Abstract
Immunohistochemical studies previously revealed the presence of the peptide transmitter N-acetylaspartylglutamate (NAAG) in spinal motor neurons, axons and presumptive neuromuscular junctions (NMJ). At synapses in the CNS, NAAG has been shown to activate the type 3 metabotropic glutamate receptor (mGluR3) and is inactivated by an extracellular peptidase, glutamate carboxypeptidase II (GCPII). The present study tested the hypothesis that NAAG meets the criteria for classification as a cotransmitter at the vertebrate NMJ. Confocal microscopy confirmed the presence of NAAG immunoreactivity and extended the resolution of the peptide's location in the lizard (Anolis carolinensis) NMJ. NAAG was localized to a presynaptic region immediately adjacent to postsynaptic acetylcholine receptors. NAAG was depleted by potassium-induced depolarization and by electrical stimulation of motor axons. The NAAG receptor, mGluR3, was localized to the presynaptic terminal consistent with NAAG's demonstrated role as a regulator of synaptic release at central synapses. In contrast, glutamate receptors, mGluR2 and NMDA, were closely associated with acetylcholine receptors in the postsynaptic membrane. GCPII, the NAAG inactivating enzyme, was identified exclusively in perisynaptic glial cells. This localization was confirmed by the loss of immunoreactivity when these cells were selectively eliminated. Finally, electrophysiological studies showed that exogenous NAAG inhibited evoked neurotransmitter release by activating a group II metabotropic glutamate receptor (mGluR2 or mGluR3). Collectively, these data support the conclusion that NAAG is a co-transmitter at the vertebrate NMJ.
Keywords: vertebrate neuromuscular junction, N-acetylaspartylglutamate, NAAG, mGluR3, GCPII, NMDA, Anolis carolinensis
Introduction
N-Acetylaspartylglutamate (NAAG) is found throughout the mammalian nervous system and meets the standard criteria of a bona fide neurotransmitter (reviewed in Neale et al., 2000; 2005; 2011). It is concentrated in synaptic vesicles and acts as a co-transmitter with the classical amine neurotransmitters, glutamate, GABA and acetylcholine (Neale et al., 2005). Considerable direct and indirect evidence have established that NAAG activates type 3 metabotropic glutamate receptor (mGluR3) in vitro (neurons, astrocytes and transfected cells) (Wroblewska et al., 1997; 1998; 2006; Neale, 2011) and in vivo (Olszewski et al., 2012). Although it has been reported to activate NMDA receptors, more rigorous analysis indicates that this is not the case (Losi et al., 2004; Fricker et al., 2009). In contrast, NAAG is efficiently hydrolyzed to N-acetylaspartate and L-glutamate by the enzyme glutamate carboxypeptidase II (GCPII), which is expressed exclusively on the extracellular face of astrocytic glial cells in the mammalian central nervous system and perisynaptic Schwann cells in the periphery (Berger, Carter, McKee, et al., 1995; Berger et al., 1999).
Acetylcholine (ACh) has long been recognized as the primary neurotransmitter at the vertebrate neuromuscular junction (NMJ), activating muscle cells via nicotinic receptors. In addition to this primary activity of ACh, the actions of several additional molecules have been described at the NMJ, including the co-transmitter ATP (Redman & Silinsky, 1994), Substance P (Bourque & Robitaille, 1998), nitric oxide (Lindgren & Laird, 1994; Thomas & Robitaille, 2001), the endocannabinoid 2-arachidonylgycerol (Newman et al., 2007), and glutamate (Pinard et al., 2003; Pinard & Robitaille, 2008).
Although glutamate has been shown to be present at the NMJ (Waerhaug & Ottersen, 1993), along with glutamate transporters (Pinard et al., 2003; Boulland et al., 2004; Rinholm et al., 2007) and ionotropic (Berger, Carter, & Coyle, 1995; Grozdanovic & Gossrau, 1998; Todd et al., 2004) and metabotropic glutamate receptors (Pinard et al., 2003), the source of synaptic glutamate and its mechanism of release or production has not been established. The discovery of NAAG-like immunoreactivity in motor neurons and ventral root axons and at the mammalian NMJ along with that of its hydrolyzing enzyme, GCPII (Ory-Lavollée et al., 1987; Moffett et al., 1993; Berger, Carter, & Coyle, 1995), suggests that NAAG might be a source of glutamate at this synapse. In addition, the discovery that a member of the group II metabotropic glutamate receptor, mGluR3, is the NAAG receptor offers the possibility that some of the modulatory effects attributed to glutamate at the NMJ may instead be mediated directly by NAAG.
The present study was carried out to determine whether NAAG is a co-transmitter at the vertebrate NMJ. The Ceratomandibularis muscle in the lizard, Anolis carolinensis, was specifically chosen for this study because of its accessibility for electrophysiology and microscopy (Lindgren & Moore, 1989; David et al., 1997; Lindgren et al., 1997; Graves et al., 2004; Newman et al., 2007). The data presented here establish the synaptic localization of NAAG, group II metabotropic glutamate receptors (mGluR2 and mGluR3), NMDA receptors, and GCPII at the vertebrate NMJ. We also show that depolarization depletes NAAG from presynaptic motor nerve terminals and that the application of exogenous NAAG inhibits evoked ACh release, with the latter effect dependent on a group II metabotropic glutamate receptor.
Methods
Dissection and Solutions
Lizards were anesthetized by briefly lowering body temperature in a −20 C freezer. They were then quickly decapitated and the lower jaw muscle, the Ceratomandibularis, was exposed and placed in normal lizard Ringers (158 mM NaCl, 2 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 5 mM HEPES, and 2 g/L d-glucose, pH 7.3). Muscle and connective tissue were cleared so that only a single layer of innervated muscle remained. For NAAG depletion studies, the preparations were incubated for 30 min in different [K+] Ringers solutions during NAAG staining (low = 0.5mM KCl, 0mM CaCl2; normal = 2mM KCl, 2mM CaCl2; high = 60mM KCl, 2mM CaCl2) while osmolarity was kept constant by modifying [NaCl]. For all electrophysiological measurements, the lizard Ringers solution was modified slightly; the concentration of CaCl2 was reduced from 2.0 mM to 1.6 mM (to reduce the probability of neurotransmitter release) and the concentration of NaCl was increased by 0.6 mM (to maintain normal osmolarity. The resulting composition was as follows: 158.6 mM NaCl, 2 mM KCl, 2 mM MgCl2, 1.6 mM CaCl2, 5 mM HEPES, and 2 g/L d-glucose, pH 7.3).
The Institutional Animal Use and Care Committees at Grinnell College and Georgetown University approved all of the procedures used in this research.
Perisynaptic Schwann Cell Ablation
Ceratomandibularis muscles were rinsed thoroughly with normal Ringers and incubated in anti-2A12 antibodies (1:100 dilution, antibody kindly provided by Dr. Chien-Ping Ko, Department of Biological Sciences, University of Southern California, Los Angeles, CA) for 3 hours at room temperature. The primary antibody was rinsed off using normal lizard Ringers solution followed by incubation with guinea pig complement (Innovative Research; Novi, MI) for 1.5 hours at 30°C. To assay ablation success, α-bungarotoxin (1:200; Invitrogen) and ethidium-homodimer-1 (1:1000; Invitrogen) were applied for 30 minutes at room temperature. To remove cellular material remaining from the ablated Schwann cells, collagenase (1 mg/ml) was applied for 30 minutes followed by 5 minutes of high tilt perfusion using normal lizard ringers solution (i.e. the perfusion dish was tilted 80° from the horizontal). The vitality of the nerve-muscle preparation was confirmed by electrically stimulating the nerve and observing full muscle twitching.
Presynaptic Staining
For back-loading the presynaptic nerve endings, the cut end of the motor axon was placed into a well containing a 10 mM solution (pH 7.0) of dextran (3,000 MW)-conjugated to either Alexa Fluor 555 or Alexa Fluor 594 (Invitrogen) overnight at 8°C to allow the dextran to fill the axons and the nerve terminals by anterograde transport. The tissue was then incubated in Ringers solution at 4° C for 3 hrs to allow for additional transport of dextran from the axons into the nerve terminals. The tissues were then processed with an immuno target as described below.
To stain the readily recyclable pool of synaptic vesicles, 8μM AM1-44 (Biotium Inc., Hayward, CA) was added to the solution bathing the tissue; the nerve was subsequently stimulated at 0.5 Hz for 48 sec. A standard FITC cube was used to quantify AM1-44 in stained preparations.
Protein Immunohistochemical Staining
Ceratomandibularis muscles (both ablated and non-ablated) were fixed in 3% paraformaldehyde (Sigma) and permeabilized in 0.3% Triton X-100. To reduce non-specific staining, the tissue was then blocked in 1% Bovine Serum Albumin for 1 hr. Tissue was incubated in the appropriate primary antibody followed by Alexa-Fluor 350, FITC, or TRITC-conjugated secondary antibody (1:500, Invitrogen). The muscle was then removed from its bone and cartilage attachments and mounted on slides with SlowFade +/− DAPI (Invitrogen).
Primary antibodies included: rabbit anti-NAAG (Moffett & Namboodiri, 1995; Olszewski et al., 2012); goat anti-mGluR2 (1:300; Santa Cruz) and anti-mGluR3 (1:300; Santa Cruz); mouse anti-NMDAR1 (1:200; Millipore); rabbit anti-GCPII (1:100; Aviva); and rabbit anti GCPIII prepared against a peptide containing 221 amino acids directly downstream from the transmembrane region of mouse GCPIII (this antibody does not cross react with membranes from C6 cells stably transfected with GCPII, DeStefano and Bzdega, unpublished). Co-localization molecules used were alpha-bungarotoxin TRITC conjugated (1:200, Invitrogen) and Yoyo-1 (1:2000, Invitrogen).
