Abstract
Objectives
To determine the histological patterns of posterior cruciate ligament (PCL) degeneration during aging and in relation to changes in articular cartilage and anterior cruciate ligament (ACL) across the entire adult age spectrum.
Methods
Human knee joints (n=120 from 65 donors) were processed within 72 hours postmortem. Articular cartilage surfaces were graded macroscopically. Each PCL was histologically evaluated for inflammation, mucinous changes, chondroid metaplasia, cystic changes and orientation of collagen fibers. Severity of PCL degeneration was classified as normal, mild, moderate or severe. PCL scores were compared to ACL and cartilage scores from the same knees.
Results
All knees had intact PCL. Histologically, 6% were normal, 76% showed mild, 12% moderate and 9% severe degeneration. Fiber disorientation was the most prevalent and severe change. Histological grades of PCL and ACL correlated but significantly fewer PCL than ACL showed severe changes. There was weaker correlation between aging and total histological PCL scores (R=0.26) compared to aging and ACL scores (R=0.42). ACL scores correlated with cartilage scores (R=0.54) while PCL scores increased with severity of OA from grades 0 to III but not between OA grades III to IV (R=0.32). In knees with ruptured ACL, the PCL scores correlated with cartilage scores of the lateral compartment.
Conclusions
PCL histopathological changes are less severe than in the ACL. PCL degeneration was associated with ACL and cartilage damage. The lack of correlation with age indicates independent pathways for PCL versus ACL degeneration.
Keywords: Posterior cruciate ligament, aging, osteoarthritis
INTRODUCTION
Osteoarthritis (OA), the most common joint disorder,[1] has a multifactorial etiology with systemic factors that predispose to the disease and local factors that determine its distribution and severity.[2] Aging and trauma are two of the strongest risk factors for OA.[2] It is not yet established if the OA process begins in a specific joint structure, but it is known that in later stages it manifests in all joint tissues.[3–6]
The posterior cruciate ligament (PCL) is an important knee structure. Together with the anterior cruciate ligament (ACL) they are essential for knee kinematics in antero-posterior tibial translation, tibial rotation, and as secondary restraints to valgus/varus forces.[7–9] Rupture of the PCL is most commonly associated with severe knee trauma and multiligament injury[10] but rarely occurs spontaneously or in isolation. This is in contrast to degeneration of the ACL, which has been reported to occur in up to 25% of the patients with severe knee OA.[4, 11] Besides the importance of the PCL in knee biomechanics, it is an important knee structure in patients with end stage OA undergoing total knee arthroplasty (TKA). Posterior cruciate ligament retaining TKA designs rely on the integrity of the PCL.[12]
Histological changes in the PCL are observed in knees undergoing TKA for severe OA.[11] These changes include mucoid degeneration, disorganization of the collagen fibers, and cystic changes.[11, 13] Moreover, during TKA a large number of knees without prior known trauma were diagnosed with ruptured or absent ACL whereas the PCL was macroscopically intact.[11] These knees are characterized by an increased severity of medial and lateral compartment OA[14]. The concomitant histopathological changes in the PCL and their interplay with changes in the ACL and cartilage are not well understood. Even less is known about intrisic age related changes in the PCL and their relationship with ACL and cartilage at early stages of the disease process.
The objectives of this study were to 1) identify patterns and severity of a comprehensive set of histological changes in the PCL from a large number of human knee joints across the entire adult age range and at all stages of cartilage degradation in donors with no history of prior joint trauma and 2) determine the correlation of changes in the PCL with the ACL and the articular cartilage.
MATERIALS AND METHODS
Procurement of human knee joints
Human knee joints were obtained from tissue banks with approval of the Scripps Human Subjects Committee. Joints were processed within 72 hours post-mortem. In this study, 120 human knee joints were analyzed from 30 male and 35 female donors, with a mean age of 66.7 ± 19 years (range, 23 – 92 years) and mean BMI 24.8 ± 7 (range, 12.5 – 43.8). The ethnic distribution of the donors was as follows: Hispanic (n=26), Caucasian (n=80), Black (n=2) and unknown (n=12). From 55 of 65 donors we obtained both knees. Subjects with a history of knee trauma or surgery were excluded.
Ligament analysis
Evaluation of the ligaments was performed as described previously.[11, 15–17] The PCL and the ACL were resected at the insertion sites on the femur and tibia. The macroscopic appearance of the ligaments was classified in 3 stages: normal, abnormal (thinner than normal and sclerotic), or ruptured.[15] For histology, the samples were immediately fixed in Z-Fix (Anatech, Battle Creek, MI). From each ACL and PCL two cuts were prepared and used for histological evaluation, a transverse cut at the proximal one third of the ligament and a longitudinal cut through the center of the ligament. The samples were from the proximal one third of the ACL substance and femur attachment site where ACL tears frequently occur.[18, 19] We used only the ligament region and not the nonmineralized interface, mineralized interface, or bone for histological analysis. Samples were embedded in paraffin. Four micrometer thick sections were cut and stained with hematoxylin and eosin (H&E).
