Abstract
Background
Transcranial near-infrared laser therapy (TLT) improves behavioral outcome in animal stroke models when applied as single treatment within the 24 h of the stroke onset. It is unknown if multiple TLT treatments have an added beneficial effect. We aim to determine whether multiple irradiations with TLT would have further improvement in behavioral outcomes in the rabbit small clot embolic stroke model (RSCEM).
Methods
Using the RSCEM, two and three TLT treatments (7.5–20 mW/cm2) were compared against single laser treatment alone (7.5–10.8 mW/cm2). Two sham irradiation groups were added for control curves. The double treatment group received TLT at 3hrs and 5hrs and the triple treatment group at 2hr, 3hrs and 4hrs after embolization. Behavioral analysis was conducted 24 hours after embolization using a dichotomized behavioral score. Determination of the effective clot amount (mg) that produces neurological deficits in 50% of the rabbits (P50) was used to compare TLT treatments with sham.
Results
The P50 for double treatment was 5.47±0.90, n=39, the corresponding P50 value for a single treatment was 3.87±0.73, n=38, and the corresponding control curve was 3.25±0.4, n=32. The P50 for triple treatment was 5.91±0.49, n=23, the corresponding P50 value for a single treatment was 3.09±0.59, n=15, and the corresponding control curve was 1.71±0.26, n=17. Triple treatment had a 91% of improvement when compared with single treatment and 245% improvement when compared with sham.
Conclusions
The present study suggests that additional TLT treatments provide further behavioral improvement when given during the acute ischemic stroke phase.
Keywords: Acute ischemic stroke, Lower level laser therapy, Neuroprotection, Photobiology
INTRODUCTION
Low-level laser therapy (LLLT) uses low-powered laser light, at wavelengths from approximately 600–1100 nm to induce a photochemical reaction in the cell, without generating heat, by a process referred to as biostimulation or photobiomodulation (1).
Transcranial infrared laser therapy (TLT) is a form of LLLT that is able to penetrate the skull at full strength and is capable of photostimulate the brain tissue located few centimeters below the skull (2).
The effects of TLT in acute ischemic stroke have been widely study. Lapchak et al used the rabbit small clot embolic stroke model (RSCEM) to demonstrate long term behavioral improvement with TLT when treating animals at 3 and 6 hours after embolization (3). Using the middle cerebral artery occlusion (MCAO) rat model, Oron et al demonstrated that TLT applied at 24 hours after the ischemic injury produced a significant improvement of neurological severity score as compared with controls when measured 14 days after the stroke (4). De Taboda et al replicated these results in the same rat MCAO model (5). Likewise, human studies have showed safety and favorable effects when TLT is applied as single treatment within the 24 hours of the stroke onset (6,7). NeuroThera Effectiveness and Safety Trials (NEST) 1 and 2 demonstrated safety for human treatment in acute ischemic stroke (6,7) and the pooled analysis of both trials showed efficacy of TLT treatment for reduction in long-term disability measured by the modified Rankin Scale (mRS) (8).
Different treatment times, irradiation doses and energy levels of TLT tested in acute ischemic stroke models have frequently been successful in reaching good outcomes (3–6,9,10). However, the optimal timing and irradiation dose for acute ischemic stroke has not yet been established. Some studies have shown an incremental benefit of LLLT with higher irradiation power and energy levels. But applying additional LLLT energy can have minimal or no effect on the target tissue due to the biphasic response of LLLT (11). Further irradiation can even be detrimental to the tissue itself due to thermal effect (12).
Most of neuroprotective therapies tested in animals have shown better efficacy when used early in the ischemic injury process, eventually losing their efficacy as time from onset to initiation of therapy increases (13). In contrast TLT appears to be equally effective when applied at different times within the first 24hrs of the ischemic brain injury. It is possible; therefore, that TLT has the potential to photomodulate at different stages of the brain ischemic cascade. We postulate that multiple treatments of TLT during the acute ischemia will act synergically enhancing furthermore the behavioral outcome. The purpose for this study was to determine whether a second and a third irradiation treatment with TLT during the ischemic phase of the stroke would result in additional improvement in behavioral outcomes using the RSCEM.