Peptide Immunohistological Staining
Using a tyramide signal amplification kit (Invitrogen), muscles first had their peptides cross-linked using 6% EDAC and 4% NHS dissolved in DMSO and diluted in Ringers without glucose. The tissue was then fixed in 3% Paraformaldehyde (Sigma) and permeabilized in 0.3% Triton X-100, blocked in 1% Bovine Serum Albumin for 30 min, followed by blocking in TSA Blocking Solution for 1 hour at 30°C. Tissue was incubated in NAAG antibody (Moffett and Namboodiri, 1995) followed by HRP conjugated immunoglobulin secondary antibody (1:200 in Blocking Solution, American Qualex) for 2 hours at room temp. After rinsing, tyramide amplification buffer was applied with TRITC or FITC conjugated anti-dinitrophenyl antibody (0.0015% H2O2, 1:100 in tyramide solution in amplification buffer) for 20 min. The tissues were then rinsed and alpha-bungarotoxin conjugated to TRITC (1:200, Invitrogen) was applied for 20 min. Muscles were removed from their bone and cartilage attachments and mounted on slides with SlowFade (Invitrogen).
Microsopy and Digital Imaging
All images were collected using a 60× oil immersion objective (n.a. 1.4) on an Olympus IX81 microscope with a DSU confocal attachment (disk #2) and an Orca-ER digital CCD camera (Hamamatsu Photonics, Japan). The following filter sets were used to image fluorophores: (i) a standard FITC filter set (Ex 470/90 nm; DM 495 nm; Em 525/50 nm) for FITC, Alexa 488 or YOYO-1, (ii) a standard TRITC filter set (Ex 545/30 nm; DM 570 nm; Em 620/60 nm) for TRITC or Alexa Fluor 555, (iii) a Texas Red filter set (Ex 560/40 nm; DM 585 nm; Em 630/75 nm) for Alexa Fluor 594 or Texas Red, and (iv) a DAPI filter set (Ex 350/50 nm; DM 400 nm; Em 460/50 nm) for DAPI or Alexa Fluor 350. All of the images were analyzed using SlideBook (Intelligent Imaging Innovations, Inc.; Denver, CO). Some of the images were further processed for 3D rendering using Metamorph (Molecular Devices, Inc., Sunnyvale, CA). For all figures in which an image collected using DIC optics was superimposed onto images collected using epifluorescence, the DIC image was shifted slightly (5 pixels) from the epifluorescence image to compensate for the offset created by a 45° mirror in the filter turret. This offset was calibrated previously using prepared slides containing structures that can be unambiguously identified using either DIC or epifluorescence.
Electrophysiology
Evoked end-plate potentials (EPPs) and spontaneous miniature end-plate potentials (MEPPs) were recorded using a glass micropipette filled with 3 M K Acetate (resistance 30–70 MΩ). Membrane potentials were amplified with a Model 1600 Neuroprobe Amplifier (A-M Systems, Sequim, WA), filtered with a HumBug noise eliminator (Quest Scientific, North Vancouver, BC, Canada), and digitized with a Powerlab data acquisition system (ADInstruments, Colorado Springs, CO). EPPs were evoked by stimulating the motor nerve axon with square pulses using a Grass S88 stimulator (frequency, 0.2 Hz; pulse duration, 0.2 ms). The stimulus amplitude was set to approximately twice the level needed to elicit maximal muscle twitching. When measuring EPPs, 4 μM d-tubocurarine chloride (dTC) was added to the Ringer solution to preclude action potentials in the muscle and muscle contraction. The amplitude of each EPP was measured after averaging 20 individual sweeps. In a few cases, EPPs were recorded at a single endplate during the entire experiment. For most of the experiments, EPPs were recorded from four or five randomly chosen synapses under each recording condition (e.g. baseline, drug, wash). Each experiment or measurement was repeated the number of times indicated in the text or figure legends, where N (uppercase) indicates the number of muscles examined and n (lowercase) indicates the total number of end-plate recordings (i.e. muscle fibers) across all muscles. Miniature End-Plate Potentials (MEPPs) were measured in the absence of stimulation and dTC. Only muscles with resting membrane potentials of at least −85 mV were included in this study.
All drugs were applied in the same manner. Stock aliquots were prepared ahead of time in dH2O as follows: NAAG, 50 mM; LY341495, 10 mM; ZJ-43, 10 mM; Glutamate, 10 mM. Immediately prior to each experiment the aliquots were diluted with Ringer solution to create the final concentration of drug. The preparation was continuously perfused (at a rate of 2 ml/minute), in normal Ringer solution or Ringer solution containing the dissolved drug(s). All drugs, except glutamate, were purchased from Tocris Bioscience (Bristol, UK); glutamate was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.).
Statistical Analysis
Unless stated otherwise, data are presented as Mean ± SEM and the Student's t-test (2-tailed assuming equal variance, unpaired) was used to analyze the data, taking p<0.05 as significant.
Western Blot Analysis
Brain
Whole brains were rinsed twice with normal Ringers and then homogenized and lysed using an ice-cold buffer: 1% Triton X-100, 50mM Tris pH 7.4, 150 mM NaCl, and protease inhibitor mixture (Roche). The lysate was centrifuged at 14,000 rpm for 20 min at 4°C to obtain total cellular protein in the supernatant. Samples were denatured and separated using a Tris-HCl 10% Bis-Tris SDS-PAGE gel (Invitrogen) and transferred to PVDF membrane (Whatman). The membrane was blocked with Tris-buffered saline and 0.1% Tween (TBST) with 5% nonfat milk for 1 hour at room temperature, incubated in primary antibody (1:1000) overnight at 4°C, washed for 1 hour with TBST, and then incubated in horseradish peroxidase conjugated secondary antibody (1:500, American Qualex) for 2 hours at room temperature. Immunoreactive protein was detected using chemiluminescence (ECL-Plus, Perkin Elmer), and images were captured with a gel documentation camera (Alpha Innotech). Blots were stripped (Thermo Fisher) and re-probed for other antibodies (Inoue et al., 2006).
Muscle
Muscle samples were homogenized in 50 mM Tris-HCL buffer pH 7.5 containing protease inhibitors. Homogenate was sedimented briefly at 500 × g, and the resulting supernatant was sedimented twice at 14,000 × g. Protein was quantified using BCA method (Thermo Scientific). For Western blots, membrane protein (50 μg) was incubated for 10 min at 70°C in LDS reducing sample buffer (Invitrogen) and then loaded on 4/12 % NuPage Bis-Tris gels (Invitrogen). Protein bands were transferred to nitrocellulose membranes (Invitrogen) according to the manufacturer instructions. The membranes were probed with anti-mGluR 2/3 receptor antibody (Santa Cruz Biotechnology) or anti-Folh1 (GCPII) antibody (Aviva Systems Biology) for one hour at room temperature followed by an hour with Amersham ECL horseradish peroxidase linked secondary antibody (GE Healthcare). Amersham ECL Detection Reagents (GE Healthcare) were used for the detection of immunoreactive proteins on the blot.
Transfected cells
Chinese Hamster Ovary (CHO) cells were transfected with rat GCPII cDNA or mouse GCPIII cDNA inserted into pCI-neo vector (Promega) as previously described (Bzdega et al., 1997; 2004).
Results
NAAG is localized to the presynaptic terminals at the lizard neuromuscular junction
To establish the presence of NAAG at the lizard neuromuscular junction (NMJ), a carbodiimide-based prefixation procedure was used to immunostain lizard Ceratomandibularis muscle preparations with a highly specific polyclonal NAAG antibody (Moffett et al., 1993). Figure 1A shows a typical NMJ, in which NAAG-like immunofluorescence is superimposed on an image of a NMJ captured using differential interference contrast (DIC) optics. NAAG appears to be localized to the presynaptic motor nerve terminal. To further clarify its localization, a series of confocal images were collected from muscles co-stained with anti-NAAG antibodies (green) and fluorescent α-bungarotoxin (red), which binds to postsynaptic nicotinic acetylcholine receptors (nAChRs). Since the nAChRs are aligned along the clefts of the postjunctional folds directly beneath the motor nerve terminals, the pattern of α-bungarotoxin staining serves to outline the position of the overlying presynaptic release zones. Figure 1B depicts 6 confocal image planes collected at 1μm intervals throughout a NMJ. At each vertical position, NAAG immunofluorescence is surrounded by α-bungarotoxin labeled nAChRs. The failure to detect NAAG outside the boundaries formed by the nAChRs (see the zoom from one image plane in figure 1B) is consistent with NAAG being within the nerve terminal release zone and not within the closely associated perisynaptic Schwann cells (PSCs).