The PCL and ACL sections were graded histologically using a modification of previous scoring systems.[11] The following categories were examined and scored for each ligament: 1) inflammation in the ligament substance, 2) mucoid degeneration, 3) chondroid metaplasia, 4) cystic changes and 5) orientation of collagen fibers. Each category was scored on both transverse and longitudinal sections and a single score was assigned for each ligament. The histological changes were scored and graded as follows: 0: no changes, 0.5: minimal changes, 1: mild changes, 2: moderate changes, 3: severe changes. The highest summed score of ligament degeneration (total PCL or ACL score) was 15 if all 5 histological categories were scored severe.[20]
The PCL were graded by two independent readers to assess intra-observer and inter-observer reliability. At the time of sample evaluation the readers were blinded and unaware of the age, sex and cartilage or ACL scores of the same knees. Each reader performed two readings of the entire sample set. The intra-class correlation of the first reader was 0.85, and 0.91 for the second reader. The inter-class correlation between readers was 0.87.
Morphologic analysis of articular cartilage
All cartilage surfaces (femoral condyles, trochlea and tibial plateaus) were graded macroscopically using a modified Outerbridge scoring system.[21] We established a more detailed scoring method based on the International Cartilage Repair Society (ICRS) knee map (Supplementary Figure 1).[22] The articular cartilage of the knee was divided into several compartments: 3 areas in the trochlea, and 9 areas each in each femoral condyle and tibial plateau. Each of these 39 areas was scored individually, and total compartment and total knee scores were calculated. For each area a score ranging from 1 – 4 was assigned as follows: 1=intact surface; 2=fibrillation; 3=fissuring; 4=erosion. The total knee cartilage scores range from 39 (normal) to 156 (maximum severity). For statistical analyses, the total knee cartilage scores were translated into grades 0–IV (grade 0: normal when total score was 39, grade I: minimal change when total score was 40 to 58, grade II: mild change when total score was 59 to 78, grade III: moderate change when total score was 79 to 97, and grade IV: severe change when total score was higher than 98). Donor distribution among the different cartilage grades was as follows: grade 0 (n=9), grade I (n=51), grade II (n=31), grade III (n=18) and grade IV (n=11).
Statistical analysis
Summary statistics are reported as mean ± standard deviation. Spearman’s nonparametric correlation coefficients were used to assess strengths of bivariate associations. 95% confidence intervals for the reported Spearman correlation coefficients were obtained from the bootstrap percentile method, based on 1000 samples. Levels of the confidence intervals are unadjusted, as our emphasis is on estimation of the strength of bivariate associations rather than inference. We used general linear models (GLMs) to investigate associations between total PCL scores and severity of macroscopic changes, and between total PCL and ACL scores. Similarly, we used GLMs to examine relationships between PCL and ACL histopathology and total cartilage scores, with age, gender, and BMI. In these latter analyses, gender was dichotomous, and age and BMI were continuous variables. Stratified paired t-tests were also used to compare component and total PCL and ACL scores; the stratification factor was either cartilage grade (O through IV) or separately age, categorized into quartiles. These analysis simultaneously allow assessment of how mean scores differ across the cartilage grades, or age quartiles. Two-sided p-values of .05 or less were considered indicative of statistical significance. We utilized a nonparmetric techinique, LOESS smoothing,[23] to depict the relationship between PCL, ACL, and cartilage scores with age. Here, we fitted first degree loess curves, with tricube kernel and 25 grid points. Calculations were performed in Stata 9.2 (Statacorp, College Station, TX) and SPSS 16.0 (SPSS Inc., Chicago, IL).
RESULTS
Relationship between PCL and ACL histopathology and age, gender, and BMI
On macroscopic evaluation all PCL in the 120 knees were intact. The mean total PCL and ACL histological scores were 3.82 ± 2.3 and 4.02 ± 2.7, respectively. The total PCL histological grades were as follows: 3% (n=4) categorized as normal, 76% (n=91) mild, 12% (n=14) moderate and 9% (n=11) severe. The total ACL histological grades were as follows: 3% (n=4) normal, 69% (n=83) mild, 15% (n=18) moderate and 13% (n=15) severe changes. Total PCL histological scores significantly increased with the severity of macroscopic ACL changes (normal, 3.39±1.89, n=68; abnormal, 4.24±2.59, n=40; ruptured, 4.83±2.72, n=12; F2,117 =3.21, p=0.044).
From multivariable GLM analyses, the total PCL histopathological scores differed significantly by gender and age, but not BMI (Supplementary Table 1A). In particular, mean PCL scores for females were significantly lower than mean PCL scores for males (females, 3.45±2.15; males, 4.28±2.18). In comparison, the total ACL histopathological scores, as well as the total cartilage scores, differed significantly by age, but not by gender or BMI (Supplementary Tables 1B, 1C). The trends of increasing total PCL, ACL, and cartilage scores with age are depicted in Figure 1. Total cartilage score shows the strongest correlation with age (Spearman’s r = 0.75, 95% CI 0.66 to 0.82), followed by total ACL score (r = 0.42, 95% CI 0.23 to 0.58) and then total PCL score (r = 0.26, 95% CI 0.08 to 0.42). The highest scores were seen in the 80–89 year old group for both ligaments (PCL 5±3, ACL 6.1±3.7). The total PCL score was consistently higher in each decade until 60 years. This trend reversed after age 60 where the mean total ACL score continued to increase more than the PCL score.
