Abstract
Repeated exposure to distraction requires attentional effort to restore task performance. However, the impact of repeated distracter exposure and exertion of attentional effort on new learning has not been examined. In the present experiment, rats were trained in a two-lever sustained attention task. Rats then continued to train, for 12 sessions, in this task either with or without a flashing houselight distracter throughout the session. The flashing houselight transiently decreased attentional performance. Trials that were part of a new light-location discrimination task were then interspersed within the sustained attention task sessions. The frequency of these new light-location discrimination trials increased with additional training. Rats exposed to the distracter exhibited higher accuracy levels during some blocks of sessions in the new light-location discrimination task trials and in the remaining sustained attention task trials compared to rats that were not exposed to the distracter. The effects of repeated distracter exposure are interpreted in the context of an “occasion-setting” model that has been used to describe performance in this task.
Keywords: attention, cognitive flexibility, distracter, occasion-setting
1. Introduction
Distracting stimuli disrupt the ability to focus on cues that indicate important outcomes for an organism. Attentional effort has been described as the process to restore task performance following a variety of demands, such as distracting stimuli (Sarter et al. 2006). Maintaining attentional performance under cognitively demanding conditions is recognized as a critical factor in applied research areas (e.g, Banbury et al. 2001; Zhang et al. 2006) and is thought to be disrupted in several neuropsychiatric conditions (Sarter et al. 2005). However, the implications of restoring task performance under taxing conditions have not been well characterized.
A sustained attention task, developed based on a taxonomy of sustained attentiondemanding task parameters (Parasuraman et al. 1987), has been validated for use with humans (Bushnell et al. 2003; Demeter et al. 2008), rats (Bushnell et al. 1994; McGaughy & Sarter, 1995) and mice (St Peters et al. 2011). In this task, one response alternative is considered correct after brief signal presentation. An alternative response is considered correct on trials with no signal presentation. One model of performance in this task predicts that the light serves as an “occasion-setter” for a conditioned approach response to the appropriate alternative, typically a lever with rats (Schmajuk & Bushnell, 2009). Increases in background noise have been induced in this task by flashing a houselight throughout the session (McGaughy & Sarter, 1995). Because increasing background noise decreases accuracy in this task, we predicted that flashing the houselight would disrupt the ability of the signal light to serve as an occasion-setter for the approach response to the appropriate lever. This disruption of the occasion-setting property of the visual signal could allow re-allocation of attentional resources from the central panel light to other stimuli within the chamber and facilitate the formation of new associations between stimuli and responses within the chamber. We tested whether exposure to the flashing houselight during this sustained attention task would facilitate the ability to learn a new light-location discrimination task, involving panel lights that had not been used during sustained attention task training.
2. Material and Method
2.1. Subjects
Fifteen male Long-Evans rats weighing between 151–175g at the beginning of the experiment were used (Charles River Laboratories, Inc., Wilmington, MA). Rats were individually housed with a 14:10-h light/dark cycle. Behavioral testing took place between 0900 and 1200, five days per week. Water access was given for 30 min after each testing session and for a minimum of one hour on days without behavioral testing. Food was available ad libitum throughout the experiment. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the College of William and Mary. Animals were treated according to the Guidelines for the Care and Use of Laboratory Animals.
2.2. Apparatus
Rats were trained in one of 12 chambers. One side of each chamber contained two retractable levers and a port with a water delivery dipper (0.01 ml) between the levers. A panel light was positioned above each retractable lever and the water port. A houselight was located on the other side of the chamber. The illumination levels of these chambers have been reported (Burk, 2004). Behavioral testing programs were controlled by a PC using Med-PC version IV.