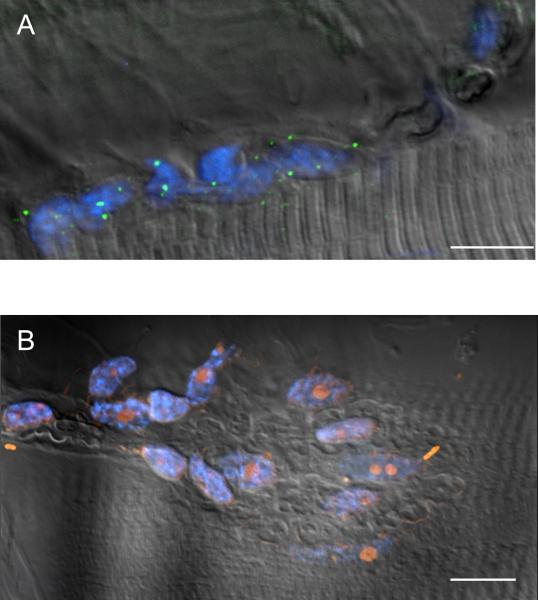
Figure 1. NAAG is present at the lizard NMJ.
A. NAAG (green) is present at the lizard NMJ junction, imaged using differential interference contrast (DIC), and is not in the muscle cells (N=11, n=165). B. Separate planes from a 3D Z-stack of a terminal stained for NAAG (green) and nicotinic ACh receptors using α-bungarotoxin (red). The Z-stack is presented in 1μm increments vertically through the field. Zoom of B shows that NAAG is consistently encapsulated within the nerve terminal. C. Two neighboring synapses show variability in presence of NAAG (green) within the nerve terminal (red, α-bungarotoxin). 73% of synapses had NAAG present at time of fixation (see figure 2). The image shown is a maximum projection of 16 images collected at 0.5 μm increments vertically through the field containing the NMJ. D. NAAG (green) does not always fill the whole terminal (red, α-bungarotoxin). The middle set of boutons still retains its NAAG, while the upper and lower sets of boutons are devoid of NAAG immunofluorescence. The image shown is a maximum projection of 21 images collected at 0.27 μm increments vertically through the field containing the NMJ. Calibration bars, 10 μm.
Surprisingly, careful inspection of hundreds of NMJs co-stained with anti-NAAG antibodies and fluorescent α-bungarotoxin revealed several NMJs completely lacking NAAG. For example, Figure 1C reveals two NMJs on adjacent muscle fibers where only one of the junctions stains for NAAG and the other does not contain any detectable NAAG. Rarely, junctions were observed similar to the one shown in Figure 1D, in which NAAG was detected in only a portion of a single NMJ. Interestingly, in cases such as these, there was a sharp demarcation between those branches that contained NAAG and those that didn't, rather than a gradation of staining over the NMJ. To rule out optical sectioning artifacts, we used the optimal vertical spacing of optical z-sections (0.27 μm for the 60× objective) and made sure to image the full vertical extent of each NMJ.
One possible explanation for the nerve terminals devoid of NAAG depicted in Figure 1C is that these NMJs had undergone extensive neurotransmitter release immediately prior to fixation and had therefore released all their stored NAAG. During the dissection and preparation of muscles, individual fibers are occasionally observed to contract even though the motor nerve is not being stimulated. These contractions are presumably due to presynaptic transmitter release, possibly caused by inadvertent damage of the fine branches of the motor axon, since they are abolished by the nAChR antagonist d-tubocurarine. If the rate of replenishment of nerve terminals with NAAG is slow relative to the time required to fix the tissue (several minutes), such presynaptic activity could deplete the NMJs of NAAG (e.g. Figure 1C). Furthermore, if the spontaneous activity were limited to one or more of the nerve terminal branches within an NMJ, this could lead to the staining pattern observed in Figure 1D.
NAAG is released by depolarization at the NMJ
To test this hypothesis, we induced neurotransmitter release by depolarizing NMJ preparations with a modified Ringer solution containing 60 mM K+ for 15 minutes prior to fixation and staining for NAAG and nAChRs. Previous work in this preparation has demonstrated that this treatment leads to extensive exocytosis of synaptic vesicles, which is sufficient to deplete the terminals of synaptic vesicles when endocytosis is blocked (Lindgren et al., 1997). As shown in Figure 2A, exposure of muscles to elevated [K+] significantly reduced the proportion of NMJs with detectable NAAG-like immunoreactivity from 74 ± 13 % (N=5, n=128) in normal Ringers to 19 ± 1 % (N=5, n=257) in high [K+] (t8 = 4.27, P = 0.0027). Furthermore, reduction of [K+] to 0.5 mM and elimination of Ca2+ from the bathing solution, conditions that should prevent synaptic transmission, caused an increase in the proportion of NMJs with detectable NAAG to 95 ± 2 % (N=4, n= 134). This percentage was significantly different from muscles bathed in high [K+] (t7 = 38.5, P = 2.8×10−9) but not from muscles bathed in normal Ringers (t7 = 1.44, P = 0.19). These data support the hypothesis that NAAG is released from nerve terminals by depolarization-induced exocytosis of synaptic vesicles and, under the conditions of these experiments, the restoration of NAAG occurs relatively slowly. Since this hypothesis is supported by functional data, we are unable to distinguish between small, clear vesicles and large, dense-core vesicles. Moreover, our data do not exclude other less likely possibilities, such as release directly from the cytoplasm (see Discussion).
Figure 2. NAAG depletion and immunohistochemical staining in synaptic vesicles.
A. In contrast to muscles bathed in normal saline (N=5), muscles incubated in high [K+] saline for 15 minutes prior to fixation (N=5) contained significantly fewer synapses with detectable NAAG. The difference in the percentage of synapse containing NAAG after being incubated for 15 minutes in saline with low [K+] and no Ca2+ (N=4) was highly significantly different from the percentage observed in muscles incubated in high [K+] saline. Asterisks indicate the means are significantly different. * P=0.0027, **P=2.8×10−9. B. The distribution of NAAG (red) is different from AM1-44 (green) following modest stimulation (0.5 Hz, 48 seconds). Inset shows a zoomed image in which NAAG can be seen preferentially located in the interior of the nerve terminal, whereas AM1-44 is concentrated in the periphery (N=2, n=40). For clarity of presentation, the color of the NAAG immunofluorescence was changed from blue to red; however, the secondary antibody was actually conjugated to AlexaFluor 350. The AM-1-44 emission spans both green and red. For clarity, only the green channel is displayed here; however, the pattern of staining is identical in the red channel. C. NAAG (green) is co-distributed with Alexa 595 Dextran (red) back-loaded into the nerve terminal (N=2, n=20). The panels display the fluorescence emission using a Texas Red filter cube (TR), a FITC filter cube (NAAG), and the superimposed images of both (merge). Calibration bars, 10 μm.
This hypothesis was tested in one further set of experiments. Just prior to fixation and staining for NAAG, the motor axon of a muscle cell was electrically stimulated at 0.5 Hz for 48 sec in the presence of the lipophilic dye AM1-44 to label the readily releasable and recyclable pool of synaptic vesicles (Pieribone et al., 1995; Rizzoli & Betz, 2005; Gaffield et al., 2006). The stimulation parameters were chosen after determining that neurotransmitter release in this preparation remains stable for over an hour at a stimulation rate of 0.5 Hz, indicating there remains a readily releasable pool of synaptic vesicles (data not shown). Preliminary measurements of AM1-44 loading indicated that 48 seconds was the minimum time necessary to produce detectable fluorescence in the nerve terminal. Interestingly, muscles treated in this way contained nerve terminals in which NAAG (red) could still be detected but only in the interior of the nerve terminal, the area occupied by the reserve pool of synaptic vesicles (Tabares et al., 2007), whereas the AM1-44 (green) was concentrated in the more peripheral regions of the nerve terminal, the area occupied by the recently recycled synaptic vesicles. This differential staining of the nerve terminal can be seen best in the zoom image in Figure 2B. In contrast to this pattern of staining, muscles in which the motor nerve terminals were back-loaded with dextran (MW 10,000) conjugated to Alexa Fluor 594 (red), a procedure which does not require any nerve stimulation, the NAAG (green) staining was co-extensive with the dextran, consistent with NAAG filled vesicles throughout the presynaptic nerve terminal (Figure 2C). Collectively, these data support the conclusion that NAAG resides in synaptic vesicles of the NMJ and is released during synaptic transmission.
Glutamate Carboxypeptidase-II
Since NAAG appears to be synaptically released at the NMJ, we wondered whether its receptor, mGluR3, and inactivating enzyme, GCPII, were also present. To test this, antibodies against GCPII and GCPIII, two NAAG peptidases cloned from rat and mouse cDNA libraries (Bzdega et al., 1997; 2004) were applied to the NMJ. Although the anti-GCPIII antibodies gave no immunoreactive signal associated with structures at the NMJ (data not shown), anti-GCPII antibodies recognized molecules on the perisynaptic Schwann cells (PSCs). Figure 3A shows a NMJ from a muscle that had been fixed and exposed to rabbit anti-CGPII primary antibody, followed by Alexa 488 secondary antibody (green). Since the only nuclei present at the NMJ are either in PSCs or the muscle, the nuclear stain DAPI was applied to reveal the location of the PSCs (the PSC and muscle nuclei can be readily distinguished since muscle nuclei have a distinct elongated, spindle shape.) Overlaying the fluorescent images of GCPII (green) and DAPI-stained PSC nuclei (blue) on a DIC image that highlights the topography of the NMJ demonstrated the location of GCPII on the surfaces of the PSCs (Figure 3A). The resolution of light micrographic images, however, is not sufficient to rule out the possibility that GCPII is projecting from the presynaptic nerve terminals, which are closely apposed to the PSC processes. To eliminate this inherent ambiguity, muscles were stained as above after selective ablation of the PSCs by a procedure that employs the combination of a complement system (from Guinea pig serum) and a monoclonal antibody specific to the PSCs (Reddy et al., 2003). Successful ablation of the PSCs was assessed by application of ethidium homodimer-1 (EthD-1) to stain dead cells. A typical example is shown in figure 3B. The residual PSC nuclei, which are stained by both DAPI (blue) and EthD-1 (red), are clearly visible; however, NMJs with dead PSCs did not display any GCPII immunoreactivity (note absence of green in Fig. 3B). The presence and location of GCPII immunoreactivity in normal NMJs and its absence when PSCs were ablated strongly supports the conclusion that GCPII is expressed by the PSCs.