Figure 1.
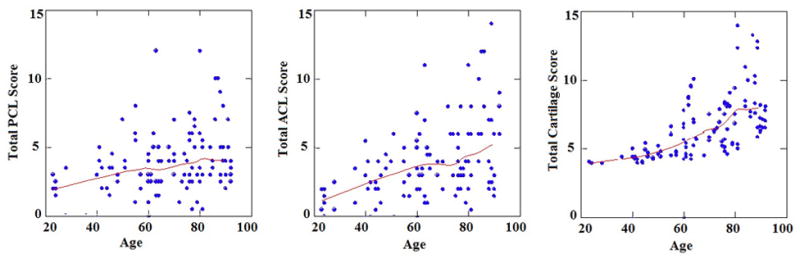
Bivariate scatterplots of total PCL, total ACL, and total cartilage scores, and associated ages. Also depicted are the LOESS regressions (smoothers), indicative of increasing scores with age. Spearman’s correlations r between age and total PCL (left panel), total ACL (middle panel), and total cartilage scores (right panel) are 0.26 (95% confidence interval 0.08 to 0.42), 0.42 (95% CI 0.23 to 0.58), and 0.75 (95% CI 0.66 to 0.82) respectively.
PCL, ACL and cartilage changes
Total knee cartilage scores were more strongly correlated with total ACL scores (Spearman’s r= 0.54, 95% CI 0.39 to 0.66) than with total PCL scores (r=0.32, 95% CI 0.13 to 0.49) (Figure 2). Both ACL scores and PCL scores differed significantly by grade (severity of cartilage changes): ACL, F4, 112 = 13.02, p<0.001; PCL, F4, 115 = 4.67, p=0.002. PCL scores increased steadily with the severity of cartilage changes from Grade 0 to Grade III, but there was no further increase in Grade IV, whereas ACL scores increased with each cartilage grade (Figure 2A). Components of ACL and PCL scores also tended to increase with increasing OA grade (Figure 2B–2F): inflammation, F4,111 = 2.63, p=0.038; mucoid, F4,111 = 4.34, p=0.027; chondroid, F4,111 = 2.54, p=0.044; cystic changes, F4,111 = 4.36, p=0.0026; fiber orientation, F4,111 = 11.10, p<0.001; total scores, F4,111 = 11.42, p<0.001. ACL but not PCL cystic change scores increased significantly at Grade IV compared to the less severe grades; and, ACL fiber orientation scores increased more rapidly than the corresponding PCL scores as cartilage grade severity increased.
Figure 2.
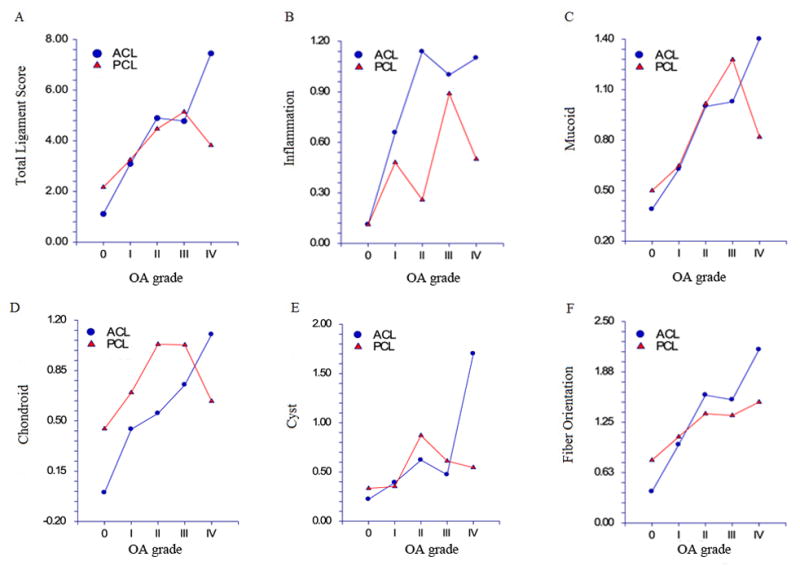
Total and specific PCL and ACL scores versus cartilage grade. Valuess depicted are the mean ligament scores at each specific cartilage grade, separately for PCL and ACL. Total PCL and ACL histological score (A), inflammation (B), mucoid changes (C), chondroid changes (D), cystic changes (E) and fiber orientation (F). Mean total and component ACL and PCL scores tended to increase with increasing OA grade: total scores, F4,111 = 11.42, p<0.001; inflammation, F4,111 = 2.63, p=0.038; mucoid, F4,111 = 4.34, p=0.027; chondroid, F4,111 = 2.54, p=0.044; cystic changes, F4,111 = 4.36, p=0.0026; fiber orientation, F4,111 = 11.10, p<0.001.