2.3. Behavioral testing procedures
Sustained attention task performance was shaped as described in previous publications from our laboratory (Boschen et al. 2009; Robinson et al. 2012). In the final task, discrimination of visual signals (500, 100 or 25-ms illumination of the central panel light) or no signal was required for water access. The relatively short and variable signal durations and inter-trial intervals (9±3 s) were designed to increase attentional demands (Koelega et al. 1990; Parasuraman et al. 1987). After a signal or no signal, the levers were extended into the chamber. For half of the animals, after a signal, a right lever press was considered correct, the dipper was raised for 3-s, and the trial was scored as a hit. If the left lever was pressed after a signal, the trial was scored as a miss. On nonsignal trials, a left lever press was rewarded and the trial was scored as a correct rejection while a right lever press was scored as a false alarm. These rules were reversed for half of the animals. The levers were retracted after a lever press or after failure to press either lever within 3 s after they were extended into the chamber (scored as an omission). There were 162 trials in each session. The trials were presented in blocks of 18 (9 nonsignal and 9 signal, 3 of each signal duration) and each trial type was chosen randomly without replacement. Training continued in this task until a criterion of at least 70% accuracy on 500-ms signal and nonsignal trials was reached. Rats were then randomly assigned to continue testing in the sustained attention task with the houselight remaining illuminated throughout the testing session (n=8) or with the houselight flashed (1.0s on/off) throughout the test session (n=7). Training continued with these conditions for 12 sessions.
After initial distracter exposure, light-location discrimination trials were interspersed within a session. Light-location discrimination trials began with 500-ms illumination of the left or right panel light. The levers were then extended and pressing the lever under the illuminated light was rewarded. These light-location discrimination trials were randomly interspersed with the attention task trials within a session. Specifically, the number of trials in each block was increased from 18 to 20, with the two additional trials being light-location discrimination trials (one trial with left and one trial with right light illumination). Thus, 10% of the trials were light-location discrimination trials. The total number of trials in each session was increased to 180 (9 blocks of 20 trials). After nine sessions, the number of light-location discrimination trials was increased to eight per block of 20 trials. After training in this condition for nine sessions, the number of light-location discrimination trials was increased to 14 per block of 20 trials. Animals trained for nine sessions with 70% of the trials involving light-location discrimination.
2.4 Behavioral measures and statistical analyses
For sustained attention task trials, the number of hits (h), misses (m), correct rejections (cr) and false alarms (fa) were determined. The relative hits (h/(h+m)) and correct rejections (cr/(cr+fa)) were calculated. A sustained attention index (SI) was calculated based upon false alarms (fa/(cr+fa)) and hits (SI=(h-fa)/(2X(h+fa)-(h+fa)2)) (McGaughy et al. 1996). The values for SI range from 1 to -1, with 1 indicating perfect accuracy on signal and nonsignal trials and a value of 0 indicating an inability to discriminate between signals and nonsignals. The relative number of correct responses (correct responses/(correct responses+incorrect responses)) was also determined for the light-location discrimination trials. Omissions were analyzed separately from measures of accuracy.
SI and relative hits were analyzed with repeated measures ANOVAs that included condition (exposed to the distracter or not) and signal duration. Relative correct rejections, relative correct responses for light-location discrimination trials and omissions were analyzed with ANOVAs that included condition. When light-location discrimination trials were introduced, the factor session was included, which consisted of the average of blocks of three sessions. All p values for ANOVAs involving within-subjects factors were corrected with the Huynh-Feldt procedure. Multiple t-tests were corrected with the Bonferroni procedure. A level of α=0.05 was considered statistically significant.
