, 5B). C2 is present in the photoreceptor outer segments (Figure 5C); an increase of this protein during light-stress makes reasonable sense because photoreceptor outer segments receive the primary insult of light stress.Nevet et al. (2012) showed that CERKL plays a role in calcium signaling pathway in photoreceptor outer segments (Nevet et al., 2012). Our study supports the presence of CERKL in the outer segments and its role in the functioning of the photoreceptor cells. CERKL increased under light stress may also indicate its protective role in the stressed retina. CERKL was previously reported to have a protective effect from oxidative stress in cell cultures (Tuson et al., 2009).
CERKL can localize in the nucleus and some CERKL protein contains a nuclear localization signal and nuclear export signals (Inagaki et al., 2006; Tuson et al., 2009), suggesting that CERKL might be a signal transporter or carrier protein that communicates between the nucleus and cytoplasm. However, we did not detect any alteration in the nuclear localization of the CERKL protein in various nuclear layers under light stress (Figure 4). Similarly, we did not detect any changes in the level of the C1 protein (Figure 5B) and when we subjected the insoluble fraction (containing nucleus) of the proteins from the same samples used in the experiment represented in Figure 5A, we detected the presence of only C1 protein in those fractions (data not shown), indicating the nuclear fraction does not contain C2. This provides an indication that the soluble C1 CERKL may contain nuclear export and localization signals and may have an independent role that may or may not be related to the C2 CERKL.
We noticed a significant down-regulation of Cerk in the light-stressed retina and both Cerk and Cerkl in light-stressed eyecups (containing RPE) (Figure 5). We tested some known retinal genes and found a reduction in the expression of those related to rhodopsin functioning and the expression of all those genes related to photoreceptor maintenance goes significantly down after induction of apoptosis. The increase in Cerkl expression in light-stressed retina may therefore indicate an additional role other than maintaining the normal retinal function, which could be a role in combating cellular oxidative stress as proposed earlier (Tuson et al., 2009). However, the decrease in Cerk expression may be related to general down-regulation of other genes involved in normal functioning. Further, we can’t rule out the possibility that increased Cerkl expression in the light-stressed retina with concomitant reduction in Cerk expression may indicate a compensatory role of CERKL for some CERK-related functioning during retinal stress.
The BETA2/NueroD1 gene is tightly linked to CERKL and is conserved in mammalian genomes. BETA2 is a transcription factor located immediately before CERKL on the 5’ end. We studied the expression of CERKL in the NeuroD1 knock-out retina with a speculation that these linked genes might be functionally related and that BETA2 may regulate the transcription of CERKL. In the one-month-old NeuroD1 retina, we found significant reductions in the Cerkl mRNA (Figure 6A), a reduction in cone arrestin to a similar extent, and a decrease in the level of C2 CERKL protein (Figure 6B); however, the photoreceptor cells (ONL thickness and photoreceptor nuclear layers) appeared to not be reduced significantly at the age of 2 months (Figure 6D). The statistically significant reduction in thickness of the ONL of NeuroD1 knock-out mice was not observed by two months of age, but the thickness of ONL become significantly different after 2-month of age (Cho JH, unpublished data). The photoreceptor cells, especially cone photoreceptors in NeuroD1 knock-out mice degenerate starting as early as P10; the development and numbers of other neurons appear normal and by 3 months of age more than 50% photoreceptor cells die in the NeuroD1 knock-out mice (Pennesi et al., 2003). When this studies were underway, Garanto et al. showed that one of the major mouse Cerkl transcripts has a transcription initiation sequence from NeuroD1 gene and that is a highly expressed isoform in the mouse retina (Garanto et al., 2011). It is possible that knocking out NeuroD1 by inserting a LacZ-neo cassette in the NeuroD1 exon II (Naya et al., 1997) disrupted the promoter and transcription initiation site of the Cerkl transcripts that make the CERKL C2 protein. However, further investigation is needed to prove that the C2 CERKL is the product of the mRNA transcript that has initiation in the NeuroD1 sequence. In one-month-old retina we also observed significant reduction in cone arrestin but not in cone opsin (Figure 6A). Cone dominant expression of CERKL and a significant presence of CERKL in the cone outer segments have been demonstrated by independent studies (Garanto et al., 2011; Vekslin and Ben-Yosef, 2010). A reduction in C2 CERKL in the NeuroD1 knock-out retina might therefore reflect the loss or dysfunction of cone photoreceptor cells. Further investigation is needed, however, to rule out the possibility of NeuroD1 acting as a trans acting transcription factor for Cerkl in cone or rod photoreceptors.