Correlation of PCL changes with knee compartmental cartilage scores
We determined if ligament degeneration was linked to local degeneration in specific knee compartments. PCL scores were weakly correlated with compartmental cartilage scores (medial femoral condyle r= 0.27, 95% CI 0.07 to 0.45; medial tibial plateau r=0.30, 95% CI 0.11 to 0.47; lateral femoral condyle r=0.24, 95% CI 0.06 to 0.41; lateral tibial plateau r=0.29, 95% CI 0.09 to 0.46; trochlea r=0.28, 95% CI 0.09 to 0.45) (Supplementary Figure 2). In contrast, ACL scores were more highly correlated with compartmental scores, specifically the medial compartment (medial femoral condyle r= 0.5, 95% CI 0.38 to 0.65; medial tibial plateau r=0.46, 95% CI 0.30 to 0.60; lateral femoral condyle r=0.48, 95% CI 0.33 to 0.60; lateral tibial plateau r=0.44, 95% CI 0.27 to 0.58; trochlea r=0.43, 95% CI 0.26 to 0.58).
Specific histological changes in the PCL and the ACL
When the ACL and PCL ligament scores were compared in a pairwise fashion, ACL inflammation scores were found to be significantly higher than the corresponding PCL scores (t116 = 3.63, p<0.001), and PCL chondroid metaplasia scores were significantly greater than their ACL counterparts (t115 = −2.71, p=0.009). No significant pairwise differences between ACL and PCL scores were found with inflammation, mucoid degeneration, fiber organization, or total scores. In Figure 3, the mean ACL and PCL ligament scores are depicted, stratified by age quartiles. Mucoid scores, cystic changes scores, orientation scores, as well as total scores, significantly increase with age (mucoid, F3,112 = 4.13, p=0.008; cystic changes, F3,112 = 3.82, p=0.012; orientation, F3,112 = 11.72, p<0.0001; total, F3,112 = 8.20, p<0.0001); inflammation scores and chondroid scores tended not to differ significantly across the age quartiles (inflammation, F3,112 = 1.45, p=0.23; chondroid, F3,112 = 1.01, p=0.39)
Figure 3.
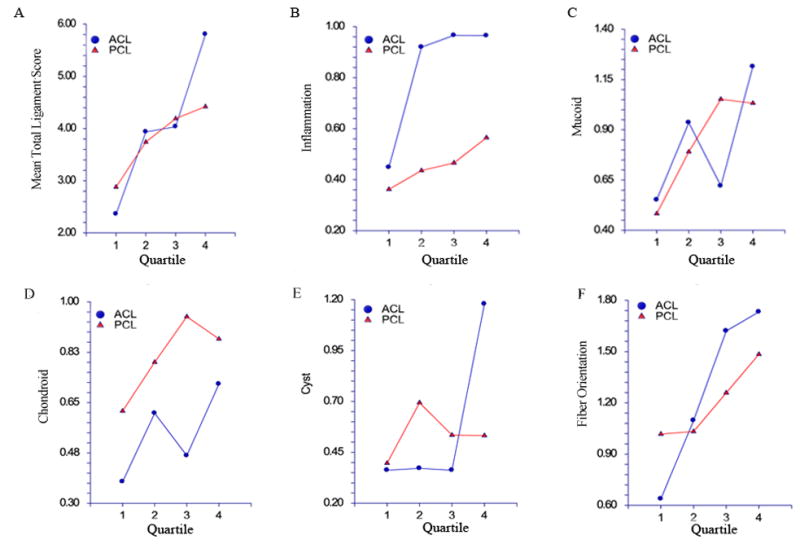
Total and specific PCL and ACL scores versus age. Ages were divided into 4 quartiles; within each quartile, means and associated 95% confidence intervals of total and specific histological scores are shown, separately for PCL (red) and ACL (blue). Total PCL and ACL histological score (A), inflammation (B), mucoid changes (C), chondroid changes (D), cystic changes (E) and fiber orientation (F). In paired analyses, ACL inflammation scores were found to be significantly higher than the corresponding PCL scores (t116 = 3.63, p<0.001), and PCL chondroid metaplasia scores were significantly greater than their ACL counterparts (t115 = −2.71, p=0.009). No significant pairwise differences between ACL and PCL scores were found with inflammation, mucoid degeneration, fiber organization, or total scores.
Inflammation
Among the different types of histopathological changes, PCL inflammation was the least apparent with an average score of 0.46 ± 0.7 and only 9 knees (8%) demonstrated moderate to severe inflammation. By contrast the average ACL inflammation score was 0.82 ± 1 and 30 knees (25%) demonstrated a moderate to severe inflammatory changes. PCL inflammation score did not significantly correlate with any of the other PCL histological criteria with the exception of PCL orientation (r=0.33, 95% CI 0.18 to 0.48), donor age, total cartilage score, or ACL inflammation score. The PCL scores were lower than the ACL scores in all OA groups, especially in OA grade II (mean PCL 0.3±0.4 mean ACL 1.1 ± 1.1) (Figure 2B).
Mucoid degeneration
Mucoid degeneration was observed in 71% of the PCL and correlated strongly with PCL chondroid metaplasia (r=0.73, 95% CI 0.59 to 0.83). When compared to the ACL mucoid score across cartilage grades, the PCL mucoid degeneration score was higher than the ACL scores in cartilage grades 0–III (Figure 2C).