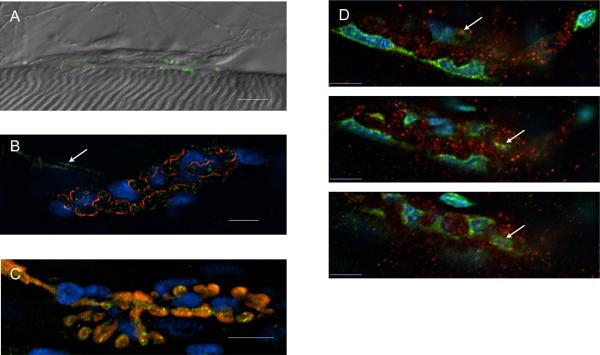
Figure 3. Immunohistochemical staining of NAAG hydrolase enzyme, GCPII.
A. Perisynaptic Schwann Cell (PSC) unablated preparation shows GCPII (green) stain at the nerve terminal (DAPI in blue indicating PSC nuclei). (N=4, n=100). B. Ablated preparation lacks GCPII (green) where ablated PSCs are marked with EthD-1 (red) and DAPI (blue) (N=2, n=50). For both panels A and B, an image collected using DIC optics is superimposed onto fluorescent images collected using DAPI, FITC and TRITC filter cubes. Calibration bars, 10 μm.
Localization of metabotropic glutamate receptor type 3 (mGluR3)
The metabotropic glutamate receptor type 3 (mGluR3) is the NAAG receptor within the central nervous system (Neale, 2011). Anti-mGluR3 polyclonal antibody was used to identify an mGluR3-like protein at the lizard NMJ (Figure 4A). To more rigorously localize this immunoreactivity, muscles were co-stained with Alexa 555 α-bungarotoxin (acetylcholine receptors, red), DAPI (PSC nuclei, blue), and anti-mGluR3 (green). Figure 4B shows a typical NMJ, in which the mGluR3 antibodies appear to bind to the motor nerve axon and its terminals; mGluR3 staining is seen within the motor axon as it approaches the muscle (see arrow) and is also within the outlines of the nicotinic acetylcholine receptors. This pattern is consistent with mGluR3 being synthesized by the motor neuron, transported down the axon, and expressed in the membrane of the nerve terminal. A similar conclusion was reached after applying anti-mGluR3 antibodies to a preparation in which the nerve terminal had been back-loaded with dextran (MW 10,000) conjugated to Alexa Fluor 594 (red). In this case, the mGluR3 receptors are even more clearly seen to be present within the motor axon and axon terminals (see Fig. 4C). However, although the majority of mGluR3 appears to be within the nerve terminal, some mGluR3 staining can be seen (Figures 4B and C) outside the vicinity of the nerve terminals, suggesting it might also be expressed by the PSCs. To determine whether this is the case, muscles were co-stained with mGluR3 antibodies and both DAPI and YOYO-1. Unlike DAPI, which appear to only bind DNA in this preparation, YOYO-1 recognizes both DNA and RNA (Glazer & Rye, 1992; Miura et al., 1996) and thus reveals both the nuclei and cytoplasm of cells, illuminating the full extent of the PSCs (see Newman et al., 2007). Figure 4D shows three confocal image planes collected at 2 μm intervals through a typical NMJ, stained with DAPI (blue), YOYO-1 (green) and the mGluR3 antibodies (red). Notice that the PSCs are double-stained, with DAPI marking their nuclei and YOYO-1 revealing the surrounding cytoplasm, including the PSC processes. Since the majority of the anti-mGluR3 (red) does not overlap with DAPI (blue) or YOYO-1 (green), this suggests that mGluR3 is primarily located on the presynaptic terminals. However, some mGluR3 appears to overlap with YOYO-1 (see arrows in 4C); thus, we cannot rule out the possibility that the PSCs also express mGluR3, a result that would be consistent with mGluR3 expression by glia in the central nervous system.
Figure 4. Immunohistochemical staining of the NAAG receptor, mGluR3.
A. mGluR3 (green) is present at the lizard NMJ. The immunofluorescence image showing mGluR3 is superimposed on an image collected at the same NMJ using DIC. (N=4, n=56). B. mGluR3 (green) is localized to the synapse, marked with α-bungarotoxin (red) and DAPI (blue) but cannot be localized to a cell type. The motor axon, marked with the arrow, appears to contain mGluR3 (N=2, n=40). C. mGluR3 (green) is contained within the motor nerve terminal, which has been back-loaded with Alexa 595 Dextran (red). The DAPI (blue) indicates PSC nuclei. The image shown is a maximum projection of 8 images collected at 0.5 μm increments vertically through the field containing the NMJ. (N=2, n=23). D. 3 planes from a Z-stack collected in 2 μm increments. mGluR3 (red) is localized primarily to the presynaptic terminal, although these images do not rule out some overlap with the PSCs (DAPI, blue and Yoyo-1, green) as indicated with the arrows. (N=2, n=50). Calibration bars, 10 μm.
Localization of Glutamate receptors: mGluR2 and NMDA
The metabotropic glutamate receptor type 2 (mGluR2) does not appear to be activated physiologically by NAAG (Wroblewska et al., 1997; 2011), but is activated by glutamate (see Conn, 2003). Since glutamate is likely produced through the GCP-catalyzed hydrolysis of NAAG, we asked whether the mGluR2 receptor is present at the NMJ. As shown in Figure 5A, anti-mGluR2 antibodies recognize a target at the lizard NMJ. To localize mGluR2 within the NMJ, muscles were co-stained with Alexa 555 α-bungarotoxin (red), DAPI (blue), and anti-mGluR2 (green). As seen in Figure 5B, the mGluR2 antibodies primarily react within the boundaries created by the α-bungarotoxin, which binds the postsynaptic nicotinic acetylcholine receptors (nAChRs). The same result was obtained in a preparation in which the PSCs had been ablated using Guinea pig complement and a monoclonal antibody specific for the PSCs (see Reddy et al., 2003). As shown in Fig. 5C, even in the absence of live PSCs, the mGluR2 antibodies bind within the outlines created by α-bungarotoxin, a pattern which is consistent with either a presynaptic or postsynaptic location. To distinguish between these two possibilities, we back-loaded nerve terminals with dextran (MW 10,000) conjugated to Alexa Fluor 594 (red) and then applied mGluR2 antibodies (green). After carefully inspecting 42 NMJs in four different muscles, we did not observe any co-localization of dextran and mGluR2 antibodies, although there was considerable anti-mGluR2 staining closely adjacent to the nerve terminals. An example of a typical NMJ is shown in Fig. 5D. The lack of overlap between the Alexa Fluor 594 (red) and the mGluR2 receptors (green) suggests that the mGluR2 receptors are primarily located on the postsynaptic side of the NMJ (see arrow).
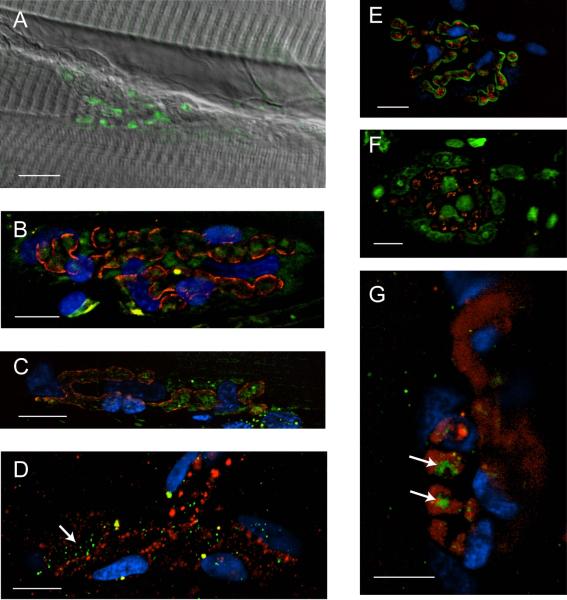
Figure 5. Immunohistochemical staining of glutamate receptors metabotropic glutamate receptor type 2 and N-Methyl-D-aspartate receptor 1.
A. mGluR2 (green) staining at the terminal. The immunofluorescence image showing mGluR2 is superimposed on an image collected at the same NMJ using DIC. B. mGluR2 (green) is localized to the synapse outlined with α-bungarotoxin (red) but its specific localization is ambiguous. The PSCs are labeled with DAPI (blue). The image shown is a maximum projection of 5 images collected at 0.5 μm increments vertically through the field containing the NMJ. (N=2, n=220). C. The distribution of mGluR2 is unaltered by PSC ablation. Ablated preparation shows mGluR2 (green) within outline created by α-bungarotoxin (red). The image shown is a maximum projection of 10 images collected at 0.5 μm increments vertically through the field containing the NMJ. (N=2, n=50). D. mGluR2 (green) is not contained within the motor nerve terminal, which has been back-loaded with Alexa 595 Dextran (red). The DAPI (blue) indicates PSC nuclei. mGluR2 receptors thought to be in the muscle membrane are marked with the arrow. (N=4, n=22). E. NMDAR1 (red) is localized to the postsynaptic end plate, outlined with α-bungarotoxin (green) (N=2, n=50) F. The NMDAR1 receptor is not localized to the PSCs. NMDAR1 (red) shows no overlap with the PSCs, which are labeled with YOYO-1 (green). (N=2, n=40). G. NMDAR1 is not localized to the motor nerve terminal, which has been back-loaded with Alexa 595 Dextran (red). The DAPI (blue) indicates PSC nuclei. The arrows mark clusters of NMDAR1 receptors that are in the end-plate of the muscle. (N=4, n=31). Calibration bars, 10 μm.