Supplementary Material
We confirmed that retina has the highest levels of expression of Cerkl mRNA among all the rat tissues we have tested. Cerkl mRNA expression is highest in the mature retina and a higher level is maintained in the ageing retina.
CERKL protein localizes to the RPE cells, photoreceptor cells, inner retinal neurons, and ganglion cells. Nuclear localization of CERKL was detected in the RPE, ganglion and the inner retinal neurons. In photoreceptor cells, CERKL protein localizes to perinuclear regions, inner segments and rod outer segments (ROS).
In light-stressed retina, Cerkl mRNA increases and the increase is specific to a CERKL protein small isoform. This is the isoform found in the ROS.
In NeuroD1 knock-out mouse retina, the expression of Cerkl mRNA and protein decreases.
ACKNOWLEDGEMENTS
The authors are thankful to Dr. Ming-Jer Tsai (Baylor College of Medicine) for providing BETA2/NeuroD1 knock-out mice for this research and Mark Dittmar (Dean McGee Eye Institute, OUHSC) for his help in animal studies. Financial support from Pediatric Ophthalmology grant from Knight’s Templar Eye Foundation (NAM), OU College of Medicine Alumni Association grant (MNM), National Eye Institute grant (EY022071)(NAM), National Center for Research Resources Grant (RR17703) (NAM); National Eye Institute Core Grant for Vision Research to OUHCS (EY12190), unrestricted grants form Research to Prevent Blindness to the Dept. of Ophthalmology, OUHSC; National Eye Institute grant (EY011930 and EY010608) and the Robert A. Welch Foundation grant (G-0010) (WHK) are acknowledged.
ABBREVIATIONS
- CERKL
ceramide kinase like
- CERK
ceramide kinase
- SD
Sprague Dawley
- qRT-PCR
quantitative RT-PCR
- ROS
rod outer segment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aleman TS, Soumittra N, Cideciyan AV, Sumaroka AM, Ramprasad VL, Herrera W, Windsor EA, Schwartz SB, Russell RC, Roman AJ, Inglehearn CF, Kumaramanickavel G, Stone EM, Fishman GA, Jacobson SG. CERKL mutations cause an autosomal recessive cone-rod dystrophy with inner retinopathy. Investigative ophthalmology & visual science. 2009;50:5944–5954. doi: 10.1167/iovs.09-3982. [DOI] [PubMed] [Google Scholar]
- Ali M, Ramprasad VL, Soumittra N, Mohamed MD, Jafri H, Rashid Y, Danciger M, McKibbin M, Kumaramanickavel G, Inglehearn CF. A missense mutation in the nuclear localization signal sequence of CERKL (p.R106S) causes autosomal recessive retinal degeneration. Molecular vision. 2008;14:1960–1964. [PMC free article] [PubMed] [Google Scholar]
- Auslender N, Sharon D, Abbasi AH, Garzozi HJ, Banin E, Ben-Yosef T. A common founder mutation of CERKL underlies autosomal recessive retinal degeneration with early macular involvement among Yemenite Jews. Investigative ophthalmology & visual science. 2007;48:5431–5438. doi: 10.1167/iovs.07-0736. [DOI] [PubMed] [Google Scholar]
- Avila-Fernandez A, Riveiro-Alvarez R, Vallespin E, Wilke R, Tapias I, Cantalapiedra D, Aguirre-Lamban J, Gimenez A, Trujillo-Tiebas MJ, Ayuso C. CERKL mutations and associated phenotypes in seven Spanish families with autosomal recessive retinitis pigmentosa. Investigative ophthalmology & visual science. 2008;49:2709–2713. doi: 10.1167/iovs.07-0865. [DOI] [PubMed] [Google Scholar]
- Bayes M, Goldaracena B, Martinez-Mir A, Iragui-Madoz MI, Solans T, Chivelet P, Bussaglia E, Ramos-Arroyo MA, Baiget M, Vilageliu L, Balcells S, Gonzalez-Duarte R, Grinberg D. A new autosomal recessive retinitis pigmentosa locus maps on chromosome 2q31-q33. Journal of medical genetics. 1998;35:141–145. doi: 10.1136/jmg.35.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornancin F, Mechtcheriakova D, Stora S, Graf C, Wlachos A, Devay P, Urtz N, Baumruker T, Billich A. Characterization of a ceramide kinase-like protein. Biochimica et biophysica acta. 2005;1687:31–43. doi: 10.1016/j.bbalip.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Brush RS, Tran JT, Henry KR, McClellan ME, Elliott MH, Mandal MN. Retinal sphingolipids and their very-long-chain fatty acid-containing species. Investigative ophthalmology & visual science. 