METHODS
All the animal procedures used in this study were approved by the Department of Veterans Affairs and the Veterans Administration San Diego Healthcare System) and met the guidelines of the National Institutes of Health. Male New Zealand white rabbits (Western Oregon Rabbit Co. Philomath, OR) weighing 2 kg to 2.5 kg were used for the experiments.
All surgical procedures and microemboli preparation were done as described previously (14). Briefly, rabbits were anesthetized with isoflurane through a facemask, 5% in 3 L/min at induction, and 3% in 3 L/min as a maintenance dose. The right internal carotid artery was exposed, and the external carotid artery and the common carotid artery were ligated. Additional branches seen originating from the internal carotid artery were also ligated. A catheter oriented toward the brain was inserted into the common carotid and secured with ligatures. The incision was closed around the catheter and the catheter was filled with heparinized saline and plugged with injection caps. Animals were allowed to recover from anesthesia for at least 2 hours before embolization. Microemboli were prepared from blood drawn from a donor rabbit and allowed to clot at 37°C for 3.5hrs. The clot was suspended in Dulbecco's PBS solution containing 0.1% bovine serum albumin and fragmented with a Polytron. Clot fragments were sized by sequential filtration through 240μm2 screen and 100μm2 nylon net. The clots retained by the nylon net were washed, suspended again in PBS and allowed to settle. The supernatant was then removed and the particles were mixed with tracer quantities of 15μm radio-labeled microspheres. An aliquot of particles was then removed for determination of specific activity. Appropriate volumes of PBS solution were then added to the particles so that a predetermined weight of particles was suspended in 1 mL, which was drawn into a syringe. Clot particles were rapidly injected through the carotid catheter and then flushed with 3 mL of normal saline. After the embolization process was complete, the catheter was ligated close to the neck, and the remaining exposed catheter and injection ports were cut off. Before returning the animals to the cages they were observed continuously for a minimum of 1 hour after the treatments. Surviving animals were euthanized 24 hours postembolization with 1 to 1.5 mL of Beuthanasia-D through the marginal ear vein.
Transcranial Laser Therapy and Treatment Allocation
Rabbits were randomly allocated into treatment groups before embolization, with concealment of the randomization sequence until all behavioral and postmortem analyses were complete. For the TLT treatments, rabbits were placed in a Plexiglas restrainer for the duration of the treatment and the laser probe was placed in direct contact with the skin overlying the skull.
An Acculaser low-energy laser, wavelength of 808.5 nm, fitted with an OZ Optics Ltd fiber optic cable and laser probe measuring 2 cm in diameter was used.
To assess the effect of multiple doses of TLT treatment during the acute ischemia two sets of experiments were planned using the RSCEM. A first group of rabbits were randomized to double TLT treatment with a power dose of 7.5mW/cm2 given at 3hrs and 5hrs or a single TLT given at 3hrs. After the completion of the first group of experiments with the double laser treatment, we adjusted settings for the triple laser dose experiments increasing the power and narrowing the treatment times. In these second group animals were randomly assigned triple TLT treatments at 2hrs, 3hrs and 4hrs post embolization or single TLT at 2hr post embolization. We used 10.8mW/cm2 for the single therapy and 20mW/cm2 for the triple therapy group. The irradiation treatment duration was 2 minutes for each dose in all laser experiments. Independent control curves were obtained for each set of experiments. Behavioral analysis was conducted after 24 hours after embolization. Each animal was rated as either normal or abnormal/dead using a dichotomized behavioral score, by an examiner blinded to original treatment assignment. Behaviorally normal rabbits did not have any signs of impairment, whereas behaviorally abnormal rabbits had loss of balance, head leans, circling, seizure-type activity, or limb paralysis. Total radioactivity in the brain was measured by placing small pieces of the dissected brain into a portable gamma counter and the amount of radiolabel present in the brain were compared with that contained in the labeled blood clot before embolization. If fewer than 10% of the total counts were found in the brain, it was assumed that the labeled blood clot had not reached the brain and the animal was excluded from analyses.
