Abstract
Objective
To analyze knee trabecular bone structure and spatial cartilage T1ρ and T2 relaxation times using 3-T MRI in subjects with and without tears of posterior horn of medial meniscus (PHMM).
Design
3-T MRI from 59 subjects (> 18 years), were used to evaluate PHMM tears based on modified WORMS scoring; and to calculate apparent trabecular bone - volume over total bone volume fraction (app. BV/TV), number (app. Tb.N), separation (app. Tb.Sp) and thickness (app. Tb.Th) for overall femur/tibia and medial/lateral femur/tibia; and relaxation times for deep and superficial layers of articular cartilage. A repeated measures analysis using GEE was performed to compare trabecular bone and cartilage relaxation time parameters between people with (n = 35) and without (n= 24) PHMM tears, while adjusting for age and knee OA presence.
Results
Subjects with PHMM tears had lower app. BV./TV and app. Tb.N, and greater app. Tb.Th, and app. Tb.Sp. They also had higher T1ρ times in the deep cartilage layer for lateral tibia and medial femur and higher T2 relaxation times for the deep cartilage layer across all compartments.
Conclusions
PHMM tears are associated with differences in underlying trabecular bone and deep layer of cartilage. Overload of subchondral bone can lead to its sclerosis and stress shielding of trabecular bone leading to the resorptive changes observed in this study. The results underline the importance of interactions of trabecular bone and cartilage in the pathogenesis of knee OA in people with PHMM tears.
Keywords: Meniscal Tears, Knee Osteoarthritis, Trabecular Bone, Cartilage, T1ρ, T2
Introduction
Menisci are integral to normal biomechanical functioning of the knee and a tear can cause a disruption of the mechanical environment, leading to altered loading patterns and subsequent degeneration of underlying cartilage. Meniscal tears have been shown to be a precursor for development of post-traumatic knee osteoarthritis (OA), even after surgical repair or resection [1]. There is a 50% reported incidence of post-traumatic knee OA after diagnosed or treated meniscus lesions at 10-20 years of follow-up [2]. There may be a six-fold higher risk of OA, 21 years after total meniscectomy, compared to age and gender matched controls [1]. Hence, meniscal repair and preservation have become the mainstay of surgical management [3, 4]. Conversely, the OA disease process can lead to degenerative tears and it has been shown that majority of people with existing knee OA have meniscal lesions [5]. In a sample with mean age of 65 years, a meniscal tear was found in 67% of asymptomatic subjects and 91% of subjects with symptomatic knee OA [5]. In people with pre-existing knee OA, meniscal pathology (displacement or tear) is associated with accelerated cartilage damage [6, 7].
Posterior horn of the medial meniscus (PHMM) is the most common site for a tear [8, 9]. Radial tears of the posterior horn have been shown to be associated with worse cartilage loss compared to other types of tears [10]. Using quantitative imaging, it has been shown that tears of the PHMM are associated with higher T1ρ and T2 relaxation times for not just the PHMM but also for the underlying cartilage [11]. T1ρ and T2 relaxation times are increased in people with knee OA and indicate loss of proteoglycans and collagen disruption [12, 13]. These studies investigated the compositional changes in the articular cartilage as a whole. It is unknown if PHMM tears are related to differential changes in the superficial (articular) and deep (bony) articular cartilage layers.
Besides degeneration of the articular cartilage and meniscus, people with knee OA show subchondral sclerosis, subchondral bone marrow edema like lesions and osteoporotic changes in the trabecular bone of distal femur and proximal tibia [14-17]. Some of the trabecular bone changes consist of a decrease in apparent trabecular bone volume fraction, apparent trabecular number and thickness and an increase in apparent trabecular separation [14-16]. It has been suggested that these changes are partly in response to the altered loading patterns commonly seen in knee OA. Day et al. showed that the elastic modulus of the medial condyle trabecular bone was reduced by 60% in presence of cartilage damage compared to control specimens. They hypothesized that the reduced modulus was related to overall decrease in mineral density due to increased rate of remodeling and bone turnover [18].
Although meniscal tears are known to cause disruption of the mechanics of the knee, and are associated with changes in underlying cartilage, it is not yet known if similar changes are seen in trabecular bone. It is also unknown if superficial and deep layers of cartilage demonstrate different relationships to PHMM tears. The aim of this study was to compare the quantitative MRI derived structural parameters of trabecular bone, and articular cartilage T1ρ and T2 relaxation times using laminar analysis, in people with and without tears of the PHMM.
Materials and Methods
Subjects
Subjects were recruited from UCSF orthopedic surgeons and the community as a part of a larger study on knee osteoarthritis. The inclusion criteria for OA patients were frequent clinical symptoms of OA (including pain, stiffness and dysfunction) and demonstration of typical signs of OA in radiographs. The controls had no history of diagnosed OA, clinical OA symptoms, previous knee injuries, or signs of OA on radiographs.
Standard standing antero-posterior radiographs of the knee were obtained in all subjects at baseline to determine the Kellgren-Lawrence (KL) grade and OA severity [19]. The 59 subjects (27 men, 32 women) that participated in this cross-sectional study had a mean age of 60.0±13.7 years and a mean BMI of 25.4±5.1 kg/m2. Of these, 35 were classified as controls (KL=0, 1, mean age= 46.1±12.8 yrs), and 24 were classified as OA (KL score >1, mean age=58.0 ±11.8 yrs).
Anatomic Alignment (AA)
AA was measured using standard weight-bearing antero-posterior radiographs as the medial angle subtended by the line of the femoral shaft as it intersects in the knee with the line of the tibial shaft. A gender neutral offset of 4.21° was used to correct for the difference between AA and mechanical axis [20].