Chondroid metaplasia
Chondroid metaplasia was observed in 75% of the PCL and correlated well with PCL mucoid degeneration (r=0.73, 95% CI 0.59 to 0.83). With respect to age, chondroid metaplasia scores increased continuously up to 6th decade, followed thereafter by slightly decreased and constant scores. The average chondroid metaplasia scores for the PCL were highest for OA grades II, and III. On the other hand, ACL chondroid metaplasia scores increased consistently with cartilage damage. PCL chondroid metaplasia scores were higher than in ACL for cartilage grade 0–III but less in cartilage grade IV (Figure 2D).
Cystic changes
Cystic changes were observed in PCL 38% of PCL. The PCL cystic score did not significantly correlate with age, other PCL histological changes, total cartilage score, or total ACL score. The PCL cystic score was equal or slight higher for cartilage grade 0–III as compared to the ACL scores. For cartilage grade IV the ACL scored more (mean 1.7 ± 1.1) than the PCL (mean 0.5 ± 0.7). Again, the ACL score for cartilage grade IV was higher than the PCL score (Figure 2E).
Collagen fiber organization
Disorganization of collagen fibers was observed in 94% of PCL. The average PCL fiber disorganization score was the highest among the different histopathological criteria (mean 1.2 ± 0.7). The PCL fiber disorganization score was significantly correlated with age (r=0.24, 95% CI 0.08 to 0.40), knee cartilage score (r=0.29, 95% CI 0.10 to 0.47), PCL inflammation score (r=0.33, 95% CI 0.18 to 0.48), and PCL mucoid score (r=0.20, 95% CI 0.01 to 0.39). With respect to age, the PCL scores were higher than the ACL up to the 6th decade, and thereafter the ACL scores were higher. PCL fiber disorganization scores increased with OA severity and were higher than the ACL for OA grade 0, but thereafter the ACL scores were higher than PCL scores. Among the different criteria for PCL evaluation, fiber disorientation was the only parameter to increase continuously with increasing cartilage grades (Figure 2F).
Aging related changes in PCL, ACL and cartilage
To identify the earliest changes in the PCL we selected 9 knees from donors younger than 45 years with normal cartilage (grade 0). In this subset the PCL histological scores were compared to the ACL scores (Figure 4). The first changes observed in the PCL were fiber disorganization, mucoid and chondroid changes. In order to differentiate between changes due to aging and those due to OA-like cartilage changes we divided knees from donors older than 60 years into 2 groups. The first group -cartilage grade I-II (n=66), representing the changes occurring during normal aging; while the second group with cartilage grade III-IV (n=29), representing knees with OA. Of the 66 knees with cartilage grade I-II the PCL histological grade was classified as 0% normal, 74% mild, 15% moderate and 11% severe. The ACL from the same knees were classified as 2% normal, 45% mild, 14% moderate and 12% severe. Therefore it appears that during aging process both ligaments have equal severity score. On the other hand, of the 29 knees with cartilage grade III-IV the PCL histological grade was classified as 69% mild, 14% moderate and 17% severe. ACL from the same knees were classified as 45% mild, 28% moderate and 28% severe grade. Therefore during arthritis progression more histopathological changes are observed in the ACL than in the PCL.
Figure 4.
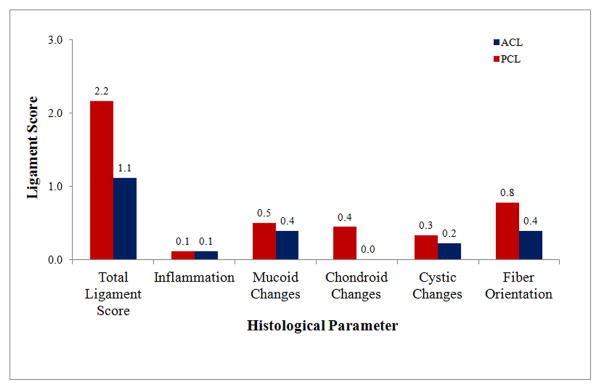
Total and specific PCL and ACL scores in donors younger than 45 years (n=9) with normal cartilage (grade 0).
DISCUSSION
Although disruption and loss of the articular cartilage is a hallmark of knee OA, the disease process results in changes in other knee tissues such as ligaments, menisci, subchondral bone and synovial membrane.[24–27] Moreover there is the unresolved question of the differences in patterns of tissue changes taking place during normal aging versus patterns observed in OA. To better understand changes in the cruciate ligaments with aging and OA, we conducted a comprehensive analysis of major histological patterns of PCL pathology in 120 knees across the adult age spectrum. Overall, our findings indicate that PCL pathology in the context of knee OA is very distinct from ACL pathology.
The study has several limitations it is a cross-sectional study in which there is a risk for reverse causality. Although there was a statistical significance between the deferent OA groups, the sample size in between groups was not equal and was determined by donor availability. Due to multiple testing there is a potential for low power. Some donor information, such as the extent of lower extremity malalignment was not available.