Quantal Response Analysis
Briefly, to evaluate the quantitative relationship between number of clots in the brain and neurological deficits, logistic S-shaped curves were fitted by computer to the quantal dose–response data. These variables are measures of the amount of milligrams of microclot that produce neurologic dysfunction in 50% of animals in a group (P50). A separate curve is generated for each TLT treatment group and a statistically significant increase in the P50 value compared with control is indicative of a behavioral improvement. The data were analyzed using the t test, which included the Bonferroni correction when appropriate.
RESULTS
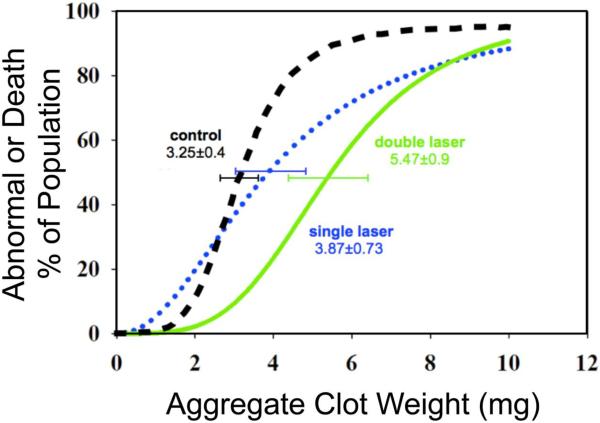
In the first randomized group of rabbits, the P50 for the sham at 5 h after embolization was 3.25±0.49 mg, slope 4.84±2.06, n=32. Rabbits treated 3 h post-embolization with a single TLT had P50 values of 3.87±0.73mg, slope 2.13±0.89, n=38. TLT double treatment, given at 3hrs and 5hrs of the stroke onset significantly increased the P50 by 69% compared to sham; P50: 5.47±0.90, slope 3.76±1.78 n=39. Double laser therapy had a non-significant increase of 41% in animal behavior when compared with the single laser treatment. (Figure 1)
Figure 1. Double Laser Curves.
The P50 for normal controls rabbits given at 3hrs is 3.25±0.4 (black curve), and for the single laser is 3.87±0.73 (blue dotted curve). The double laser (green curve) significantly shifted the P50 to 5.47±0.90, n=39. P= 0.01 vs. controls
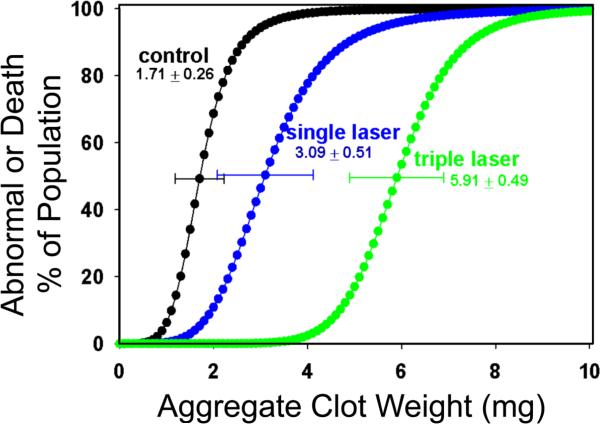
In the second randomized group of rabbits the P50 of the sham at 24 h after embolization was 1.71±0.26 mg, slope 5.02 ±3.13, n= 17. Animals treated 2hr post embolization with TLT had P50 values of 3.09±0.51, slope 4.83 ± 2.74, n=15. Triple TLT treatment given at 2, 3 and 4hrs post embolization, significantly increase the P50 to 5.91±0.49, slope 9.46 ± 5.45, n=23 which was 265% improvement when compared with sham and 91% of improvement compared with the single treatment. (Figure 2)
Figure 2. Triple Laser Curves.
The P50 for normal control rabbits (shown as black curve, n=17) is 1.71 0.26 mg, and single laser treatment (blue curve, n=15) significantly shifted P50 to 3.09 0.51 mg, P=0.02 vs. control. Triple laser treatment (green curve, n=23) further increased P50 to 5.91 0.49 mg, P=0.01 vs. single treatment.