MRI acquisition
MRI of the knee was performed using a 3T GE Signa HDx MR Scanner (General Electric, Milwaukee, WI, USA) and an eight-channel phased-array knee coil (Invivo, Orlando, FL, USA). In the OA subjects, the knee with more severe findings on the radiographs was imaged. In controls, the dominant leg was imaged. Parallel imaging was performed with an array spatial sensitivity technique (ASSET) using acceleration factor (AF) = 2. Scanning parameters are shown in Table 1 [16, 21, 22]. For quantification of trabecular bone structure, a modified sampling scheme allowed the 3D FIESTA-c sequence to be employed with auto-calibration and twofold under-sampling (-R=2), which reduced the imaging time to nearly half of that in the conventional method[16].
Table 1.
MR Acquisition Parameters
| Sequence | Parameters* | Variables |
|---|---|---|
| sagittal T2-weighted fat-saturated fast spin-echo (FSE) | TR/TE = 4300/51 ms, field of view = 14 cm, matrix = 512 × 256 slice thickness = 2.5 mm, gap = 0.5 mm, echo train length [ETL] = 9, bandwidth = 31.25 kHz, NEX = 2, acquisition time = 4 min | Semi-quantitative clinical WORMS grades |
| Sagittal 3D T1ρ quantification sequence | TR/TE = 9.3/3.7 ms, time of recovery = 1500 ms, field of view = 14 cm, matrix = 256×192, ST = 3 mm, bandwidth = 31.25 kHz, views per segment = 48, time of spin-lock (TSL) = 0/10/40/80 ms, FSL = 500 Hz, acquisition time = 13 min | Superficial and Deep Articular Cartilage T1ρ Relaxation times |
| Sagittal 3D T2 quantification sequence | same as the T1ρ quantification except for magnetization preparation TE = 3.1/13.5/23.9/44.8 ms, acquisition time = 13 min | Superficial and Deep Articular Cartilage T2 Relaxation times |
| Axial Trabecular Bone structure quantification sequence | axial fully refocused steady state free-precession (SSFP) 3D phase cycles Fast Imaging Employing Steady State Acquisition (3D FIESTA-c) sequence, TR/TE = 11/4.2 ms, acquisition matrix = 512 × 384, flip angle = 60, field of view = 10 cm, 90 slices, slice thickness = 1 mm, acquisition time = 10 min | app. BV/TV, app. Tb.Th, app. Tb.Sp, app, Tb.N |
TR = Repetition Time, TE = Echo Time
Semi-quantitative morphological MR grading
Modified Whole-Organ Magnetic Resonance Imaging Score (WORMS) [23] was used to assess cartilage and meniscus morphology on a sagittal intermediate-weighted FSE fat-saturated image by board certified radiologists (TML with 20 and LN with 4 years of experience). The radiologists were blinded to subject information and performed separate readings, with a consensus in case of disagreement.
Meniscal morphology was graded using a modified WORMS score of the knee as follows: 0 = no lesion, 1= intra-substance abnormality, 2 = non-displaced tear, 3 = displaced or complex tear without deformity, and 4 = maceration of the meniscus. Medial and lateral meniscus were graded separately for the anterior horn, body and posterior horn. Posterior horn tears were the most common and used to stratify the subjects into those without tears (PHMM grades 0-1) and those with tears (PHMM grade 2-4).
Trabecular Bone
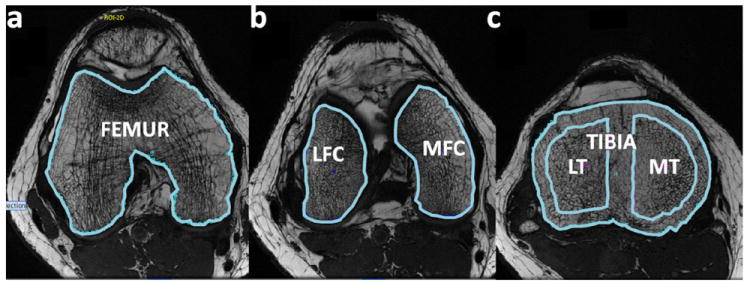
The analysis of trabecular bone structure parameters was performed using an in-house interface description language (IDL)-based (RSIm Boulder, CO, USA) developed image analysis software. Six different compartments were defined for trabecular bone analysis: femur (F), lateral and medial femoral condyle (LFC/MFC), Tibia (T). Tibia was also analyzed by separating into lateral and medial tibia (LT/MT). These regions of interest (ROIs) consisting of trabecular bone and marrow were segmented (DK, JZ) on multiple slices based on axial images similar to a previously described processing methods as illustrated in Figure 1.
Figure 1.
Trabecular bone structure post-processing: bone and marrow ROI were outlined for the femur (A), lateral and medial condyles (B), tibia (C) and LT/MT (C)
Fuzzy-C means clustering technique using bone enhancement features was used to segment the regions into bone and marrow [24]. This technique has been shown to account for partial volume effects, noise, and intensity in-homogeneities. The method is also shown to be more precise (reproducible), better validated and to better discriminate between participants with and without vertebral fractures than the other thresholding based approaches[24]. The trabecular bone parameters were then calculated using biphasic model described previously [25, 26]. The structure parameters assessed differ from those derived using histomorphometry, and therefore are considered “apparent” structure parameters, including apparent bone volume over total bone volume fraction – app. BV/TV, apparent trabecular number – app. Tb.N [1/mm], apparent trabecular separation – app. Tb.Sp [mm] and apparent trabecular thickness – app. Tb.Th [mm] [16].