PCL rupture
In the present post-mortem study all PCL were intact and the ACL were ruptured in 10% of the knees. Loss of the ACL in severe OA is attributed to mechanical impingement[28] and ligament abrasion against osteophytes.[18] As the PCL is not typically subjected to impingement and abrasion in knee OA, this may in part account for the less severe changes.
PCL degeneration and aging
The PCL begins to degenerate early but does not continue to degenerate with aging. PCL changes, particularly fiber disorganization, mucoid degeneration, and chondroid metaplasia, were the earliest to occur, even before degeneration in the articular cartilage was observed. The PCL histopathological scores were higher than the ACL scores in donors up to age 50 beyond which ACL scores became more severe. This suggests that PCL changes reflect a milder degenerative process, which is not profoundly accelerated by aging. The severity of the PCL histological changes also did not correlate with the age of patients with end stage knee OA.[29] Over age 50, PCL degeneration is more associated with the presence of cartilage damage rather than age.
Two groups of knees demonstrated distinct patterns of PCL pathology. Knees from patients older than 60 years with high-grade cartilage damage had significantly worse total PCL scores compared to the knees in the same age group with lower-grade cartilage damage. The second subset was knees with ruptured ACL. In those knees, the PCL scores were significantly higher than in knees with intact ACL. The divergent histopathologic changes in the ACL and the PCL indicate independent pathologic mechanisms.
PCL histology and correlation to cartilage degeneration
The PCL histological scores were lower in knees with cartilage grade IV compared to grade III resulting in a poor correlation with cartilage grade. In contrast, the ACL histological scores from the same donors increased continuously with increasing cartilage grades. Moreover, there was no correlation between the total PCL score and the cartilage scores of the specific knee compartments. ACL tears have been associated with an increase in lateral compartment OA,[14] presumably due to shift in the contact stress posteriorly and laterally on the surface of the tibia on MRI and fluoroscopy.[30] In dogs, transection of the PCL resulted in less severe articular cartilage degeneration and osteophyte formation than transection of the ACL.[31] PCL degeneration may contribute less to overall joint pathology than ACL degeneration.
Histological changes in PCL versus ACL
Changes in collagen fibrils affect the biomechanical properties of the ligament[32–34] and there is a decrease in collagen fibrils in the PCL in individuals older than 60.[35] In our study, fiber disorganization was the earliest and most common change in the extracellular matrix. It was also the most common histopathological change in patients younger than age 45 with normal appearing cartilage and was more common in the PCL than the ACL. It was also the only histopathological change in the PCL that increased continuously with the changes in the articular cartilage. In contrast, there was a weak correlation between PCL fiber disorganization and donor age. The ACL exhibited overall higher levels of fiber disorientation, which did correlate with donor age.
Chondroid metaplasia, a shift in ligament cell phenotype towards a more chondrocytic morphology and cartilaginous matrix[36, 37] was noted in 75% of the PCL. Mucoid changes represent degeneration of collagen and replacement with glycosaminoglycans.[38] While clinically symptomatic mucoid degeneration is a rare condition[39] it has previously been described histologically in patients undergoing knee arthroplasty[13, 40] and been visualized by MRI in a small number of cases.[41] Based on the present findings, mucoid degeneration appears to gradually develop, first appearing in the fifth decade, with minimal increase in severity thereafter. Overall PCL inflammation scores were lower than all other histopathological changes and correlated little with age, OA grade, or any other histopathological measure. Inflammation was lower in the PCL relative to the ACL in all the age groups and in all levels of cartilage degeneration. This suggests that the inflammation in the PCL is not likely to be a significant factor in aging, degeneration, or OA.
Supplementary Material
Supplementary Table 1. Associations between total PCL scores, total ACL scores, and total cartilage scores and the factors gender, age, and BMI, from general linear model analyses. Numerator and denominator degrees of freedom for the F ratios are 1 and 112 respectively for the total PCL and total cartilage analyses, and 1 and 109 respectively for the total ACL analyses. In these general linear models (GLM analyses), gender is a dichotomous variable, and age and BMI are continuous.
Supplementary Figure 1. Macroscopic grading of cartilage.
A) ICRS map
B) Modified Outerbridge classification
C) Cartilage grading
Scattergrams of total PCL scores with total cartilage scores and specific knee compartmental cartilage scores. Spearman correlations r of total PCL scores with the other scores are: total cartilage, r=0.34, 95% CI 0.16 to 0.50; medial femoral condyle, r= 0.27, 95% CI 0.07 to 0.45; medial tibial plateau, r=0.30, 95% CI 0.11 to 0.47; lateral femoral condyle, r=0.24, 95% CI 0.06 to 0.41; lateral tibial plateau, r=0.29, 95% CI 0.09 to 0.46; trochlea, r=0.28, 95% CI 0.09 to 0.45.
Acknowledgments
Lilo Creighton and Melissa Szeto are gratefully acknowledged for their support for the histology of the specimens.