3. Results
There were no significant differences between rats that were exposed to the distracter and those that were not on any behavioral measure prior to distracter exposure. To test the effects of initial exposure to the distracter, the first day of flashing houselight exposure was examined. Initial distracter exposure decreased overall task accuracy. A condition X signal duration ANOVA for SI yielded a main effect of condition, F(1,13) = 13.03, p = 0.003 (SI averaged across signal duration±SEMs; rats that were not exposed to the distracter: 0.447±0.052; rats that were exposed to the distracter: 0.210±0.037). This effect was primarily due to a decrease in accuracy on signal trials, although the condition X signal duration ANOVA for the relative hits only yielded a trend for the main effect of condition (p = 0.056) and for the signal duration X condition interaction (p =0.073; mean percent correct ± SEMs for rats not exposed to the distracter; 500 ms: 91.4 ± 2.0; 100 ms: 65.1 ± 3.9; 25 ms: 29.3 ± 6.3; CR: 79.4 ± 6.5; Omissions per session: 9.1 ± 5.2; rats exposed to the distracter; 500 ms: 75.4 ± 4.2; 100 ms: 48.5 ± 3.6; 25 ms: 29.7 ± 7.2; CR: 69.3 ± 4.6; Omissions per session: 13.4 ± 6.5). Flashing houselight-induced decreases in accuracy did not persist with additional testing. During the final set of three sessions, there were no effects of distracter condition on accuracy (all p > 0.36 for main effects or interactions involving condition).
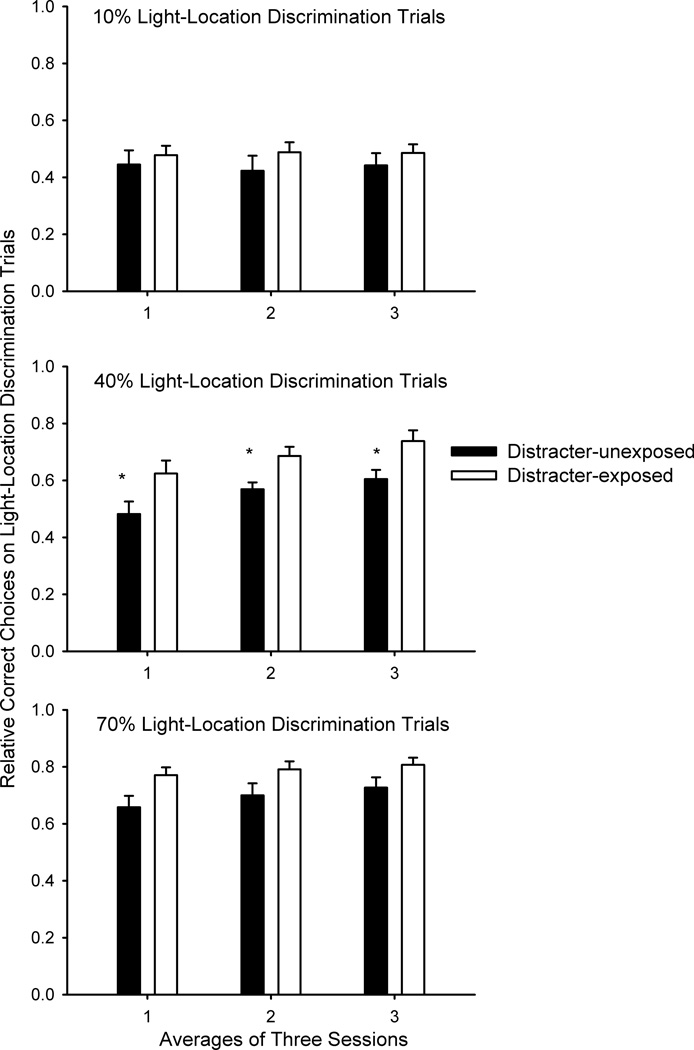
When 10% of the trials were light-location discrimination trials, there were no differences between rats that were or were not exposed to the distracter on accuracy in the light-location discrimination or the sustained attention task trials. Moreover, performance of rats that were or were not exposed to the distracter failed to significantly differ from chance (50%) performance in the light-location discrimination task. When the percentage of light-location discrimination trials was increased to 40% of the trials within a session, rats that were exposed to the distracter exhibited higher accuracy in the light-location discrimination task compared with rats not exposed to the distracter, an observation confirmed by a significant main effect of condition, F(1,13) = 8.81, p = 0.011 (Figure 1). One-sample t-tests including mean performance from all nine testing sessions with 40% light-location discrimination trials indicated that rats that were exposed to the distracter performed higher than chance (t(6) = 5.289, p = 0.002) on the light-location discrimination trials whereas rats that were not exposed to the distracter failed to perform significantly above chance (p > 0.10). There were no significant differences between rats that were or were not exposed to the distracter on any measure of sustained attention task performance when 40% of the trials involved light-location discrimination (mean percent correct ± SEMs for rats not exposed to the distracter; hits averaged across all signal durations: 55.0 ± 6.4; correct rejections: 85.0 ± 3.0; rats exposed to the distracter: hits averaged across all signal durations: 59.9 ± 4.0; correct rejections: 85.2 ± 2.5).