Laminar Analysis of Articular Cartilage T1ρ and T2 relaxation times
Cartilage sub compartments were segmented on multiple slices semi-automatically in high resolution SPGR images using the in-house software developed with Matlab (Mathworks, Natick, MA, USA) based on edge detection and Bezier splines [27]. The cartilage compartments analyzed included: lateral femoral condyle (LFC), medial femoral condyle (MFC), lateral tibia (LT), medial tibia (MT), and the patella (P). All these regions of interest were further partitioned into two equal layers: Deep (closer to the subchondral bone) and Superficial (closer to articular surface) automatically using in-house developed software [27]. T1ρ and T2 maps were reconstructed by fitting the T1ρ- and T2-weighted images pixed-by-pixel to the equations below using in-house developed software:
T1ρ and T2 maps were rigidly registered to SPGR images and cartilage contours generated from SPGR images after segmentation were overlaid to the registered T1ρ and T2 maps. Mean T1ρ and T2 values were calculated in defined regions. To reduce artifacts caused by partial volume effects with synovial fluid, pixels with relaxation time greater than 130 ms in T1ρ or 100 ms for T2 maps were removed from the data used for quantification.
Statistics
All statistical analysis were performed using IBM SPSS Statistics 19.0 (IBM Corporation, Armonk, NY 10504, USA). Independent samples Student’s t-tests were used to compare age and BMI between those with and without PHMM tear. Chi-square tests were used to compare the distribution of gender (Males and Females) and disease presence (controls and OA) between those with and without PHMM tears. To compare the differences in trabecular bone and cartilage relaxation time parameters between people with and without PHMM tears, across different knee compartments, a repeated measures analysis was performed using Generalized Estimating Equations (GEE), while adjusting for age and knee OA presence. The GEE model was first run including the interaction between group (PHMM tear vs. no tear) and knee compartment (repeated measure). If the interaction term was not found to be statistically-significant (p > 0.05), it was removed from the model and the analysis re-run, and we report the overall estimated differences between subjects with and without PHMM tears. If the interaction term was found to be statistically significant (p < 0.05), the GEE model was run separately for each compartment, and the estimated differences for each compartment are reported separately. The 2 cartilage layers were analyzed separately since it was not an aim of the paper to compare the relaxation times between the two layers.
Results
Subject Characteristics
Table 2 shows the subject characteristics. 35 subjects did not have a PHMM tear (WORMS grade < 2) and 24 subjects showed the presence of tear of PHMM (WORMS grade ≥ 2). Subjects with PHMM tears were older (p < 0.001) and had greater proportion of OA subjects where OA was defined as KL > 1 (p = 0.001). There were no differences in BMI (p = 0.33), frontal plane alignment (p = 0.19) or distribution of males and females (p = 0.99) between the groups.
Table 2.
Subject Age, BMI, Gender, Disease Presence and Alignment by presence of posterior horn medial meniscus tear
| No Tear (n= 35) | Tear (n = 24) | p-value | |
|---|---|---|---|
| Age (years)* | 45.7 (12.5) | 58.6 (11.7) | 0.000 |
| BMI (kg/m2)* | 24.8 (4.3) | 26.2 (6.1) | 0.325 |
| Gender (M:F)* | 16:19 | 11:13 | 0.993** |
| Disease Presence (C:OA)* | 27:8 | 8:16 | 0.001** |
| Alignment (Degrees)* | 179.2 (2.7) | 177.9 (4.2) | 0.191 |
Mean (SD) for both groups
Chi-Square test
Trabecular Bone Structural Parameters
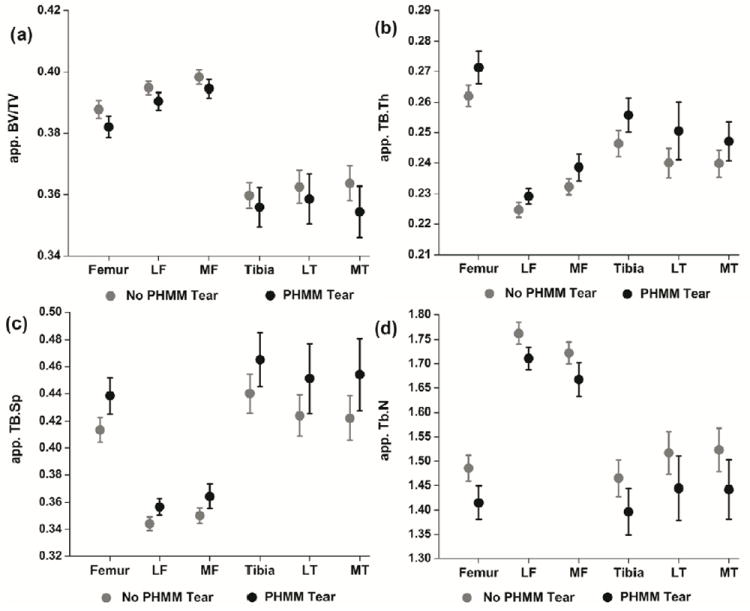
Means (95% CI) are in Figure 2. Table 3 shows the results from the GEE analysis. The interaction between group (PHMM tear vs. No tear) and compartment was not statistically-significant for app. BV./TV (p = 0.14), app. Tb.Th (p = 0.28), app. Tb.Sp (p = 0.13) and app. Tb.N (p = 0.69). Subsequent analysis without the interaction term showed a statistically significant main effect for all trabecular bone parameters. Subjects with PHMM tears had lower app. BV./TV, greater app. Tb.Th, greater app. Tb.Sp and lower app. Tb.N (Table 3) compared to subjects without PHMM tears.