This study was supported by the National Institutes of Health (AG007996), Donald and Darlene Shiley, and by an Osteoarthritis Research Society International Scholarship Award (YDL).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the manuscript and all authors approved the final version. Dr. Lotz had full access to all data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. D’Lima, Lotz, Levy, Koziol
Acquisition of data. Levy, Hasegawa, Patil
Analysis and interpretation of data. Koziol, Levy, Lotz, D’Lima
References
- 1.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–69. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett LD, Buckland-Wright JC. Meniscal and articular cartilage changes in knee osteoarthritis: a cross-sectional double-contrast macroradiographic study. Rheumatology (Oxford) 2002;41:917–23. doi: 10.1093/rheumatology/41.8.917. [DOI] [PubMed] [Google Scholar]
- 4.Hill CL, Seo GS, Gale D, et al. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005;52:794–9. doi: 10.1002/art.20943. [DOI] [PubMed] [Google Scholar]
- 5.Bergin D, Keogh C, O’Connell M, et al. Atraumatic medial collateral ligament oedema in medial compartment knee osteoarthritis. Skeletal Radiol. 2002;31:14–8. doi: 10.1007/s002560100418. [DOI] [PubMed] [Google Scholar]
- 6.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365:965–73. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 7.Grood ES, Stowers SF, Noyes FR. Limits of movement in the human knee. Effect of sectioning the posterior cruciate ligament and posterolateral structures. J Bone Joint Surg Am. 1988;70:88–97. [PubMed] [Google Scholar]
- 8.Fleming BC. Biomechanics of the anterior cruciate ligament. J Orthop Sports Phys Ther. 2003;33:A13–5. [PubMed] [Google Scholar]
- 9.Woo SL, Livesay GA, Engle C. Biomechanics of the human anterior cruciate ligament. Muscle stabilization and ACL reconstruction. Orthop Rev. 1992;21:935–41. [PubMed] [Google Scholar]
- 10.Voos JE, Mauro CS, Wente T, et al. Posterior Cruciate Ligament: Anatomy, Biomechanics, and Outcomes. Am J Sports Med. 2011 doi: 10.1177/0363546511416316. [DOI] [PubMed] [Google Scholar]
- 11.Mullaji AB, Marawar SV, Simha M, et al. Cruciate ligaments in arthritic knees: a histologic study with radiologic correlation. J Arthroplasty. 2008;23:567–72. doi: 10.1016/j.arth.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Scott RD, Volatile TB. Twelve years’ experience with posterior cruciate-retaining total knee arthroplasty. Clin Orthop Relat Res. 1986:100–7. [PubMed] [Google Scholar]
- 13.Kleinbart FA, Bryk E, Evangelista J, et al. Histologic comparison of posterior cruciate ligaments from arthritic and age-matched knee specimens. J Arthroplasty. 1996;11:726–31. doi: 10.1016/s0883-5403(96)80012-x. [DOI] [PubMed] [Google Scholar]
- 14.Stein V, Li L, Lo G, et al. Pattern of joint damage in persons with knee osteoarthritis and concomitant ACL tears. Rheumatol Int. 2011 doi: 10.1007/s00296-010-1749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allain J, Goutallier D, Voisin MC. Macroscopic and histological assessments of the cruciate ligaments in arthrosis of the knee. Acta Orthop Scand. 2001;72:266–9. doi: 10.1080/00016470152846592. [DOI] [PubMed] [Google Scholar]
- 16.Cushner FD, La Rosa DF, Vigorita VJ, et al. A quantitative histologic comparison: ACL degeneration in the osteoarthritic knee. J Arthroplasty. 2003;18:687–92. doi: 10.1016/s0883-5403(03)00256-0. [DOI] [PubMed] [Google Scholar]
- 17.Trompeter AJ, Gill K, Appleton MA, et al. Predicting anterior cruciate ligament integrity in patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2009;17:595–9. doi: 10.1007/s00167-008-0701-0. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy JC, Weinberg HW, Wilson AS. The anatomy and function of the anterior cruciate ligament. As determined by clinical and morphological studies. J Bone Joint Surg Am. 1974;56:223–35. [PubMed] [Google Scholar]
- 19.Wang IE, Mitroo S, Chen FH, et al. Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. J Orthop Res. 2006;24:1745–55. doi: 10.1002/jor.20149. [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa A, Otsuki S, Pauli C, et al. Anterior cruciate ligament changes in human joint in aging and osteoarthritis. Arthritis Rheum. 2012;64:696–704. doi: 10.1002/art.33417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752–7. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 22.ICRS Cartilage Evaluation Package. 2000 http://www.cartilage.org/_files/contentmanagement/ICRS_evaluation.pdf.