Figure 1.
The figure depicts relative correct choices on the light-location discrimination trials averaged for sets of three sessions (means ± SEMs). Light-location discrimination trials were 10% (top panel), 40% (middle panel) and then 70% (bottom panel) of the trials in each session. Rats exposed to the distracter exhibited higher relative correct choices when 40% of the trials were light-location discrimination trials (asterisk designates p < 0.05) and a similar trend when 70% of the trials were light-location discrimination trials.
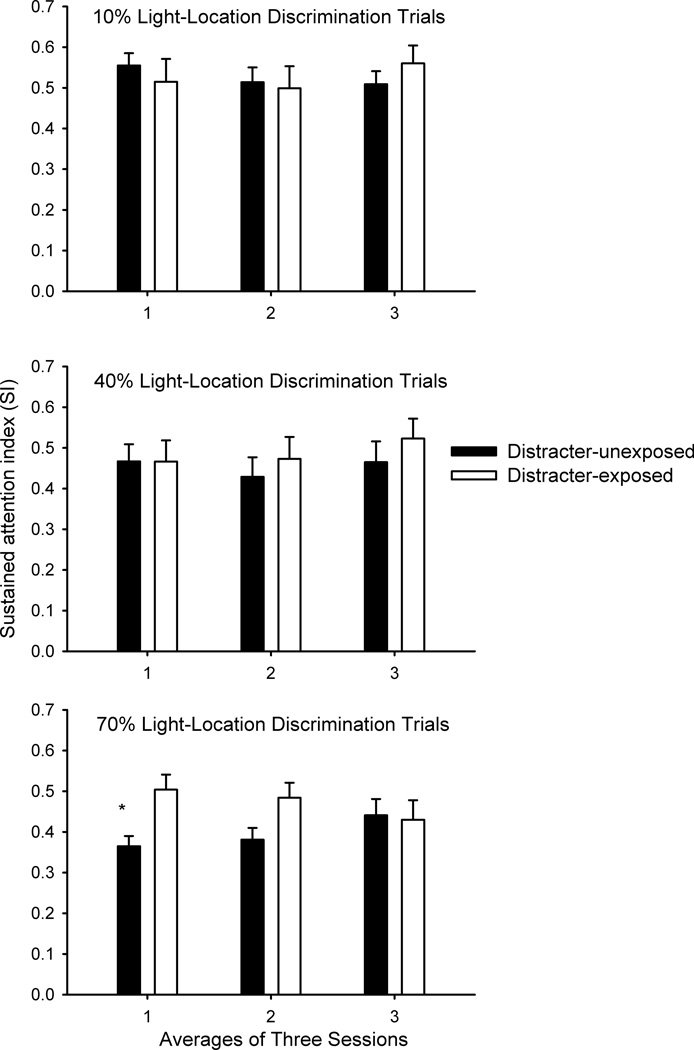
When the percentage of light-location discrimination trials was increased to 70%, there continued to be a trend for higher accuracy levels on light-location discrimination trials for the rats exposed to the distracter compared to those not exposed to the distracter (p = 0.069). Both rats that were (t(6) = 11.476, p < 0.001) and were not (t(7) = 5.079, p = 0.001) exposed to the distracter performed above chance on the light-location discrimination trials. For SI, previous distracter exposure did interact with session, F(2,26) = 7.516, p = 0.006 (Figure 2). The rats that were exposed to the distracter exhibited higher SI values than rats that were not exposed to the distracter during the first set of three sessions under these conditions, t(13) = 3.18, p = 0.007. During the second set of sessions, the group difference was no longer significant when the Bonferroni correction was applied, t(13) = 2.22, p = 0.045. The effects of previous distracter exposure could not be attributed entirely to changes in hits or correct rejections (mean percent correct ± SEMs for rats not exposed to the distracter; hits averaged across all signal durations: 51.8 ± 3.2; correct rejections: 84.0 ± 3.3; rats exposed to the distracter: hits averaged across all signal durations: 57.7 ± 3.3; correct rejections: 86.0 ± 2.6), as there were no main effects of condition nor was the condition X session interaction significant for either of these measures or for omissions (all p > 0.10).