Figure 2.
Means (95% Confidence Intervals) for trabecular bone structural parameters for subjects with and without tears of the posterior horn of medial meniscus. (A) – apparent bone volume fraction, (B)- apparent trabecular thickness, (C)- apparent trabecular separation, (D)- apparent trabecular number. GEE analysis showed that after accounting for age and OA presence, subjects with PHMM tears had lower app. BV./TV, greater app. Tb.Th, greater app. Tb.Sp and lower app. Tb.N.
Table 3.
Results from the GEE model for differences in trabecular bone parameters across all compartments in people with and without PHMM tears.
| Estimate Difference | 95 % CI | p-value | |
|---|---|---|---|
| App. BV/TV | 0.01 | 0.002, 0.011 | 0.008 |
| App. Tb.Th | -0.01 | -0.015, -0.005 | < 0.001 |
| App. Tb.Sp | -0.03 | -0.042, -0.015 | < 0.001 |
| App. Tb.N | 0.09 | 0.045, 0.125 | 0.034 |
All values are adjusted for age and disease presence.
BV/TV = Bone volume fraction; Tb.Th = Trabecular thickness; Tb.Sp = Trabecular separation ; Tb.N = Trabecular number
Laminar Analysis of Articular Cartilage T1ρ and T2 relaxation times
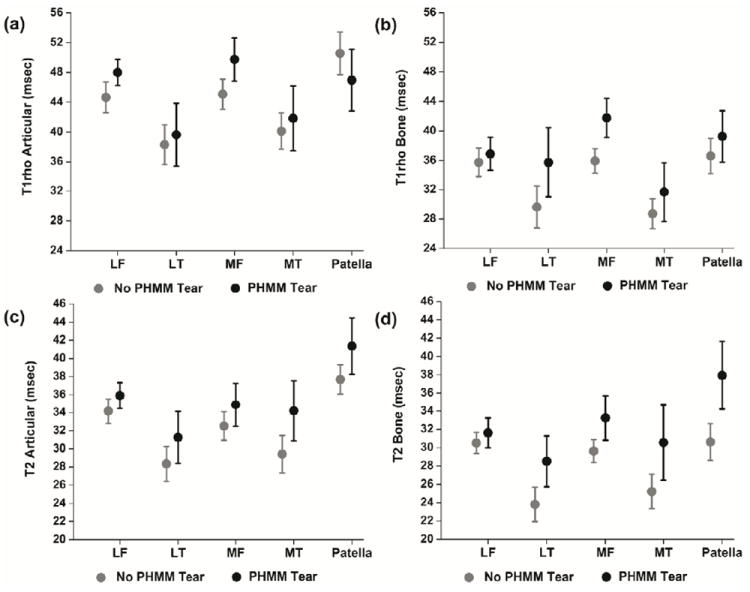
Means (95% CI) are shown in Figure 3. Results from the GEE model are in Table 4. There were significant interaction effects between group (PHMM tear vs. No tear) and compartment for T1ρ in the superficial (p < 0.001) and deep cartilage layers (p < 0.001). On analyzing each compartment separately, people with PHMM tears had higher T1ρ relaxation times in the deep cartilage layer for lateral tibia and medial femur only (Table 4). Also, T1ρ relaxation times were higher for the superficial articular layer of patella in subjects without PHMM tears (Table 4). None of the other differences were statistically significant (Table 4).
Figure 3.
Means (95% Confidence Intervals) for (A) articular (superficial) layer T1ρ relaxation times, (B) bone (deep) layer T1ρ relaxation times, (C) articular (superficial) layer T2 relaxation times and (D) bone (deep) layer T2 relaxation times. People with PHMM tears had lower superficial T1ρ for patella, and higher deep T1ρ for lateral tibia and medial femur, and higher deep T2 for all compartments.
Table 4.
Results from the GEE model for differences in articular cartilage T1ρ and T2 relaxation times in people with and without PHMM tears.
| Estimated Differences* | 95 % CI | p-value | ||
|---|---|---|---|---|
| T1ρ Articular (Superficial) Layer | Lateral Femur | -0.6 | -3.4, 2.1 | 0.648 |
| Medial Femur | -1.7 | -5.4, 1.9 | 0.348 | |
| Lateral Tibia | -0.2 | -4.5, 4.9 | 0.943 | |
| Medial Tibia | -0.162 | -5.1, 4.7 | 0.948 | |
| Patella | 6.5 | 1.9, 11.0 | 0.006 | |
| T1ρ Bone (Deep) Layer | Lateral Femur | -1.1 | -3.3, 5.5 | 0.623 |
| Medial Femur | -4.6 | -7,2, -2.0 | < 0.001 | |
| Lateral Tibia | -6.2 | -11.6, -0.9 | 0.023 | |
| Medial Tibia | -3.8 | -8.5, 0.8 | 0.105 | |
| Patella | -1.1 | -5.8, 3.6 | 0.650 | |
| T2 Superficial Layer | -1.4 | -3.2, 0.4 | 0.126 | |
| T2 Deep Layer | -2.8 | -4.9, -0.7 | 0.010 | |
All values are adjusted for age and disease presence.
T1ρ superficial and deep layer had significant interaction effects between group (PHMM tear vs. No tear) and hence the data are reported for each compartment. T2 superficial and deep layers did not have a significant interaction effect so data are reported across all compartments.