- 23.Cleveland WS. Robust Locally Weighted Regression and Smoothing Scatterplots. Journal of the American Statistical Association. 1979;74:829–36. [Google Scholar]
- 24.Kaeding CC, Pedroza AD, Parker RD, et al. Intra-articular findings in the reconstructed multiligament-injured knee. Arthroscopy. 2005;21:424–30. doi: 10.1016/j.arthro.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Fithian DC, Paxton LW, Goltz DH. Fate of the anterior cruciate ligament-injured knee. Orthop Clin North Am. 2002;33:621–36. v. doi: 10.1016/s0030-5898(02)00015-9. [DOI] [PubMed] [Google Scholar]
- 26.O’Connor DP, Laughlin MS, Woods GW. Factors related to additional knee injuries after anterior cruciate ligament injury. Arthroscopy. 2005;21:431–8. doi: 10.1016/j.arthro.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Pauli C, Grogan SP, Patil S, et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage. 2011;19:1132–41. doi: 10.1016/j.joca.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ireland ML, Ballantyne BT, Little K, et al. A radiographic analysis of the relationship between the size and shape of the intercondylar notch and anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2001;9:200–5. doi: 10.1007/s001670100197. [DOI] [PubMed] [Google Scholar]
- 29.Albert A, Forthomme JP, Vandenhooft A, et al. Are lesions of the posterior cruciate ligament predictable before knee arthroplasty? A histological study of 434 ligaments in osteoarthritic knees. Acta Orthop Belg. 2008;74:652–8. [PubMed] [Google Scholar]
- 30.Li G, Moses JM, Papannagari R, et al. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88:1826–34. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- 31.Pournaras J, Symeonides PP, Karkavelas G. The significance of the posterior cruciate ligament in the stability of the knee. An experimental study in dogs. J Bone Joint Surg Br. 1983;65:204–9. doi: 10.1302/0301-620X.65B2.6826632. [DOI] [PubMed] [Google Scholar]
- 32.Bosch U, Decker B, Moller HD, et al. Collagen fibril organization in the patellar tendon autograft after posterior cruciate ligament reconstruction. A quantitative evaluation in a sheep model. Am J Sports Med. 1995;23:196–202. doi: 10.1177/036354659502300212. [DOI] [PubMed] [Google Scholar]
- 33.Oxlund H, Andreassen TT. The roles of hyaluronic acid, collagen and elastin in the mechanical properties of connective tissues. J Anat. 1980;131:611–20. [PMC free article] [PubMed] [Google Scholar]
- 34.Viidik A, Danielson CC, Oxlund H. On fundamental and phenomenological models, structure and mechanical properties of collagen, elastin and glycosaminoglycan complexes. Biorheology. 1982;19:437–51. doi: 10.3233/bir-1982-19305. [DOI] [PubMed] [Google Scholar]
- 35.Sargon MF, Doral MN, Atay OA. Age-related changes in human PCLs: a light and electron microscopic study. Knee Surg Sports Traumatol Arthrosc. 2004;12:280–4. doi: 10.1007/s00167-003-0427-y. [DOI] [PubMed] [Google Scholar]
- 36.Petersen W, Tillmann B. Structure and vascularization of the cruciate ligaments of the human knee joint. Anat Embryol (Berl) 1999;200:325–34. doi: 10.1007/s004290050283. [DOI] [PubMed] [Google Scholar]
- 37.Petersen W, Tillmann B. Blood and lymph supply of the posterior cruciate ligament: a cadaver study. Knee Surg Sports Traumatol Arthrosc. 1999;7:42–50. doi: 10.1007/s001670050119. [DOI] [PubMed] [Google Scholar]
- 38.Lintz F, Pujol N, Boisrenoult P, et al. Anterior cruciate ligament mucoid degeneration: a review of the literature and management guidelines. Knee Surg Sports Traumatol Arthrosc. 2011;19:1326–33. doi: 10.1007/s00167-011-1433-0. [DOI] [PubMed] [Google Scholar]
- 39.Shoji T, Fujimoto E, Sasashige Y. Mucoid degeneration of the posterior cruciate ligament: a case report. Knee Surg Sports Traumatol Arthrosc. 2010;18:130–3. doi: 10.1007/s00167-009-0885-y. [DOI] [PubMed] [Google Scholar]
- 40.Nelissen RG, Hogendoorn PC. Retain or sacrifice the posterior cruciate ligament in total knee arthroplasty?. A histopathological study of the cruciate ligament in osteoarthritic and rheumatoid disease. J Clin Pathol. 2001;54:381–4. doi: 10.1136/jcp.54.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viana SL, Fernandes JL, Mendonca JL, et al. Diffuse intrasubstance signal abnormalities of the posterior cruciate ligament: the counterpart of the mucoid degeneration of the anterior cruciate ligament? A case series. JBR-BTR. 2008;91:245–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Associations between total PCL scores, total ACL scores, and total cartilage scores and the factors gender, age, and BMI, from general linear model analyses. Numerator and denominator degrees of freedom for the F ratios are 1 and 112 respectively for the total PCL and total cartilage analyses, and 1 and 109 respectively for the total ACL analyses. In these general linear models (GLM analyses), gender is a dichotomous variable, and age and BMI are continuous.
Supplementary Figure 1. Macroscopic grading of cartilage.
A) ICRS map
B) Modified Outerbridge classification
C) Cartilage grading
Scattergrams of total PCL scores with total cartilage scores and specific knee compartmental cartilage scores. Spearman correlations r of total PCL scores with the other scores are: total cartilage, r=0.34, 95% CI 0.16 to 0.50; medial femoral condyle, r= 0.27, 95% CI 0.07 to 0.45; medial tibial plateau, r=0.30, 95% CI 0.11 to 0.47; lateral femoral condyle, r=0.24, 95% CI 0.06 to 0.41; lateral tibial plateau, r=0.29, 95% CI 0.09 to 0.46; trochlea, r=0.28, 95% CI 0.09 to 0.45.