2010;51:4422–4431. doi: 10.1167/iovs.09-5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Klein WH, Tsai MJ. Compensational regulation of bHLH transcription factors in the postnatal development of BETA2/NeuroD1-null retina. Mech Dev. 2007;124:543–550. doi: 10.1016/j.mod.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Tsai MJ. Preferential posterior cerebellum defect in BETA2/NeuroD1 knockout mice is the result of differential expression of BETA2/NeuroD1 along anterior-posterior axis. Dev Biol. 2006;290:125–138. doi: 10.1016/j.ydbio.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Garanto A, Riera M, Pomares E, Permanyer J, de Castro-Miro M, Sava F, Abril JF, Marfany G, Gonzalez-Duarte R. High transcriptional complexity of the retinitis pigmentosa CERKL gene in human and mouse. Investigative ophthalmology & visual science. 2011;52:5202–5214. doi: 10.1167/iovs.10-7101. [DOI] [PubMed] [Google Scholar]
- Garanto A, Vicente-Tejedor J, Riera M, de la Villa P, Gonzalez-Duarte R, Blanco R, Marfany G. Targeted knockdown of Cerkl, a retinal dystrophy gene, causes mild affectation of the retinal ganglion cell layer. Biochimica et biophysica acta. 2012;1822:1258–1269. doi: 10.1016/j.bbadis.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Gordon WC, Casey DM, Lukiw WJ, Bazan NG. DNA damage and repair in light-induced photoreceptor degeneration. Investigative ophthalmology & visual science. 2002;43:3511–3521. [PubMed] [Google Scholar]
- Graf C, Niwa S, Muller M, Kinzel B, Bornancin F. Wild-type levels of ceramide and ceramide-1-phosphate in the retina of ceramide kinase-like-deficient mice. Biochem Biophys Res Commun. 2008;373:159–163. doi: 10.1016/j.bbrc.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Hafezi F, Marti A, Munz K, Reme CE. Light-induced apoptosis: differential timing in the retina and pigment epithelium. Experimental eye research. 1997;64:963–970. doi: 10.1006/exer.1997.0288. [DOI] [PubMed] [Google Scholar]
- Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, Sieving PA, Sheils DM, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi MR, Humphries P. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- Inagaki Y, Mitsutake S, Igarashi Y. Identification of a nuclear localization signal in the retinitis pigmentosa-mutated RP26 protein, ceramide kinase-like protein. Biochem Biophys Res Commun. 2006;343:982–987. doi: 10.1016/j.bbrc.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Littink KW, Koenekoop RK, van den Born LI, Collin RW, Moruz L, Veltman JA, Roosing S, Zonneveld MN, Omar A, Darvish M, Lopez I, Kroes HY, van Genderen MM, Hoyng CB, Rohrschneider K, van Schooneveld MJ, Cremers FP, den Hollander AI. Homozygosity mapping in patients with cone-rod dystrophy: novel mutations and clinical characterizations. Investigative ophthalmology & visual science. 2010;51:5943–5951. doi: 10.1167/iovs.10-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal MN, Ambasudhan R, Wong PW, Gage PJ, Sieving PA, Ayyagari R. Characterization of mouse orthologue of ELOVL4: genomic organization and spatial and temporal expression. Genomics. 2004;83:626–635. doi: 10.1016/j.ygeno.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Mandal MN, Ayyagari R. Complement factor H: spatial and temporal expression and localization in the eye. Investigative ophthalmology & visual science. 2006;47:4091–4097. doi: 10.1167/iovs.05-1655. [DOI] [PubMed] [Google Scholar]
- Mandal MN, Moiseyev GP, Elliott MH, Kasus-Jacobi A, Li X, Chen H, Zheng L, Nikolaeva O, Floyd RA, Ma JX, Anderson RE. Alpha-phenyl-N-tertbutylnitrone (PBN) prevents light-induced degeneration of the retina by inhibiting RPE65 protein isomerohydrolase activity. The Journal of biological chemistry. 2011;286:32491–32501. doi: 10.1074/jbc.M111.255877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal MN, Patlolla JM, Zheng L, Agbaga MP, Tran JT, Wicker L, Kasus-Jacobi A, Elliott MH, Rao CV, Anderson RE. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic Biol Med. 2009;46:672–679. doi: 10.1016/j.freeradbiomed.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal MN, Vasireddy V, Jablonski MM, Wang X, Heckenlively JR, Hughes BA, Reddy GB, Ayyagari R. Spatial and temporal expression of MFRP and its interaction with CTRP5. Investigative ophthalmology & visual science. 2006a;47:5514–5521. doi: 10.1167/iovs.06-0449. [DOI] [PubMed] [Google Scholar]