DISCUSSION
Using the RSCEM, we found an effective enhancement of behavioral outcome with additional treatments of laser therapy during the acute brain ischemic phase. Such better outcome was coupled with the total laser energy dose applied to the animals. When the triple treatment was applied there was a 245% of behavioral improved compared to the controls. Such beneficial incremental treatment response has not been observed with any neuroprotective agent tested on the RSCEM, including recombinant tissue plasminogen activator (t-PA). (Table 1) We noted during the experiments that some animals, which were embolized with very high clot weight, surprisingly survived. These animals are usually expected to die from clot burden a few hours after embolization, but when given a triple laser dose, they had a normal behavior 24hrs after the embolization. In our experience, such treatment response using the RSCEM has only been observed after treatment with tPA.
TABLE 1.
Neuroprotective effect of agents tested on the RSCEM.
| Treatment | Time of treatment | P50 increment | Reference |
|---|---|---|---|
| t-PA | 1 hr. | 145% | (14) |
| TNK | 1 hr. | 144% | (22) |
| NXY-059 | 5 min | 144% | (23) |
| Memantine | 1 hr. | 180% | (24) |
| Edaravone | 3 hr. | 133% | (25) |
| Laser Single 25mW/cm2 | 1 hr. | 108% | (3) |
| Laser Double 7.5mW/cm2 |
2 & 5hr. | 68% | Current Study |
| Laser Triple 20mW/cm2 | 2, 3 & 4hr. | 245% | Current study |
t-PA: tissue plasminogen activator, TNK: Tenecteplase, NXY: Disodium-[(tert-butylimino)methyl]benzene-1,3-disulfonate N-Oxide
Although the neuroprotective effects of LLLT in acute brain ischemia are not fully understood, research progress in cellular and molecular biology has revealed some of its mechanisms of actions. LLLT is absorbed by the mitochondrial cytochrome c oxydase causing an upregulation of the cellular respiratory chain. A host of downstream cellular responses involving nitric oxide, reactive oxygen species, adenosine monophosphate and increase of ATP ensues, which ultimately might dictate the LLLT effects (1).
Lapchak et al using the same RSCEM have confirmed an increase of ATP production after TLT in the rabbit ischemic brain (9). Such increase appears to be energy dose dependent (9). However, LLLT studies have also showed a bell shaped curve response to light therapy with detrimental effects at higher energies (11). High energy levels could initiate a bigger increase in ROS and a decrease in mitochondrial membrane potential, which can induce damage mitochondria and lead to toxicity (15).
It seems plausible that the improvement of the rabbit behavioral outcome was not only due to the delivery of higher quantities of LLLT energy but also by the photostimuation of different biological events during the ischemic cascade itself. In general, the temporal profile of the main pathophysiological mechanism underlying the acute focal brain ischemia begins with cellular bioenergetic failure followed by excitotoxicity, oxidative stress, blood brain barrier dysfunction, and post ischemic inflammation (16). Augmentation of ATP by LLLT not only provides a bioenergetic relief but it can also modulate separate biological reactions.
Several other different mechanism of LLLT photoactivation has been postulated. Under hypoxic conditions the cytochrome C oxidase reduces nitrite to nitric oxide (NO) (17). Such reaction is also enhanced by LLLT increasing the local production of NO (18) improving the local cerebral blood flow (CBF) in the brain ischemic tissue (19). Other ischemic stroke animal models have shown that LLLT also decreases apoptosis (20), leads to the production of endogenous neurotrophic factors and enhancing recovery of function via neurogenesis (4,21).
Single TLT dose has shown an improvement in outcomes in different animal models (3,5) and in human clinical trials (6,7). Conversely, in our first set of experiments, despite the apparent incremental effect of the double treatment, single laser treatment was not significant better than the sham. While there was considerable variability in the outcome of the animals within the similar clot weight, we believe that mainly the use lower power density of 7.5mW/cm2 accounted for such results. After we observed that a double TLT irradiation dose safely improved animal outcome, we increased the LLLT power settings and reduced the treatment times for the triple TLT treatments experiments. The change of the setting for the triple dose might be considered a methodological limitation in our study. However the decision of changing the settings and irradiation times was made to obtain a more robust response of the multiple laser treatments. Yet, our study was not designed to define the maximal optimal dose of radiation or total energy tolerated by the ischemic brain. Neither did we set out to determine the specific number of treatments at specific treatment times that could result in optimal behavioral outcomes. This work is currently planned.