For T2 relaxation times, the interaction effect between group (PHMM tear vs. No tear) and compartment was not statistically significant for superficial (p = 0.27) or deep (p = 0.08) cartilage layer. Subsequent GEE model without the interaction term showed that people with PHMM tear had higher T2 relaxation times for the deep cartilage layer across all compartments (Table 4), while the difference between T2 relaxation times in the superficial cartilage layer were not statistically significant.
Discussion
The aim of this paper was to compare trabecular bone structural parameters at the peri-articular femur and tibia in subjects with and without tears of the posterior horn of medial meniscus. The results show that the subjects who have PHMM tears have global structural differences in the peri-articular trabecular bone consisting of decreased bone volume fraction and trabecular number and increased trabecular thickness and separation, even after accounting for age and presence of knee osteoarthritis. The results also showed that the differences in the articular cartilage T1ρ and T2 times are driven by the differences in the deep bone layer, indicating a role of the bone-cartilage interface in the pathogenesis of knee OA in people with PHMM tears. This study demonstrates the relationship between meniscal tears and trabecular bone structure and the findings have significance towards understanding the pathogenesis of post-traumatic knee osteoarthritis.
We found that people with PHMM tears show degradation of underlying trabecular bone in all compartments. Balanced interaction between all major knee tissues is critical to a healthy knee and a disruption of the balance could be related to the degenerative knee process. Early degenerative changes in the articular cartilage are characterized by a loss of proteoglycans and a disruption of the collagen matrix[28]. These changes can be detected by quantitative MR imaging of the articular cartilage T1ρ and T2 relaxation times. It has been shown the people with knee OA have elevated T1ρ and T2 relaxation times [12, 13, 29]. Furthermore, presence of cartilage or meniscal lesions is also associated with elevated quantitative MR parameters in the articular cartilage indicating degeneration [11, 30]. Finally, loss of cartilage thickness and increase in cartilage T1ρ and T2 relaxation time parameters has been shown to be related to degradation of underlying trabecular bone [14-16]. All these results highlight the importance of evaluating all major knee tissues to completely characterize the knee environment. Results from this study support and enhance the earlier findings, indicating that the degenerative cascade initiated due to meniscal tears also encompasses the underlying trabecular bone. The PHMM tear group had a larger number of individuals with knee OA and this was adjusted statistically in the analyses. To further investigate the effect of PHMM tear on trabecular bone structure, a sub-analysis (not shown) was performed comparing the trabecular bone parameters between people with and without PHMM tears, separately in control and knee OA groups. The results showed that the observed pattern of differences between those with and without PHMM tears, persisted in the control group, but not in the OA group. These findings provide additional evidence to support the speculation that the presence of PHMM tear might be causative of bone changes.
Meniscal tears are associated with risk of accelerated cartilage loss due to increase in contact stresses which can also affect the underlying bone. The trabecular bone structure of subjects with PHMM tears in this study primarily indicates bone resorption, with loss of bone volume, increased trabecular separation and decreased trabecular number. Menisci transmit 45-60% of the compressive load at the knee [31] by converting the axial compressive load into radial tensile strain through generation of hoop stress [32]. It has been shown that menisci can carry 45-70% of the knee load and a radial incision can lead to failure of generation of the hoop stresses [33]. Therefore a tear can lead to increase in the contact stresses by 40-700% [34-36]. Such high stress on the degenerated articular cartilage can lead to deterioration of the underlying cartilage at a rate more rapid than in knees without meniscal tears. In a sheep model, radial meniscal tears resulted in intense and fast OA in the articular surface next to the tear [37]. Other animal models have also shown similar results with tears, meniscectomies and repairs leading to OA [38, 39]. Trabecular bone also responds to changes in articular loading. Previous research has shown that altered loading patterns during walking seen in people with knee OA like high knee adduction moment are associated with increased bone subchondral bone area and density besides greater loss of medial knee cartilage [40, 41]. Also, high adduction moment has been shown to be associated with greater incidence of medial meniscus pathology [42]. It is possible that the sclerosis of the subchondral bone leads to stress shielding of the trabecular bone and result in trabecular bone degeneration. In 2000, Fukuda et al. reported findings from a study where they inserted mini-pressure transducers in cadaveric pig knees in the medial and lateral tibia at subchondral, epiphyseal and diaphysial levels [43]. They then recorded the loads transmitted to these areas in intact knees and knees with meniscectomies under static and impact loading with varus and valgus alignment. They found that meniscectomy, in presence of varus alignment, led to an 4-5.2 times increase in the compressive stress in the subchondral bone while the increase in the epiphyseal and diaphyseal regions was only doubled. They concluded that the meniscus mainly protects the underlying subchondral bone by load dissipation and attenuation. Based on these results, it could be hypothesized that with an increase in compressive load, the subchondral bone would remodel by becoming thicker and sclerotic. It is plausible that meniscal tears could lead to similar changes if the tear is severe enough to compromise the function of the meniscus. Traditionally, at least radial tears of the medial meniscus extending to the periphery have been considered functionally equivalent to a total meniscectomy due to the failure of generation of hoop stresses [44]. Hence, a meniscectomy, or a meniscal tear could lead to similar increases in the subchondral bone with consequent stress shielding of the underlying trabecular bone. Stress shielding of the trabecular bone could lead to bone resorption since the bone responds to over-load as well as unloading. In this study we found loss of bone volume fraction, increased trabecular thickness but decrease in number, and increased trabecular separation. These findings indicate bone attrition which support the idea of stress shielding.