Lastly, the glutamate formed by the hydrolysis of NAAG also could activate NMDA receptors, which have been reported at the NMJs of amphibians, fish, and mammals (Berger, Carter, & Coyle, 1995; Grozdanovic & Gossrau, 1998; Todd et al., 2004; Malomouzh et al., 2011). To determine whether these receptors are also present at the lizard NMJ, we applied polyclonal antibodies created against the NMDAR1 subunit of the NMDA receptor. As shown in Fig. 5E, which presents a muscle co-stained with Alexa 488 α-bungarotoxin (green), DAPI (blue), and anti-NMDAR1 (red), NMDA receptors, similar to mGluR2, are primarily localized to the muscle end plate, adjacent to the nAChRs. To see if any NMDA receptors are present on the nearby PSCs, another muscle was co-stained with YOYO-1, which highlights the PSCs by staining their nucleic acids. As seen in Fig. 5F, anti-NMDAR1 staining (red) does not overlap with YOYO-1 (green), thus ruling out the possible localization of NMDA receptors on the PSCs. Finally, Fig. 5G shows that anti-NMDAR1 staining (green) does not co-localize with nerve terminals back-loaded with dextran (MW 10,000) conjugated to Alexa Fluor 594 (red). Clusters of NMDAR1 receptors (indicated with the arrows) are seen directly adjacent to the nerve terminal boutons, consistent with these receptors being localized to the end-plate region of the muscle membrane.
Western Blots
To confirm that antibodies were recognizing proteins of the appropriate molecular weight in membrane preparations of Anolis carolinensis, Western blots were probed with antibodies against mGluR2/3, GCPII (FOLH1); mGluR2; mGluR3; NMDAR1 (Fig. 6). In membranes from lizard brain (Fig. 6A), antibodies against mGluR2, mGluR3 and NMDAR1 reacted with proteins at the appropriate molecular weight for these receptors. As seen in Fig. 6B, GCPII (FOLH1) antibody recognized protein at the appropriate molecular weight for this enzyme in lizard muscle, mouse brain, Chinese Hamster Ovary (CHO) cells transfected with GCPII, but not CHO cells transfected with GCPIII. Membranes from lizard muscle and mouse brain gave immunoreactive bands at the level of mGluR2/3 monomers and dimers while CHO cells transfected with GCPII did not (Fig. 6C).
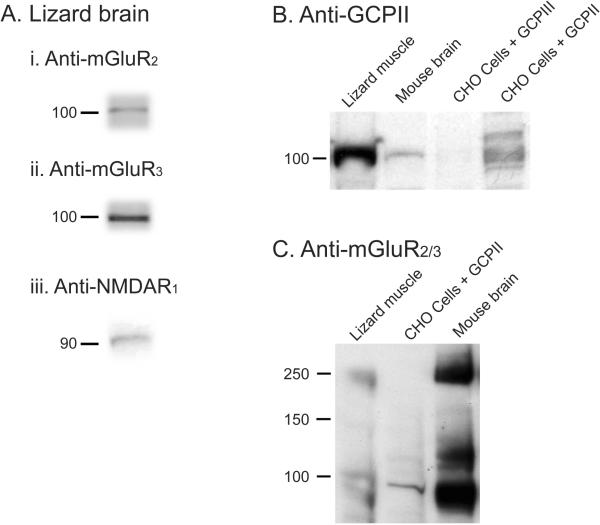
Figure 6. Western Blots - mGluR2/3, GCPII (FOLH1), mGluR2, mGluR3, NMDAR1.
A. Western blots of membranes from lizard brain. (i) Blot was probed with anti mGluR2 antibody (Santa Cruz); (ii) Blot was probed with anti mGluR3 antibody (Santa Cruz). (iii) Blot was probed with anti NMDAR1 antibody (Abcam). B. Western blot analysis of GCPII. Blot was probed with anti FOLH1 antibody (Aviva). From left to right, the lanes contained: lizard muscle, mouse brain, CHO cells transfected with GCPIII, CHO cells transfected with GCPII. C. Western blot analysis for presence of type II metabotropic glutamate receptors. Blot was probed with anti mGluR 2/3 (Santa Cruz). From left to right, the lanes contained: lizard muscle, CHO cells transfected with GCPII, mouse brain.
NAAG reduces evoked neurotransmitter release
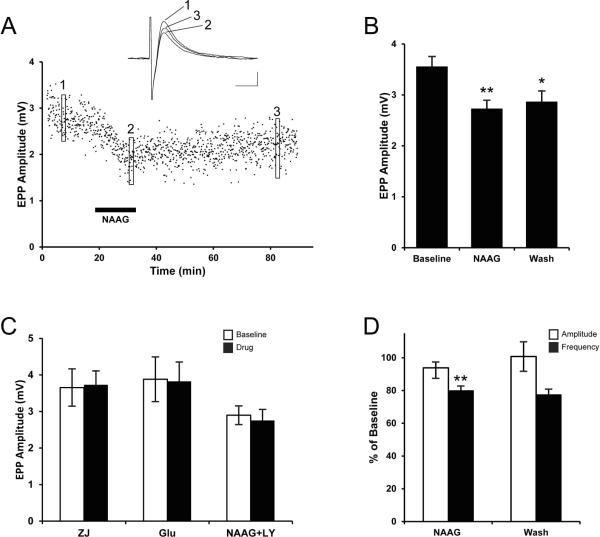
Given that NAAG is located in presynaptic motor nerve endings (Fig. 1), is released by depolarization (Fig. 2), and its receptor (Fig. 3) and inactivating enzyme (Fig. 4) are present at the lizard NMJ, we asked whether application of exogenous NAAG modifies synaptic transmission. To prevent the NAAG from being inactivated by endogenous GCPII, the GCP inhibitor ZJ-43 was applied along with NAAG. While electrically stimulating the motor nerve at a low frequency (0.2 Hz), end-plate potentials (EPPs) were recorded at single neuromuscular junctions with intracellular microelectrodes. To ensure the EPP remained below the action potential threshold in the muscle, the nicotinic ACh receptor antagonist, d-tubocurarine chloride, was added (4 μM) to the perfusate. As seen in Fig. 7a, application of NAAG and ZJ-43 produced a steady decrease in EPP amplitude over a ten-minute application and the EPP amplitude remained depressed for at least one hour after washing out the NAAG (and ZJ-43) with normal Ringers solution. To statistically analyze this effect, EPPs were measured at multiple end plates before, during and after the application of NAAG and ZJ-43. The results from 62 end plates recorded in 7 different muscles are summarized in Fig. 7b. NAAG (100 μM) decreased EPP amplitude by ~25% (baseline, 3.55 ± 0.20 mV; NAAG, 2.72 ± 0.17 mV; t122 = 3.089, P = 0.0025). Following wash out of NAAG, the EPP amplitude increased slightly, but this change was not statistically significant. Instead, the EPP amplitude remained significantly different from baseline (wash, 2.86 ± 0.22 mV; comparing NAAG to wash:t122 = 0.466, P = 0.63; comparing baseline to wash:t127 = 2.22, P = 0.024,). To see if any of the above changes might be due to ZJ-43 per se, independent of the exogenous NAAG, the GCP inhibitor was applied by itself. Application of ZJ-43 at the same concentration used above (20 μM) had no effect on EPP amplitude (Fig. 7C; baseline, 3.65 ± 0.51 mV; ZJ-43, 3.73 ± 0.38 mV; t22 = 0.118, P = 0.91). This result was expected given the evidence gained at other synapses that high frequency stimulation is required to release endogenous NAAG (Neale et al., 2005).
Figure 7. NAAG presynaptically inhibits the release of acetylcholine.
A. Time course of EPP amplitude recorded from a single muscle cell. Each point represents the amplitude of the EPP response to a supramaximal stimulus to the motor nerve at 0.2 Hz. NAAG (100 μM) and NAAG peptidase inhibitor ZJ-43 (20 μM) were applied to the bathing solution during the time indicated by the horizontal bar. Averages of 15 traces, taken at three different times (as indicated by the boxes) are shown in the inset. Calibration bars, 1 mV, 5 ms. B. Mean EPP amplitudes are presented before (baseline), during (NAAG), and after (wash) the application of NAAG (100 μM) and ZJ-43 (20 μM). Asterisks indicate the means are significantly different from Baseline. * P=0.024, **P=0.0025. C. Mean EPP amplitudes before (baseline) and during application of one of the following drug treatments (drug): 20 μM ZJ-43 by itself (ZJ, n=11), ZJ-43 with 1 μM glutamate (Glu, n=16), and ZJ-43 with a combination of 100 μM NAAG and 10 μM group II mGluR antagonist LY341495 (NAAG+LY, n=39). None of these drug treatments were significantly different from baseline. D. Mean MEPP amplitude and frequency are shown as percent of baseline during (NAAG) and after (wash) application of 100 μM NAAG and 20 μM ZJ-43. Asterisks (**) indicate the mean is significantly different from baseline (P=0.026, N=4, paired t-test).