Based on the preliminary data from animal studies and the successful NEST 1 and 2 studies, the current phase III clinical trial of TLT, NEST-3, uses a single dose of TLT of approximately 10mW/cm2. Based on our results, higher irradiation settings and multiple treatments could be safely applied to humans during acute ischemia leading to further clinical improvement. To move forward to new clinical trials, our studies need to be replicated and the questions raised above have to be addressed in future animal studies.
CONCLUSIONS
In the present study, incremental treatments with TLT significantly improve behavioral outcome in this stroke animal model. Further studies are needed to improve TLT treatment times, irradiation and energy levels for optimal human acute ischemic stroke TLT treatment.
Acknowledgments
GRANT SUPPORT: NIH Grant: SPOTRIAS 5P50NS044148 to UCSD Stroke Center.
Photothera Inc. supplied the laser for use in the studies and supplying partial funding.
Footnotes
DISCLOSURES J.A.Z. is the principal investigator for NEST-III clinical trial and is on the Photothera Scientific Advisory Board.
B.H.G., Y.C.M., B.C.M., and G.M.T. have nothing to disclose.
REFERENCES
- 1.Karu TI. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB life. 2010;62(8):607–610. doi: 10.1002/iub.359. [DOI] [PubMed] [Google Scholar]
- 2.Stemer AB, Huisa BN, Zivin JA. The evolution of transcranial laser therapy for acute ischemic stroke, including a pooled analysis of NEST-1 and NEST-2. Current cardiology reports. 2010;12(1):29–33. doi: 10.1007/s11886-009-0071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lapchak PA, Wei J, Zivin JA. Transcranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbits. Stroke; a journal of cerebral circulation. 2004;35(8):1985–1988. doi: 10.1161/01.STR.0000131808.69640.b7. [DOI] [PubMed] [Google Scholar]
- 4.Oron A, Oron U, Chen J, Eilam A, Zhang C, Sadeh M, Lampl Y, Streeter J, DeTaboada L, Chopp M. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke; a journal of cerebral circulation. 2006;37(10):2620–2624. doi: 10.1161/01.STR.0000242775.14642.b8. [DOI] [PubMed] [Google Scholar]
- 5.Detaboada L, Ilic S, Leichliter-Martha S, Oron U, Oron A, Streeter J. Transcranial application of low-energy laser irradiation improves neurological deficits in rats following acute stroke. Lasers in surgery and medicine. 2006;38(1):70–73. doi: 10.1002/lsm.20256. [DOI] [PubMed] [Google Scholar]
- 6.Zivin JA, Albers GW, Bornstein N, Chippendale T, Dahlof B, Devlin T, Fisher M, Hacke W, Holt W, Ilic S, Kasner S, Lew R, Nash M, Perez J, Rymer M, Schellinger P, Schneider D, Schwab S, Veltkamp R, Walker M, Streeter J. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke; a journal of cerebral circulation. 2009;40(4):1359–1364. doi: 10.1161/STROKEAHA.109.547547. [DOI] [PubMed] [Google Scholar]
- 7.Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, Borenstein P, Andersson B, Perez J, Caparo C, Ilic S, Oron U. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke; a journal of cerebral circulation. 2007;38(6):1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 8.Huisa B, Stemer A, Walker M, Rapp K, Meyer B, Zivin J. Transcranial laser therapy for acute ischemic stroke: a pooled analysis of NEST-1 and NEST-2. International journal of stroke : official journal of the International Stroke Society. 2012 doi: 10.1111/j.1747-4949.2011.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5'-triphosphate (ATP) content following embolic strokes in rabbits. Brain research. 2010;1306:100–105. doi: 10.1016/j.brainres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Lapchak PA, Salgado KF, Chao CH, Zivin JA. Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: an extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience. 2007;148(4):907–914. doi: 10.1016/j.neuroscience.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose-response : a publication of International Hormesis Society. 2009;7(4):358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilic S, Leichliter S, Streeter J, Oron A, DeTaboada L, Oron U. Effects of power densities, continuous and pulse frequencies, and number of sessions of low-level laser therapy on intact rat brain. Photomedicine and laser surgery. 2006;24(4):458–466. doi: 10.1089/pho.2006.24.458. [DOI] [PubMed] [Google Scholar]