Imaging studies have demonstrated increased bone volume fraction and trabecular thickness of medial tibia and decreased bone volume fraction, trabecular thickness and increase separation in lateral tibia, in people with knee OA [14, 16]. The authors hypothesized that overloading of medial compartment due to varus malalignment and unloading of the lateral compartment could be the reason for the observed pattern. Cartilage loss in the medial compartment was also found to be inversely related to trabecular bone changes in medial tibia and directly related to bone attrition in lateral tibia. A few studies have analyzed trabecular bone changes after meniscal pathology using destabilization of medial meniscus (DMM) animal models. DMM is achieved by transecting the medial menisco-tibial ligament. Botter et al. found that 8 weeks after the DMM surgery, there was an increase in the subchondral plate thickness but no changes were seen in the underlying trabecular bone as assessed by micro-CT imaging [45]. On the other hand, Moodie et al. used the DMM mice model to demonstrate that after 8 weeks of the surgery, the trabecular bone in medial tibia had lower app. BV/TV and higher app. Tb.Th compared to the contralateral extremity, indicating loss of trabecular bone [46]. The subchondral plate thickness was also increased. 3-dimensional micro-CT reconstruction showed extensive bone loss and remodeling in the tibiae. There was also a concomitant loss of medial tibial cartilage. Moodie et al, attributed the difference in the findings of their study and the one performed by Botter et al., to the difference in mice strains used. The authors also mentioned that the observed results could also be due to the DMM surgery having affected the ACL to varying extents. Our in-vivo study demonstrated similar findings as the animal study done by Moodie et al. with trabecular bone loss in the medial tibia. In fact, we found deterioration in not just the medial compartments but also in lateral compartments in both tibia and femur indicating a more global loss of trabecular bone structure. This could be due to the fact that our sample had subjects with all stages of knee osteoarthritis. Also our subjects with PHMM tears were not more malaligned compared to those without tears, which could also be a reason for similar changes in both compartments.
An earlier study showed that tears of the PHMM are associated with higher T1ρ and T2 relaxation times for the PHMM [11]. The authors did not evaluate the regional variation in cartilage T1ρ and T2 times in their study. We found that PHMM tears are associated with an increase of T1ρ in the deep layer of lateral tibia and medial femur, and an increase in T2 for deep cartilage layer of all compartments. Such differences were not seen for the superficial layer. Increase in T1ρ and T2 indicates loss of proteoglycans and collagen disruption, which based on the results from this study, appear to be more prominent in the bone layer. Taken together with the results from the trabecular bone analysis, these data suggest that there is an interaction between the underlying bone and cartilage in the pathogenesis of knee degeneration in people with PHMM tears. Another study had earlier shown that in subjects with anterior cruciate ligament (ACL) tears who had undergone reconstruction, the T1ρ times were elevated in the superficial layer of the weight-bearing areas of articular cartilage were increase an year after the surgery [47]. The authors did not find a difference in the deep layer. Hence, it is possible that different mechanisms are involved in the degeneration of the articular cartilage in people with ligament injuries and meniscal injuries. Ligaments are primarily responsible for load sharing at end range, proprioception and guiding motion but do not contribute to load dissipation. It has been shown that even after a year of ACL reconstruction knee arthrokinematics is not restored in the medial compartment when compared with the contralateral knee [48]. Subjects still demonstrated increased internal rotation of the tibia which could alter the loading patterns and also increase shear forces leading to greater damage to superficial cartilage layer. On the other hand, meniscal tears lead to increase in contact stresses which can cause changes in underlying bone and lead to damage of the cartilage starting in the deep layers. Further longitudinal research would be needed to confirm these speculations. The 2-6 msec differences in cartilage relaxation times are similar to previous reports comparing healthy and diseased cartilage, and people with and without knee pain, indicating the clinical significance of these findings [11, 29, 47, 49, 50].
The study has some limitations which need to be taken into consideration while interpreting the results. Since it’s a cross-sectional study, it is difficult to derive conclusions about causality regarding meniscal tears leading to trabecular bone changes. Additionally, information on weight-bearing physical activity levels, which we did not have, would enhance the interpretation of these findings since bone adapts to loading. Also, it is a relatively small sample size and the results would need to be replicated in larger samples with longitudinal data. Finally, the analyses focused on PHMM tears, and it is likely that tears in other parts of medial meniscus, and /or tears of lateral meniscus would be associated with different patterns of cartilage and trabecular bone changes. Due to the small sample size, we could not evaluate the relationship of different type of tears with trabecular bone parameters.
In conclusion, tears of the posterior horn of medial meniscus are associated with degeneration of the trabecular bone structure and of the deep layer of articular cartilage. Since knee OA is a multi-tissue disorder, it needs to be seen if surgical management of meniscal tears with repair, resection and biological supplementation leads to improvement of not only cartilage morphology but also underlying trabecular bone. These results also highlight the growing importance of studying the interactions of trabecular bone and cartilage in pathogenesis of knee OA in people with tears of posterior horn of medial meniscus.
Acknowledgments
The authors thank Zinta Zarins and S. Paran Yap for their technical support, and T. Munoz and M. Guan for their help in recruiting and consenting patients for the study. This study was supported by NIH RO1 AR46905.
Footnotes
AUTHOR CONTRIBUTIONS:
Conception and Design: Kumar, Link, Li, Majumdar
Acquisition of data: Kumar, Schooler, Zuo
Analysis and Interpretation of Data: Kumar, Schooler, Zuo, McCulloch, Nardo, Link, Li, Majumdar
Drafting of article or revising it critically for important intellectual content: Kumar, Schooler, Nardo, Zuo, McCulloch, Link, Li, Majumdar
Final approval of the version of the article to be published: Kumar, Schooler, Zuo, McCulloch, Nardo, Link, Li, Majumdar
Authors Kumar (Deepak.kumar@ucsf.edu) and Majumdar (sharmila.majumdar@ucsf.edu) take full responsibility for the integrity of this work as a whole.