Although ZJ-43 was applied to inhibit the GCP-catalyzed hydrolysis of NAAG, it is possible that some glutamate was present in the NAAG solution applied to the muscles. For example, Losi et al. (2004) reported the need to repurify commercial NAAG in order to reduce the contaminating glutamate concentration to less than 0.1% and Fricker et al. (2009) subsequently reported up to 0.4% glutamate contamination of commercial NAAG. To see whether glutamate contamination could account for the effects we observed following NAAG application, we measured the effect of 1 μM glutamate, the concentration expected if the glutamate contamination was as high as 1%, on EPP amplitude. Unlike NAAG, 1 μM glutamate did not have a significant effect on EPP amplitude (Fig. 7C; baseline, 3.88 ± 0.61 mV; glutamate, 3.83 ± 0.53 mV; t29 = 0.071, P = 0.94). Thus, glutamate contamination is not a likely explanation for the effect of exogenous NAAG on EPP amplitude.
To test whether the change in EPP amplitude by NAAG was due to a decrease in the amount of ACh released (i.e. a presynaptic effect) or due to a change in the sensitivity of the nicotinic ACh receptors in the muscle (i.e. a postsynaptic effect), spontaneous miniature end-plate potentials (MEPPs) were recorded before and after application of NAAG. The results are summarized in Fig. 7D. Although NAAG did not alter the mean MEPP amplitude (93.9 ± 6.5 % of baseline; comparing NAAG to baseline using a paired t-test, t3 = 0.181, P = 0.52), application of NAAG significantly decreased MEPP frequency (79.9 ± 2.8 % of baseline; comparing NAAG to baseline using a paired t-test, t3 = 1.03, P = 0.026). Interestingly, although the difference from baseline is only marginally significant, the decrease in MEPP frequency persists following the washout of NAAG (77.4 ± 3.4 % of baseline; comparing baseline to wash using a paired t-test, t3 = 0.78, P = 0.052), which is similar to the persistent decrease of evoked ACh release (see Fig. 7B). Taken together, these results indicate that NAAG has a presynaptic effect, decreasing the quantal content of evoked ACh release at the NMJ.
Finally, to determine if the NAAG-induced decrease in neurotransmitter release was mediated by activating a Group II mGluR receptor, the mGluR2/3 antagonist LY341495 was applied along with NAAG and ZJ-43. In these tests, the EPP amplitude was unchanged (Fig. 7C; baseline, 2.90 ± 0.26 mV; NAAG & LY341495, 2.75 ± 0.30 mV; t75 = 0.363, P = 0.72). Thus, as seen elsewhere in the nervous system, NAAG presynaptically reduces neurotransmitter release via activation of a group II mGluR at the vertebrate NMJ.
Discussion
While NAAG immunoreactivity has long been observed in rat motor neurons (Ory-Lavollée et al., 1987; Moffett et al., 1993) and NAAG-like immunoreactivity has been reported at the rat NMJ (Berger, Carter, & Coyle, 1995), the role it plays in synaptic transmission at this peripheral synapse has not been rigorously documented. The data presented here meet several of the central criteria required to establish NAAG's role as a cotransmitter at this synapse: 1) localization at the presynaptic release zone adjacent to the post-synaptic nAChRs of the synaptic cleft, 2) release from this zone upon depolarization by elevated potassium or electrical induction of action potentials, 3) the presence of NAAG's receptor (mGluR3) on the presynaptic terminal, 4) the expression of its inactivating enzyme GCPII by the perisynaptic glia, and 5) the inhibition of evoked acetylcholine release by exogenous NAAG acting via an mGluR2/3 (group II) receptor.
Using ultrastructural immunocytochemistry, we and others previously localized NAAG in synaptic vesicles in the central nervous system (Williamson & Neale, 1988; Renno et al., 1997). The localization of NAAG within the presynaptic terminal and the loss of immunoreactivity following depolarization in high K+ (Fig. 2A) or moderate stimulation (0.5 Hz) of the motor axons is consistent with a similar localization at the NMJ. The failure of NAAG to rapidly reoccupy the release zone that is reestablished by recycled vesicles (Fig. 2B) indicates that the synthesis or vesicle packaging of NAAG is a relatively slow process compared to the process of recycling ACh into synaptic vesicles. This is consistent with the observation that NAAG, like other neuropeptides, is released selectively under high levels of neuronal activity (Neale et al., 2011) and is resynthesized relatively slowly(Arun et al., 2006).
Although our results strongly suggest that NAAG is localized to synaptic vesicles at the NMJ, we are unable to distinguish between the small vesicles that have been shown to contain ACh (see Review by Ceccarelli & Hurlbut, 1980) and the large, dense-core vesicles that contain peptides, such as substance P and calcitonin gene-related peptide (Matteoli et al., 1988; 1990). Our results could also support the presence of NAAG exclusively within the cytoplasm of the nerve terminal and its release via a membrane transporter. This would, however, be a very novel mechanism for a peptide and is inconsistent with NAAG's vesicular localization in central synapses and studies demonstrating its calcium-dependent release from neurons (Neale et al., 2000). Unlike most neuropeptides, which are synthesized on ribosomes in the cell body, NAAG is enzymatically produced from N-acetylaspartate and glutamate (Becker et al., 2010; Collard et al., 2010), suggesting it is synthesized locally in the nerve terminal. Thus, it is likely that NAAG is present in both the cytoplasm and within a vesicular compartment, from which it is released upon depolarization. Confirmation of the details of NAAG release at the NMJ awaits further studies.
The distribution of glutamate carboxypeptidase II (GCPII) in the mammalian nervous system parallels that of NAAG (Fuhrman et al., 1994). This enzyme, GCPII, is expressed at much higher levels in the nervous system than GCPIII (Bzdega et al., 2004; Hlouchová et al., 2007). Consistent with this, the data presented here indicate that GCPII, but not GCPIII, is the NAAG peptidase present at the lizard NMJ. A similar punctate pattern of GCPII staining, which we observed on the perisynaptic Schwann cells (PSCs) at the lizard NMJ (Fig. 3A), has been reported in the mammalian CNS (Slusher et al., 1992). Since all immunoreactivity against GCPII disappeared after PSC ablation, we conclude that the PSCs are the exclusive source of this enzyme at the NMJ. GCPII similarly is expressed by astrocytes and Schwann cells in the mammalian nervous system (Berger, Carter, & Coyle, 1995; Berger et al., 1999).
Pinard and colleagues (2003) provided the first evidence that metabotropic glutamate receptors are present and modulate synaptic transmission at the NMJ. Consistent with the model of NAAG function (Neale et al., 2005; 2011), they found that a group I/II mGluR agonist reduced transmitter release at the frog NMJ while a group I/II antagonist reduced synaptic depression evoked by release of an endogenous cotransmitter under conditions of high but not low level excitation. This has been confirmed and extended by the current study showing that the group II mGluR3, but not the mGluR2 receptor, is present primarily on motor nerve terminals in the lizard NMJ, a distribution that parallels its distribution in the CNS, where mGluR3 receptors typically are located on presynaptic nerve terminals and their activation inhibits neurotransmitter release (Niswender & Conn, 2010).
In this study we also found the presence of NMDA and mGluR2 receptors at the lizard NMJ. Either of these receptors are likely targets for glutamate, which may explain its modulatory effects at the NMJ (Pinard et al., 2003). NMDA receptors have been reported previously in the end-plates of amphibian, fish and mammalian muscles (Berger, Carter, & Coyle, 1995; Grozdanovic & Gossrau, 1998; Todd et al., 2004; Mays et al., 2009; Malomouzh et al., 2011). The results from the lizard NMJ confirm with a high level of resolution that the NMDAR1 subunit protein is in the post-synaptic end plate, lying immediately below the pre-synaptic nerve terminal (see Fig. 5G). Additionally, we provide the first rigorous identification of mGluR2 in this same location (see Fig. 5B,C). Interestingly, the NMDA and mGluR2 receptors were consistently observed to be just inside the clusters of dense nAChRs, which are located on the ridges created by the post-junctional folds (see Fig. 5 B,C,E). These finding are consistent with a recent study by Malomouzh et al. (2011) who localized the NMDA receptor NR1 subunit to the depths of the post-junctional folds in rat neuromuscular junctions. The presence of glutamate receptors (NMDA and mGluR2) adjacent to nAChRs strongly suggests that glutamate has a role in regulating the function of the vertebrate NMJ.
Our observation that exogenous NAAG, in the presence of the GCPII inhibitor ZJ-43, inhibits evoked neurotransmitter release at the lizard NMJ and that this inhibition is blocked by a group II mGluR antagonist, supports the hypothesis that NAAG is a cotransmitter at the vertebrate NMJ and is consistent with numerous studies demonstrating a similar role for this peptide at synapses in the central nervous system (Neale et al., 2005; 2011). Since exogenous glutamate was without effect when applied at more than twice the concentration expected as a contaminant in our NAAG solution (see Losi et al., 2004; Fricker et al., 2009), we conclude that NAAG can modulate neurotransmitter release at the vertebrate NMJ independent of glutamate. Although both glutamate and NAAG fulfill the characteristics of a cotransmitter at the NMJ, the novel result reported here is that NAAG can function as a neuromodulator regulating acetylcholine release at this synapse.