- 13.Zivin JA. Factors determining the therapeutic window for stroke. Neurology. 1998;50(3):599–603. doi: 10.1212/wnl.50.3.599. [DOI] [PubMed] [Google Scholar]
- 14.Zivin JA, Lyden PD, DeGirolami U, Kochhar A, Mazzarella V, Hemenway CC, Johnston P. Tissue plasminogen activator. Reduction of neurologic damage after experimental embolic stroke. Archives of neurology. 1988;45(4):387–391. doi: 10.1001/archneur.1988.00520280033012. [DOI] [PubMed] [Google Scholar]
- 15.Sharma SK, Kharkwal GB, Sajo M, Huang YY, De Taboada L, McCarthy T, Hamblin MR. Dose response effects of 810 nm laser light on mouse primary cortical neurons. Lasers in surgery and medicine. 2011;43(8):851–859. doi: 10.1002/lsm.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clinical neurology and neurosurgery. 2009;111(6):483–495. doi: 10.1016/j.clineuro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell metabolism. 2006;3(4):277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Ball KA, Castello PR, Poyton RO. Low intensity light stimulates nitrite-dependent nitric oxide synthesis but not oxygen consumption by cytochrome c oxidase: Implications for phototherapy. Journal of photochemistry and photobiology B, Biology. 2011;102(3):182–191. doi: 10.1016/j.jphotobiol.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Uozumi Y, Nawashiro H, Sato S, Kawauchi S, Shima K, Kikuchi M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers in surgery and medicine. 2010;42(6):566–576. doi: 10.1002/lsm.20938. [DOI] [PubMed] [Google Scholar]
- 20.Liang HL, Whelan HT, Eells JT, Wong-Riley MT. Near-infrared light via light-emitting diode treatment is therapeutic against rotenone- and 1-methyl-4-phenylpyridinium ion-induced neurotoxicity. Neuroscience. 2008;153(4):963–974. doi: 10.1016/j.neuroscience.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung MC, Lo SC, Siu FK, So KF. Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1. Lasers in surgery and medicine. 2002;31(4):283–288. doi: 10.1002/lsm.10096. [DOI] [PubMed] [Google Scholar]
- 22.Lapchak PA, Araujo DM, Zivin JA. Comparison of Tenecteplase with Alteplase on clinical rating scores following small clot embolic strokes in rabbits. Experimental neurology. 2004;185(1):154–159. doi: 10.1016/j.expneurol.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Lapchak PA, Araujo DM, Song D, Wei J, Zivin JA. Neuroprotective effects of the spin trap agent disodium-[(tert-butylimino)methyl]benzene-1,3-disulfonate N-oxide (generic NXY-059) in a rabbit small clot embolic stroke model: combination studies with the thrombolytic tissue plasminogen activator. Stroke; a journal of cerebral circulation. 2002;33(5):1411–1415. doi: 10.1161/01.str.0000015346.00054.8b. [DOI] [PubMed] [Google Scholar]
- 24.Lapchak PA. Memantine, an uncompetitive low affinity NMDA open-channel antagonist improves clinical rating scores in a multiple infarct embolic stroke model in rabbits. Brain research. 2006;1088(1):141–147. doi: 10.1016/j.brainres.2006.02.093. [DOI] [PubMed] [Google Scholar]
- 25.Lapchak PA, Zivin JA. The lipophilic multifunctional antioxidant edaravone (radicut) improves behavior following embolic strokes in rabbits: a combination therapy study with tissue plasminogen activator. Experimental neurology. 2009;215(1):95–100. doi: 10.1016/j.expneurol.2008.09.004. [DOI] [PubMed] [Google Scholar]