- Mandal MN, Vasireddy V, Reddy GB, Wang X, Moroi SE, Pattnaik BR, Hughes BA, Heckenlively JR, Hitchcock PF, Jablonski MM, Ayyagari R. CTRP5 is a membrane-associated and secretory protein in the RPE and ciliary body and the S163R mutation of CTRP5 impairs its secretion. Investigative ophthalmology & visual science. 2006b;47:5505–5513. doi: 10.1167/iovs.06-0312. [DOI] [PubMed] [Google Scholar]
- Martin RE, Elliott MH, Brush RS, Anderson RE. Detailed characterization of the lipid composition of detergent-resistant membranes from photoreceptor rod outer segment membranes. Investigative ophthalmology & visual science. 2005;46:1147–1154. doi: 10.1167/iovs.04-1207. [DOI] [PubMed] [Google Scholar]
- Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997;11:2323–2334. doi: 10.1101/gad.11.18.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevet MJ, Vekslin S, Dizhoor AM, Olshevskaya EV, Tidhar R, Futerman AH, Ben-Yosef T. Ceramide kinase-like (CERKL) interacts with neuronal calcium sensor proteins in the retina in a cation-dependent manner. Investigative ophthalmology & visual science. 2012;53:4565–4574. doi: 10.1167/iovs.12-9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noell WK, Albrecht R. Irreversible effects on visible light on the retina: role of vitamin. A Science (New York, N.Y. 1971;172:76–79. doi: 10.1126/science.172.3978.76. [DOI] [PubMed] [Google Scholar]
- Noell WK, Delmelle MC, Albrecht R. Vitamin A deficiency effect on retina: dependence on light. Science (New York, N.Y. 1971;172:72–75. doi: 10.1126/science.172.3978.72. [DOI] [PubMed] [Google Scholar]
- Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Investigative ophthalmology. 1966;5:450–473. [PubMed] [Google Scholar]
- Papermaster DS, Dreyer WJ. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974;13:2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Pennesi ME, Cho JH, Yang Z, Wu SH, Zhang J, Wu SM, Tsai MJ. BETA2/NeuroD1 null mice: a new model for transcription factor-dependent photoreceptor degeneration. J Neurosci. 2003;23:453–461. doi: 10.1523/JNEUROSCI.23-02-00453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovina P, Schanzer A, Graf C, Mechtcheriakova D, Jaritz M, Bornancin F. Subcellular localization of ceramide kinase and ceramide kinase-like protein requires interplay of their Pleckstrin Homology domain-containing N-terminal regions together with C-terminal domains. Biochimica et biophysica acta. 2009;1791:1023–1030. doi: 10.1016/j.bbalip.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Mitchell R, Li H, Graham DR, Ash JD. Leukemia inhibitory factor inhibits neuronal development and disrupts synaptic organization in the mouse retina. J Neurosci Res. 2005;82:316–332. doi: 10.1002/jnr.20619. [DOI] [PubMed] [Google Scholar]
- Smith RS, Kao WWY, John SWM. Ocular development. In: Smith RS, John SWM, Nishina PM, Sundberg JP, editors. Systematic Evaluation of the Mouse Eye: Anatomy, Pathology, and Biomethods. New York: CRC Press; 2002. pp. 45–63. [Google Scholar]
- Tang Z, Wang Z, Ke T, Wang QK, Liu M. Novel compound heterozygous mutations in CERKL cause autosomal recessive retinitis pigmentosa in a nonconsanguineous Chinese family. Archives of ophthalmology. 2009;127:1077–1078. doi: 10.1001/archophthalmol.2009.207. [DOI] [PubMed] [Google Scholar]
- Tuson M, Garanto A, Gonzalez-Duarte R, Marfany G. Overexpression of CERKL, a gene responsible for retinitis pigmentosa in humans, protects cells from apoptosis induced by oxidative stress. Molecular vision. 2009;15:168–180. [PMC free article] [PubMed] [Google Scholar]
- Tuson M, Marfany G, Gonzalez-Duarte R. Mutation of CERKL, a novel human ceramide kinase gene, causes autosomal recessive retinitis pigmentosa (RP26) Am J Hum Genet. 2004;74:128–138. doi: 10.1086/381055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekslin S, Ben-Yosef T. Spatiotemporal expression pattern of ceramide kinase-like in the mouse retina. Molecular vision. 2010;16:2539–2549. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.