Two enzymes, NAAG synthetase I and II, have been identified in the mammalian central nervous system (Becker et al., 2010; Collard et al., 2010). While NAAG synthetase I mediates the synthesis of NAAG, NAAG Synthetase II produces NAAG and a sister peptide N-acetylaspartylglutamylglutamate (NAAG2) from NAAG (Lodder-Gadaczek et al., 2011). NAAG2 is present in the rat spinal cord at concentrations that are 75-fold less than NAAG. More immediately relevant to this research, message for this enzyme was detected by in situ hybridization in large neuronal cell bodies in the ventral horn of the rat spinal cord. While having several-fold greater affinity for NAAG, our rabbit anti-NAAG serum cross-reacts with NAAG2. This leaves open the possibility that NAAG2 also is present in motor neuron synaptic endings. The putative role of NAAG2 as a cotransmitter at the NMJ and elsewhere remains to be tested, specifically with respect to its synaptic release and its ability to activate a receptor.
These data in themselves do not fully resolve the question as to which molecule, NAAG, glutamate (produced by GCPII hydrolysis of NAAG), or possibly NAAG2, activates pre- or perisynaptic mGluR3 and whether the postsynaptic NMDA and mGluR2 receptors are activated by glutamate released from NAAG, NAAG2 or by a separate cotransmitter pool of glutamate. The presence of both presynaptic mGluR3 and postsynaptic NMDA and mGluR2 at the NMJ is consistent with both NAAG and glutamate acting as cotransmitters at this synapse. In the central nervous system, NAAG is often co-expressed in glutamatergic circuits (Neale et al., 2000; 2005). In these neurons, there would appear to be no purpose for NAAG release unless it is to act on mGluR3 receptors that are not accessible to the coreleased primary transmitter, glutamate. A rationale for the corelease of glutamate and NAAG is that glutamate acts on receptors immediately within the synaptic cleft, such as post-synaptic NMDA or mGluR2 receptors, and is rapidly removed from this cleft without reaching significant concentrations in the perisynaptic region. In contrast, GCPII expression by perisynaptic glia rather than within the immediate synaptic space permits the peptide to diffuse from the synapse and act at presynaptic mGluR3 outside of the release zone prior to its inactivation by GCPII expressed on perisynaptic glial membranes. In this model (Neale et al., 2005; Neale, 2011) and consistent with mGluR agonist and antagonist data at the neuromuscular junction (Pinard et al., 2003), NAAG's cotransmitter role can be seen as moderating synaptic transmission under high levels of excitation. Since NAAG is colocalized with each of the different small amine transmitters in some neurons (Neale et al., 2000), it is proposed to function broadly to regulate transmitter release across a spectrum of nerve cell circuits, including now the NMJ.
Acknowledgments
The 2A12 antibody was kindly provided by Dr. Chien-Ping Ko, Department of Biological Sciences, University of Southern California, Los Angeles, CA. This work was supported by the National Institutes of Health (NS072735, CL; NS038080, JN) and by an endowment and generous gifts from Nancy and Daniel Paduano.
References
- Arun P, Madhavarao CN, Moffett JR, Namboodiri MAA. Regulation of N-acetylaspartate and N-acetylaspartylglutamate biosynthesis by protein kinase activators. J Neurochem. 2006;98:2034–2042. doi: 10.1111/j.1471-4159.2006.04068.x. [DOI] [PubMed] [Google Scholar]
- Becker I, Lodder J, Gieselmann V, Eckhardt M. Molecular characterization of N-acetylaspartylglutamate synthetase. J Biol Chem. 2010;285:29156–29164. doi: 10.1074/jbc.M110.111765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger UV, Carter RE, Coyle JT. The immunocytochemical localization of N-acetylaspartyl glutamate, its hydrolysing enzyme NAALADase, and the NMDAR-1 receptor at a vertebrate neuromuscular junction. Neuroscience. 1995;64:847–850. doi: 10.1016/0306-4522(95)92578-8. [DOI] [PubMed] [Google Scholar]
- Berger UV, Carter RE, McKee M, Coyle JT. N-acetylated alpha-linked acidic dipeptidase is expressed by non-myelinating Schwann cells in the peripheral nervous system. J Neurocytol. 1995;24:99–109. doi: 10.1007/BF01181553. [DOI] [PubMed] [Google Scholar]
- Berger UV, Luthi-Carter R, Passani LA, Elkabes S, Black I, Konradi C, Coyle JT. Glutamate carboxypeptidase II is expressed by astrocytes in the adult rat nervous system. J Comp Neurol. 1999;415:52–64. doi: 10.1002/(sici)1096-9861(19991206)415:1<52::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Boulland J-L, Qureshi T, Seal RP, Rafiki A, Gundersen V, Bergersen LH, Fremeau RT, Edwards RH, Storm-Mathisen J, Chaudhry FA. Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J Comp Neurol. 2004;480:264–280. doi: 10.1002/cne.20354. [DOI] [PubMed] [Google Scholar]
- Bourque MJ, Robitaille R. Endogenous peptidergic modulation of perisynaptic Schwann cells at the frog neuromuscular junction. J Physiology. 1998;512(Pt 1):197–209. doi: 10.1111/j.1469-7793.1998.197bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdega T, Crowe SL, Ramadan ER, Sciarretta KH, Olszewski RT, Ojeifo OA, Rafalski VA, Wroblewska B, Neale JH. The cloning and characterization of a second brain enzyme with NAAG peptidase activity. J Neurochem. 2004;89:627–635. doi: 10.1111/j.1471-4159.2004.02361.x. [DOI] [PubMed] [Google Scholar]
- Bzdega T, Turi T, Wroblewska B, She D, Chung HS, Kim H, Neale JH. Molecular cloning of a peptidase against N-acetylaspartylglutamate from a rat hippocampal cDNA library. J Neurochem. 1997;69:2270–2277. doi: 10.1046/j.1471-4159.1997.69062270.x. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol Rev. 1980;60:396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- Collard F, Stroobant V, Lamosa P, Kapanda CN, Lambert DM, Muccioli GG, Poupaert JH, Opperdoes F, Van Schaftingen E. Molecular identification of N-acetylaspartylglutamate synthase and beta-citrylglutamate synthase. J Biol Chem. 2010;285:29826–29833. doi: 10.1074/jbc.M110.152629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ. Physiological roles and therapeutic potential of metabotropic glutamate receptors. Ann N Y Acad Sci. 2003;1003:12–21. doi: 10.1196/annals.1300.002. [DOI] [PubMed] [Google Scholar]
- David G, Barrett JN, Barrett EF. Stimulation-induced changes in [Ca2+] in lizard motor nerve terminals. J Physiology. 1997;504(Pt 1):83–96. doi: 10.1111/j.1469-7793.1997.083bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker A-C, Mok MHS, la Flor, de R, Shah AJ, Woolley M, Dawson LA, Kew JNC. Effects of N-acetylaspartylglutamate (NAAG) at group II mGluRs and NMDAR. Neuropharmacology. 2009;56:1060–1067. doi: 10.1016/j.neuropharm.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Fuhrman S, Palkovits M, Cassidy M, Neale JH. The regional distribution of N-acetylaspartylglutamate (NAAG) and peptidase activity against NAAG in the rat nervous system. J Neurochem. 1994;62:275–281. doi: 10.1046/j.1471-4159.1994.62010275.x. [DOI] [PubMed] [Google Scholar]
- Gaffield MA, Rizzoli SO, Betz WJ. Mobility of synaptic vesicles in different pools in resting and stimulated frog motor nerve terminals. Neuron. 2006;51:317–325. doi: 10.1016/j.neuron.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Glazer AN, Rye HS. Stable dye-DNA intercalation complexes as reagents for high-sensitivity fluorescence detection. Nature. 1992;359:859–861. doi: 10.1038/359859a0. [DOI] [PubMed] [Google Scholar]
- Graves AR, Lewin KA, A Lindgren C. Nitric oxide, cAMP and the biphasic muscarinic modulation of ACh release at the lizard neuromuscular junction. J Physiology. 2004;559:423–432. doi: 10.1113/jphysiol.2004.064469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanovic Z, Gossrau R. Co-localization of nitric oxide synthase I (NOS I) and NMDA receptor subunit 1 (NMDAR-1) at the neuromuscular junction in rat and mouse skeletal muscle. Cell Tissue Res. 1998;291:57–63. doi: 10.1007/s004410050979. [DOI] [PubMed] [Google Scholar]
- Hlouchová K, Barinka C, Klusák V, Sácha P, Mlcochová P, Majer P, Rulísek L, Konvalinka J. Biochemical characterization of human glutamate carboxypeptidase III. J Neurochem. 2007;101:682–696. doi: 10.1111/j.1471-4159.2006.04341.x. [DOI] [PubMed] [Google Scholar]
- Inoue T, Hammaker D, Boyle DL, Firestein GS. Regulation of JNK by MKK-7 in fibroblast-like synoviocytes. Arthritis Rheum. 2006;54:2127–2135. doi: 10.1002/art.21919. [DOI] [PubMed] [Google Scholar]
- Lindgren CA, Laird MV. Nitroprusside inhibits neurotransmitter release at the frog neuromuscular junction. Neuroreport. 1994;5:2205–2208. doi: 10.1097/00001756-199410270-00054. [DOI] [PubMed] [Google Scholar]