CONFLICT OF INTEREST : No conflict of interest for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joseph Schooler, Email: Joseph.schooler@ucsf.edu.
Jin Zuo, Email: jinzuo@yahoo.com.
Charles E. McCulloch, Email: CMcCulloch@epi.ucsf.edu.
Lorenzo Nardo, Email: Lorenzo.nardo@ucsf.edu.
Thomas M. Link, Email: Thomas.link@ucsf.edu.
Xiaojuan Li, Email: Xiaojuan.li@ucsf.edu.
Sharmila Majumdar, Email: Sharmila.majumdar@ucsf.edu.
References
- 1.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41:687–93. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 3.Rockborn P, Gillquist J. Results of open meniscus repair. Long-term follow-up study with a matched uninjured control group. J Bone Joint Surg Br. 2000;82:494–8. doi: 10.1302/0301-620x.82b4.9942. [DOI] [PubMed] [Google Scholar]
- 4.Hede A, Larsen E, Sandberg H. The long term outcome of open total and partial meniscectomy related to the quantity and site of the meniscus removed. Int Orthop. 1992;16:122–5. doi: 10.1007/BF00180200. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale ME, McLaughlin S, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am. 2003;85-A:4–9. doi: 10.2106/00004623-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 7.Berthiaume MJ, Raynauld JP, Martel-Pelletier J, Labonte F, Beaudoin G, Bloch DA, et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005;64:556–63. doi: 10.1136/ard.2004.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bin SI, Kim JM, Shin SJ. Radial tears of the posterior horn of the medial meniscus. Arthroscopy. 2004;20:373–8. doi: 10.1016/j.arthro.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Smith JP, 3rd, Barrett GR. Medial and lateral meniscal tear patterns in anterior cruciate ligament-deficient knees. A prospective analysis of 575 tears. Am J Sports Med. 2001;29:415–9. doi: 10.1177/03635465010290040501. [DOI] [PubMed] [Google Scholar]
- 10.Kan A, Oshida M, Oshida S, Imada M, Nakagawa T, Okinaga S. Anatomical significance of a posterior horn of medial meniscus: the relationship between its radial tear and cartilage degradation of joint surface. Sports Med Arthrosc Rehabil Ther Technol. 2:1. doi: 10.1186/1758-2555-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarins ZA, Bolbos RI, Pialat JB, Link TM, Li X, Souza RB, et al. Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthritis Cartilage. 2010;18:1408–16. doi: 10.1016/j.joca.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, et al. Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011;29:324–34. doi: 10.1016/j.mri.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med. 2009;61:1310–8. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsey CT, Narasimhan A, Adolfo JM, Jin H, Steinbach LS, Link T, et al. Magnetic resonance evaluation of the interrelationship between articular cartilage and trabecular bone of the osteoarthritic knee. Osteoarthritis Cartilage. 2004;12:86–96. doi: 10.1016/j.joca.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Blumenkrantz G, Lindsey CT, Dunn TC, Jin H, Ries MD, Link TM, et al. A pilot, two-year longitudinal study of the interrelationship between trabecular bone and articular cartilage in the osteoarthritic knee. Osteoarthritis Cartilage. 2004;12:997–1005. doi: 10.1016/j.joca.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Bolbos RI, Zuo J, Banerjee S, Link TM, Ma CB, Li X, et al. Relationship between trabecular bone structure and articular cartilage morphology and relaxation times in early OA of the knee joint using parallel MRI at 3 T. Osteoarthritis Cartilage. 2008;16:1150–9. doi: 10.1016/j.joca.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986:34–40. [PubMed] [Google Scholar]
- 18.Day JS, Ding M, van der Linden JC, Hvid I, Sumner DR, Weinans H. A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J Orthop Res. 2001;19:914–8. doi: 10.1016/S0736-0266(01)00012-2. [DOI] [PubMed] [Google Scholar]
- 19.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraus VB, Vail TP, Worrell T, McDaniel G. A comparative assessment of alignment angle of the knee by radiographic and physical examination methods. Arthritis Rheum. 2005;52:1730–5. doi: 10.1002/art.21100. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Han ET, Busse RF, Majumdar S. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magn Reson Med. 2008;59:298–307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pai A, Li X, Majumdar S. A comparative study at 3 T of sequence dependence of T2 quantitation in the knee. Magn Reson Imaging. 2008;26:1215–20. doi: 10.1016/j.mri.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Folkesson J, Carballido-Gamio J, Eckstein F, Link TM, Majumdar S. Local bone enhancement fuzzy clustering for segmentation of MR trabecular bone images. Med Phys. 37:295–302. doi: 10.1118/1.3264615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majumdar S, Genant HK. A review of the recent advances in magnetic resonance imaging in the assessment of osteoporosis. Osteoporos Int. 1995;5:79–92. doi: 10.1007/BF01623308. [DOI] [PubMed] [Google Scholar]
- 26.Majumdar S, Newitt D, Jergas M, Gies A, Chiu E, Osman D, et al. Evaluation of technical factors affecting the quantification of trabecular bone structure using magnetic resonance imaging. Bone. 1995;17:417–30. doi: 10.1016/s8756-3282(95)00263-4. [DOI] [PubMed] [Google Scholar]