Figure 2.
The figure depicts performance as measured by the sustained attention index (SI) averaged across signal duration and across sets of three sessions on the remaining sustained attention trials when the light-location discrimination was 10% (top panel), 40% (middle panel) and then 70% (bottom panel) of the trials in each session (means ± SEMs). Rats that were exposed to the distracter demonstrated a higher level of accuracy during the initial set of three sessions when 70% of the trials were light-location discrimination (asterisk designates p < 0.05) and a similar trend during the subsequent set of three sessions.
4. Discussion
Distracter exposure transiently decreased attentional performance. Subsequently, rats that were exposed to the distracter exhibited higher performance levels and reached above-chance performance more quickly in a new light-location discrimination task compared to rats that were not exposed to the distracter. Schmajuk & Bushnell (2009) argue that when light intensity is in the “signal range” a conditioned response to the signal lever is elicited whereas if the light intensity is in the “blank range” a conditioned response occurs to the blank (nonsignal) lever location in this attention task. The strength of the associations between signals and nonsignals and the appropriate conditioned response may make it more difficult for new associations to form between other stimuli and the levers. However, distracter presentation likely decreased the strength of the associations between signal intensity and the conditioned responses, providing a better opportunity to associate novel stimuli (illumination of the left and right lights) with responses to the respective levers. We cannot rule out the possibility that the continued presentation of the flashing houselight also developed an occasion-setting property and further facilitated acquisition of the light-location task. Indeed, other findings have suggested that noise can serve as an occasion-setter during an operant discrimination task (Maes & de Groot, 2003). Moreover, we cannot exclude the possibility that additional testing sessions with 10% light-location discrimination trials would have altered the observed results.
The addition of the light-location discrimination trials within a session may have increased spatial attentional demands, as there were not cues about whether the discrimination would be a light-location trial or a sustained attention task trial. Thus, the rats needed to attend to the three panel lights to observe which light would be illuminated (or not illuminated on nonsignal trials). Moreover, the increased uncertainty about which stimulus will be presented may contribute to attentional demands (Drummond & Shomstein, 2010). However, increases in attentional demands cannot entirely account for the differences between rats that were or were not exposed to the distracter because the procedures for presenting the new light-location discrimination trials was the same for rats that were or were not exposed to the distracter. The extent to which increases in attentional demands are necessary for demonstrating effects of prior distracter exposure will require further investigation.
Performance in the remaining sustained attention task trials by rats that were exposed to the distracter was higher for one set of three sessions when 70% of the trials were light-location trials compared with rats that were not exposed to the distracter. One interesting speculation is that as the rats that were exposed to the distracter learned (at least above chance performance) the light-location discrimination task, they were able to more quickly re-allocate resources to perform better on the remaining sustained attention task trials. The extent to which decreasing the percentage of sustained attention task trials from 60% to the 30% may have had on the higher performance by rats that were exposed to the distracter cannot be determined with the present experimental design. A number of factors, such as the modality, severity and duration of the distracter as well as the nature of the information to be learned, are likely to be critical factors to study in order to further understand the effects of distraction on subsequent learning.
Highlights.