- Lindgren CA, Moore JW. Identification of ionic currents at presynaptic nerve endings of the lizard. J Physiology. 1989;414:201–222. doi: 10.1113/jphysiol.1989.sp017684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren CA, Emery DG, Haydon PG. Intracellular acidification reversibly reduces endocytosis at the neuromuscular junction. J Neurosci. 1997;17:3074–3084. doi: 10.1523/JNEUROSCI.17-09-03074.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder-Gadaczek J, Becker I, Gieselmann V, Wang-Eckhardt L, Eckhardt M. N-acetylaspartylglutamate synthetase II synthesizes N-acetylaspartylglutamylglutamate. J Biol Chem. 2011;286:16693–16706. doi: 10.1074/jbc.M111.230136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losi G, Vicini S, Neale J. NAAG fails to antagonize synaptic and extrasynaptic NMDA receptors in cerebellar granule neurons. Neuropharmacology. 2004;46:490–496. doi: 10.1016/j.neuropharm.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Malomouzh AI, Nurullin LF, Arkhipova SS, Nikolsky EE. NMDA receptors at the endplate of rat skeletal muscles: precise postsynaptic localization. Muscle Nerve. 2011;44:987–989. doi: 10.1002/mus.22250. [DOI] [PubMed] [Google Scholar]
- Matteoli M, Haimann C, De Camilli P. Substance P-like immunoreactivity at the frog neuromuscular junction. Neuroscience. 1990;37:271–275. doi: 10.1016/0306-4522(90)90213-n. [DOI] [PubMed] [Google Scholar]
- Matteoli M, Haimann C, Torri-Tarelli F, Polak JM, Ceccarelli B, De Camilli P. Differential effect of alpha-latrotoxin on exocytosis from small synaptic vesicles and from large dense-core vesicles containing calcitonin gene-related peptide at the frog neuromuscular junction. Proc Natl Acad Sci USA. 1988;85:7366–7370. doi: 10.1073/pnas.85.19.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays TA, Sanford JL, Hanada T, Chishti AH, Rafael-Fortney JA. Glutamate receptors localize postsynaptically at neuromuscular junctions in mice. Muscle Nerve. 2009;39:343–349. doi: 10.1002/mus.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Ichikawa Y, Ishikawa T, Ogura M, de Fries R, Shimada H, Mitsuhashi M. Fluorometric determination of total mRNA with oligo(dT) immobilized on microtiter plates. Clin Chem. 1996;42:1758–1764. [PubMed] [Google Scholar]
- Moffett JR, Namboodiri MA. Differential distribution of N-acetylaspartylglutamate and N-acetylaspartate immunoreactivities in rat forebrain. J Neurocytol. 1995;24:409–433. doi: 10.1007/BF01181604. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Namboodiri MA, Neale JH. Enhanced carbodiimide fixation for immunohistochemistry: application to the comparative distributions of N-acetylaspartylglutamate and N-acetylaspartate immunoreactivities in rat brain. J Histochem Cytochem. 1993;41:559–570. doi: 10.1177/41.4.8450195. [DOI] [PubMed] [Google Scholar]
- Neale JH. N-acetylaspartylglutamate is an agonist at mGluR3 in vivo and in vitro. J Neurochem. 2011;119:891–895. doi: 10.1111/j.1471-4159.2011.07380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale JH, Bzdega T, Wroblewska B. N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem. 2000;75:443–452. doi: 10.1046/j.1471-4159.2000.0750443.x. [DOI] [PubMed] [Google Scholar]
- Neale JH, Olszewski RT, Gehl LM, Wroblewska B, Bzdega T. The neurotransmitter N-acetylaspartylglutamate in models of pain, ALS, diabetic neuropathy, CNS injury and schizophrenia. Trends in Pharmacological Sciences. 2005;26:477–484. doi: 10.1016/j.tips.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Neale JH, Olszewski RT, Zuo D, Janczura KJ, Profaci CP, Lavin KM, Madore JC, Bzdega T. Advances in understanding the peptide neurotransmitter NAAG and appearance of a new member of the NAAG neuropeptide family. J Neurochem. 2011;118:490–498. doi: 10.1111/j.1471-4159.2011.07338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman Z, Malik P, Wu T-Y, Ochoa C, Watsa N, Lindgren C. Endocannabinoids mediate muscarine-induced synaptic depression at the vertebrate neuromuscular junction. Eur J Neurosci. 2007;25:1619–1630. doi: 10.1111/j.1460-9568.2007.05422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski RT, Bzdega T, Neale JH. mGluR3 and not mGluR2 receptors mediate the efficacy of NAAG peptidase inhibitor in validated model of schizophrenia. Schizophrenia Research. 2012;136:160–161. doi: 10.1016/j.schres.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Ory-Lavollée L, Blakely RD, Coyle JT. Neurochemical and immunocytochemical studies on the distribution of N-acetyl-aspartylglutamate and N-acetyl-aspartate in rat spinal cord and some peripheral nervous tissues. J Neurochem. 1987;48:895–899. doi: 10.1111/j.1471-4159.1987.tb05601.x. [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Shupliakov O, Brodin L, Hilfiker-Rothenfluh S, Czernik AJ, Greengard P. Distinct pools of synaptic vesicles in neurotransmitter release. Nature. 1995;375:493–497. doi: 10.1038/375493a0. [DOI] [PubMed] [Google Scholar]
- Pinard A, Robitaille R. Nitric oxide dependence of glutamate-mediated modulation at a vertebrate neuromuscular junction. Eur J Neurosci. 2008;28:577–587. doi: 10.1111/j.1460-9568.2008.06355.x. [DOI] [PubMed] [Google Scholar]
- Pinard A, Lévesque S, Vallée J, Robitaille R. Glutamatergic modulation of synaptic plasticity at a PNS vertebrate cholinergic synapse. Eur J Neurosci. 2003;18:3241–3250. doi: 10.1111/j.1460-9568.2003.03028.x. [DOI] [PubMed] [Google Scholar]
- Reddy LV, Koirala S, Sugiura Y, Herrera AA, Ko CP. Glial cells maintain synaptic structure and function and promote development of the neuromuscular junction in vivo. Neuron. 2003;40:563–580. doi: 10.1016/s0896-6273(03)00682-2. [DOI] [PubMed] [Google Scholar]
- Redman RS, Silinsky EM. ATP released together with acetylcholine as the mediator of neuromuscular depression at frog motor nerve endings. J Physiology. 1994;477(Pt 1):117–127. doi: 10.1113/jphysiol.1994.sp020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renno WM, Lee JH, Beitz AJ. Light and electron microscopic immunohistochemical localization of N-acetylaspartylglutamate (NAAG) in the olivocerebellar pathway of the rat. Synapse. 1997;26:140–154. doi: 10.1002/(SICI)1098-2396(199706)26:2<140::AID-SYN5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Rinholm JE, Slettaløkken G, Marcaggi P, Skare Ø, Storm-Mathisen J, Bergersen LH. Subcellular localization of the glutamate transporters GLAST and GLT at the neuromuscular junction in rodents. Neuroscience. 2007;145:579–591. doi: 10.1016/j.neuroscience.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- Slusher BS, Tsai G, Yoo G, Coyle JT. Immunocytochemical localization of the N-acetylaspartylglutamate (NAAG) hydrolyzing enzyme N-acetylated alpha-linked acidic dipeptidase (NAALADase) J Comp Neurol. 1992;315:217–229. doi: 10.1002/cne.903150208. [DOI] [PubMed] [Google Scholar]
- Tabares L, Ruiz R, Linares-Clemente P, Gaffield MA, Alvarez de Toledo G, Fernandez-Chacón R, Betz WJ. Monitoring synaptic function at the neuromuscular junction of a mouse expressing synaptopHluorin. J Neurosci. 2007;27:5422–5430. doi: 10.1523/JNEUROSCI.0670-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Robitaille R. Differential frequency-dependent regulation of transmitter release by endogenous nitric oxide at the amphibian neuromuscular synapse. J Neurosci. 2001;21:1087–1095. doi: 10.1523/JNEUROSCI.21-04-01087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd K, Slatter C, Ali D. Activation of ionotropic glutamate receptors on peripheral axons of primary motoneurons mediates transmitter release at the zebrafish NMJ. J Neurophysiol. 2004;91:828–840. doi: 10.1152/jn.00599.2003. [DOI] [PubMed] [Google Scholar]
- Waerhaug O, Ottersen OP. Demonstration of glutamate-like immunoreactivity at rat neuromuscular junctions by quantitative electron microscopic immunocytochemistry. Anat Embryol. 1993;188:501–513. doi: 10.1007/BF00190144. [DOI] [PubMed] [Google Scholar]
- Williamson LC, Neale JH. Ultrastructural localization of N-acetylaspartylglutamate in synaptic vesicles of retinal neurons. Brain Res. 1988;456:375–381. doi: 10.1016/0006-8993(88)90243-0. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Santi MR, Neale JH. N-acetylaspartylglutamate activates cyclic AMP-coupled metabotropic glutamate receptors in cerebellar astrocytes. Glia. 1998;24:172–179. doi: 10.1002/(sici)1098-1136(199810)24:2<172::aid-glia2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wegorzewska IN, Bzdega T, Neale JH. Type 2 metabotropic glutamate receptor (mGluR2) fails to negatively couple to cGMP in stably transfected cells. Neurochem Int. 2011;58:176–179. doi: 10.1016/j.neuint.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewska B, Wegorzewska IN, Bzdega T, Olszewski RT, Neale JH. Differential negative coupling of type 3 metabotropic glutamate receptor to cyclic GMP levels in neurons and astrocytes. J Neurochem. 2006;96:1071–1077. doi: 10.1111/j.1471-4159.2005.03569.x. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69:174–181. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]