- 27.Carballido-Gamio J, Bauer JS, Stahl R, Lee KY, Krause S, Link TM, et al. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal. 2008;12:120–35. doi: 10.1016/j.media.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dijkgraaf LC, de Bont LG, Boering G, Liem RS. The structure, biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. J Oral Maxillofac Surg. 1995;53:1182–92. doi: 10.1016/0278-2391(95)90632-0. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789–97. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stahl R, Luke A, Li X, Carballido-Gamio J, Ma CB, Majumdar S, et al. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients--a 3.0-Tesla MRI study. Eur Radiol. 2009;19:132–43. doi: 10.1007/s00330-008-1107-6. [DOI] [PubMed] [Google Scholar]
- 31.Seedhom BB, Dowson D, Wright V. Proceedings: Functions of the menisci. A preliminary study. Ann Rheum Dis. 1974;33:111. doi: 10.1136/ard.33.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bullough PG, Munuera L, Murphy J, Weinstein AM. The strength of the menisci of the knee as it relates to their fine structure. J Bone Joint Surg Br. 1970;52:564–7. [PubMed] [Google Scholar]
- 33.Shrive NG, O’Connor JJ, Goodfellow JW. Load-bearing in the knee joint. Clin Orthop Relat Res. 1978:279–87. [PubMed] [Google Scholar]
- 34.Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14:270–5. doi: 10.1177/036354658601400405. [DOI] [PubMed] [Google Scholar]
- 35.Fukubayashi T, Kurosawa H. The contact area and pressure distribution pattern of the knee. A study of normal and osteoarthrotic knee joints. Acta Orthop Scand. 1980;51:871–9. doi: 10.3109/17453678008990887. [DOI] [PubMed] [Google Scholar]
- 36.Kurosawa H, Fukubayashi T, Nakajima H. Load-bearing mode of the knee joint: physical behavior of the knee joint with or without menisci. Clin Orthop Relat Res. 1980:283–90. [PubMed] [Google Scholar]
- 37.Burger C, Mueller M, Wlodarczyk P, Goost H, Tolba RH, Rangger C, et al. The sheep as a knee osteoarthritis model: early cartilage changes after meniscus injury and repair. Lab Anim. 2007;41:420–31. doi: 10.1258/002367707782314265. [DOI] [PubMed] [Google Scholar]
- 38.Hede A, Svalastoga E, Reimann I. Articular cartilage changes following meniscal lesions. Repair and meniscectomy studied in the rabbit knee. Acta Orthop Scand. 1991;62:319–22. doi: 10.3109/17453679108994461. [DOI] [PubMed] [Google Scholar]
- 39.Cummins JF, Mansour JN, Howe Z, Allan DG. Meniscal transplantation and degenerative articular change: an experimental study in the rabbit. Arthroscopy. 1997;13:485–91. doi: 10.1016/s0749-8063(97)90128-6. [DOI] [PubMed] [Google Scholar]
- 40.Creaby MW, Wang Y, Bennell KL, Hinman RS, Metcalf BR, Bowles KA, et al. Dynamic knee loading is related to cartilage defects and tibial plateau bone area in medial knee osteoarthritis. Osteoarthritis Cartilage. 18:1380–5. doi: 10.1016/j.joca.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 41.Thorp LE, Wimmer MA, Block JA, Moisio KC, Shott S, Goker B, et al. Bone mineral density in the proximal tibia varies as a function of static alignment and knee adduction angular momentum in individuals with medial knee osteoarthritis. Bone. 2006;39:1116–22. doi: 10.1016/j.bone.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Vanwanseele B, Eckstein F, Smith RM, Lange AK, Foroughi N, Baker MK, et al. The relationship between knee adduction moment and cartilage and meniscus morphology in women with osteoarthritis. Osteoarthritis Cartilage. 18:894–901. doi: 10.1016/j.joca.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Fukuda Y, Takai S, Yoshino N, Murase K, Tsutsumi S, Ikeuchi K, et al. Impact load transmission of the knee joint-influence of leg alignment and the role of meniscus and articular cartilage. Clin Biomech (Bristol, Avon) 2000;15:516–21. doi: 10.1016/s0268-0033(00)00013-9. [DOI] [PubMed] [Google Scholar]
- 44.Messner K, Gao J. The menisci of the knee joint. Anatomical and functional characteristics, and a rationale for clinical treatment. J Anat. 1998;193(Pt 2):161–78. doi: 10.1046/j.1469-7580.1998.19320161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Botter SM, Glasson SS, Hopkins B, Clockaerts S, Weinans H, van Leeuwen JP, et al. ADAMTS5-/- mice have less subchondral bone changes after induction of osteoarthritis through surgical instability: implications for a link between cartilage and subchondral bone changes. Osteoarthritis Cartilage. 2009;17:636–45. doi: 10.1016/j.joca.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 46.Moodie JP, Stok KS, Muller R, Vincent TL, Shefelbine SJ. Multimodal imaging demonstrates concomitant changes in bone and cartilage after destabilisation of the medial meniscus and increased joint laxity. Osteoarthritis Cartilage. 19:163–70. doi: 10.1016/j.joca.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2--initial experience with 1-year follow-up. Radiology. 2011;258:505–14. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carpenter RD, Majumdar S, Ma CB. Magnetic resonance imaging of 3-dimensional in vivo tibiofemoral kinematics in anterior cruciate ligament-reconstructed knees. Arthroscopy. 2009;25:760–6. doi: 10.1016/j.arthro.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 49.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 64:248–55. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolbos RI, Ma CB, Link TM, Majumdar S, Li X. In vivo T1rho quantitative assessment of knee cartilage after anterior cruciate ligament injury using 3 Tesla magnetic resonance imaging. Invest Radiol. 2008;43:782–8. doi: 10.1097/RLI.0b013e318184a451. [DOI] [PMC free article] [PubMed] [Google Scholar]