Rats were trained in a sustained attention task and then exposed (or not) to a distracter
Attentional performance was transiently disrupted by the distracter
Rats were tested with light-location discrimination trials interspersed with attention task trials
Rats that were exposed to the distracter performed more accurately in the light-location discrimination and attention task during some blocks of trials
Acknowledgements
The authors wish to thank Lauren Clifford, Kathryn Easterling, Samantha Jayasinghe, Brittany Minor, Mason Montgomery, Paige Roseman and Elsia Yoo for technical assistance. This work was supported by AG030646.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banbury SP, Macken WJ, Tremblay S, Jones DM. Auditory distraction and short-term memory: phenomena and practical implications. Hum. Factors. 2001;43:12–29. doi: 10.1518/001872001775992462. [DOI] [PubMed] [Google Scholar]
- Boschen KE, Fadel JR, Burk JA. Systemic and intrabasalis administration of the orexin-1 receptor antagonist, SB-334867, disrupts attentional performance in rats. Psychopharmacology (Berl.) 2009;206:231–238. doi: 10.1007/s00213-009-1596-2. [DOI] [PubMed] [Google Scholar]
- Burk JA. Introduction of a retention interval in a sustained attention task in rats: Effects of a visual distracter and increasing the inter-trial interval. Behav. Processes. 2004;67:521–531. doi: 10.1016/j.beproc.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Benignus VA, Case MW. Signal detection behavior in humans and rats: a comparison with matched tasks. Behav. Processes. 2003;64:121–129. doi: 10.1016/s0376-6357(03)00146-3. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Kelly KL, Crofton KM. Effects of toluene inhalation on detection of auditory signals in rats. Neurotoxicol. Teratol. 1994;16:149–60. doi: 10.1016/0892-0362(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Demeter E, Sarter M, Lustig C. Rats and humans paying attention: cross-species task development for translational research. Neuropsychology. 2008;22:787–799. doi: 10.1037/a0013712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond L, Shomstein S. Object-based attention: shifting or uncertainty? Atten. Percept. Psychophys. 2010;72:1743–1755. doi: 10.3758/APP.72.7.1743. [DOI] [PubMed] [Google Scholar]
- Koelega HS, Brinkman JA, Zwep B, Verbaten MN. Dynamic vs static stimuli and their effect on visual vigilance performance. Percept. Mot. Skills. 1990;70:823–831. doi: 10.2466/pms.1990.70.3.823. [DOI] [PubMed] [Google Scholar]
- Maes JHR, de Groot G. Effects of noise on the performance of rats in an operant discrimination task. Behav. Processes. 2003;61:57–68. doi: 10.1016/s0376-6357(02)00163-8. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: Selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav. Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: Task validation and effects of, age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl.) 1995;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Warm JS, Dember WN. Vigilance: Taxonomy and utility. In: Mark LS, Warm JS, Huston RL, editors. Ergonomics and human factors. New York, NY: Springer-Verlag; 1987. [Google Scholar]
- Robinson AM, Mangini DF, Burk JA. Task demands dissociate the effects of muscarinic M1 receptor blockade and protein kinase C inhibition on attentional performance in rats. J. Psychopharmacol. 2012;26:1153–1160. doi: 10.1177/0269881111415732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res. Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: Interactions between signal-driven and cognitive modulation of signal detection. Brain Res. Brain Res. Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Schmajuk NA, Bushnell PJ. A computational model reveals classical conditioning mechanisms underlying visual signal detection in rats. Behav. Processes. 2009;82:340–351. doi: 10.1016/j.beproc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- St Peters M, Cherian AK, Bradshaw M, Sarter M. Sustained attention in mice: expanding the translational utility of the SAT by incorporating the Michigan Controlled Access Response Port (MICARP) Behav. Brain Res. 2011;225:574–583. doi: 10.1016/j.bbr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Smith MR, Witt GJ. Identification of real-time diagnostic measures of visual distraction with an automatic eye-tracking system. Hum. Factors. 2006;48:805–821. doi: 10.1518/001872006779166307. [DOI] [PubMed] [Google Scholar]